Abstract
Colorectal cancer (CRC) is one of the most common malignancies worldwide. As current therapies toward CRC, including chemotherapy and radiotherapy, pose limitations, such as multidrug resistance (MDR) as well as the intrinsic and potential cytotoxic effects, necessitating to find more effective treatment options with fewer side effects, traditional Chinese medicine (TCM) has an advantage in complementary therapies. In the present study, 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide (MTT assays), trypan blue staining, colony formation, 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) staining, cell cycle determination, and Annexin V-FITC/PI staining were used to examine the efficacy of Sanjie Yiliu Formula (SJYLF) against CRC proliferation and to investigate its underlying molecular mechanisms through protein expression of various proapoptotic factors by quantitative polymerase chain reaction (q-PCR) and Western blotting. This four-herb-TCM SJYLF can be suggested as one of the decoctions clinically effective in late-stage cancer treatment. Our results suggest that SJYLF robustly decreased the viability of only CRC cell lines (HCT-8, SW-480, HT-29, and DLD-1) and not the normal human kidney cells (HK-2). Moreover, SJYLF significantly suppressed proliferation and induced apoptosis in HCT-8 and downregulated cyclin D1, CDK4, and BCL-2, while Bax expression was upregulated at both mRNA and protein expression levels.
Introduction
Colorectal cancer (CRC) is one of the most common and fatal malignancies worldwide. An increasing number of CRC cases, late diagnosis, high risk of tumor recurrence, and multidrug resistance (MDR) make it the fourth cause of cancer-related deaths globally.1,2 It is estimated that the number of new CRC cases may increase to 2.5 million annually by 2035.3 Despite multiple available treatments like chemotherapy, surgical resection, targeted therapy, and radiotherapy, CRC diagnosis remains poor in patients. CRC is mostly diagnosed in the last stages of the disease, making eradicating cancer impossible.3 Conventional chemotherapy, making use of 5-fluorouracil (5-FU), FOLFOX, and platinum-based drugs, e.g., cisplatin and oxaliplatin,4,5 has its limitations, such as low efficacy, multidrug resistance (MDR), intrinsic and potential cytotoxic effects, and relapse in more than 50% cases.5,6 Therefore, finding more selective and effective chemotherapeutic options with fewer side effects remains an urgent need and a challenge to treat CRC patients.
Traditional Chinese medicines (TCMs) have been appreciated for more than 5000 years and have evolved their diagnosis methods, treatment procedures, and therapies.7 Since the past decade, TCM has gained considerable attention as a complementary and alternative treatment for various types of cancers.8,9 Recent studies appreciate the contribution of TCM for the cure of CRC with certain benefits, including toxicity attenuation, inhibition of tumorigenesis, effective remedy, and decreased risks of metastasis.10,11 TCM herbals in a discrete dose and form of concoction/formula have proved to be a better long-term remedy in stage II and III CRC patients.12,13 Furthermore, TCM-based remedies in combination with chemotherapy, radiotherapy, and conventional medicine were found more effective in cancer patients.14−16 For these reasons, TCM can be considered as an advantageous and alternative remedy for CRC.
Several TCM is being used as a concoction or formula composed of various herbs, minerals, and animal extracts or their components. Their synergistic action seems to be more effective against cancer and other related diseases.17 Xiaotan Sanjie and Ruanjian Sanjie are multiherb decoctions found effective in gastric and breast cancers, respectively. These formulae were found to induce apoptosis in cancer cells.18,19 Another multiherb decoction, BushenShugan Formula (BSF), was found effective in treating middle- and late-stage lung cancers,20 while Ginseng Radix and Astragali Radix showed promising therapeutic results against CRC.21,22
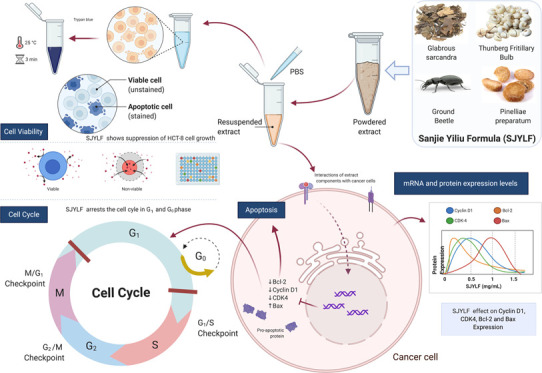
TCM Sanjie Yiliu Formula (SJYLF) is a four-component anticolon cancer decoction composed of Rhizoma Pinelliae Preparatum (Fabanxia), Glabrous Sarcandra herb, Thunberg Fritillary bulb, and ground beetle species (Figure 1). Both meta-analysis and systematic review show that individual components of SJYLF may play an important role in enhancing life quality in cancer patients by relieving symptoms and decreasing the harmful effects of undergoing chemotherapy23,24 (China patents CN101744849A, CN102688404A). Nevertheless, no detailed studies are available about the precise anticancer activities and mechanisms of the SJYLF component decoction. In the present study, we have investigated the anti-CRC effects of SJYLF. 3-(4,5-Dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, 4,6-diamidino-2-phenylindole dihydrochloride (DAPI) staining, cell cycle analysis, and Annexin V-FITC/PI staining were used to investigate an antitumor activity for SJYLF by assessing cell morphology and viability. We have also evaluated the efficacy of SJYLF against CRC progression and examined its underlying molecular mechanisms by assessing the protein expression of various proapoptotic factors by quantitative polymerase chain reaction (q-PCR) and Western blotting. This investigation will stipulate the significance of SJYLF in monitoring the growth of the colorectal tumor and suggests itself as a novel complementary therapy for CRC treatment.
Figure 1.
Four-component Sanjie Yiliu Formula (SJYLF) and its effects on cancer cells. SJYLF is a four-component TCM consisting of Glabrous Sarcanda, Thenberg Fritillary bulb, Pinelliae Preparatum, and ground beetle species. The four constituents are powdered and suspended in phosphate-buffered saline (PBS) to use as a formula to treat colorectal cancer (CRC) cell lines. Cell viability, cell cycle arrest, and apoptosis processes are studied with respect to mRNA and protein expression levels for cyclin D1, CDK4, Bcl-2, and Bax proteins (figure created with biorender).
Results
SJYLF Suppresses Colorectal Tumor Growth
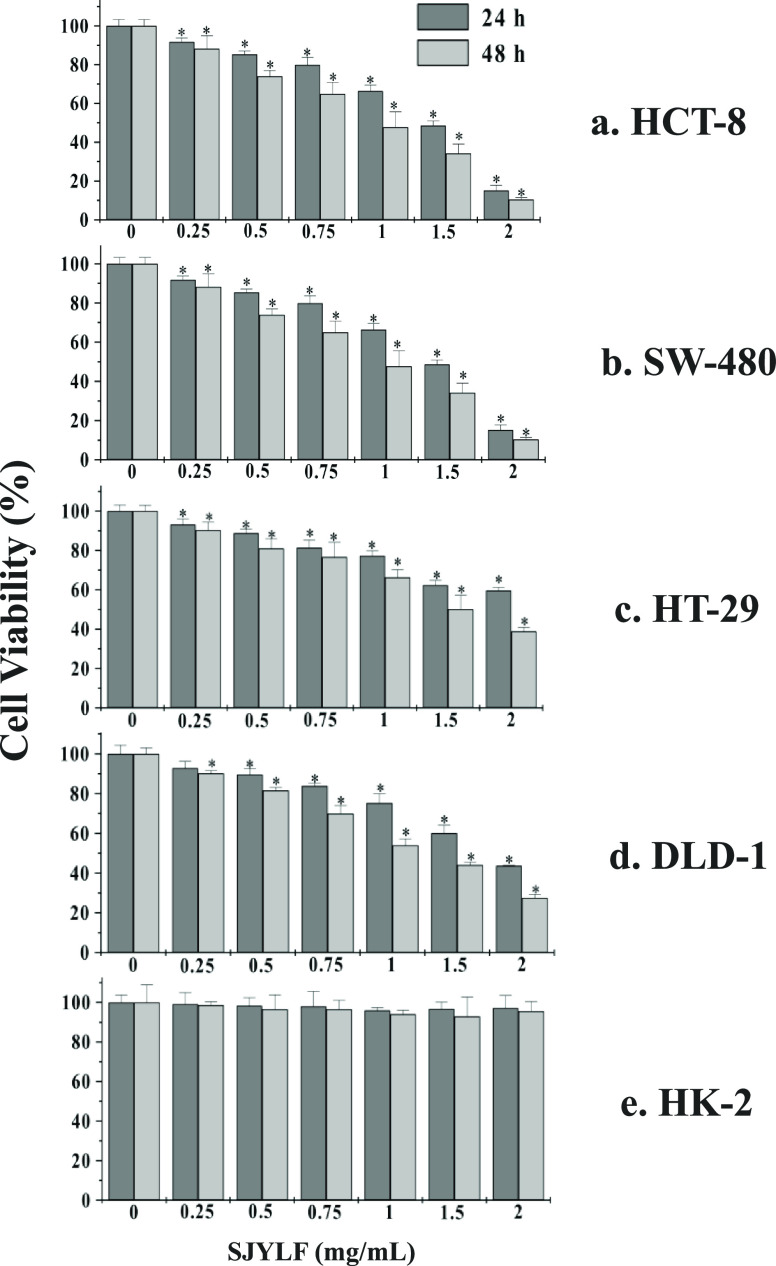
For 24 and 48 h, the anticancer effect of SJYLF was evaluated with the help of MTT assays using CRC cell lines (HCT-8, SW-480 HT-29, and DLD-1). An increasing SJYLF extract concentration (0–2 mg/mL) was added to the cells. Cell viability was also monitored for human kidney cell lines (HK-2). Figure 2a–d shows a marked reduction of the CRC cell line viability by SJYLF, in terms of both time- and dose-dependent manner. Figure 2e shows that SJYLF is selectively toxic to the CRC cell line and not toward normal human cells (HK-2).
Figure 2.
Suppression of HCT-8, SW-480, HT-29, DLD-1, and HK-2 cell viability in the presence of SJYLF. The viability of all CRC cell lines in the presence of SJYLF was markedly reduced by SJYLF in both a dose- and time-dependent manner, where panels (a)–(d) show the effect of 0, 0.5, 1, 1.5, and 2 mg/mL SJYLF, while HK-2 cell viability was not affected by SJYLF (e).
Table 1 shows that IC50 values for SJYLF were 1.25 ± 0.20 and 0.91 ± 0.11 mg/mL for HCT-8 cells; 1.94 ± 0.09 and 1.09 ± 0.10 mg/mL for SW-480 cells; 2.64 ± 0.04 and 1.54 ± 0.08 mg/mL for HT-29 cells; and 1.90 ± 0.17 and 1.19 ± 0.08 mg/mL for DLD-1 cells at 24 and 48 h, respectively. HCT-8 cells show growth inhibition by 0.91 ± 0.11 mg/mL with the lowest effective dose of SJYLF. SJYLF antiproliferative activity was found to be concentration-dependent. As SJYLF extract showed better IC50 against HCT-8 cell line, these cells were further used to determine the mechanism of action for SJYLF. An SJYLF concentration of 0–1.5 mg/mL was selected for subsequent analysis.
Table 1. IC50 (mg/mL) Calculated for SJYLF against Five Different Cell Lines at Two Different Time Intervals.
| 24 h | 48 h | |
|---|---|---|
| HCT-8 | 1.25 ± 0.20 | 0.91 ± 0.11 |
| SW-480 | 1.94 ± 0.09 | 1.09 ± 0.10 |
| HT-29 | 2.64 ± 0.04 | 1.54 ± 0.08 |
| DLD-1 | 1.90 ± 0.17 | 1.19 ± 0.08 |
| HK-2 | 679.93 ± 9.45 | 201.16 ± 5.66 |
SJYLF Suppresses the Proliferation of HCT-8 Cells
To investigate the inhibition mechanism and confirm the proliferation suppressant effect of SJYLF toward HCT-8 cells, trypan blue staining, colony formation, and DAPI immunofluorescence staining methods were utilized. Cells were treated with 0.5, 1.0, and 1.5 mg/mL of SJYLF and stained with trypan blue to analyze proliferation factors.
As shown in Figure 3, SJYLF treatment could decrease colorectal cancer cell colony formation in a dose-dependent manner. Figure 4 shows a significant decline in cell growth after SJYLF treatment, where trypan blue exclusion staining shows a considerable increase in dead cells. Furthermore, DAPI immunofluorescence shows that SJYLF extract was found to cause apoptosis by suppressing the HCT-8 cell proliferation, as shown in Figure 5. All of these results vividly illustrate that SJYLF extract inhibits the proliferation of HCT-8 cells and declines cell growth.
Figure 3.
Suppression of the HCT-8 cell colony formation in the presence of SJYLF. HCT-8 cells were grown in the presence of increasing concentrations of SJYLF extract (0.5–1.5 mg/mL in PBS) for 24 h. The SJYLF treatment could decrease colorectal cancer cell colony formation in a dose-dependent manner where panels (a)–(d) show the 0, 0.5, 1, and 1.5 mg/mL SJYLF.
Figure 4.
Suppression of HCT-8 cell growth in the presence of SJYLF. HCT-8 cells were grown in the presence of increasing concentrations of SJYLF extract (0.5–1.5 mg/mL in PBS) for 24 h. The SJYLF treatment could decrease colorectal cancer cell growth in a dose-dependent manner where panels (a)–(d) show the 0, 0.5, 1, 1.5 mg/mL SJYLF.
Figure 5.
Suppression of HCT-8 cells in the presence of SJYLF. HCT-8 cells were grown in the presence of increasing concentrations of SJYLF extract (0.5–1.5 mg/mL in PBS) for 24 h. DAPI immunofluorescence staining was demonstrated to prove that SJYLF suppresses the proliferation of HCT-8 cells in a dose-dependent manner where panels (a)–(d) show the 0, 0.5, 1, 1.5 mg/mL SJYLF.
SJYLF Stimulates Apoptosis in HCT-8 Cells
Flow cytometric analysis was used to confirm the apoptotic effects of SJYLF on HCT-8 cells. For 48 h, the cells were treated with an increasing concentration of SJYLF (0.5, 1.0, and 1.5 mg/mL). Annexin V-FITC and PI stains were used for labeling. Figure 6a–d indicates that after the treatment of SJYLF, there was a considerable increase in the percentage of apoptotic cells in a dose-dependent manner. At a concentration of 1.5 mg/mL, SJYLF induced 23.42 ± 2.58% apoptotic events in comparison to that of 0.87 ± 1.71% apoptotic events in the negative control (Figure 6e). Consequently, it described that SJYLF induced apoptosis in HCT-8 cells.
Figure 6.
SJYLF-induced apoptotic events. The percentage of apoptotic cells was significantly increased in a dose-dependent manner after SJYLF treatment, where panels (a)–(d) show the 0, 0.5, 1, and 1.5 mg/mL SJYLF. At a concentration of 1.5 mg/mL, SJYLF induced 23.42 ± 2.58% apoptotic events in comparison to that of 0.87 ± 1.71% apoptotic events in the negative control (e).
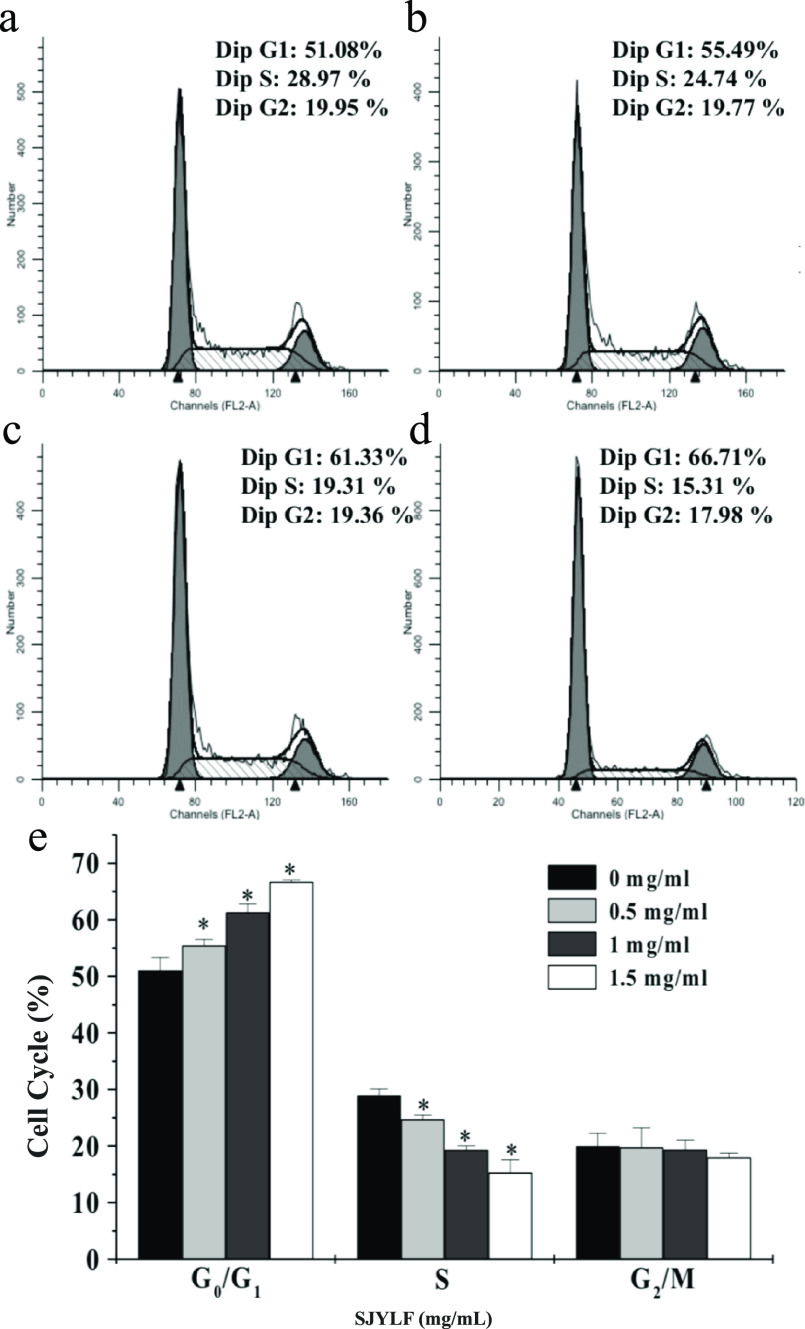
Effects of SJYLF on Cell Cycle Progression in HCT-8 Cells
Cell cycle involves a sequential process starting from the cell’s last to current mitosis. It comprises four phases including the Gap 1 phase (G1 phase), deoxyribonucleic acid (DNA) synthesis phase (S phase), Gap 2 phase (G2 phase), and M phase (mitosis phase). Interphase includes G1, S, and G2 phases. After the interphase and mitotic phase, a parental cell divides into two progeny cells with similar genetic material. Figure 7a–d shows that cell apoptosis was induced by SJYLF through cell cycle arrest. Treatment with SJYLF enhanced the cells’ concentration in the G0 and G1 phases, while the decreased concentration of cells in the G2 phases was also observed. SJYLF treatment (at a concentration of 1.5 mg/mL) induced the cell cycle in the G0/G1 phase considerably; the HCT-8 cell population was increased from 51.08 to 66.71% (Figure 7a–e). These results propose that SJYLF inhibits the progression of the cell cycle to induce apoptosis.
Figure 7.
Effect of SJYLF on cell cycle. SJYLF increased the ratio of cells in the G0 and G1 phases in a dose-dependent manner after SJYLF treatment, where panels (a)–(d) show the 0, 0.5, 1, and 1.5 mg/mL SJYLF. At the same time, it decreased the ratio of cells in the G2 phases, suggesting that SJYLF inhibits cell cycle progression to stimulate apoptosis. The population of HCT-8 cells was increased from 51.08 to 66.71% in the G0/G1 phase (e).
Expression of Upstream and Downstream Apoptosis and Cycle-Related Proteins of the Cyclin D-CDK4 Signaling Pathway
The key controlling point of the cell reproductive cycle where proliferation starts is between the G1 and S phases. The classical approach to relate the signals and the cell restriction point is the signal pathway whose core is cyclin-CDK4/6.
Different proteins control the signaling of apoptosis. Significant proteins of apoptosis include proapoptotic Bax and antiapoptotic Bcl-2. As compared to the control group (0 mg/mL), SJYLF (0.5, 10, and 1.5 mg/mL) downregulated the expression of Bcl-2 protein and upregulated the expression of Bax protein. A considerable increase in the Bax/Bcl-2 ratio suggests that SJYLF has a profound induction effect on apoptosis proteins (Figure 7a–c). Apoptosis is caused by the activation of caspase waterfall and elevated Bax/Bcl-2 that result in the permeability changes in the mitochondrial membrane. Proteins related to the cell cycle declined the expression of CDK4 and cyclin D1 proteins, and the inhibition of the G0/G1 phase progression was also noticed.
As seen in Figure 8a, SJYLF-treated HCT-8 cells show a considerable decrease (>50%) in the relative mRNA expression for cyclin D1, CDK4, and Bcl-2, while Bax mRNA level was increased about 25% (Figure 8a). When protein levels of cyclin D1, CDK4, Bcl-2, and Bax were measured with Western blotting, similar protein expression patterns were observed (Figure 8b,c). Therefore, the induction of apoptosis seems to be associated with the increased expression ratio of proapoptotic Bax/Bcl-2 proteins, suggesting that the SJYLF treatment makes the HCT-8 cells sensitive to apoptotic stimuli.
Figure 8.
Quantitative PCR and Western blot analyses in SJYLF-treated HCT-8 cells. Determination of mRNA and protein expression levels of cyclin D1, CDK4, Bcl-2, and BAX in SJYLF-treated HCT-8 cells (0.5–1.5 mg/mL extract) shows significantly reduced levels of cyclin D1, CDK4, and Bcl-2 (a, b). Western blot analysis of four proteins, cyclin D1, CDK4, Bcl-2, and Bax, in SJYLF-treated HCT-8 cells shows that SJYLF has a significant induction effect on the expression of Bax. In contrast, Bcl-2, CDK4, and cyclin D1 protein expression were significantly reduced in the presence of 1.5 mg/mL of SJYLF (c).
Discussion
Traditional Chinese medicines (TCMs) are suitable, effective, and usually pose less harmful effects.25 These medicines are being used for thousands of years while improving clinical symptoms and life quality in many fatal diseases, including cancer. In this aspect, TCM as a single herb formulation or in the form of decoction/formula has gathered worldwide attention for the treatment of various types of cancers, including CRC.7,26 Ruanjian Sanjie (RJSJ), Huanglian Jiedu Tang, Yanshu Injection, Feiji Recipe, Jianpi Yangzheng Xiaozheng Recipe, and Jiedu Xiaozheng Yin are few reported formulas showing antitumor activities in various cancers.19,27−31 TCM Sanjie Yiliu Formula (SJYLF) is a four-component anti-CRC decoction involved in relieving the symptoms and can work in synergism with chemotherapy. This study has reported the antitumor, antiproliferative, and apoptosis-inducing activity of SJYLF (Figure 1).
Apoptosis, no doubt, is a significant self-regulating mechanism32 to maintain stability in the internal tissue environment. One common method in cancer therapy is the induction of apoptosis,33−35 and it rendered an important index of evaluating the anticancer efficacy of drugs.36 SJYLF shows the antitumor and antiproliferative activities against CRC cell lines, i.e., HCT-8 cells with minimal effects on normal cells, i.e., HK-2 cells, in a time- and dose-dependent manner (Figures 2 and 3). MTT, colony-forming, trypan blue, and DAPI immunofluorescence assays are in good agreement with the anticancer activity of SJYLF. Selective cytotoxicity, effectiveness, and efficacy against HCT-8 cells make it important to elucidate the SJYLF mechanism of action. A herbal formulation named C168, in the form of methanol extract (CME), was also found to show the antiproliferative activity against CRC by halting the cell cycle in G2/M and inducing apoptosis.34
SJYLF components, including Rhizoma Pinelliae Preparatum (Fabanxia), Glabrous Sarcandra herb, Thunberg Fritillary bulb, and ground beetle species, have already been reported to exert anticancer activity against colorectal cancer cells. Rhizoma Pinelliae Preparatum (Fabanxia) and Glabrous Sarcandra herb have been reported to show antitumor and antiproliferative properties by arresting the cell cycle and inducing apoptosis in human cell lines.37,38 Thunberg Fritillary bulb has also been reported to inhibit uncontrolled cell growth.39 Many TCMs prepared from insects have been reported to possess immune-modulating antitumor, antiproliferative, and apoptosis-inducing properties.40 Extracts from Paederus beetle (Paederus fuscipes) have been found to inhibit cancer cell growth by MTT and colony-forming assay.41 Pertinent to that, SJYLF components, Glabrous Sarcandra herb and Thunberg Fritillary bulb, were previously reported as nontoxic.42,43 Though Rhizoma Pinelliae Preparatum (Fabanxia) is considered a toxic herb, its amount and ratio in SJYLF are nontoxic.44 Increasing drug concentration levels caused a considerable increase in the apoptotic cell proportion while downregulating the antiapoptosis protein, Bcl-2 (Figures 6 and 8).
SJYLF was found to induce apoptosis via cyclin D1, CDK4, Bcl-2, and Bax proteins that have been reported to involve in many cancers, including CRC. Regulation of cyclin D1 and CDK4 in tumor cells may make SJYLF an effective complementary therapy to combat CRC. Many anticancer agents have been reported to arrest the cell cycle at the G0/G1 phase by regulating Bcl-2 and Bax proteins.45,46
Bcl-2 belongs to a special protein family47 in which certain members promote apoptosis, including Bax, Bid, and Bad,48 whereas certain members like Bcl-2 have an inhibition effect on the release of cytochrome c in the cytoplasm from mitochondria, resulting in apoptosis inhibition.49 Bcl-2 protein is chiefly localized in mitochondria, nuclear membrane, endoplasmic reticulum, and various tumor cells. It has the potential to increase the potential of mitochondrial membrane,50 inhibiting the calcium ion release, preventing endonuclease activation, resulting in an antiapoptotic effect. It is reported that p53 can inhibit Bcl-2, while it also activates Bax and Bak proteins. Bax protein tends to activate the death effect factor caspase or change the cell membrane permeability,51 resulting in the release of small molecules and ions, by cytochrome C, through the cell membrane, therefore promoting the apoptosis of the cell. When Bcl-2 is dominant, then the change in Bcl-2/Bax can regulate apoptosis, and cell possesses antiapoptotic effects. On the other hand, the overexpression of Bax makes the cells a victim of apoptosis.52
An important role is played by CDK4 and cyclin D153 in regulating the progression of the cell cycle. Western blotting, q-PCR, and cell cycle test showed that SJYLF could block A549 cells in the G0/G1 phase and significantly reduce CDK4 and cyclin expression level D1 (Figures 3 and 7). Cell cycle is regulated by CDKs and cyclins to pass from the G1-to-S phase. CDK4 and CDK6 act as switches for cell cycle entry from G1 to S phase.54 Although some studies report that CDK4 is not involved in the mammalian cell cycle, it still plays an important role in the control of cancer proliferation.55 In SJYLF-treated cells, CDK4 levels were found to significantly decrease, while cyclin D1 levels were also marginally decreased (Figure 8b,c). These results propose that SJYLF initiates apoptosis by arresting the cell cycle, where Bcl-2, cyclin D1, and CDK4 protein are downregulated, and Bax protein expression was induced. This preclinical study shows that some additional investigation for SJYLF is required regarding p53 and other proteins, and characterization of active components will help a better understanding of the SJYLF mechanism.
Conclusions
Our results demonstrate that SJYLF possesses remarkable anti-CRC activity with fewer toxic effects on normal cells. Moreover, SJYLF is involved in the suppression of proliferation and induction of apoptosis in cancer cells.
Materials and Methods
Ethical Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the institutional and/or national research committee’s ethical standards and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for Publication
Informed consent was obtained from all individual participants included in the study.
Reagents and Cell Lines
CRC cell lines (HCT-8, SW-480, HT-29, and DLD-1) and normal human kidney cell line HK-2 were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and subjected to short tandem repeat profiling to determine their authenticity. Cell lines were cultured and maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) containing 10% fetal serum of calf (Invitrogen, Carlsbad, CA), 1 U/mL of penicillin G, and 1 μg/mL of streptomycin at 37 °C in a humidified atmosphere containing 5% CO2.
Sanjie Yiliu Formula (SJYLF) Preparation
Herbs and beetle species used in the SJYLF formula were purchased from Pharmaceutical Market (Shanghai, China). SJYLF is a four-component decoction of Thunberg Fritillary bulb, Glabrous Sarcandra herb, Rhizoma Pinelliae Preparatum, and ground beetle species mixed in a ratio of 1:5:10:15. A fine powder of mixture was extracted with distilled water at 100 °C twice for 2 h and centrifuged at 4000g to obtain the supernatant that was further evaporated to a dried powder using a rotary evaporator. This powder was used as the SJYLF extract in further experiments by dissolving it in phosphate-buffered saline. Multiple extractions were performed to evaluate the SJYLF extract’s efficacy, and at least three different batches of SJYLF were used to replicate the results as statistically significant.
Cell Viability-MTT Assay
Cell viability was assessed using a modified MTT colorimetric assay with 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Calbiochem) as described elsewhere.34 Cells (100 μL, 4 × 105 cells) were seeded in 6-well plates and incubated in the presence of 5% carbon dioxide at 37 °C. Cell viability was examined after 24 and 48 h by adding the increased concentration of SJYLF extract (0.25–2 mg/mL in PBS). IC50 was determined for all cell lines, and one with potent IC50 was used in further experiments to elucidate the mechanism of action for SJYLF.
SJYLF was found to be selectively toxic to the CRC cell line and did not affect normal human cells (HK-2). For the subsequent experiments, including the suppression of proliferation, apoptosis stimulation, cell cycle progression, expression of apoptosis-related genes and proteins, HCT-8 cells (most affected by SJYLF) were used.
Trypan Blue and Colony Formation Assays
To determine the percentage of dead and live cells, the trypan blue assay was performed as described by Yao et al.,56 where “number of the trypan blue-stained cells divided by the total cell number”. HCT-8 cells were grown in the presence of increasing concentrations of SJYLF extract (0.5–1.5 mg/mL in PBS) for 24 h. Trypan blue (0.4%) was added to 100 μL of cells and mixed thoroughly before examining the cells under a light microscope. Stained cells were considered dead cells. For the formation of a cell colony, a 6-well plate was used to culture cells. After 2 weeks, crystal violet was used to stain the cell colony and count them.
Immunofluorescence Staining
Immunofluorescence staining was performed as described in a previous study.57 HCT-8 cells were treated with increasing SJYLF extract concentrations (0.5–1.5 mg/mL in PBS). Cells were harvested and washed with PBS. To avoid the heat shock, paraffin sections were used to fix the cells on glass coverslips, followed by a methanol treatment for permeabilization. Cells were incubated at 4 °C with a 1:800 dilution ratio of rabbit and antimouse CCR2 antibody (cat. no. ab203128, Abcam, London, U.K.). Secondary antibodies were labeled for fluorescent visualization using Texas Red-labeled antirabbit IgG (Zsbio, Beijing, China) for 1 h at 37 °C, whereas 4,6-diamidino-2-phenylindole dihydrochloride (DAPI, Beyotime, Beijing, China) was used to examine the nuclei. SPOT Flex camera (Tokyo, China) and Olympus BX600 microscope were used to evaluate all specimens.
Flow Cytometric Analysis
HCT-8 cell lines were cultured in DMEM containing 10% fetal calf serum in the presence of increasing concentrations of SJYLF extract (0.5–1.5 mg/mL in PBS). Harvested cells were washed with ice-cold PBS (Invitrogen, MA) having 0.2% ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, MA) and resuspended in the same buffer containing 3% fetal bovine serum to obtain a cell suspension (FBS, Gibco, MA). Annexin V-FITC/PI labeling was performed through BioLegend as fluorescent-conjugated antibodies, as described elsewhere.58 A minimum of 30 000 cells were analyzed on a NovoCyte flow cytometer (ACEA Biosciences), and NovoExpress software was implied to analyze the data. Annexin V and PI double-positive cells were considered late apoptotic or necrotic cells where PI fluorescence was observed at >625 nm.
Quantitative PCR
To assess the gene expression levels of cyclin D1, CDK4 (cyclin-dependent kinases), Bcl-2 (B cell leukemia/lymphoma 2), BAX in SJYLF-treated HCT-8 cells (0.5–1.5 mg/mL extract), quantitative PCR, and Western blot analyses were performed. Trizol reagent (Invitrogen, Carlsbad, CA) was used to extract the total RNA from HCT-8 cells according to the manufacturer’s instruction. Reverse transcription was performed using an RT kit (cat. no. RR014A; Takara, Dalian, China). Complementary DNA was employed for quantitative PCR analysis using an SYBR kit (cat. no. RR840A; Takara, Dalian, China) on a CFX96 machine (Bio-Rad, MA) with programmed cycles following a cycle of 90 s at 95 °C and after that 40 cycles of 95 °C for 10 s and 58 °C for 30 s. GAPDH was utilized as a control. Each experiment was conducted three times, and the 2–ΔΔCq method was used to analyze the relative expressions of mRNA.59 The primers for cyclin D1, CDK4, Bcl-2, and BAX are given in Table 2.
Table 2. Primers Synthesized for Amplification of Cyclin D1, CDK4, Bcl-2, and BAX Genes.
| cyclin D1 | forward primer | 5′-ATGTTCGTGGCCTCTAAGATGA-3′ |
| reverse primer | 5′-CAGGTTCCACTTGAGCTTCTTC-3′ | |
| CDK4 | forward primer | 5′-ATGGCTACCTCTCGATATGAGC-3′ |
| reverse primer | 5′-CATTGGGGACTCTCACACTCT-3′ | |
| Bcl-2 | forward primer | 5′-GGTGGGGTCATGTGTGTGG-3′ |
| reverse primer | 5′-CGGTTCAGGTACTCAGTCATCC-3′ | |
| BAX | forward primer | 5′-CCCGAGAGGTCTTTTTCCGAG-3′ |
| reverse primer | 5′-CCAGCCCATGATGGTTCTGAT-3′ |
Western Blotting
SJYLF-treated HCT-8 cells (0.5–1.5 mg/mL extract) were lysed in an ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Beijing, China) having phosphatase inhibitors (Merck Millipore, MA) and protease inhibitor cocktails (Merck Millipore, MA) for about 30 min. Lysed cells were centrifuged (6000g, 15 min) at 4 °C, and the clear supernatant was obtained.
Bicinchoninic acid (BCA) kit was used to check protein concentration. Proteins were denatured by adding a 5× loading buffer to 20 μL of sample followed by sample boiling for 5 min at 90 °C. Tris–glycine SDS-PAGE (12%, 120 V) was used to separate the protein. Proteins were then transferred to poly(vinylidene fluoride) (PVDF) membrane (Millipore, MA) by electroblotting. To avoid nonspecific binding, 5% bovine serum albumin (BSA) in TBS/T buffer was used as a blocking buffer. After washing with PBS, membranes were incubated overnight with primary antibodies of β-actin, Bcl-2, Bax, CDK4, and cyclin D1 at 4 °C. After primary incubation, horseradish peroxidase-conjugated secondary antibody (Zsbio, Beijing, China) was added, and membranes were again incubated for 1 h. Chemiluminescent substrate ECL kit (Merck Millipore, MA) was used to detect the protein bands that were visualized by using X-ray film exposure.
Statistical Analysis
After three times the conduction of all experiments, the values were obtained as a mean ± standard deviation. Three batches of SJYLF were used to replicate the results as statistically significant. Student’s t test estimated group comparisons. Two-sided p-values were reported, and a p-value < 0.05 was designated as statistically significant. SPSS statistical software package (SPSS 19.; SPSS, Chicago, IL) was used to conduct all analyses.
Acknowledgments
The authors acknowledge Dr. Lu Changrui for helpful discussions and guidance. The present study was supported by the Medical Key Subject Construction Project of Shanghai District (ZK2019B08).
Author Contributions
R.Z.T., M.F.R., and C.B.Y. conceptualized the project. R.Z.T., F.K., and Z.Z.L performed the experiments. A.I.B. and M.M. analyzed the data. All authors were involved in the preparation of the manuscript. R.Z.T., Z.Z.L., and C.B.Y. provided the resources. F.K., M.F.R., and C.B.Y analyzed the results and reviewed/edited the manuscript. All authors have read and agreed to the published version of the manuscript.
The authors declare no competing financial interest.
References
- Mármol I.; Sánchez-de-Diego C.; Pradilla Dieste A.; Cerrada E.; Rodriguez Yoldi J. M. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197 10.3390/ijms18010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviano L. F.; Li X.; Murray M.; Frye J. T.; Lung B. E.; Zhang Y. Y.; Yang J.; Taub E. M.; Bucobo J. C.; Buscaglia J. M.; Li E.; Miller J. D. Type 2 diabetes impacts colorectal adenoma detection in screening colonoscopy. Sci. Rep. 2020, 10, 7793 10.1038/s41598-020-64344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y.-H.; Chen Y.-X.; Fang J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduction Targeted Ther. 2020, 5, 22 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfner M. F.; Debus J. Radiotherapy for Colorectal Cancer: Current Standards and Future Perspectives. Visc. Med. 2016, 32, 172–177. 10.1159/000446486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarni W.; Dutta R.; Green R.; Katiri S.; Patel B.; Mohapatra S. S.; Mohapatra S. Mithramycin A Inhibits Colorectal Cancer Growth by Targeting Cancer Stem Cells. Sci. Rep. 2019, 9, 15202 10.1038/s41598-019-50917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neo J. H.; Ager E. I.; Angus P. W.; Zhu J.; Herath C. B.; Christophi C. Changes in the renin angiotensin system during the development of colorectal cancer liver metastases. BMC Cancer 2010, 10, 134 10.1186/1471-2407-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferth T.; Li P. C. H.; Konkimalla V. S. B.; Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol. Med. 2007, 13, 353–361. 10.1016/j.molmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Hawkins D. S.; Arndt C. A. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer 2003, 98, 2447–2456. 10.1002/cncr.11799. [DOI] [PubMed] [Google Scholar]
- Ling C.-q.; Yue X.-q.; Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J. Integr. Med. 2014, 12, 331–335. 10.1016/S2095-4964(14)60038-8. [DOI] [PubMed] [Google Scholar]
- Rivera-Valentin R. K.; Zhu L.; Hughes D. P. Bone sarcomas in pediatrics: progress in our understanding of tumor biology and implications for therapy. Pediatr. Drugs 2015, 17, 257–271. 10.1007/s40272-015-0134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. Y.; Liu C. B.; Chen A. H.; Ding Y. J.; Jin H. Y.; Seow-Choen F.; Nicholls R. J. The role of traditional Chinese medicine in colorectal cancer treatment. Tech. Coloproctol. 2008, 12, 1. 10.1007/s10151-008-0392-z. [DOI] [PubMed] [Google Scholar]
- Liu X.; Ji Q.; Deng W.; Chai N.; Feng Y.; Zhou L.; Sui H.; Li C.; Sun X.; Li Q. JianPi JieDu recipe inhibits epithelial-to-mesenchymal transition in colorectal cancer through TGF-β/Smad mediated Snail/E-cadherin expression. BioMed Res. Int. 2017, 2017, 1–11. 10.1155/2017/2613198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Weber M. Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett. 2013, 332, 304–312. 10.1016/j.canlet.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Efferth T.; Kahl S.; Paulus K.; Adams M.; Rauh R.; Boechzelt H.; Hao X.; Kaina B.; Bauer R. Phytochemistry and pharmacogenomics of natural products derived from traditional Chinese medicine and Chinese materia medica with activity against tumor cells. Mol. Cancer Ther. 2008, 7, 152–161. 10.1158/1535-7163.MCT-07-0073. [DOI] [PubMed] [Google Scholar]
- Qi F.; Li A.; Inagaki Y.; Gao J.; Li J.; Kokudo N.; Li X.-K.; Tang W. Chinese herbal medicines as adjuvant treatment during chemoor radio-therapy for cancer. BioSci. Trends 2010, 4, 297–307. [PubMed] [Google Scholar]
- Lam W.; Bussom S.; Guan F.; Jiang Z.; Zhang W.; Gullen E. A.; Liu S.-H.; Cheng Y.-C. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci. Transl. Med. 2010, 2, 45ra59 10.1126/scitranslmed.3001270. [DOI] [PubMed] [Google Scholar]
- Cao R.; Zhang H.; Guo J.; Liu X.-h.; Liu C.; Zhu C.-h.; Wu X.-z. A novel pharmacological method to study the Chinese medicinal formula Hua-Zheng-Hui-Sheng-Dan. Evidence-Based Complementary Altern. Med. 2015, 2015, 436807 10.1155/2015/436807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.; Wei P.-K. Xiaotan Sanjie decoction inhibits interleukin-8-induced metastatic potency in gastric cancer. World J. Gastroenterol. 2015, 21, 1479. 10.3748/wjg.v21.i5.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Zhao J.; Hu R.; Yao Q.; Zhang G.; Shen H.; Yagüe E.; Hu Y. Ruanjian Sanjie decoction exhibits antitumor activity by inducing cell apoptosis in breast cancer. Oncol. Lett. 2017, 13, 3071–3079. 10.3892/ol.2017.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z.; Xue W.; Dou M.; Li L.; Lu J.; Ma B.; Deng X.; Zhang M.; Zhai Y.; Wang S.; Zhao J. Bushenshugan Formula Attenuates the Development of Lung Cancer by Inhibiting Epithelial-Mesenchymal Transition. Cell. Physiol. Biochem. 2018, 47, 1977–1988. 10.1159/000491466. [DOI] [PubMed] [Google Scholar]
- Kang K. A.; Kim H. S.; Kim D. H.; Hyun J. W. The role of a ginseng saponin metabolite as a DNA methyltransferase inhibitor in colorectal cancer cells. Int. J. Oncol. 2013, 43, 228–236. 10.3892/ijo.2013.1931. [DOI] [PubMed] [Google Scholar]
- Law P.-C.; Auyeung K. K.; Chan L.-Y.; Ko J. K. Astragalus saponins downregulate vascular endothelial growth factor under cobalt chloride-stimulated hypoxia in colon cancer cells. BMC Complementary Altern. Med. 2012, 12, 160 10.1186/1472-6882-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X.; Huang B.; Wang G.; Zhang C. The ethnobotanical, phytochemical and pharmacological profile of the genus Pinellia. Fitoterapia 2014, 93, 1–17. 10.1016/j.fitote.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Ahn M. Y.; Kim B. J.; Kim H. J.; Jin J. M.; Yoon H. J.; Hwang J. S.; Park K.-K. Anti-cancer effect of dung beetle glycosaminoglycans on melanoma. BMC Cancer 2019, 19, 9 10.1186/s12885-018-5202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V.; Patel S.; Tcyganov E.; Gabrilovich D. I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends immunol. 2016, 37, 208–220. 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung V. C.; Wu X.; Lu P.; Hui E. P.; Zhang Y.; Zhang A. L.; Lau A. Y.; Zhao J.; Fan M.; Ziea E. T.; et al. Chinese herbal medicine for symptom management in cancer palliative care: systematic review and meta-analysis. Medicine 2016, 95, e6650 10.1097/01.md.0000484108.92666.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. L.; Kuo P. L.; Tzeng T. F.; Sung S. C.; Yen M. H.; Lin L. T.; Lin C. C. Huang-lian-jie-du-tang, a traditional Chinese medicine prescription, induces cell-cycle arrest and apoptosis in human liver cancer cells in vitro and in vivo. J. Gastroenterol. Hepatol. 2008, 23, e290–e299. 10.1111/j.1440-1746.2008.05390.x. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Li G.; Huang H. Clinical observation on treatment of 75 mid-late stage cancer patients with yanshu injection. Chin. J. Integr. Tradit. West. Med. 2006, 26, 681–684. [PubMed] [Google Scholar]
- Huang Y.-S.; Shi Z.-M. Intervention effect of Feiji Recipe on immune escape of lung cancer. Chin. J. Integr. Tradit. West. Med. 2007, 27, 501–504. [PubMed] [Google Scholar]
- Wu J.; Liu S.; Zhang X.; Chen M.; Zou X. Effect of Jianpi Yangzheng Xiaozheng recipe on apoptosis and autophagy of subcutaneous transplanted tumor in nude mice: an experimental study on mechanism. Chin. J. Integr. Tradit. West. Med. 2015, 35, 1113–1118. [PubMed] [Google Scholar]
- Cao Z.; Lin W.; Huang Z.; Chen X.; Zhao J.; Zheng L.; Ye H.; Liu Z.; Liao L.; Du J. Ethyl acetate extraction from a Chinese herbal formula, Jiedu Xiaozheng Yin, inhibits the proliferation of hepatocellular carcinoma cells via induction of G0/G1 phase arrest in vivo and in vitro. Int. J. Oncol. 2013, 42, 202–210. 10.3892/ijo.2012.1703. [DOI] [PubMed] [Google Scholar]
- Ségaliny A. I.; Mohamadi A.; Dizier B.; Lokajczyk A.; Brion R.; Lanel R.; Amiaud J.; Charrier C.; Boisson-Vidal C.; Heymann D. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int. J. Cancer 2015, 137, 73–85. 10.1002/ijc.29376. [DOI] [PubMed] [Google Scholar]
- Kimura Y.; Sumiyoshi M.; Baba K. Antitumor and antimetastatic activity of synthetic hydroxystilbenes through inhibition of lymphangiogenesis and M2 macrophage differentiation of tumor-associated macrophages. Anticancer Res. 2016, 36, 137–148. [PubMed] [Google Scholar]
- Leong L. M.; Chan K. M.; Hamid A.; Latip J.; Rajab N. F. Herbal Formulation C168 Attenuates Proliferation and Induces Apoptosis in HCT 116 Human Colorectal Carcinoma Cells: Role of Oxidative Stress and DNA Damage. Evidence-Based Complementary Altern. Med. 2016, 2016, 2091085 10.1155/2016/2091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.-R.; Li H.-M.; Zhu M.; Li J.; Ma T.; Huo Q.; Hong Y.-S.; Wu C.-Z. Non-benzoquinone geldanamycin analog, WK-88-1, induces apoptosis in human breast cancer cell lines. J. Microbiol. Biotechnol. 2018, 28, 542–550. 10.4014/jmb.1710.10063. [DOI] [PubMed] [Google Scholar]
- Wiebke E. A.; Grieshop N. A.; Loehrer P. J.; Eckert G. J.; Sidner R. A. Antitumor effects of 5-fluorouracil on human colon cancer cell lines: antagonism by levamisole. J. Surg. Res. 2003, 111, 63–69. 10.1016/S0022-4804(03)00053-2. [DOI] [PubMed] [Google Scholar]
- Li W.; Chiu L.; Lam W.; Wong W.; Chan Y.; Ho Y.; Wong E. Y.; Wong Y.; Ooi V. E. Ethyl acetate extract of Chinese medicinal herb Sarcandra glabra induces growth inhibition on human leukemic HL-60 cells, associated with cell cycle arrest and up-regulation of pro-apoptotic Bax/Bcl-2 ratio. Oncol. Rep. 2007, 17, 425–431. 10.3892/or.17.2.425. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Cai Y.; Wang L.; Liu H.; Wang X. Optimization of processing technology of Rhizoma Pinelliae Praeparatum and its anti-tumor effect. Afr. Health Sci. 2015, 15, 101–106. 10.4314/ahs.v15i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai H.; Rencun Y.. Cancer Management with Chinese Medicine; World Scientific, 2012. [Google Scholar]
- Shi Z.; Song T.; Wan Y.; Xie J.; Yan Y.; Shi K.; Du Y.; Shang L. A systematic review and meta-analysis of traditional insect Chinese medicines combined chemotherapy for non-surgical hepatocellular carcinoma therapy. Sci. Rep. 2017, 7, 4355 10.1038/s41598-017-04351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani F.; Monfared A. S.; Zabihi E.; Khafri S.; Karimi M.; et al. Evaluation of the effects of Paederus beetle extract and gamma irradiation on HeLa cells. Iran. J. Basic Med. Sci. 2014, 17, 303–306. [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Qiao Y.; Li J.; An C.; Hu K.; Tang M. Acute and sub-chronic toxicity studies of the extract of Thunberg Fritillary Bulb. Regul. Toxicol. Pharmacol. 2014, 68, 370–377. 10.1016/j.yrtph.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Cao H.-J.; Tan R.-R.; He R.-R.; Tang L.-P.; Wang X.-L.; Yao N.; Duan W.-J.; Hu Y.-A.; Yao X.-S.; Kurihara H. Sarcandra glabra extract reduces the susceptibility and severity of influenza in restraint-stressed mice. Evidence-Based Complementary Altern. Med. 2012, 2012, 236539 10.1155/2012/236539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Cheng Z.; He S.; Shi J.; Liu S.; Zhang G.; Zhu L.; Liu L.; Liu Z.; Lin N.; Lu L. Pinelliae rhizoma, a toxic chinese herb, can significantly inhibit CYP3A activity in rats. Molecules 2015, 20, 792–806. 10.3390/molecules20010792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q.; You C.; Zheng C.; Gu Y.; Gu H.; Zhang R.; Wu H.; Sun B. 3-Nitroacridine derivatives arrest cell cycle at G0/G1 phase and induce apoptosis in human breast cancer cells may act as DNA-target anticancer agents. Life Sci. 2018, 206, 1–9. 10.1016/j.lfs.2018.05.010. [DOI] [PubMed] [Google Scholar]
- Wang X.; Tian J.; Jiao X.; Geng J.; Wang R.; Liu N.; Gao X.; Griffin N.; Gao Y.; Shan F. The novel mechanism of anticancer effect on gastric cancer through inducing G0/G1 cell cycle arrest and caspase-dependent apoptosis in vitro and in vivo by methionine enkephalin. Cancer Manage. Res. 2018, 10, 4773–4787. 10.2147/CMAR.S178343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q.; Shi H.; Liu F. CD163+ M2-type tumor-associated macrophage support the suppression of tumor-infiltrating T cells in osteosarcoma. Int. Immunopharmacol. 2016, 34, 101–106. 10.1016/j.intimp.2016.01.023. [DOI] [PubMed] [Google Scholar]
- Wolfs I. M.; Donners M. M.; de Winther M. P. Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb. Haemostasis 2011, 106, 763–771. 10.1160/TH11-05-0320. [DOI] [PubMed] [Google Scholar]
- Han J. J.; Yu M.; Houston N.; Steinberg S. M.; Kohn E. C. Progranulin is a potential prognostic biomarker in advanced epithelial ovarian cancers. Gynecol. Oncol. 2011, 120, 5–10. 10.1016/j.ygyno.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L.; Wang Y. C.; Li W. S.; Du Y. The role of mTOR and phospho-p70S6K in pathogenesis and progression of gastric carcinomas: an immunohistochemical study on tissue microarray. J. Exp. Clin. Cancer Res. 2009, 28, 152 10.1186/1756-9966-28-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K.; Wang Y.; Hu Y.; Zheng L.; Xu W.; Li G. Specific tumor-derived CCL2 mediated by pyruvate kinase M2 in colorectal cancer cells contributes to macrophage recruitment in tumor microenvironment. Tumor Biol. 2017, 39, 1010428317695962 10.1177/1010428317695962. [DOI] [PubMed] [Google Scholar]
- Hultgren E. M.; Patrick M. E.; Evans R. L.; Stoos C. T.; Egland K. A. SUSD2 promotes tumor-associated macrophage recruitment by increasing levels of MCP-1 in breast cancer. PLoS One 2017, 12, e0177089 10.1371/journal.pone.0177089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.; Sun W.; Liao Y.; Zeng H.; Shan L.; Yin F.; Wang Z.; Zhou Z.; Hua Y.; Cai Z. Monocyte chemotactic protein-1 promotes the proliferation and invasion of osteosarcoma cells and upregulates the expression of AKT. Mol. Med. Rep. 2015, 12, 219–225. 10.3892/mmr.2015.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science 1989, 246, 603–608. 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Malumbres M.; Sotillo Ro.; Santamari′a D.; Galán J.; Cerezo A.; Ortega S.; Dubus P.; Barbacid M. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 2004, 118, 493–504. 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Yao C.; Wu S.; Li D.; Ding H.; Wang Z.; Yang Y.; Yan S.; Gu Z. Co-administration phenoxodiol with doxorubicin synergistically inhibit the activity of sphingosine kinase-1 (SphK1), a potential oncogene of osteosarcoma, to suppress osteosarcoma cell growth both in vivo and in vitro. Mol. Oncol. 2012, 6, 392–404. 10.1016/j.molonc.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L.; Cui X.; Zhang X.; Cheng L.; Liu Y.; Yang Y.; Fan P.; Wang Q.; Lin Y.; Zhang J. SARI inhibits angiogenesis and tumour growth of human colon cancer through directly targeting ceruloplasmin. Nat. Commun. 2016, 7, 11996 10.1038/ncomms11996. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wambecke A.; Laurent-Issartel C.; Leroy-Dudal J.; Giffard F.; Cosson F.; Lubin-Germain N.; Uziel J.; Kellouche S.; Carreiras F. Evaluation of the potential of a new ribavirin analog impairing the dissemination of ovarian cancer cells. PLoS One 2019, 14, e0225860 10.1371/journal.pone.0225860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J.; Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2– ΔΔCT method. Methods 2001, 25, 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]