Abstract
Background: Arthritis is a cartilage degenerative disease that is mainly induced by the degradation of the cartilage extracellular matrix (ECM), which is found to be regulated by the expression level of a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMT-5), an enzyme degrading Aggrecans in the ECM. Feprazone is a classic nonsteroidal anti-inflammatory drug with promising efficacy in arthritis. The present study aims to investigate the protective effect of Feprazone on the degraded Aggrecan in the human chondrocytes induced with tumor necrosis factor-α (TNF-α) and to clarify the underlying mechanism. Methods: To investigate the effect of Feprazone, the CHON-001 chondrocytes were stimulated with TNF-α (10 ng/mL) in the presence or absence of Feprazone (3, 6 μM) for 24 h. Mitochondrial membrane potential was evaluated using the Rhodamine 123 assay. The gene expressions of interleukin-1β (IL-1β), interleukin-8 (IL-8), monocyte chemotactic protein 1 (MCP-1), and ADAMTS-5 in the treated chondrocytes were detected using real-time quantitative polymerase chain reaction (qRT-PCR), and the protein levels of these targets were determined using enzyme-linked immunosorbent assay (ELISA). SOX-4 was knocked down by transfecting the siRNA into the chondrocytes. Western blot analysis was utilized to evaluate the expression levels of SOX-4, Aggrecan, and protein kinase C (PKCα). Results: First, the reduced mitochondrial membrane potential (ΔΨm) and secretion of proinflammatory factors (IL-1β, IL-8, and MCP-1) induced by TNF-α were significantly reversed by treatment with Feprazone. Second, the expression of Aggrecan was significantly decreased by stimulation with TNF-α via upregulation of ADAMTS-5 but was dramatically reversed by the introduction of Feprazone. Third, we found that TNF-α elevated the expression of ADAMTS-5 by upregulating SOX-4, which was observed to be related to the activation of PKCα. Lastly, the elevated expression of SOX-4 induced by TNF-α was significantly reversed by Feprazone. Conclusions: Feprazone might ameliorate TNF-α-induced loss of Aggrecan via the inhibition of the SOX-4/ADAMTS-5 signaling pathway.
Introduction
Arthritis is a common disease that follows degenerative changes of the articular cartilage and is clinically characterized by articular dysfunction.1 It is statistically reported that in America, the morbidity of arthritis in the population with age over 75 is 80%, which brings a great burden for families and society.2 Currently, the pathological mechanism underlying arthritis remains unknown. Increasing evidence indicates that excessive production of proinflammatory cytokines and degradation of extracellular matrix (ECM) in chondrocytes are involved in the development and processing of arthritis.3,4 The imbalance between the synthesis and metabolism of inflammatory factors is the basic inducer responsible for the articular cartilage damage.5 It is reported that tumor necrosis factor-α (TNF-α) can suppress the synthesis of cartilage matrix and induce the degradation of cartilage matrix by upregulating the expression of matrix metalloproteinases (MMPs). The result of immunohistochemistry indicates that cartilage damage in arthritis is closely related to the production of TNF-α.6 ECM mainly consists of collagens and proteoglycans. Proteoglycans, mainly the cartilage Aggrecans, keep sufficient water in the ECM to enable cartilage tissues to be resistant to the compression from articular cartilage and distribute the load,7,8 and they can be degraded by Aggrecanase. In early 1999, science first reported the cloned and purified Aggrecanase-1,9 named disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMT-4). Later, Aggrecanase-2 was discovered by the same team and termed ADAMTS-5.10 In the cartilage of arthritic patients, the expressions of ADAMTS-4 and ADAMTS-5 are significantly elevated.11 The SOX family is a group of newly discovered transcriptional factors that play an important role in regulating the expression of ECM-related genes, such as collagen type I α1 (Col2α1).12,13 It has been recently reported that SOX-4 induces osteoarthritic cartilage deterioration by upregulating the expression level of ADAMTS-5.14 Therefore, SOX-4 might become a promising target for clinical treatment of arthritis via maintaining the structure of ECM.
Feprazone, a nonsteroidal anti-inflammatory drug (NSAID), is derived from phenylbutazone with prenylated modification of an n-butyl group on phenyl rings. The molecular structure of Feprazone is shown in Figure 1A. Feprazone is reported to have a 10-fold higher affinity to bind cyclooxygenase-2 (COX-2) than cyclooxygenase-1 (COX-1). It also reduces the production of prostaglandin E2 (PGE2) in endothelial cells.15 Feprazone has been used for the treatment of multiple kinds of inflammatory diseases.16 In clinical trials, Feprazone has been utilized to treat osteoarthritis (OA)17 and rheumatoid arthritis18 with positive data. However, the antiarthritis mechanism remains unknown. To provide a sufficient theoretical foundation for the clinical treatment of arthritis using Feprazone, the present study investigates the effects of Feprazone on the regulation of inflammatory factor production and the degradation of ECM to claim the possible mechanism.
Figure 1.
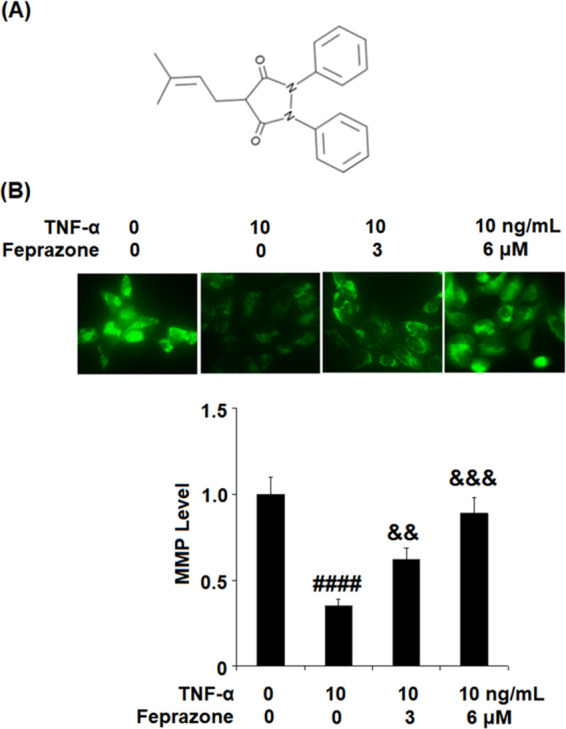
Feprazone ameliorated TNF-α-induced reduction of mitochondrial membrane potential (ΔΨm) in human CHON-001 chondrocytes. Cells were stimulated with TNF-α (10 ng/mL) in the presence or absence of Feprazone (3, 6 μM) for 24 h. (A) Molecular structure of Feprazone. (B) Mitochondrial membrane potential (ΔΨm) was assayed by Rhodamine 123 (####, P < 0.0001 vs vehicle group; &&, &&&, P < 0.01, 0.001 vs TNF-α group, N = 6).
Results
Feprazone Ameliorated TNF-α-Induced Reduction of Mitochondrial Membrane Potential (ΔΨm) in Human CHON-001 Chondrocytes
To evaluate the effect of Feprazone on the mitochondrial membrane potential, the CHON-001 chondrocytes were stimulated with TNF-α (10 ng/mL) in the presence or absence of Feprazone (3, 6 μM) for 24 h, and Rhodamine 123 staining assay was performed. As shown in Figure 1B, the mitochondrial membrane potential in the chondrocytes was significantly suppressed by stimulation with TNF-α but greatly elevated by the introduction of Feprazone in a dose-dependent manner, indicating a possible inhibitory effect of Feprazone against apoptosis in chondrocytes induced with TNF-α.
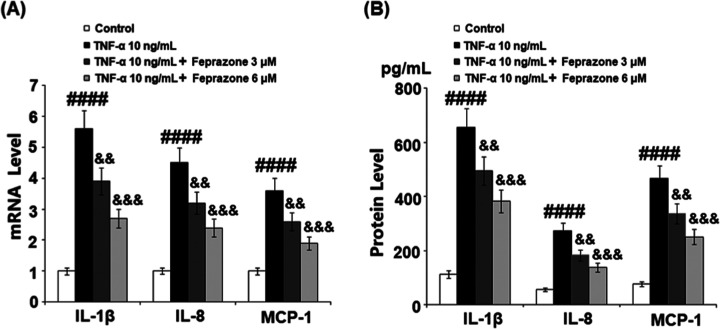
Feprazone Inhibited TNF-α-Induced Expressions and Secretions of Interleukin-1β (IL-1β), Interleukin-8 (IL-8), and Monocyte Chemotactic Protein 1 (MCP-1) in Human CHON-001 Chondrocytes
High concentrations of inflammatory cytokines have been reported in the synovial fluid of joints with cartilage defects and OA. Among them, IL-1β, IL-8, and MCP-1 are three of the major proinflammatory cytokines involved in joint inflammation.19 Therefore, we investigated the effects of Feprazone on TNF-α-induced expressions and secretions of IL-1β, IL-8, and MCP-1 in human CHON-001 chondrocytes. As shown in Figure 2A, the gene expressions of IL-1β, IL-8, and MCP-1 in human CHON-001 chondrocytes were significantly elevated by treatment with TNF-α, but dramatically suppressed by the introduction of Feprazone in a dose-dependent manner. We further checked the secretions of these inflammatory factors. As shown in Figure 2B, the concentrations of IL-1β in the control, TNF-α, TNF-α + 3 μM Feprazone, and TNF-α + 6 μM Feprazone groups were 112.3, 655.9, 495.4, and 382.1 pg/mL, respectively. Approximately 56.8, 272.7, 182.6, and 137.5 pg/mL IL-8 were detected in the chondrocytes incubated with blank medium, TNF-α, TNF-α in the presence of 3 μM Feprazone, and TNF-α in the presence of 6 μM Feprazone, respectively. Lastly, the concentrations of MCP-1 in the control, TNF-α, TNF-α + 3 μM Feprazone, and TNF-α + 6 μM Feprazone groups were 76.6, 466.0, 335.7, and 251.2 pg/mL, respectively. These data indicate that the expressions of inflammatory factors induced by stimulation with TNF-α were significantly inhibited by Feprazone.
Figure 2.
Feprazone inhibited TNF-α-induced expression and secretions of IL-1β, IL-8, and MCP-1 in human CHON-001 chondrocytes. Cells were stimulated with TNF-α (10 ng/mL) in the presence or absence of Feprazone (3, 6 μM) for 24 h. (A) mRNA of IL-1β, IL-8, and MCP-1. (B) Secretions of IL-1β, IL-8, and MCP-1 (####, P < 0.0001 vs vehicle group; &&, &&&, P < 0.01, 0.001 vs TNF-α group, N = 5).
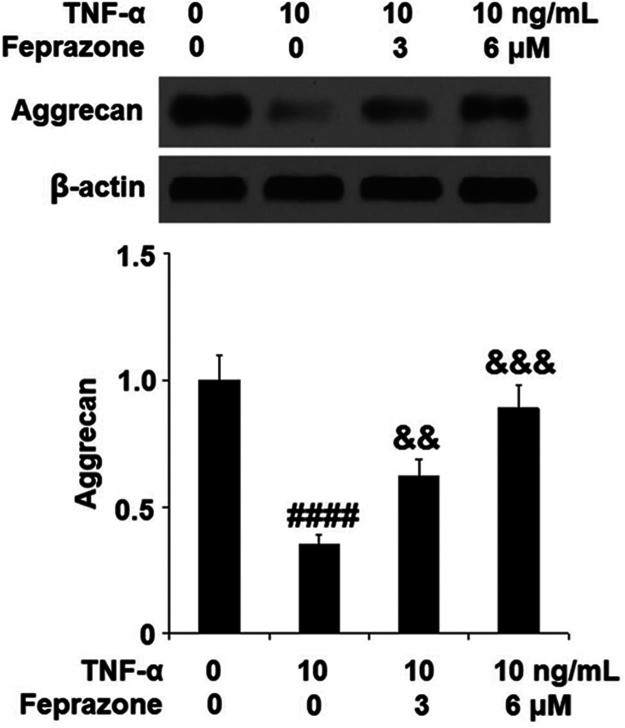
Feprazone Attenuated TNF-α-Induced Expression of Aggrecan in Human CHON-001 Chondrocytes
To further investigate the effect of Feprazone on the degradation of ECM, the amount of Aggrecan protein, an important component of chondrocyte ECM, was determined using Western blot analysis. As shown in Figure 3, Feprazone dose responsively ameliorated the reduction of Aggrecan caused by TNF-α, indicating a protective effect of Feprazone against ECM degradation induced by TNF-α.
Figure 3.
Feprazone attenuated TNF-α-induced reduction of Aggrecan in human CHON-001 chondrocytes. Cells were stimulated with TNF-α (10 ng/mL) in the presence or absence of Feprazone (3, 6 μM) for 24 h. Expression of Aggrecan was measured (####, P < 0.0001 vs vehicle group; &&, &&&, P < 0.01, 0.001 vs TNF-α group, N = 6).
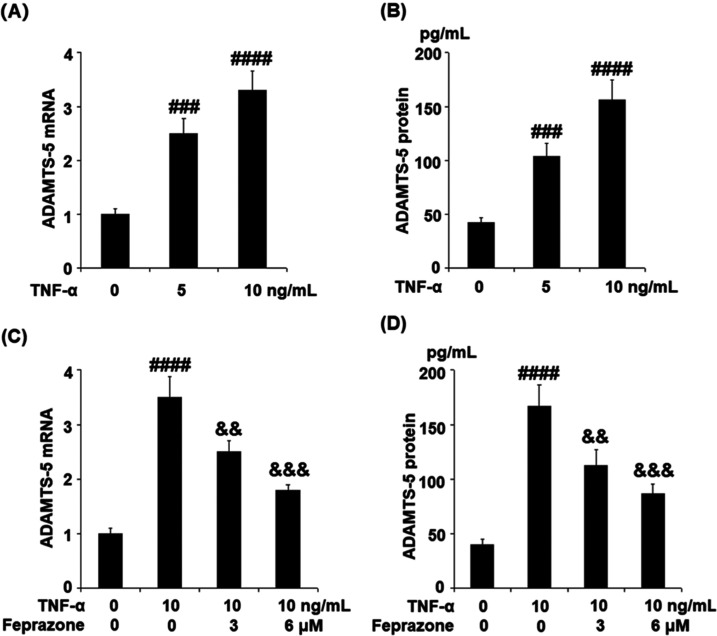
Feprazone Suppressed TNF-α-Induced Expression of ADAMTS-5 in Human CHON-001 Chondrocytes
We further explored the mechanism underlying the protective property of Feprazone against the decreased expression of Aggrecan induced by TNF-α. The dose proportionality of TNF-α against the expression level of ADAMTS-5 in the human chondrocytes was first evaluated. The cells were stimulated with TNF-α (5 and 10 ng/mL) for 24 h. As shown in Figure 4A,B, ADAMTS-5 was significantly upregulated by stimulation with TNF-α in a dose-dependent manner. Subsequently, the chondrocytes were stimulated with TNF-α (10 ng/mL) in the presence or absence of Feprazone (3, 6 μM) for 24 h. As shown in Figure 4C, the presence of Feprazone dose responsively alleviated the elevated expression of ADAMTS-5. The concentrations of ADAMTS-5 (Figure 4D) in the control, TNF-α, TNF-α + 3 μM Feprazone, and TNF-α + 6 μM Feprazone group were 40.3, 166.8, 112.6, and 86.5 pg/mL, respectively. These data indicate that Feprazone might ameliorate the decrease in the protein level of Aggrecan in the chondrocytes by suppressing the expression level of ADAMTS-5, which is a catabolic enzyme for Aggrecan.
Figure 4.
Feprazone suppressed TNF-α-induced expression of ADAMTS-5 in human CHON-001 chondrocytes. (A, B) Cells were stimulated with TNF-α (5 and 10 ng/mL) for 24 h. mRNA and protein levels of ADAMTS-5 as measured by real-time polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA). (C, D) Cells were stimulated with TNF-α (10 ng/mL) in the presence or absence of Feprazone (3, 6 μM) for 24 h. mRNA and protein levels of ADAMTS-5 as measured by real-time PCR and ELISA (###, ####, P < 0.001, 0.0001 vs vehicle group; &&, &&&, P < 0.01, 0.001 vs TNF-α group, N = 5).
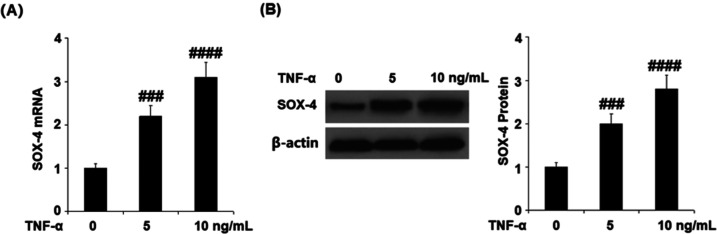
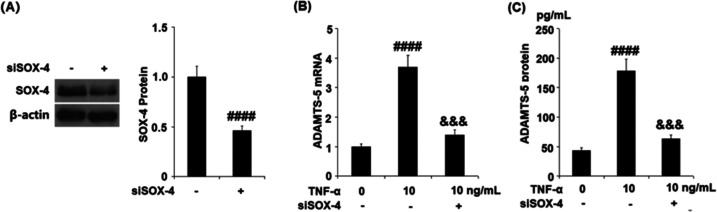
TNF-α Elevated the Expression of ADAMTS-5 by Upregulating SOX-4
To further explore the potential regulatory mechanism of TNF-α against the expression of ADAMTS-5, we detected the expression of SOX-4 following stimulating the cells with 5 and 10 ng/mL TNF-α for 24 h. As shown in Figure 5, the gene and protein expressions of SOX-4 were significantly promoted by stimulation with TNF-α in a dose-dependent manner. Subsequently, the chondrocytes were transfected with SOX-4 siRNA followed by stimulation with 10 ng/mL TNF-α. As shown in Figure 6A, the expression of SOX-4 was dramatically suppressed in the siRNA group, indicating that the SOX-4 knockdown was successfully established. The gene expression of ADAMTS-5 (Figure 6B) was significantly elevated by stimulation with TNF-α, but this was reversed by the transfection with SOX-4 siRNA. As shown in Figure 6C, the protein concentrations of ADAMTS-5 in the control, TNF-α, and TNF-α + SOX-4 siRNA group were 43.6, 178.1, and 63.3 pg/mL, respectively. These data indicate that TNF-α might elevate the expression of ADAMTS-5 by upregulating SOX-4.
Figure 5.
TNF-α increased expression of SOX-4 in human CHON-001 chondrocytes. Cells were stimulated with TNF-α (5 and 10 ng/mL) for 24 h. (A) mRNA of SOX-4. (B). Protein of SOX-4 (###, ####, P < 0.001, 0.0001 vs vehicle group, N = 5).
Figure 6.
Effects of TNF-α on the expression of ADAMTS-5 is mediated by SOX-4. Cells were transfected with SOX-4 RNA, followed by stimulation with TNF-α (10 ng/mL). (A) Western blot analysis revealed successful knockdown of SOX-4. (B) mRNA of ADAMTS-5. (C) Protein of ADAMTS-5 (####, P < 0.0001 vs vehicle group; &&&, P < 0.001 vs TNF-α group, N = 5).
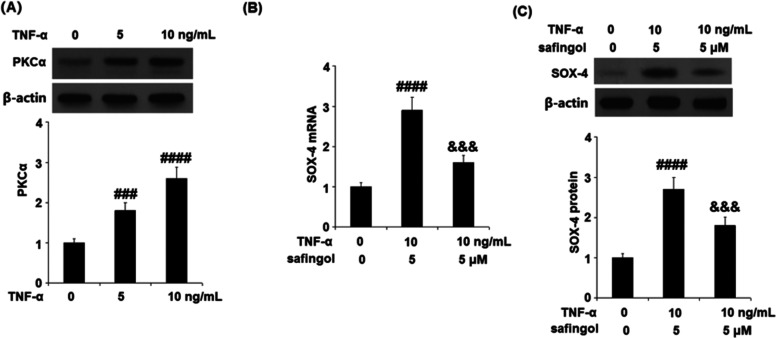
Effects of TNF-α on SOX-4 Expression Were Mediated by Protein Kinase C (PKCα)
To answer how TNF-α regulated the expression of SOX-4, PKCα was introduced into the system. As shown in Figure 7A, the expression level of PKCα was significantly elevated by stimulation with TNF-α in a dose-dependent manner. Subsequently, the cells were stimulated with TNF-α (10 ng/mL) or the PKCα inhibitor safingol (5 μM) for 24 h. As shown in Figure 7B, we found that the elevated expression of SOX-4 induced by TNF-α was dramatically suppressed by the co-administration of the PKCα inhibitor safingol, indicating that the effects of TNF-α on SOX-4 expression might be mediated by PKCα.
Figure 7.
Effects of TNF-α on SOX-4 expression are mediated by PKCα. (A) Cells were stimulated with TNF-α (5 and 10 ng/mL) for 24 h. Expression of PKCα was measured. (B, C) Cells were stimulated with TNF-α (10 ng/mL) or the PKCα inhibitor safingol (5 μM) for 24 h. mRNA and protein levels of SOX-4 were measured (####, P < 0.0001 vs vehicle group; &&&, P < 0.001 vs TNF-α group, N = 5).
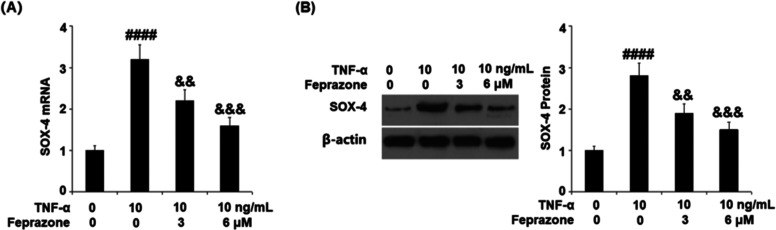
Feprazone Reduced the Expression of SOX-4
We further investigated the effects of Feprazone against TNF-α-induced expression of SOX-4. As shown in Figure 8, the expression of SOX-4, which was significantly elevated by stimulation with TNF-α, was dramatically suppressed by the treatment with Feprazone in a dose-dependent manner, indicating a regulatory effect of Feprazone on the SOX-4/ADAMTS-5 signaling pathway.
Figure 8.
Feprazone reduced the expression of SOX-4. Cells were stimulated with TNF-α (10 ng/mL) in the presence or absence of Feprazone (3, 6 μM) for 24 h. (A) mRNA of SOX-4. (B) Protein of SOX-4 (####, P < 0.0001 vs vehicle group; &&, &&&, P < 0.01, 0.001 vs TNF-α group, N = 5).
Discussion
Currently, the pathological mechanism underlying arthritis remains unknown; it is believed to be induced by the combined function of systemic and local elements. In the several stages of arthritis, the imbalance between the synthesis and metabolism of articular cartilage are observed to be the common pathological characteristics that contribute to the destruction of cartilage structure and function. Articular cartilage mainly consists of chondrocytes and extracellular matrix (ECM). ECM is mainly composed of type II collagen and Aggrecan, which occupy approximately 90% of the total ECM components. Aggrecan contains multiple hydrophilic branched chains and exerts the function of lubrication and pressure resistance for the chondrocytes by maintaining water molecules and metal ions.20,21 As the collagens are protected by the Aggrecans located on the surface of the collagen skeleton, the loss of Aggrecan in the cartilage ECM is regarded as a key step for the destruction of articular cartilage and is commonly observed in the early stages of arthritis.22 Previous reports indicate that MMPs and Aggrecanases are the two main enzymes that induce the degradation of Aggrecan.23 Aggrecanase plays an important role in the early stage of the degradation of human articular cartilage Aggrecans.24 It is reported that Aggrecanase degrades its substrate Aggrecan in the synovial joint fluid of arthritis patients.25 ADAMTS-5 is the main Aggrecanase discovered recently and is found to be expressed on multiple types of tissues, including cartilage, the uterus, bladder, brain, and heart. It is reported that the expression of ADAMTS-5 is regulated by various metabolic factors, such as IL-1α,26 IL-1β,27 TNF-α,28 and other cytokines. However, the regulatory mechanism remains unclear. We found that the mitochondrial membrane potential in human chondrocytes was suppressed and the production of inflammatory factors was significantly elevated by the stimulation with TNF-α, indicating induction of mitochondrial dysfunction and inflammation in the chondrocytes. By treatment with Feprazone, the mitochondrial dysfunction and inflammation were significantly alleviated. These data indicate that Feprazone showed a promising protective property against the damage to chondrocytes induced by TNF-α. In our future work, the in vivo antiarthritis property of Feprazone will be further investigated using the animal arthritis model. Subsequently, we found that the expression of Aggrecan was significantly suppressed by stimulation with TNF-α, possibly by upregulating ADAMTS-5. Feprazone suppressed the degradation of Aggrecan by inhibiting the expression of ADAMTS-5, indicating a promising protective property against injured Aggrecan in the cartilage ECM induced by TNF-α.
In the present study, we further investigated the regulatory effect of TNF-α on the expression of ADAMTS-5 and thereafter that of Aggrecan. The SOX family is a group of transcriptional factors with powerful cellular functions29 reported to be involved in the formation of cartilage.30 Rodriguez-Leon reported that in the development of cartilage, mutation of SOX-9 occurs and impacts the production of type II collagen, finally contributing to the abnormal development of chondrocytes.31,32 SOX-5 and SOX-6 are also reported to be implicated in the formation of cartilage by activating the Col2α1 gene.33 Recently, SOX-4 has been found to regulate the expressions of both ADAMTS-4 and ADAMTS-5 in the chondrocytes.14 We explored whether SOX-4 is involved in the regulatory effect of TNF-α on the expression of ADAMTS-5. First, we found that SOX-4 was significantly upregulated by stimulation with TNF-α. After knocking down the expression of SOX-4 in the chondrocytes, the upregulation on the expression of ADAMTS-5 by TNF-α was dramatically abolished, indicating that SOX-4 might be involved in the regulatory effect of TNF-α on ADAMTS-5 expression. PKCα is a key protein kinase that regulates the expression of various transcriptional factors.34,35 In the joint tissue, PKC isoenzymes are identified as an essential regulator of chondrogenesis. Previous studies show that the expression of PKCα is increased in human OA tissue, and its expression is required for the TNF-α-activated inflammatory pathway and reactive oxygen species (ROS) production in chondrocytes.35,36 Additionally, PKCα is necessary for the activation of SOX-9 protein in chondrogenesis.37 We demonstrated that TNF-α induces the expressions of both PKCα and SOX-4, and the deficiency of SOX-4 completely abolished ADAMTS-5 expression, while the blockage of PKCα activity reduced the TNF-α-increased SOX-4 expression, suggesting PKCα activation is required for SOX-4 expression. The presence of Feprazone also remarkably reduced SOX-4 expression, suggesting its amelioration on Aggrecanase (ADAMTS-5) and that inflammatory factors could be mediated via its action on SOX-4 and PKCα. ROS-induced PKCα activation is linked to mitochondrial dysfunction in human cells.38 However, the relative contribution of PKCα, inflammation, and mitochondrial metabolism to chondrocyte dysfunction is unknown. A further study will help to provide a complete picture of the underlying mechanism. Lastly, we found that SOX-4 could be downregulated by treatment with Feprazone, indicating that SOX-4 might be involved in the protective effect of Feprazone on the damaged Aggrecan induced by TNF-α. In our future work, the mechanism will be further confirmed by introducing the SOX-4-overexpressed chondrocytes. Feprazone has been used for pain control for a long time, but its toxicity has been reported.39 The current study provides molecular evidence of its therapeutic potential, but its effect in vivo remains to be investigated.
Taken together, our data indicate that Feprazone might ameliorate TNF-α-induced loss of Aggrecan via inhibition of the SOX-4/ADAMTS-5 signaling pathway.
Materials and Methods
Cell Culture and Treatment
The human chondrocyte cell line, CHON-001 cells, was purchased from ATCC (Rockville, Maryland) and cultured in the DMEM complete medium containing 10% fetal bovine serum and penicillin/streptomycin at 37 °C and 5% CO2. Cells were stimulated with TNF-α (10 ng/mL)40 in the presence or absence of Feprazone (3, 6 μM) for 24 h.
Rhodamine 123 Staining
The mitochondrial membrane potential (ΔΨm) was determined using the Rhodamine 123 staining kit (Beyotime, Shanghai, China). Briefly, the cells were seeded on the 24-well plates, followed by being washed three times using phosphate-buffered saline (PBS) buffer. Subsequently, the cells were stained with 2 μM Rhodamine 123 for 15 min and further washed several times with the PBS buffer. Finally, the images were taken using a fluorescence microscope (Olympus, Tokyo, Japan). The dissipated ΔΨm was represented by the reduction of green Rhodamine 123 fluorescence.
Real-Time PCR Analysis
The total RNA was extracted from the treated chondrocytes using the TRI Reagent RNA Isolation Reagent (Sigma, Massachusetts) and transformed into cDNA utilizing a First-Strand cDNA Synthesis kit (Pharmacia LKB, Uppsala, Sweden). The real-time PCR was conducted with the TaqMan system (Thermo Fisher Scientific). The PCR amplification and product detection were performed using the ABI PRISM 7300 Sequence Detection System (Thermo Fisher Scientific). The relative gene expression was normalized to GAPDH and the 2–ΔΔCt method was used to calculate the relative expression.
Western Blot Assay
The total protein from the chondrocytes was extracted utilizing the cellular lysis buffer (Thermo Fisher Scientific) and quantified using the bicinchoninic acid (BCA) protein quantitative kit (Beyotime, Shanghai, China). Subsequently, 40 μg of protein were loaded and separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto the poly(vinylidene difluoride) (PVDF) membrane (Beyotime, Shanghai, China). The membrane was then incubated with 5% bovine serum albumin (BSA) to remove the nonspecific binding proteins, followed by incubation with primary antibodies against Aggrecan (CST, 1:1000, Massachusetts), SOX-4 (CST, 1:1000, Massachusetts), and PKCα (CST, 1:1000, Massachusetts), with β-actin as a negative control (CST, 1:1000, Massachusetts). Finally, the membrane was mixed with enhanced chemiluminescent (ECL) solution and exposed to the Tanon 5200 (Tanon, Shanghai, China), followed by washing with the Tris-buffered saline Tween (TBST). Image J software (National Institutes of Health) was used to perform the densitometric analysis.
ELISA Assay
The released levels of IL-1β, IL-8, MCP-1, and ADAMTS-5 in the treated chondrocytes were evaluated using ELISA assay. Briefly, the supernatant or cell lysis was mixed with 5% BSA to remove the nonspecific binding proteins. Following immobilizing the antibodies against IL-1β, IL-8, MCP-1, or ADAMTS-5 onto the 96-well microtiter plates, the samples were added to be incubated for approximately 30 min. Subsequently, the horseradish peroxidase (HRP)-conjugated antimouse immunoglobulin was added into the system, followed by washing three times with the PBS buffer. Finally, the samples were incubated with a TMB substrate solution for half an hour to yield the blue product of the oxidation reaction. A spectrophotometer (Thermo, Massachusetts) was used to measure the absorbance at 450 nm.
Statistical Analysis
Data are shown as mean ± standard deviation (SD). The statistical analysis was conducted using GraphPad Prism software version 7.0 (San Diego, CA). Analysis of variance (ANOVA) was used to analyze the experimental data. P < 0.05 was regarded as statistically significant.
Acknowledgments
This work was supported by the Kunshan Health Bureau (KSHB-003050021).
Author Contributions
† X.X. and L.L. contributed equally to this work.
The authors declare no competing financial interest.
References
- Alcaraz M. J.; Megias J.; Garcia-Arnandis I.; Clerigues V.; Guillen M. I. New molecular targets for the treatment of osteoarthritis. Biochem. Pharmacol. 2010, 80, 13–21. 10.1016/j.bcp.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Neogi T.; Zhang Y. Epidemiology of osteoarthritis. Rheum. Dis. Clin. North Am. 2013, 39, 1–19. 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haleagrahara N.; Hodgson K.; Miranda-Hernandez S.; Hughes S.; Kulur A. B.; Ketheesan N. Flavonoid quercetin-methotrexate combination inhibits inflammatory mediators and matrix metalloproteinase expression, providing protection to joints in collagen-induced arthritis. Inflammopharmacology 2018, 26, 1219–1232. 10.1007/s10787-018-0464-2. [DOI] [PubMed] [Google Scholar]
- Lemos G. A.; Rissi R.; de Souza Pires I. L.; de Oliveira L. P.; de Aro A. A.; Pimentel E. R.; Palomari E. T. Low-level laser therapy stimulates tissue repair and reduces the extracellular matrix degradation in rats with induced arthritis in the temporomandibular joint. Lasers. Med. Sci. 2016, 31, 1051–1059. 10.1007/s10103-016-1946-3. [DOI] [PubMed] [Google Scholar]
- Hedbom E.; Hauselmann H. J. Molecular aspects of pathogenesis in osteoarthritis: the role of inflammation. Cell. Mol. Life Sci. 2002, 59, 45–53. 10.1007/s00018-002-8404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring M. B. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000, 43, 1916–1926. . [DOI] [PubMed] [Google Scholar]
- Vázquez-Portalatín N.; Kilmer C. E.; Panitch A.; Liu J. C. Characterization of Collagen Type I and II Blended Hydrogels for Articular Cartilage Tissue Engineering. Biomacromolecules 2016, 17, 3145–3152. 10.1021/acs.biomac.6b00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R.; Nelson F.; Dahlberg L.; Tchetina E.; Kobayashi M.; Yasuda T.; Laverty S.; Squires G.; Kojima T.; Wu W.; Billinghurst R. C. Proteolysis of the collagen fibril in osteoarthritis. Biochem. Soc. Symp. 2003, 70, 115–123. 10.1042/bss0700115. [DOI] [PubMed] [Google Scholar]
- Tortorella M. D.; Burn T. C.; Pratta M. A.; Abbaszade I.; Hollis J. M.; Liu R.; Rosenfeld S. A.; Copeland R. A.; Decicco C. P.; Wynn R.; Rockwell A.; Yang F.; Duke J. L.; Solomon K.; George H.; Bruckner R.; Nagase H.; Itoh Y.; Ellis D. M.; Ross H.; Wiswall B. H.; Murphy K.; Hillman M. C. Jr.; Hollis G. F.; Newton R. C.; Magolda R. L.; Trzaskos J. M.; Arner E. C. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science 1999, 284, 1664–1666. 10.1126/science.284.5420.1664. [DOI] [PubMed] [Google Scholar]
- Abbaszade I.; Liu R. Q.; Yang F.; Rosenfeld S. A.; Ross O. H.; Link J. R.; Ellis D. M.; Tortorella M. D.; Pratta M. A.; Hollis J. M.; Wynn R.; Duke J. L.; George H. J.; Hillman M. C. Jr.; Murphy K.; Wiswall B. H.; Copeland R. A.; Decicco C. P.; Bruckner R.; Nagase H.; Itoh Y.; Newton R. C.; Magolda R. L.; Trzaskos J. M.; Burn T. C.; et al. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J. Biol. Chem. 1999, 274, 23443–23450. 10.1074/jbc.274.33.23443. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P.; Boileau C.; Boily M.; Brunet J.; Mineau F.; Geng C.; Reboul P.; Laufer S.; Lajeunesse D.; Martel-Pelletier J. The protective effect of licofelone on experimental osteoarthritis is correlated with the downregulation of gene expression and protein synthesis of several major cartilage catabolic factors: MMP-13, cathepsin K and aggrecanases. Arthritis Res. Ther. 2005, 7, R1091 10.1186/ar1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami D.; Akiyama H.; Suzuki A.; Nakamura T.; Nakano T.; Yoshikawa H.; Tsumaki N. Sox9 sustains chondrocyte survival and hypertrophy in part through Pik3ca-Akt pathways. Development 2011, 138, 1507–1519. 10.1242/dev.057802. [DOI] [PubMed] [Google Scholar]
- Akiyama H.; Chaboissier M. C.; Martin J. F.; Schedl A.; de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata Y.; Nakamura E.; Hata K.; Wakabayashi M.; Murakami T.; Wakamori K.; Yoshikawa H.; Matsuda A.; Fukui N.; Nishimura R. Sox4 is involved in osteoarthritic cartilage deterioration through induction of ADAMTS4 and ADAMTS5. FASEB J. 2019, 33, 619–630. 10.1096/fj.201800259R. [DOI] [PubMed] [Google Scholar]
- Hou Q.; Hu Y.; Guo Y. Effects of feprazone on cyclooxygenase in vitro. Yaoxue Xuebao 2000, 35, 481–483. [Google Scholar]
- Fletcher M. R.; Loebl W.; Scott J. T. Feprazone, a new anti-inflammatory agent. Studies of potency and gastrointestinal tolerance. Ann. Rheum. Dis. 1975, 34, 190–194. 10.1136/ard.34.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent M. R.; Berry D.; Grahame R. Feprazone in osteoarthritis: comparison of twice and 3-times daily dosage regimens. Pharmatherapeutica 1983, 3, 93–397. [PubMed] [Google Scholar]
- Bianchi G.; Cerri B.; Grasso S.; Carazzi R.; Dotti F.; Ongari R.; Cerrato O.; Viara M. Safety and efficacy of feprazone for long-term treatment of rheumatoid arthritis and osteoarthritis. Clin. Ther. 1981, 3, 349–355. [PubMed] [Google Scholar]
- Tsuchida A. I.; Beekhuizen M.; C ′t Hart M.; Radstake T. R.; Dhert W. J.; Saris D. B.; van Osch G. J.; Creemers L. B. Cytokine profiles in the joint depend on pathology, but are different between synovial fluid, cartilage tissue and cultured chondrocytes. Arthritis Res. Ther. 2014, 16, 441 10.1186/s13075-014-0441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo W.; Li B.; Zhang D. Activation of G-protein-coupled bile acid receptor Gpbar1 (TGR5) inhibits degradation of type II collagen and aggrecan in human chondrocytes. Eur. J. Pharmacol. 2019, 856, 172387 10.1016/j.ejphar.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Luo Y.; Sinkeviciute D.; He Y.; Karsdal M.; Henrotin Y.; Mobasheri A.; Onnerfjord P.; Bay-Jensen A. The minor collagens in articular cartilage. Protein Cell. 2017, 8, 560–572. 10.1007/s13238-017-0377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin H. J.; Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J. Bone Jt. Surg. 1970, 52, 424–434. 10.2106/00004623-197052030-00002. [DOI] [PubMed] [Google Scholar]
- Chen J.; Luo M.; Wang W.; Zhang Z.; He Y.; Duance V. C.; Hughes C. E.; Caterson B.; Cao J. Altered proteolytic activity and expression of MMPs and aggrecanases and their inhibitors in Kashin-Beck disease. J. Orthop. Res. 2015, 33, 47–55. 10.1002/jor.22708. [DOI] [PubMed] [Google Scholar]
- Sandy J. D. A contentious issue finds some clarity: on the independent and complementary roles of aggrecanase activity and MMP activity in human joint aggrecanolysis. Osteoarthritis Cartilage 2006, 14, 95–100. 10.1016/j.joca.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Hills R.; Mazzarella R.; Fok K.; Liu M.; Nemirovskiy O.; Leone J.; Zack M. D.; Arner E. C.; Viswanathan M.; Abujoub A.; Muruganandam A.; Sexton D. J.; Bassill G. J.; Sato A. K.; Malfait A. M.; Tortorella M. D. Identification of an ADAMTS-4 cleavage motif using phage display leads to the development of fluorogenic peptide substrates and reveals matrilin-3 as a novel substrate. J. Biol. Chem. 2007, 282, 11101–11109. 10.1074/jbc.M611588200. [DOI] [PubMed] [Google Scholar]
- Bau B.; Gebhard P. M.; Haag J.; Knorr T.; Bartnik E.; Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002, 46, 2648–2657. 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- Pratta M. A.; Scherle P. A.; Yang G.; Liu R. Q.; Newton R. C. Induction of aggrecanase 1 (ADAM-TS4) by interleukin-1 occurs through activation of constitutively produced protein. Arthritis Rheum. 2003, 48, 119–133. 10.1002/art.10726. [DOI] [PubMed] [Google Scholar]
- Yamanishi Y.; Boyle D. L.; Clark M.; Maki R. A.; Tortorella M. D.; Arner E. C.; Firestein G. S. Expression and regulation of aggrecanase in arthritis: the role of TGF-beta. J. Immunol. 2002, 168, 1405–1412. 10.4049/jimmunol.168.3.1405. [DOI] [PubMed] [Google Scholar]
- Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999, 27, 1409–1420. 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q.; Eberspaecher H.; Lefebvre V.; De Crombrugghe B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev. Dyn. 1997, 209, 377–386. . [DOI] [PubMed] [Google Scholar]
- Lefebvre V.; Behringer R. R.; de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage 2001, 9, S69–S75. 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- Kawakami Y.; Rodriguez-Leon J.; Izpisua Belmonte J. C. The role of TGFbetas and Sox9 during limb chondrogenesis. Curr. Opin. Cell Biol. 2006, 18, 723–729. 10.1016/j.ceb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Lefebvre V.; Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res., Part C 2005, 75, 200–212. 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- Cho R. L.; Lin W. N.; Wang C. Y.; Yang C. C.; Hsiao L. D.; Lin C. C.; Yang C. M. Heme oxygenase-1 induction by rosiglitazone via PKCalpha/AMPKalpha/p38 MAPKalpha/SIRT1/PPARgamma pathway suppresses lipopolysaccharide-mediated pulmonary inflammation. Biochem. Pharmacol. 2018, 148, 222–237. 10.1016/j.bcp.2017.12.024. [DOI] [PubMed] [Google Scholar]
- Kim D.; Nam H. J.; Lee W.; Yim H. Y.; Ahn J. Y.; Park S. W.; Shin H. R.; Yu R.; Won K. J.; Bae J. S.; Kim K. I.; Baek S. H. PKCalpha-LSD1-NF-kappaB-Signaling Cascade Is Crucial for Epigenetic Control of the Inflammatory Response. Mol. Cell 2018, 69, 398–411. 10.1016/j.molcel.2018.01.002. [DOI] [PubMed] [Google Scholar]
- LaVallie E. R.; Chockalingam P. S.; Collins-Racie L. A.; Freeman B. A.; Keohan C. C.; Leitges M.; Dorner A. J.; Morris E. A.; Majumdar M. K.; Arai M. Protein kinase Czeta is up-regulated in osteoarthritic cartilage and is required for activation of NF-kappaB by tumor necrosis factor and interleukin-1 in articular chondrocytes. J. Biol. Chem. 2006, 281, 24124–24137. 10.1074/jbc.M601905200. [DOI] [PubMed] [Google Scholar]
- Ma C. H.; Wu C. H.; Jou I. M.; Tu Y. K.; Hung C. H.; Hsieh P. L.; Tsai K. L. PKR activation causes inflammation and MMP-13 secretion in human degenerated articular chondrocytes. Redox Biol. 2018, 14, 72–81. 10.1016/j.redox.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvirkvelia N.; McMenamin M.; Warren M.; Jadeja R. N.; Kodeboyina S. K.; Sharma A.; Zhi W.; O’Connor P. M.; Raju R.; Lucas R.; Madaio M. P. Kidney-targeted inhibition of protein kinase C-alpha ameliorates nephrotoxic nephritis with restoration of mitochondrial dysfunction. Kidney Int. 2018, 94, 280–291. 10.1016/j.kint.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T.; Izawa Y.; Wada H.; Makita T.; Hashimoto Y.; Enomoto M. Toxicological aspects of feprazone, a new nonsteroidal anti-inflammatory drug”. Toxicol. Appl. Pharmacol. 1982, 64, 255–270. 10.1016/0041-008X(82)90222-8. [DOI] [PubMed] [Google Scholar]
- Kim H. A.; Song Y. W. TNF-alpha-mediated apoptosis in chondrocytes sensitized by MG132 or actinomycin D. Biochem. Biophys. Res. Commun. 2002, 295, 937–944. 10.1016/S0006-291X(02)00789-1. [DOI] [PubMed] [Google Scholar]