Abstract
Background:
Multidisciplinary discussion (MDD) is widely recommended for patients with interstitial lung disease (ILD), but published primary data from MDD has been scarce, and factors influencing MDD other than chest computed tomography (CT) and lung histopathology interpretations have not been well-described.
Methods:
Single institution MDD of 179 patients with ILD.
Results:
MDD consensus clinical diagnoses included autoimmune-related ILD, chronic hypersensitivity pneumonitis, smoking-related ILD, idiopathic pulmonary fibrosis, medication-induced ILD, occupation-related ILD, unclassifiable ILD, and a few less common pulmonary disorders. In 168 of 179 patients, one or more environmental exposures or pertinent features of the medical history were identified, including recreational/avocational, residential, and occupational exposures, systemic autoimmune disease, malignancy, medication use, and family history. The MDD process demonstrated the importance of comprehensively assessing these exposures and features, beyond merely noting their presence, for rendering consensus clinical diagnoses. Precise, well-defined chest CT and lung histopathology interpretations were rendered at MDD, including usual interstitial pneumonia, nonspecific interstitial pneumonia, and organizing pneumonia, but these interpretations were associated with a variety of MDD consensus clinical diagnoses, demonstrating their nonspecific nature in many instances. In 77 patients in which MDD consensus diagnosis differed from referring diagnosis, assessment of environmental exposures and medical history was found retrospectively to be the most impactful factor.
Conclusions:
A comprehensive assessment of environmental exposures and pertinent features of the medical history guided MDD. In addition to rendering consensus clinical diagnoses, MDD presented clinicians with opportunities to initiate environmental remediation, behavior modification, or medication alteration likely to benefit individual patients with ILD.
Keywords: multidisciplinary discussion, interstitial lung disease, idiopathic pulmonary fibrosis, hypersensitivity, smoking, autoimmune, occupation
Introduction
Diagnosing the etiology of interstitial lung disease (ILD) can be a challenging aspect of caring for pulmonary patients and a barrier to initiating appropriate and efficacious treatment. Part of the challenge is that ILD encompasses a large number of separate disease entities with overlapping findings on diagnostic imaging and lung tissue sampling. In a recent survey of patients with ILD, many reported at least one misdiagnosis and experienced a median delay in treatment of approximately one year 1. Early diagnosis of ILD and idiopathic pulmonary fibrosis (IPF) in particular, along with timely initiation of treatment and lung transplantation referral, has been shown to have a survival benefit 2. One approach to facilitating a timely and accurate diagnosis of ILD has been presentation of patients for multidisciplinary ILD discussion (MDD). Generally, MDD incorporates input from expert pulmonary clinicians, thoracic radiologists, and thoracic pathologists regarding clinical information, chest imaging studies, and lung biopsy samples 3,4. Although MDD has now become widely recommended and often incorporated into clinical practice, the evidence for this recommendation is very limited and is likewise recognized as such in the published ILD practice guidelines 5–7 To the best of our knowledge, fewer than 15 studies have been published over the past two decades assessing primary data within an MDD and its impact on MDD consensus diagnoses 3,4,7–16.
In the small number of prior publications which have described primary data from MDD 3,4,7–16, almost all have focused on either interpretation of chest CT imaging, interpretation of surgical lung biopsy, or a comparison of MDD consensus clinical diagnoses compared to initial referring clinical diagnoses. Several publications focused on interobserver variability within MDD of chest CT imaging and/or surgical lung biopsy interpretation by experts in their respective fields, and how such interobserver variability may affect MDD consensus clinical diagnoses 10,13–15. Studies comparing MDD consensus diagnoses to initial referring diagnoses have generally demonstrated fewer patients with unclassifiable ILD following the MDD process 3,4,7,9,12.
Remarkably, no prior MDD studies have been published to the best of our knowledge which have focused on the importance of assessing environmental exposures and pertinent features of the medical history within MDD. These observations are surprising, given the well-known role of environmental exposures in the development of ILD, and since clinical assessment of these exposures along with the medical history is widely recognized by expert ILD clinicians as likely the most important components of the evaluation of a patient with ILD. Reasons for lack of published data in this regard are likely multifactorial, and may result from the difficulty in scientifically assessing how such input alters the MDD process and potentially alters MDD consensus diagnoses.
Based on the published guidelines and recommendations described above regarding MDD and ILD, we established an MDD process as part of the University of Maryland ILD program. As our MDD progressed in assessing individual patients, we observed that in addition to the well-known importance of expert radiology and pathology interpretations, assessments of environmental exposures (recreational, avocational, occupational and residential) along with pertinent features of the medical history guided the MDD process, and these assessments were impactful on the consensus clinical diagnoses rendered at MDD. The goal of this report is to present our findings with the hope of providing information which can be beneficial to health care providers caring for patients with ILD and ultimately to ILD patients.
Methods
Patients and MDD Data Collection
This study was reviewed and approved by the University of Maryland Institutional Review Board (HP-44077). Data were analyzed from 179 patients that were presented to the University of Maryland multidisciplinary ILD conference (MDD) between October 2016 and April 2019. Patients were presented to MDD at the discretion of the pulmonary physician who performed the initial ILD consultation at our institution. Standard demographic and logistical data which was transcribed and recorded from MDD included date of presentation to MDD, age, gender, race, date of CT chest, type and date of lung biopsy if applicable, MDD chest CT interpretation, MDD lung biopsy histopathologic interpretation and MDD consensus clinical diagnosis. Additionally, information was recorded at MDD regarding pertinent features of the medical history, which included past medical history (particularly in regard to the presence of systemic autoimmune disease or prior malignancy), medication use, family history of either lung disease or autoimmune disease, and autoimmune serologies which had been obtained prior to MDD. Similarly, detailed information was recorded at MDD regarding environmental exposures, which for the purposes of this study, were characterized as recreational/avocational (cigarettes, marijuana, heroin or cocaine use, or inhalation of vapors, gases, or fumes recreationally), residential (exposure to mold or avian proteins), or occupational (asbestos, silica, cement, concrete, wood, metal, or paper dust, or vapors, gases, or fumes).
MDD interpretation of chest CT imaging
All 179 patients had chest CT imaging performed prior to MDD and available for review at MDD. MDD chest CT interpretation was performed by thoracic radiologists and was rendered based on established radiologic terms and patterns according to published literature 5,17–21. Of note, we used the term smoking-related ILD when a broad spectrum of smoking-related parenchymal abnormalities were present on chest CT, including patterns consistent with respiratory bronchiolitis (RB), desquamative interstitial pneumonia (DIP), pulmonary Langerhans’ cell histiocytosis (PLCH), and emphysematous spaces with thickened fibrotic walls (emphysema with fibrosis) 19,22–25. The term unclassifiable fibrosis was rendered when findings of interstitial pulmonary fibrosis (reticulation, traction bronchiectasis, honeycomb change, volume loss) were present, but more specific radiologic patterns of pulmonary fibrosis such as usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), chronic hypersensitivity pneumonitis (HP), or smoking-related ILD were absent 17,26,27.
MDD interpretation of lung histopathology
In 56 patients, surgical lung biopsy (SLB) was performed prior to MDD and was available for review at MDD. MDD histopathology interpretation was performed by thoracic pathologists and was rendered based on established radiologic terms and patterns according to published literature 5,17,26,28–31. The term unclassifiable fibrosis was rendered when findings of histologic fibrosis were present on SLB, but more specific pathologic patterns of pulmonary fibrosis such as UIP, NSIP, OP, or chronic HP were absent 17,26.
Referring and MDD consensus clinical diagnoses
For the purposes of this study, we additionally accessed the electronic medical record (EMR) retrospectively to obtain the referring clinical diagnoses as documented in the EMR by the referring pulmonary physician. MDD consensus clinical diagnoses were rendered at MDD based on established published literature 5,17,20,28,32,33. We used the term smoking-related ILD to encompass a broad spectrum of smoking-related processes on imaging, including RB, DIP, PLCH, and emphysema with fibrosis, in association clinically with the presence of cigarette smoking or other substance use associated with smoke inhalation 19,22–25,34. The term unclassifiable ILD was assigned when ILD was present, but either two or more ILD diagnoses remained under consideration or the ILD remained unclassifiable 17,26,27.
In patients in which MDD consensus diagnosis differed compared to referring diagnosis, two authors (ND, NWT) retrospectively analyzed information provided at MDD regarding the discussion and assessment of the environmental exposures and pertinent features of the medical history, the specificity of chest CT interpretations rendered at MDD, and the specificity of SLB interpretations rendered at MDD. These two authors then retrospectively made a determination as to the primary factor or factors (assessment of environmental exposures and pertinent features of the medical history, chest CT interpretation or SLB interpretation) which were most impactful on the MDD consensus clinical diagnosis in each of these individual patients.
Statistical analyses
Referring clinical diagnoses and MDD consensus clinical diagnoses were compared by chi-square (Fisher’s exact test). P-value <0.05 denoted statistical significance.
Results
Patient Characteristics
We analyzed 179 patients presented at MDD between October 2016 and April 2019. The characteristics of the patients are shown in Table 1. There were slightly more males than females, and more Caucasian patients than patients of African American, Asian, or Hispanic ethnicity.
Table 1.
Characteristics of patients at time of presentation for multidisciplinary discussion (MDD) and findings of referring and MDD consensus clinical diagnoses (n = 179 total)
| Characteristics | |||
|---|---|---|---|
| Age, years, median [1st, 3rd] | 66 [57,70] | ||
| Gender | |||
| Male, n | 99 (55%) | ||
| Female, n | 80 (45%) | ||
| Ethnicity | |||
| Caucasian, n | 113 (63%) | ||
| African American, n | 51 (28%) | ||
| Asian, n | 8 (5%) | ||
| Hispanic, n | 7 (4%) | ||
| Clinical Diagnosis | Referring clinical diagnosis, n (%) | MDD consensus clinical diagnosis, n (%) | Chi-square |
| Unclassifiable ILD * | 67 (38%) | 29 (16%) | p < 0.001 |
| Autoimmune-related ILD | 32 (18%) | 40 (22%) | |
| Chronic hypersensitivity pneumonitis | 21 (12%) | 31 (17%) | |
| Idiopathic pulmonary fibrosis (IPF) | 16 (9%) | 17 (10%) | |
| Smoking-related ILD | 14 (8%) | 19 (11%) | |
| Occupation-related ILD | 7 (4%) | 6 (3%) | |
| Medication-induced ILD | 5 (3%) | 12 (7%) | |
| Other | 17 (9%) † | 25 (14%) ‡ | |
unclassifiable ILD indicates two or more ILD diagnoses remained under consideration or ILD remained unclassifiable.
cryptogenic organizing pneumonia (n = 4), sarcoidosis (n = 3), idiopathic nonspecific interstitial pneumonia (n = 2), constrictive bronchiolitis (n = 2), follicular bronchiolitis (n = 1), lymphangitic carcinoma (n = 1), chronic eosinophilic pneumonia (n = 1), pulmonary veno-occlusive disease (n = 1), congenital bronchopulmonary dysplasia (n = 1), and idiopathic pulmonary hemosiderosis (n = 1).
sarcoidosis (n = 2), constrictive bronchiolitis (n = 2), acute interstitial pneumonia (n = 2), diffuse pleural thickening (n = 2), emphysema (n = 2), no ILD (n = 2), pleuroparenchymal fibroelastosis (n = 1), congenital bronchopulmonary dysplasia (n = 1), idiopathic pulmonary hemosiderosis (n = 1), diffuse panbronchiolitis (n = 1), chronic eosinophilic pneumonia (n = 1), pulmonary capillary hemangiomatosis (n = 1), idiopathic nonspecific interstitial pneumonia (n = 1), lymphangitic carcinoma (n = 1), pulmonary veno-occlusive disease (n = 1), post-infectious ILD (n = 1), lymphocytic interstitial pneumonia (n = 1), chronic aspiration (n = 1), interstitial lung abnormalities (n = 1).
The referring clinical diagnoses and MDD consensus clinical diagnoses are also shown in Table 1. The most common referring diagnosis was unclassifiable ILD (67 patients, 38%) and fewer patients (29 patients, 16%) were rendered this diagnosis following MDD. Autoimmune-related ILD, chronic hypersensitivity pneumonitis, smoking-related ILD, and medication-induced ILD were each observed to be more frequent clinical diagnoses following MDD compared to referring diagnosis. Idiopathic pulmonary fibrosis (IPF) was perhaps less frequent than anticipated in both referring diagnosis (16 patients, 9%) and MDD consensus diagnosis (17 patients, 10%). A minority of patients had variety of less common pulmonary disorders both at referral and with MDD, and these are listed in detail in the table legend of Table 1.
Presence of environmental exposures and pertinent features of the medical history
As seen in Table 2, almost all patients, 168 of 179 patients, had one or more environmental exposure or pertinent feature of the medical history which was identified. A majority of patients (132 patients, 73%) had concerning environmental exposures, with many having had more than one exposure which required an assessment at MDD. Smoke inhalation due to cigarette use (84 patients, 47%, median pack-years 20 [10,35]) or other substance use was very prevalent in this cohort, along with inhalation of other recreational substances in a minority of patients. Exposure to organic antigens such as mold or avian proteins within the residential environment was likewise common (52 patients, 29%). Similarly, many concerning exposures related to the patients’ occupation were observed, including exposure to silicates (asbestos and silica), wood, metal, or paper dust, and a variety of exposures to vapors, gases or fumes.
Table 2.
Environmental exposures and pertinent features of the medical history which required assessment at MDD
| n (% of 179 total patients) * | |
|---|---|
| Environmental Exposures | 132 (73%) |
| Avocational / Recreational | 87 (49%) |
| Cigarette smoking | 84 (47%) |
| Pack-years, median [1st, 3rd] | 20 [10,35] |
| Marijuana | 10 (6%) |
| Heroin or cocaine | 7 (4%) |
| Wood, metal, or paper dust | 4 (2%) |
| Vapors, gases, or fumes | 2 (1%) |
| Other † | 2 (1%) |
| Residential | 52 (29%) |
| Mold | 37 (21%) |
| Avian proteins | 24 (13%) |
| Occupational | 42 (23%) |
| Vapors, gases, or fumes | 14 (8%) |
| Silica, cement, or concrete | 13 (7%) |
| Asbestos | 12 (7%) |
| Wood, metal, or paper dust | 7 (3%) |
| Other ‡ | 4 (2%) |
| Medical History | 66 (35%) |
| Autoimmune disease | 53 (29%) |
| Anti-synthetase syndrome | 15 (8%) |
| Undifferentiated connective tissue disease | 13 (7%) |
| Rheumatoid arthritis | 11 (6%) |
| Systemic lupus erythematosus | 5 (3%) |
| Sjogren’s syndrome | 3 (2%) |
| Other ¶ | 6 (3%) |
| Medication use | 20 (11%) |
| Amiodarone or Dronedarone | 6 (3%) |
| Methotrexate or Leflunomide | 5 (3%) |
| TNF inhibitors | 3 (2%) |
| Systemic chemotherapy (antineoplastic) | 2 (1%) |
| Other § | 5 (3%) |
| Malignancy | 6 (3%) |
| Hematologic | 2 (1%) |
| Solid organ | 4 (2%) |
| Family history of ILD or autoimmune disease | 6 (3%) |
note: many individual patients had more than one environmental exposure and/or pertinent feature of the medical history which required assessment at MDD, thus numbers of patients in the sub-categories could exceed those in the respective categories.
vaping (n = 1), organic animal proteins (n = 1).
hard metals (n = 1), beryllium (n = 1), burn pits (n = 1), dioxin (agent orange, n = 1).
psoriasis (n = 3), antineutrophilic cymiddlelasmic antibody-associated vasculitis (n = 2), and systemic sclerosis (n = 1).
rituximab (n = 1), sirolimus (n = 1), basiliximab (n = 1), ibrutinib (n = 1), evolocumab (n = 1).
A history of systemic autoimmune disease was common (53 patients, 29%) and consisted of a variety of well-defined autoimmune diseases. Anti-synthetase syndrome was the most common autoimmune process identified, and many different myositis–specific and myositis–associated antibodies were observed in these patients. A history of prior malignancy was noted in a few patients, and similarly a family history of either lung disease or autoimmune disease was noted in a few patients. Medications which have been well-associated with pulmonary toxicity were either currently being used or had been previously taken in 20 patients (11%). Amiodarone (or dronedarone) was the most common concerning medication observed, but a rather diverse group of medications were noted, described in the body and table legend of Table 2. As previously shown in Table 1, the percentage of patients rendered with medication-induced ILD was higher following MDD.
Association of environmental exposures and pertinent features of the medical history with MDD consensus clinical diagnoses
When examining the information in Tables 1 and 2, it can be observed and concluded that concerning environmental exposures or pertinent features of the medical history did not necessarily equate with an individual patient’s lung disease being directly related to those exposures or features, and these observations are further detailed in Table 3. For example, as seen in Table 3, there were 84 patients with a history of cigarette smoking, but only 18 of these patients had a rendered MDD consensus clinical diagnosis of smoking-related ILD, with the remaining patients having a variety of other consensus diagnoses rendered. In the 52 patients with concerning residential mold and/or avian exposures, a majority did have an MDD consensus diagnosis of chronic HP, whereas in the 42 patients with concerning occupational exposures, only a minority had an MDD consensus diagnosis of occupation-related ILD, with a variety of other forms of ILD rendered in the remainder.
Table 3.
Association of environmental exposures and pertinent features of the medical history with MDD consensus clinical diagnoses
| Environmental Exposures * | MDD Consensus Clinical Diagnosis | n |
|---|---|---|
| Recreational: cigarette smoking (n = 84) |
Smoking-related ILD | 18 |
| Autoimmune-related ILD | 12 | |
| Chronic hypersensitivity pneumonitis (HP) | 12 | |
| Idiopathic pulmonary fibrosis (IPF) | 5 | |
| Occupation-related ILD | 5 | |
| Medication-induced ILD | 5 | |
| Other ‡ | 13 | |
| Unclassifiable ILD † | 14 | |
| Residential: mold or avian proteins (n = 52) |
Chronic hypersensitivity pneumonitis (HP) | 29 |
| Smoking-related ILD | 6 | |
| Autoimmune-related ILD | 5 | |
| Medication-induced ILD | 2 | |
| Idiopathic pulmonary fibrosis (IPF) | 1 | |
| Occupation-related ILD | 1 | |
| Other ¶ | 3 | |
| Unclassifiable ILD † | 5 | |
| Occupational: gases, silica, asbestos, dust, hard metals (n = 42) |
Idiopathic pulmonary fibrosis (IPF) | 8 |
| Occupation-related ILD | 6 | |
| Chronic hypersensitivity pneumonitis (HP) | 6 | |
| Smoking-related ILD | 5 | |
| Autoimmune-related ILD | 2 | |
| Other § | 6 | |
| Unclassifiable ILD † | 9 | |
| Medical History * | MDD Consensus Clinical Diagnosis | n |
| Autoimmune disease (n = 53) |
Autoimmune-related ILD | 39 |
| Medication-induced ILD | 5 | |
| Occupation-related ILD | 1 | |
| Idiopathic pulmonary fibrosis (IPF) | 1 | |
| Other # | 2 | |
| Unclassifiable ILD † | 5 | |
| Medication use (n = 20) |
Medication-induced ILD | 12 |
| Autoimmune-related ILD | 3 | |
| Other ** | 1 | |
| Unclassifiable ILD † | 4 | |
note: many patients had more than one environmental exposure or pertinent feature of the medical history requiring assessment at MDD, thus total numbers of patients in this column add to greater than 179
unclassifiable ILD indicates two or more ILD diagnoses remained under consideration or ILD remained unclassifiable.
diffuse pleural thickening (n = 2), emphysema (n = 2), diffuse panbronchiolitis (n = 1), idiopathic nonspecific interstitial pneumonia (n = 1), interstitial lung abnormalities (n = 1), lymphangitic carcinoma (n = 1), lymphocytic interstitial pneumonia (n = 1), sarcoidosis (n = 1), pulmonary veno-occlusive disease (n = 1), post-infectious ILD (n = 1), pleuroparenchymal fibroelastosis (n = 1).
chronic aspiration (n = 1), post-infectious ILD (n = 1), sarcoidosis (n = 1).
diffuse pleural thickening (n = 2), acute interstitial pneumonia (n = 1), constrictive bronchiolitis (n = 1), lymphocytic interstitial pneumonia (n = 1), post-infectious ILD (n = 1).
emphysema (n = 1), normal lung parenchyma (n = 1).
normal lung parenchyma (n = 1).
In regard to features of the medical history, as seen in Table 3, 53 patients had a history of systemic autoimmune disease, but only 39 of these patients were rendered an MDD consensus clinical diagnosis of autoimmune-related ILD. Similar findings were observed in the 20 patients who had taken medications which have been well-associated with pulmonary toxicity. These observations overall demonstrate that it is not merely the presence of environmental exposures and pertinent features of the medical history, but it’s their assessment at MDD that is important, assessing the propensity, intensity and duration of the exposures themselves and within the context of expert chest CT imaging and lung histopathology interpretations.
Association of chest CT imaging interpretations with MDD consensus clinical diagnoses
All 179 patients had chest CT imaging available for interpretation at MDD. Table 4 shows an overview of 150 patients in which the most frequently observed radiologic interpretations were rendered at MDD, and the associated MDD consensus clinical diagnoses. Three common radiologic patterns observed were UIP, NSIP, and OP, and Table 4 demonstrates that each of these precise radiologic patterns was associated with a variety of MDD consensus clinical diagnoses. In the 31 patients with UIP on imaging, 17 had an MDD consensus clinical diagnosis of IPF, but other forms of ILD were found in the remaining 14 patients. Imaging interpretations of NSIP and OP were observed in 21 and 16 patients, respectively, and were often associated with autoimmune-related ILD, but these radiologic patterns were observed in other forms of ILD as well. Interpretations of multifocal GGO, widespread mosaicism, and unclassifiable fibrosis were observed in many patients and were associated with many different forms of ILD. Thus, although precise, well-defined radiologic interpretations including UIP, NSIP, and OP were rendered at MDD, they were associated with a variety of clinical forms of ILD, indicating their nonspecific nature in many instances. Panels A and B in Figure 1 demonstrate representative associations of chest CT imaging interpretations with MDD consensus clinical diagnoses.
Table 4.
Association of chest CT imaging interpretations with MDD consensus clinical diagnoses in 150 of 179 patients
| Chest CT Imaging Interpretation | MDD Consensus Clinical Diagnosis | n |
|---|---|---|
| Usual interstitial pneumonia (UIP) (n = 31) |
Idiopathic pulmonary fibrosis (IPF) | 17 |
| Autoimmune-related ILD | 8 | |
| Chronic hypersensitivity pneumonitis (HP) | 2 | |
| Medication-induced ILD | 2 | |
| Occupation-related ILD | 1 | |
| Unclassifiable ILD * | 1 | |
| Nonspecific interstitial pneumonia (NSIP) (n = 21) |
Autoimmune-related ILD | 11 |
| Chronic hypersensitivity pneumonitis (HP) | 4 | |
| Medication-induced ILD | 1 | |
| Acute interstitial pneumonia (AIP) | 1 | |
| Idiopathic NSIP | 1 | |
| Unclassifiable ILD * | 3 | |
| Organizing pneumonia (OP) (n = 16) |
Autoimmune-related ILD | 10 |
| Chronic hypersensitivity pneumonitis (HP) | 2 | |
| Medication-induced ILD | 1 | |
| Acute interstitial pneumonia (AIP) | 1 | |
| Unclassifiable ILD * | 2 | |
| Multifocal ground glass opacities (GGO) (n = 16) |
Medication-induced ILD | 4 |
| Autoimmune-related ILD | 2 | |
| Smoking-related ILD | 2 | |
| Chronic hypersensitivity pneumonitis (HP) | 1 | |
| Other ‡ | 4 | |
| Unclassifiable ILD * | 3 | |
| Widespread mosaicism (n = 13) |
Chronic hypersensitivity pneumonitis (HP) | 4 |
| Autoimmune-related ILD | 2 | |
| Medication-induced ILD | 1 | |
| Occupation-related ILD | 1 | |
| Other ¶ | 3 | |
| Unclassifiable ILD * | 2 | |
| Unclassifiable fibrosis † (n = 25) |
Chronic hypersensitivity pneumonitis (HP) | 8 |
| Occupation-related ILD | 3 | |
| Medication-induced ILD | 2 | |
| Smoking-related ILD | 1 | |
| Unclassifiable ILD * | 11 | |
| Smoking-related ILD (n = 17) |
Smoking-related ILD | 14 |
| Chronic hypersensitivity pneumonitis (HP) | 2 | |
| Autoimmune-related ILD | 1 | |
| Chronic hypersensitivity pneumonitis (HP) (n = 11) |
Chronic hypersensitivity pneumonitis (HP) | 8 |
| Occupation-related ILD | 1 | |
| Unclassifiable ILD * | 2 | |
unclassifiable ILD indicates two or more ILD diagnoses remained under consideration or ILD remained unclassifiable.
unclassifiable fibrosis indicates radiologic findings of pulmonary fibrosis were present, but more specific radiologic patterns of fibrosis were absent.
chronic aspiration (n = 1), constrictive bronchiolitis (n = 1), post-infectious ILD (n = 1), chronic eosinophilic pneumonia (n = 1).
idiopathic pulmonary hemosiderosis (n = 1), constrictive bronchiolitis (n = 1), congenital bronchopulmonary dysplasia (n = 1).
Figure 1.
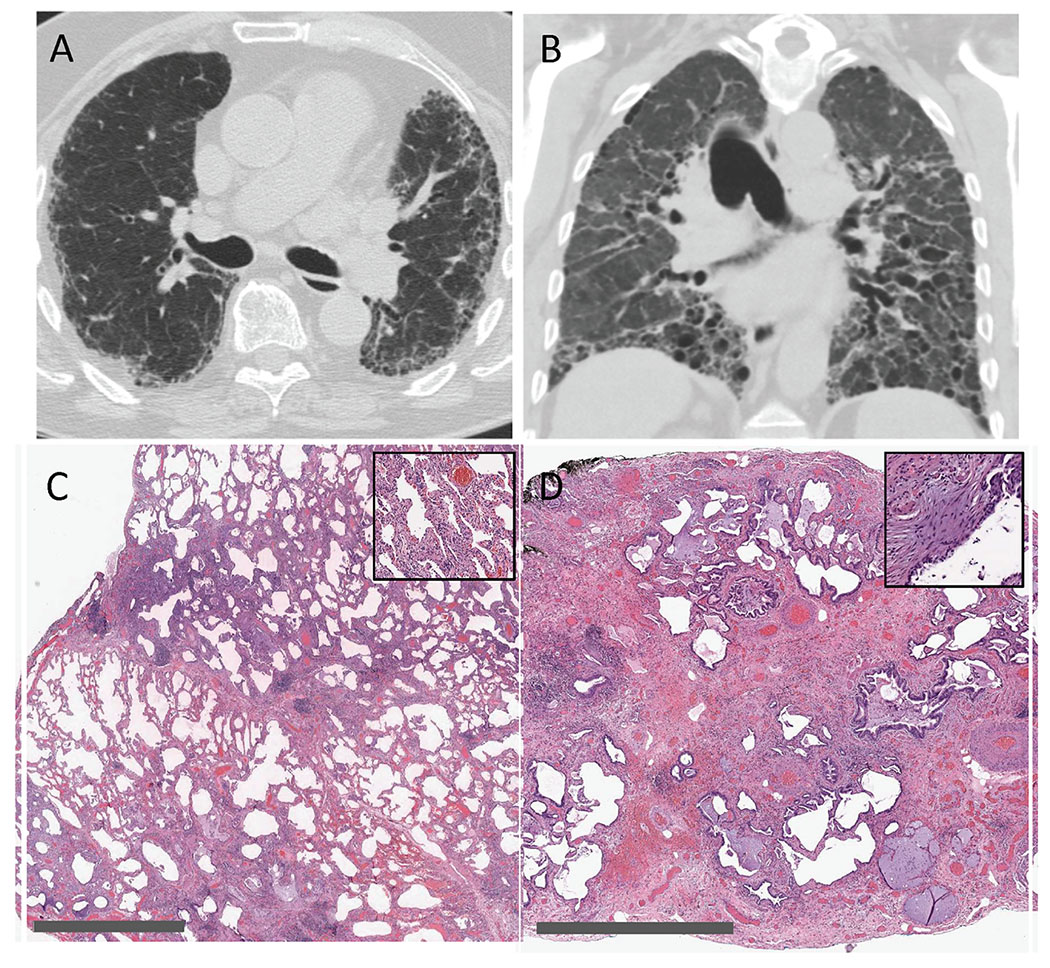
Representative images demonstrating the association of environmental exposures and medical history assessment with radiologic and histopathologic interpretations. (A) Axial chest CT image demonstrating peripheral reticulation, traction bronchiectasis (more apparent on coronal reformatting) and honeycombing in a basilar-predominant distribution; MDD chest CT interpretation was typical UIP. The pleural-based opacities seen in the periphery of the right hemithorax are consistent with pleural fat on mediastinal windowing, a finding often observed with pulmonary fibrosis. Medical history was pertinent for clinical and laboratory findings consistent with antisynthetase syndrome, including the serologic presence of myositis-specific antibodies anti-NXP-2 and anti-MDA5. MDD consensus clinical diagnosis was autoimmune-related ILD. (B) Coronal CT image demonstrating widespread traction bronchiectasis and lower lobe volume loss consistent with severe pulmonary fibrosis, but lacking the typical radiologic features and distribution of UIP, NSIP, or OP; MDD chest CT interpretation was unclassifiable fibrosis. Exposure history indicated many years as a machinist in the steel industry, with longterm exposure to stone grinding wheels and hard-metals. MDD consensus clinical diagnosis was occupation-related ILD. (C) Low magnification SLB histopathology image demonstrating widespread thickening of interalveolar septae in a geographically homogenous pattern, and with high magnification (inset) demonstrating both cellular and fibrotic components; MDD histopathologic interpretation was NSIP. Exposure history indicated long-term avian exposures, and medical history indicated intermittent fever, myalgias, dyspnea, and serologic testing consistent with avian protein exposure, all consistent with HP, despite the lack of bronchiolocentric or granulomatous inflammation on SLB. MDD consensus clinical diagnosis was chronic HP. (D) Low magnification SLB histopathology image demonstrating pulmonary fibrosis, collapse of secondary pulmonary lobules, honeycombing, and fibroblastic foci (inset); MDD histopathologic interpretation was UIP. Medical history indicated treatment with infliximab, and her clinical course was most consistent with subacute and chronic lung injury as a result of infliximab therapy. MDD consensus clinical diagnosis was medication-induced ILD. Gray scale bars in lower left corners of panels C and D represent 3 mm and 2 mm, respectively.
Although Table 4 demonstrates that many chest CT interpretations may be nonspecific, the imaging interpretations of chronic HP and smoking-related ILD were more closely associated with MDD consensus clinical diagnoses of chronic HP and smoking-related ILD, respectively. It should be noted that even these relatively specific radiologic patterns were in a few instances associated with alternative MDD consensus clinical diagnoses.
Association of surgical lung biopsy interpretations with MDD consensus clinical diagnoses
Surgical lung biopsy (SLB) had been performed and was available for review at MDD in 56 patients. Table 5 shows an overview of 41 patients in which the most frequently observed histopathologic interpretations were rendered at MDD, and the associated MDD consensus clinical diagnoses. Common histologic patterns observed on SLB were UIP (12 patients) and NSIP (12 patients), but similar to the chest CT imaging observations, both of these well-defined histopathologic patterns were associated with a variety of MDD consensus clinical diagnoses, demonstrating their nonspecific nature in many instances. Unclassifiable fibrosis (9 patients) was likewise associated with several different MDD consensus clinical diagnoses. Table 5 does show that the histopathologic interpretations of OP and HP were closely associated with autoimmune-related ILD and chronic HP, respectively, although the number of patients with these patterns on SLB was small in our group of patients. Panels C and D in Figure 1 demonstrate representative associations of SLB interpretations with MDD consensus clinical diagnoses.
Table 5.
Association of surgical lung biopsy (SLB) interpretations with MDD consensus clinical diagnoses in 41 of 56 patients
| SLB Histopathologic Interpretation | MDD Consensus Clinical Diagnosis | n |
|---|---|---|
| Usual interstitial pneumonia (UIP) (n = 12) |
Idiopathic pulmonary fibrosis (IPF) | 4 |
| Medication-induced ILD | 2 | |
| Autoimmune-related ILD | 1 | |
| Chronic hypersensitivity pneumonitis (HP) | 1 | |
| Occupation-related ILD | 1 | |
| Unclassifiable ILD * | 3 | |
| Nonspecific interstitial pneumonia (NSIP) (n = 12) |
Chronic hypersensitivity pneumonitis (HP) | 5 |
| Autoimmune-related ILD | 3 | |
| Idiopathic pulmonary fibrosis (IPF) | 1 | |
| Acute interstitial pneumonia (AIP) | 1 | |
| Idiopathic NSIP | 1 | |
| Unclassifiable ILD * | 1 | |
| Unclassifiable fibrosis † (n = 9) |
Smoking-related ILD | 5 |
| Chronic hypersensitivity pneumonitis (HP) | 1 | |
| Unclassifiable ILD * | 3 | |
| Organizing pneumonia (OP) (n = 4) |
Autoimmune-related ILD | 4 |
| Hypersensitivity pneumonitis (n = 4) |
Chronic hypersensitivity pneumonitis (HP) | 4 |
unclassifiable ILD indicates two or more ILD diagnoses remained under consideration or ILD remained unclassifiable.
unclassifiable fibrosis indicates histologic findings of pulmonary fibrosis were present, but more specific histopathologic patterns of fibrosis were absent.
Relative contribution of factors when MDD consensus clinical diagnoses differed from referring clinical diagnoses
As shown in Table 1, referring clinical diagnoses and MDD consensus clinical diagnoses differed (p < 0.001). Overall, MDD consensus clinical diagnosis was identical to the referring clinical diagnosis in 102 patients (57%) and differed in 77 patients (43%). The four most common MDD consensus clinical diagnoses in these 77 patients were chronic HP (14 patients), autoimmune-related ILD (12 patients), medication-induced ILD (9 patients), and IPF (9 patients).
In these 77 patients in whom MDD consensus clinical diagnoses differed compared to referring clinical diagnoses, a retrospective determination was made as to the primary factor or factors that were most impactful on individual MDD consensus clinical diagnoses: assessment of environmental exposures and medical history, chest CT interpretation or SLB interpretation. Overall, the results of these determinations are shown in the Venn diagram in Figure 2. The comprehensive assessment of environmental exposures and pertinent features of the medical history had the greatest impact on MDD consensus clinical diagnoses in these patients, being the sole primary factor in 28 of 77 patients and a factor in combination with chest CT interpretation in an additional 25 patients.
Figure 2.
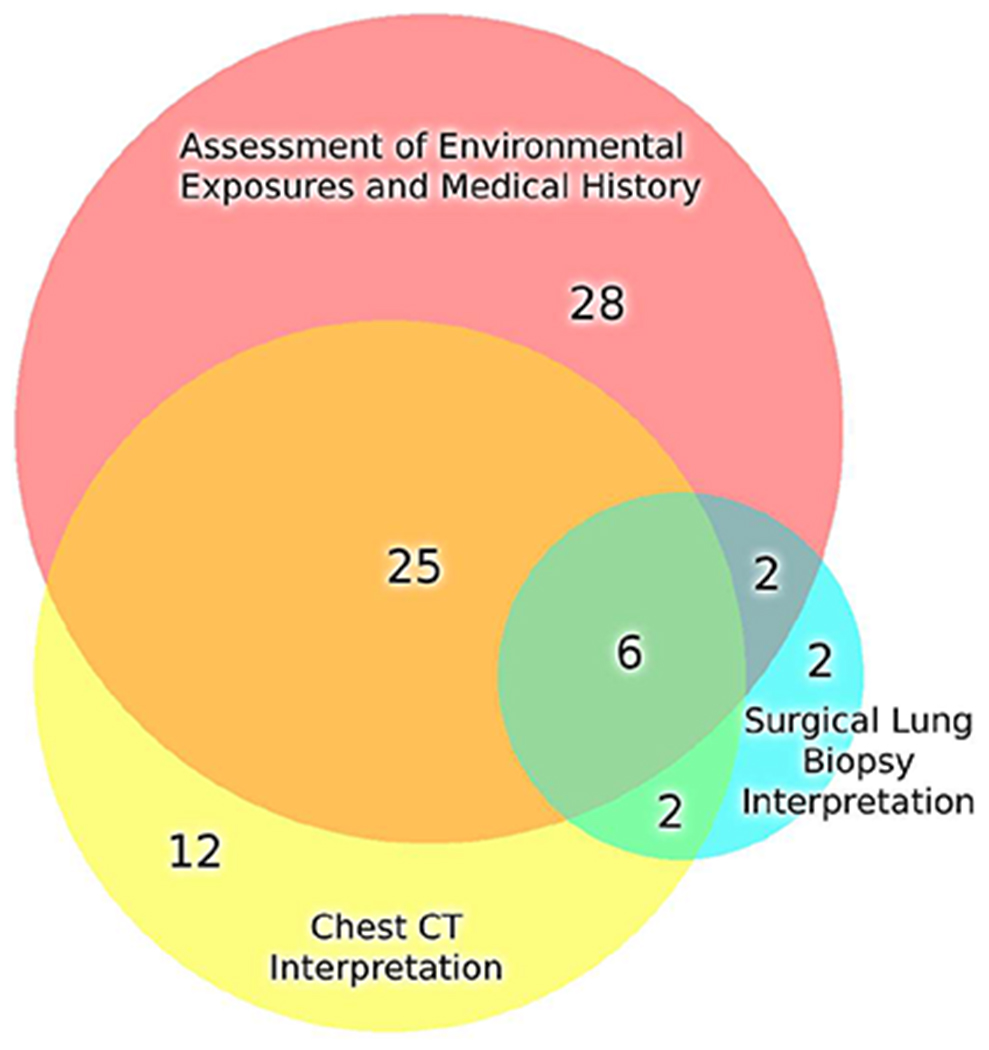
Venn diagram of 77 patients in which MDD consensus clinical diagnosis differed from referring clinical diagnosis, and demonstrating the relative contribution of individual factors (assessment of environmental exposures and pertinent features of the medical history, chest CT interpretation, and SLB interpretation) that were most impactful on MDD consensus clinical diagnoses. Of note, the overall size of the three circles reflects the numbers of patients enclosed within the circles. Assessment of environmental exposures and medical history had the greatest impact on MDD consensus clinical diagnoses in these 77 patients.
Discussion
The major observation of our study is the importance of comprehensively assessing recreational, avocational, residential, and occupational exposures along with pertinent features of the medical history in patients with ILD presented for MDD. It is not merely the presence of these exposures and features that is important, but it is their expert assessment that guides MDD and allows for precise and accurate rendering of MDD consensus clinical diagnoses. Some of this assessment is related to expertise regarding specifics of the exposure, such as its propensity to cause pulmonary disease, intensity and duration of the exposure, or presence of alternative exposures, and to the particular specifics of the medical history, whereas other portions relate to expert interpretation of chest CT imaging and lung histopathology. In our subgroup of 77 patients in which MDD consensus clinical diagnoses differed from referring clinical diagnoses, we found that a comprehensive assessment of environmental exposures and medical history was the most impactful factor on MDD consensus clinical diagnoses in this group of patients.
Our findings do not negate the fact that precise and well-defined interpretations of chest CT imaging and SLB histopathology are essential in the accurate diagnosis of all forms of ILD. In some of the uncommon pulmonary disorders seen in the legend of Table 1, imaging or histopathology likely provides the precise diagnosis in the absence of any assessment of exposure or medical history. Nevertheless, our data demonstrates that well-defined radiologic and histopathologic patterns are often associated with a wide variety of clinical forms of ILD (Tables 4 and 5), demonstrating their nonspecific nature in many instances. Recent published guidelines have also recognized the nonspecific nature of chest CT imaging in selected instances, stating that chronic HP, for example, may present with imaging patterns which are “neither suggestive nor compatible with features of HP” 35.
Previous studies describing primary MDD data 3,4,7–16 have focused almost exclusively on such chest CT and lung histopathology interpretations. This approach may appear somewhat surprising to ILD clinicians, since an assessment of exposures within the patient’s environment as well as pertinent features of the medical history are widely recognized as likely the most important components of the clinical evaluation of a patient with ILD. Some of those earlier studies did focus more on the “idiopathic” interstitial pneumonias, and thus may have assumed that these exposures and features would play a lesser role. Our data do support active involvement in the MDD process by pulmonary ILD clinicians with extensive experience in assessing the significance and potential role of a multitude of environmental exposures. As availability of these experts may be limited, pulmonary physicians may benefit from implementing standardized tools to increase recognition of potentially relevant exposures, including the use of a standardized exposure questionnaire or the integration of smart phrases and automated clinical decision support into electronic health records 33,36,37.
It is interesting to note that none of our 179 patients had an MDD consensus clinical diagnosis of cryptogenic (idiopathic) organizing pneumonia (COP) and only one patient had idiopathic NSIP. As shown in Tables 4 and 5, many patients did have MDD radiologic and histologic interpretations of NSIP and OP, but these patients were rendered with well-recognized clinical forms of ILD following MDD rather than idiopathic disease. A prior MDD publication found similar results, in which no patients (out of 90) had an MDD clinical diagnosis of idiopathic NSIP, leading the authors to state that idiopathic NSIP is a “very rare condition” 9. Similarly in regard to idiopathic disease, only ten percent of our patients (17 of 179) had an MDD consensus clinical diagnosis of IPF, which is perhaps surprising since IPF has generally been described as one of the most common ILDs overall and at MDD 3,7,9–11,14. This discrepancy is likely due to our thoracic radiologists utilizing precise and strict radiologic criteria for typical or probable UIP which have evolved over the past 5 years 5, as well as the comprehensive and detailed assessment of environmental exposures and medical history within our MDD. In a recent Fleischner Society publication, the authors stated that a diagnosis of IPF “requires an inquiring mind, a clear understanding of the differential diagnosis for IPF, and a comprehensive and structured approach to help exclude known causes and associations of fibrosing lung disease” 20. In our experience, the greater the expertise in pulmonary, occupational, and environmental medicine present at MDD and the greater depth in which a patient’s environmental exposures and medical history are explored, the more likely that a diagnosis other than idiopathic disease (IPF, COP, idiopathic NSIP) will be rendered.
One potential interpretation of our results is in conjunction with the recently published study on the efficacy of antifibrotic therapy (nintedanib) in progressive fibrosing ILD 38. That study demonstrated beneficial use of antifibrotic therapy not only for patients with IPF, but also for patients with a wide variety of other forms of fibrosing ILD. It could therefore be asked whether establishing a precise MDD consensus diagnosis is actually impactful. However, currently approved antifibrotics have not to date been demonstrated to prolong survival, although they have been shown to slow pulmonary function decline. Thus within this context, a major benefit of assessing environmental exposures and the medical history within MDD will be the opportunity for clinicians to initiate environmental remediation, behavior modification, and/or a medication alteration in selected patients, which perhaps may be one of the most beneficial and efficacious interventions in many patients with ILD 33,39–41.
We fully recognize the limitations of the present study. First, the retrospective nature of this study does not allow us to have longitudinal or outcome data that would support or refute our MDD consensus clinical diagnoses, a limitation which can be overcome in future prospective research after more time has elapsed for follow up. Second, in the group of 77 patients in which MDD consensus diagnosis differed from referring, our determination that assessment of environmental exposures and the medical history had the greatest impact was a retrospective judgement determination. It is interesting to note that almost half of these patients (35 of 77) had an MDD consensus diagnosis of either chronic HP, autoimmune-related ILD, or medication-induced ILD, forms of ILD which required a major contribution from the exposure and medical history assessment. Additionally, although precise, well-defined radiologic and histologic patterns were rendered, these patterns were often nonspecific as shown in Tables 4 and 5, leading to CT and SLB interpretations being less impactful when MDD consensus diagnosis differed compared to referring. Conversely, in other patients, rather specific radiologic (e.g., pleuroparenchymal fibroelastosis) or histologic (e.g., diffuse panbronchiolitis) patterns were rendered, instances in which chest CT interpretation and SLB interpretation, respectively, were determined to be the most impactful factors. Overall, we concluded that the assessment of environmental exposures and the medical history had the greatest impact on MDD consensus diagnoses in these 77 patients, but this was a retrospective judgment and clearly open to critique by other expert pulmonary clinicians, radiologists, and pathologists. Third, the possibility of selection bias exists, as patients were presented to MDD at the discretion of the pulmonary physician who performed the initial ILD consultation at our institution. The pulmonary physician may have thus been swayed to present patients to MDD only if the ILD diagnosis was particularly confusing or complex, and it is possible that patients with more straight-forward ILD were simply not presented to MDD. Fourth, it was difficult to numerically quantify many of the environmental exposures which were described and assessed in Table 2. For cigarette smoking, quantifying this exposure was rather straight-forward and was expressed as pack-years, but for most of the other exposures, we relied on information presented at MDD, in which exposures were most often characterized with terms such as substantial or multi-year. As part of the MDD process, the group of expert pulmonologists, radiologists, and pathologists provided their best expertise as to the significance and potential causal relationship in regard to each of the exposures which were assessed.
MDD has now become widely recommended by expert guidelines and is often incorporated into clinical practice. In our current study, a comprehensive assessment of environmental exposures and pertinent features of the medical history guided the MDD process, as some of the most vital input came from clinicians with expertise in pulmonary medicine and as well as expertise in occupational and environmental exposures. Due to the large number of patients requiring expert assessment of occupational and environmental exposures, implementation of a formal education process in this regard to pulmonary trainees as well as the use of standardized tools, such as an exposure questionnaire or integration of smart phrases into the EMR, should be considered to promote an accurate consensus diagnosis at MDD and to clinically benefit all patients with ILD 33,36,37. An accurate MDD consensus clinical diagnosis likely provides the most appropriate, patient-centered, therapeutic strategies, and perhaps most importantly, MDD may be able to determine whether initiation of an environmental remediation, behavior modification or medication alteration would likely provide a beneficial effect in individual patients with ILD.
Highlights.
Multidisciplinary discussion (MDD) is endorsed for interstitial lung disease (ILD)
Prior published MDD data has focused on chest CT and lung biopsy interpretations
Our MDD showed the value of assessing environmental exposures and medical history
MDD may offer opportunities for environmental, behavior, or medication modification
Acknowledgments
This work was supported by the NIH R01HL126897 and VA Merit Awards I01CX000101 and I01BX002499.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Cosgrove GP, Bianchi P, Danese S, Lederer DJ. Barriers to timely diagnosis of interstitial lung disease in the real world: the INTENSITY survey. BMC Pulm Med. 2018;18(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med. 2011;184(7):842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Sadeleer LJ, Meert C, Yserbyt J, et al. Diagnostic Ability of a Dynamic Multidisciplinary Discussion in Interstitial Lung Diseases: A Retrospective Observational Study of 938 Cases. Chest. 2018;153(6):1416–1423. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri N, Spencer L, Greaves M, Bishop P, Chaturvedi A, Leonard C. A Review of the Multidisciplinary Diagnosis of Interstitial Lung Diseases: A Retrospective Analysis in a Single UK Specialist Centre. J Clin Med. 2016;5(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. [DOI] [PubMed] [Google Scholar]
- 6.Prasad JD, Mahar A, Bleasel J, et al. The interstitial lung disease multidisciplinary meeting: A position statement from the Thoracic Society of Australia and New Zealand and the Lung Foundation Australia. Respirology. 2017;22(7):1459–1472. [DOI] [PubMed] [Google Scholar]
- 7.Jo HE, Glaspole IN, Levin KC, et al. Clinical impact of the interstitial lung disease multidisciplinary service. Respirology. 2016;21(8):1438–1444. [DOI] [PubMed] [Google Scholar]
- 8.Borie R, Kannengiesser C, Gouya L, et al. Pilot experience of multidisciplinary team discussion dedicated to inherited pulmonary fibrosis. Orphanet J Rare Dis. 2019;14(1):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai GT, Tan TC, Lee YS, et al. Impact of an interstitial lung disease service in the diagnosis and management of interstitial lung disease in Singapore. Singapore Med J. 2020;61(6):302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flaherty KR, King TE Jr., Raghu G, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med. 2004;170(8):904–910. [DOI] [PubMed] [Google Scholar]
- 11.Fujisawa T, Mori K, Mikamo M, et al. Nationwide cloud-based integrated database of idiopathic interstitial pneumonias for multidisciplinary discussion. Eur Respir J. 2019;53(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grewal JS, Morisset J, Fisher JH, et al. Role of a Regional Multidisciplinary Conference in the Diagnosis of Interstitial Lung Disease. Ann Am Thorac Soc. 2019;16(4):455–462. [DOI] [PubMed] [Google Scholar]
- 13.Walsh SLF, Wells AU, Desai SR, et al. Multicentre evaluation of multidisciplinary team meeting agreement on diagnosis in diffuse parenchymal lung disease: a case-cohort study. Lancet Respir Med. 2016;4(7):557–565. [DOI] [PubMed] [Google Scholar]
- 14.Flaherty KR, Andrei AC, King TE Jr., et al. Idiopathic interstitial pneumonia: do community and academic physicians agree on diagnosis? Am J Respir Crit Care Med. 2007;175(10):1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomeer M, Demedts M, Behr J, et al. Multidisciplinary interobserver agreement in the diagnosis of idiopathic pulmonary fibrosis. Eur Respir J. 2008;31(3):585–591. [DOI] [PubMed] [Google Scholar]
- 16.Burge PS, Reynolds J, Trotter S, Burge GA, Walters G. Histologist’s original opinion compared with multidisciplinary team in determining diagnosis in interstitial lung disease. Thorax. 2017;72(3):280–281. [DOI] [PubMed] [Google Scholar]
- 17.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva CI, Muller NL, Lynch DA, et al. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology. 2008;246(1):288–297. [DOI] [PubMed] [Google Scholar]
- 19.Galvin JR, Franks TJ. Smoking-related lung disease. J Thorac Imaging. 2009;24(4):274–284. [DOI] [PubMed] [Google Scholar]
- 20.Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6(2):138–153. [DOI] [PubMed] [Google Scholar]
- 21.Galvin JR, Frazier AA, Franks TJ. Collaborative radiologic and histopathologic assessment of fibrotic lung disease. Radiology. 2010;255(3):692–706. [DOI] [PubMed] [Google Scholar]
- 22.Franks TJ, Galvin JR. Smoking-Related “Interstitial” Lung Disease. Arch Pathol Lab Med. 2015;139(8):974–977. [DOI] [PubMed] [Google Scholar]
- 23.Attili AK, Kazerooni EA, Gross BH, Flaherty KR, Myers JL, Martinez FJ. Smoking-related interstitial lung disease: radiologic-clinical-pathologic correlation. Radiographics. 2008;28(5):1383–1396; discussion 1396-1388. [DOI] [PubMed] [Google Scholar]
- 24.Churg A, Hall R, Bilawich A. Respiratory bronchiolitis with fibrosis-interstitial lung disease: a new form of smoking-induced interstitial lung disease. Arch Pathol Lab Med. 2015;139(4):437–440. [DOI] [PubMed] [Google Scholar]
- 25.Flaherty KR, Fell C, Aubry MC, et al. Smoking-related idiopathic interstitial pneumonia. Eur Respir J. 2014;44(3):594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones KD. Unclassifiable interstitial lung disease: a pathologist’s perspective. Eur Respir Rev. 2018;27(147). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryerson CJ, Urbania TH, Richeldi L, et al. Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J. 2013;42(3):750–757. [DOI] [PubMed] [Google Scholar]
- 28.Vasakova M, Morell F, Walsh S, Leslie K, Raghu G. Hypersensitivity Pneumonitis: Perspectives in Diagnosis and Management. Am J Respir Crit Care Med. 2017;196(6):680–689. [DOI] [PubMed] [Google Scholar]
- 29.Hashisako M, Fukuoka J. Pathology of Idiopathic Interstitial Pneumonias. Clin Med Insights Circ Respir Pulm Med. 2015;9(Suppl 1):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leslie KO. My approach to interstitial lung disease using clinical, radiological and histopathological patterns. J Clin Pathol. 2009;62(5):387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang P, Jones KD, Urisman A, et al. Pathologic Findings and Prognosis in a Large Prospective Cohort of Chronic Hypersensitivity Pneumonitis. Chest. 2017;152(3):502–509. [DOI] [PubMed] [Google Scholar]
- 32.Schwarz MI, King TE. Interstitial lung disease. 4th ed. Hamilton, Ont. ; Lewiston, N.Y.: B.C. Decker; 2003. [Google Scholar]
- 33.Kalchiem-Dekel O, Galvin JR, Burke AP, Atamas SP, Todd NW. Interstitial Lung Disease and Pulmonary Fibrosis: A Practical Approach for General Medicine Physicians with Focus on the Medical History. J Clin Med. 2018;7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada T, Nakanishi Y, Homma T, et al. Airspace enlargement with fibrosis shows characteristic histology and immunohistology different from usual interstitial pneumonia, nonspecific interstitial pneumonia and centrilobular emphysema. Pathol Int. 2013;63(4):206–213. [DOI] [PubMed] [Google Scholar]
- 35.Raghu G, Remy-Jardin M, Ryerson CJ, et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(3):e36–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filios MS, Storey E, Baron S, Luensman GB, Shiffman RN. Enhancing Worker Health Through Clinical Decision Support (CDS): An Introduction to a Compilation. J Occup Environ Med. 2017;59(11):e227–e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baron S, Filios MS, Marovich S, Chase D, Ash JS. Recognition of the Relationship Between Patients’ Work and Health: A Qualitative Evaluation of the Need for Clinical Decision Support (CDS) for Worker Health in Five Primary Care Practices. J Occup Environ Med. 2017;59(11):e245–e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med. 2019;381(18):1718–1727. [DOI] [PubMed] [Google Scholar]
- 39.Sack C, Raghu G. Idiopathic pulmonary fibrosis: unmasking cryptogenic environmental factors. Eur Respir J. 2019;53(2). [DOI] [PubMed] [Google Scholar]
- 40.Jacobs RL, Andrews CP, Coalson J. Organic antigen-induced interstitial lung disease: diagnosis and management. Ann Allergy Asthma Immunol. 2002;88(1):30–41. [DOI] [PubMed] [Google Scholar]
- 41.Salisbury ML, Myers JL, Belloli EA, Kazerooni EA, Martinez FJ, Flaherty KR. Diagnosis and reatment of Fibrotic Hypersensitivity Pneumonia. Where We Stand and Where We Need to Go. Am J Respir Crit Care Med. 2017;196(6):690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]


