Abstract
SARS-CoV-2 is the causal agent of COVID-19 disease. Currently, infection with SARS-CoV-2 has been the cause of death of over 2.5 million people globally, and there is still no effective curative treatment. Clinically, the severe symptoms caused by COVID-19, in addition to pneumonia, are associated with the development of hyperinflammatory syndrome and thrombosis. It is urgent to expand our understanding of the molecular mechanisms involved in the pathophysiology of COVID-19. This article discusses the potential role that the chemokine CX3CL1 could have in the development of COVID-19-associated thrombosis. CX3CL1 is abundantly expressed by activated endothelium and is an important regulator of many aspects of endothelial function and dysfunction, including thrombosis. The generation of hypotheses about molecules that could be relevant in well-defined aspects of the pathophysiology of COVID-19 encourages the development of basic and clinical studies, that could help find effective and much needed treatments.
Keywords: CX3CL1, COVID-19, Thrombosis
Introduction
The World Health Organization declared a pandemic derived from the health contingency due to the SARS-CoV-2 coronavirus on March 11, 2020. SARS-CoV-2 is the causal agent of coronavirus disease-2019 (COVID-19), which causes severe symptoms of pneumonia. Patients with co-morbidities such as systemic arterial hypertension, obesity and type 2 diabetes mellitus, have the highest risk of death from COVID-19 [1].
Lung tissue from deceased COVID-19 patients presents diffuse alveolar damage, infiltration of T cells in the perivascular area, severe endothelial damage, thrombosis, and generalized microangiopathy in the pulmonary vessels [2]. In addition to the damage at the lung level, SARS-CoV-2 is able to produce various systemic effects. Clinically, severe COVID-19 cases are frequently associated with the development of serious cardiovascular conditions such as arterial and venous thromboembolism, which significantly increase the relative risk of death in these patients [3], [4]. In this regard, it has been reported that chronic anticoagulant therapy and prophylactic treatment with sodium heparin have been effective in reducing the relative risk of death from COVID-19 [5], [6]. Studying the underlying mechanisms associated with injury and dysfunction of vascular endothelium associated with severe COVID-19 could help find more effective therapeutic options for the disease, and aid in reducing its mortality.
Hyperinflammation in COVID-19 patients is characterized by elevated peripheral levels of the cytokine IL-6. This cytokine also confers a pro-thrombotic environment by inducing the expression of fibrinogen, the precursor of fibrin [7]. Patients with severe COVID-19 have been reported to have markedly elevated levels of fibrinogen (630 mg/dl, reference range 200–400 mg/dl) and D-dimer (0.6–4 µg/ml, reference concentration < 0.4 µg/ml), a fibrin breakdown product [8]. Another molecule that could be associated with thrombosis secondary to COVID-19 is the chemokine CX3CL1, which, as will be discussed below, could be positively regulated in the endothelium during SARS-CoV-2 infection, and contribute to a pro-thrombotic loop.
The chemokine CX3CL1, also known as fractalkine (FKN), is abundantly expressed in endothelial cells after activation with pro-inflammatory cytokines such as TNF-α and IL-1β [9], [10]. It is synthesized as a membrane-bound molecule and also exists as a soluble chemotactic molecule [11]; both the membrane bound and the soluble form bind to a single receptor called CX3CR1. CX3CL1 is a relevant chemokine in endothelial dysfunction preceding atherosclerosis and other cardiovascular events [12], [13]. CX3CL1 is cleaved in response to inflammatory stimulus such as atherosclerosis, diabetes and post-transplant vasculopathy [14]. As discussed in the Evaluation of Hypothesis section, CX3CL1 could be up-regulated in the endothelium during SARS-CoV-2 infection and could contribute to the perpetuation of a pro-thrombotic loop.
In human cells, Angiotensin Converting Enzyme 2 (ACE2) mediates SARS-CoV-2 internalization through interaction with the coronavirus spike protein (S) [15]. Indeed, human recombinant soluble ACE2 has been used to inhibit SARS-CoV-2 infection of endothelial cells [16]. It has been suggested that after endocytosis of the viral complex, ACE2 is down-regulated [17], [18]. ACE2 is a vasoprotective regulator of the renin angiotensin system (RAS), which catalyzes the conversion of Angiotensin II (Ang II) to the heptapeptide Ang 1–7, a molecule that has vasodilatory, antioxidant and anti-inflammatory activity [19]. In an experimental model of induced thrombosis in vena cava, in spontaneously hypertensive rats it was demonstrated that a low activity of ACE2 is a prothrombotic factor [20]. In other studies it has been described that Ang 1–7 decreases vascular inflammation and the aggregation of platelets to blood vessels [20], [21]. Therefore, SARS-CoV-2 mediated downregulation of ACE2 could cause the accumulation of angiotensin II, which ultimately diminishes the capacity to counteract RAS activation contributing to the pathology of COVID-19. Additionally, there is in vivo evidence that ACE2 depletion induces a robust upregulation of CX3CL1 [22]
Hypothesis
A potential pathophysiological mechanism of SARS-CoV-2 infection in endothelial cells could be the blocking or neutralization of the natural function of the ACE2 receptor after endocytosis of viral particles. This condition would lead to the overexpression of CX3CL1, which would promote endothelial damage due to the excessive recruitment of immune cells, and have a pro-thrombotic effect derived from platelet activation that may contribute to the physiopathology of COVID-19.
Evaluation of hypothesis
Coronavirus Spike protein has a high affinity towards ACE2 in the soluble and membrane-bound forms [23]. At the cell membrane, SARS-CoV-2 spike protein binding to ACE2 triggers the receptor-mediated endocytosis of viral particles after activation by TMPRSS2, a cellular serine protease [40]. It has been proposed that sequestration of ACE2 in acidic endosomes depletes ACE2 from plasma membrane, countering its protective effects [17], [18], [24]. So far, there is no direct evidence that SARS-CoV-2 negatively regulates the expression of ACE2; however, this has been demonstrated in other coronaviruses that use ACE2 as an entry pathway. For example, VERO cells infected with SARS-CoV dramatically reduced by shedding the expression of ACE2 from 24 h post-infection [25], and depletion of ACE2 has also been documented in lung tissue of animals infected with SARS-CoV [26]. Furthermore, the human coronavirus NL63 has been reported to negatively regulate the expression of the ACE2 protein during its replication [27]. Recently Kumar et al., have proposed as a hypothesis that the entry of SARS-CoV-2 virus into the endothelial cell downregulates the expression of the ACE2 receptor, causing an imbalance in the RAS which leads to endothelial damage and activation of the prothrombotic cascade in COVID-19 patients [28].
Although the biological consequences of negative regulation of ACE2 expression, or a decrease in its activity in endothelial cells, would have severe thrombogenic effects, chemokine CX3CL1 could contribute to perpetuating the thrombotic loop as discussed below. A direct indication that CX3CL1 expression might be increased by SARS-CoV-2 infection is found in mice genetically deficient in ACE2 that exhibit increased CX3CL1 expression, especially when hypertrophy and myocardial damage are induced through chronic cardiac administration of Ang-II [22]. Although the molecular mechanism responsible for CX3CL1 upregulation upon ACE2 ablation should be investigated, the link between RAS and this chemokine in vivo is clear.
The initial overexpression of CX3CL1 could favor the recruitment of CX3CR1+ immune cells, such as monocytes [29] and cytotoxic T cells, which could produce an inflammatory environment, as has been described in atherosclerosis [30], and rheumatoid arthritis [31]. In addition, studies carried out in rats indicate that the increase in the levels of CX3CL1 could have a pro-thrombotic effect [32]. Lung tissue of rats subjected to the acute pulmonary thromboembolism model shows increased levels of CX3CL1 mRNA, however, the mechanisms of these increases remain unknown. Interestingly, anticoagulation treatment with aspirin was found to decrease CX3CL1 mRNA levels, in addition to controlling pulmonary arterial pressure and damage caused by pulmonary embolism [32]. These findings suggest that CX3CL1 overexpression accompanies the damage caused by pulmonary thromboembolism. CX3CL1 could promote thrombosis through platelet activation. It has been shown that when platelets are stimulated with CX3CL1 they increase the expression of P-selectin, adhesion to collagen and fibrinogen is promoted in laminar flow, and degranulation is increased [33].
In COVID-19 patients, treatment with heparin has had favorable results in preventing thrombosis [6]. Evidence from in vitro studies indicates that, in human endothelial cells, the expression of CX3CL1 might also be decreased with heparin treatment, e.g. in IFN-γ stimulated human umbilical cord endothelial cells, treatment with heparin (4 mg/ml) resulted in CX3CL1 downregulation at the transcriptional and protein levels, and it also decreased the adhesion of mononuclear cells [34], which play a central role in vascular injury.
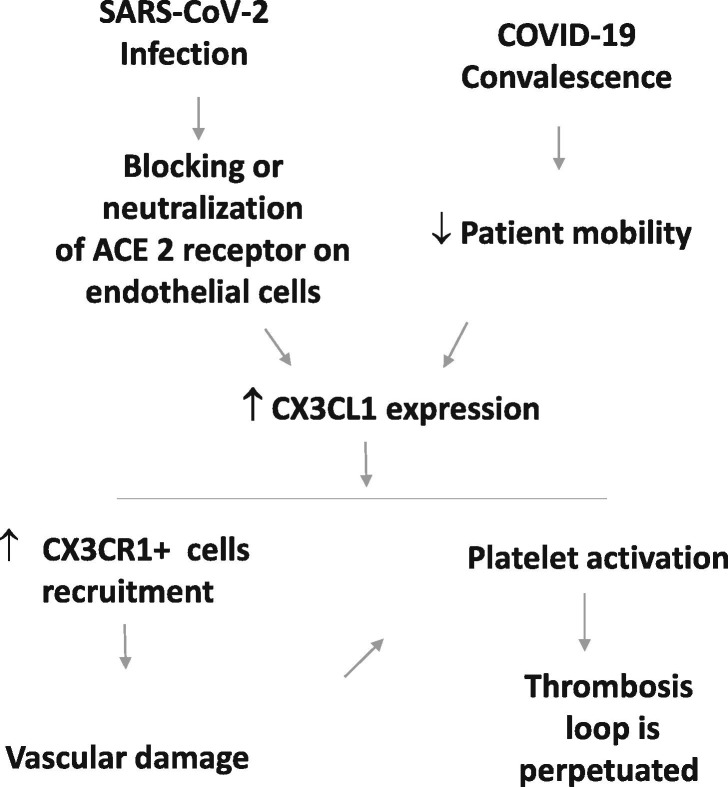
Finally, during convalescence from COVID-19 disease, mainly in hospitalized patients, there is a decrease in blood flow due to the lack of mobility, which is known to promote thrombosis. Ruze et al reported that the expression of CX3CL1 in endothelial cells is increased when blood flow is decreased [35]. Therefore, it is possible that an increase in the expression of CX3CL1 is favored at the initial moment of infection and could be aggravated later with patient immobility (Fig. 1 ).
Fig. 1.
Proposed mechanistic link between SARS-CoV-2 infection and CX3CL1. In the early stage of COVID-19, viral particle binding to the ACE2 receptor on the cell membrane of endothelial cells, induces its internalization and intracellular degradation. The decrease in ACE2 function increases the expression of CX3CL1, favoring the recruitment of CX3CR1+ cells, causing an increase in local inflammatory mediators and vascular damage due to immune cells with cytotoxic or inflammatory activity. Vascular damage could also lead to platelet activation and thrombosis by mechanisms dependent on or independent of CX3CL1. Additionally, during hospitalization and/or convalescence, a decrease in blood flow due to patient immobility could also increase CX3CL1 levels.
Empirical data
In a recent study, direct evidence of upregulation of CX3CL1 was observed by transcriptional profiling of two pulmonary cell lines (Calu-1 and A549) upon infection with SARS-CoV-2 [36]. This upregulation seems to be specific as infection with Influenza A or other coronaviruses such as SARS-CoV or MERS did not show a significant change in CX3CL1 expression [36]. In vivo data correlating CX3CL1 upregulation and SARS-CoV-2 infection is currently missing; it will be especially relevant to characterize transcriptional changes upon infection of endothelial cells where expression of CX3CL1 is relevant. However, as mentioned early, ACE2 deficiency triggers an increase in the expression of CX3CL1 in mice [22] and coronavirus spike protein induces downregulation of ACE2 in the cell membrane[26], [27], therefore it is plausible that a mechanistic link exists between SARS-CoV-2 infection and CX3CL1 upregulation in endothelial cells.
On the other hand, a small clinical study evaluated the plasma levels of a panel of adhesion molecules in 39 patients with COVID-19; measured molecules included intercellular adhesion molecule (ICAM-1), vascular cell adhesion molecule-1 (VCAM), vascular adhesion protein-1 (VAP-1) and CX3CL1. The samples were grouped according to illness severity: 9 samples from patients with mild symptoms and 30 samples from patients with severe symptoms were analyzed. The authors found a global increase in plasma levels of adhesion molecules in patients with severe COVID-19, compared to patients with mild symptoms or control individuals. In the case of CX3CL1, patients with severe symptoms presented a 2.1-fold increase in this chemokine in relation to patients with mild symptoms, presenting medians of 1457.5 pg/ml and 684.6 pg/ml respectively [37].
Consequences of the hypothesis
Although more evidence should be collected regarding the upregulation of CX3CL1 in endothelial cells as well as its levels in the plasma of COVID-19 patients, different experimental studies and direct clinical evidence may suggest that the pro-thrombotic cytokine CX3CL1 could be increased and play a role in the pathophysiology of COVID-19. Increased levels of CX3CL1 have been described in cardiovascular disorders, and a recent report found that this chemokine is increased in patients with COVID-19, especially in patients with the severe manifestation of the disease. Indirect studies suggest potential mechanisms by which CX3CL1 could promote vascular damage and thrombosis. Reduction of ACE2 abundance in the cell membrane induced by the spike-mediated endocytosis may not only contribute to aggravate serious cardiovascular conditions such as arterial and venous thromboembolism, but also induce CX3CL1 upregulation in the endothelium, contributing to a pro-thrombotic environment and immune cell recruitment contributing to the harmful cycle associated with severe COVID-19 and its lethality.
Overall, it is highly relevant to study the role of CX3CL1 in the pathophysiology of COVID-19, especially as in vivo pharmacological inhibition of CX3CL1 can be achieved with mAb or chemical compounds [38], [39]. Finally, soluble CX3CL1 can be readily detected in serum, and thus may be a potential predictive marker for thrombotic complications to identify COVID-19 patients requiring a more aggressive anti-thrombotic management (Fig. 1).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement.
VJV acknowledges support from CDMX grant SECTEI/277/2019.
This article had a Departamental funding from Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas.
References
- 1.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189(5):846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z., Fuentes N.L., Fuller G.M. Characterization of the IL-6 responsive elements in the gamma fibrinogen gene promoter. J Biol Chem. 1995;270:24287–24291. doi: 10.1074/jbc.270.41.24287. [DOI] [PubMed] [Google Scholar]
- 8.Webb B.J., Peltan I.D., Jensen P., Hoda D., Hunter B., Silver A., et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2(12):e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imaizumi T., Yoshida H., Satoh K. Regulation of CX3CL1/fractalkine expression in endothelial cells. J Atheroscler Thromb. 2004;11(1):15–21. doi: 10.5551/jat.11.15. [DOI] [PubMed] [Google Scholar]
- 10.Ahn S.Y., Cho C.H., Park K.G., Lee H.J., Lee S., Park S.K., et al. Tumor necrosis factor-alpha induces fractalkine expression preferentially in arterial endothelial cells and mithramycin A suppresses TNF-alpha-induced fractalkine expression. Am J Pathol. 2004;164:1663–1672. doi: 10.1016/s0002-9440(10)63725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazan J.F., Bacon K.B., Hardiman G., Wang W., Soo K., Rossi D., et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385(6617):640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 12.Hildemann S., Schulz C., Fraccarollo D., Schöpp C., Flierl U., Wissel K., et al. Fractalkine promotes platelet activation and vascular dysfunction in congestive heart failure. Thromb Haemost. 2014;111(04):725–735. doi: 10.1160/TH13-08-0640. [DOI] [PubMed] [Google Scholar]
- 13.Flierl U., Bauersachs J., Schäfer A. Modulation of platelet and monocyte function by the chemokine fractalkine (CX3 CL1) in cardiovascular disease. Eur J Clin Invest. 2015;45(6):624–633. doi: 10.1111/eci.12443. [DOI] [PubMed] [Google Scholar]
- 14.Wong B.W.C., Wong D., McManus B.M. Characterization of fractalkine (CX3CL1) and CX3CR1 in human coronary arteries with native atherosclerosis, diabetes mellitus, and transplant vascular disease. Cardiovasc Pathol. 2002;11(6):332–338. doi: 10.1016/s1054-8807(02)00111-4. [DOI] [PubMed] [Google Scholar]
- 15.Wan Y., Shang J., Graham R., Baric R.S., Li F., Gallagher T. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS Coronavirus. J Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson A.M., Wysocki J., Batlle D. Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor: therapeutic implications. Hypertension. 2020;76(5):1339–1349. doi: 10.1161/HYPERTENSIONAHA.120.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Datta P.K., Liu F., Fischer T., Rappaport J., Qin X. SARS-CoV-2 pandemic and research gaps: understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020;10(16):7448–7464. doi: 10.7150/thno.48076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraga-Silva R.A., Sorg B.S., Wankhede M., deDeugd C., Jun J.Y., Baker M.B., et al. ACE2 activation promotes antithrombotic activity. Mol Med. 2010;16(5-6):210–215. doi: 10.2119/molmed.2009.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas M.C., Pickering R.J., Tsorotes D., Koitka A., Sheehy K., Bernardi S., et al. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res. 2010;107(7):888–897. doi: 10.1161/CIRCRESAHA.110.219279. [DOI] [PubMed] [Google Scholar]
- 22.Song B., Zhang Z.-Z., Zhong J.-C., Yu X.-Y., Oudit G.Y., Jin H.-Y., et al. Loss of angiotensin-converting enzyme 2 exacerbates myocardial injury via activation of the CTGF-fractalkine signaling pathway. Circ J. 2013;77(12):2997–3006. doi: 10.1253/circj.cj-13-0805. [DOI] [PubMed] [Google Scholar]
- 23.Du L., He Y., Zhou Y., Liu S., Zheng B.-J., Jiang S. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groß S., Jahn C., Cushman S., Bär C., Thum T. SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: From basic science to clinical implications. J Mol Cell Cardiol. 2020;144:47–53. doi: 10.1016/j.yjmcc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O., et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84(2):1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dijkman R, Jebbink MF, Deijs M, Milewska A, Pyrc K, Buelow E, et al. Replication-dependent downregulation of cellular angiotensin-converting enzyme 2 protein expression by human coronavirus NL63. J Gen Virol 2012,93:1924-1929. [DOI] [PubMed]
- 28.Kumar A., Narayan R.K., Kumari C., Faiq M.A., Kulandhasamy M., Kant K., et al. SARS-CoV-2 cell entry receptor ACE2 mediated endothelial dysfunction leads to vascular thrombosis in COVID-19 patients. Med Hypotheses. 2020;145:110320. doi: 10.1016/j.mehy.2020.110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park Y, Lee J, Kwak JY, Noh K, Yim E, Kim HK, et al. Fractalkine induces angiogenic potential in CX3CR1-expressing monocytes. J Leukoc Biol 2018,103:53-66. [DOI] [PubMed]
- 30.Stolla M., Pelisek J., von Brühl M.-L., Schäfer A., Barocke V., Heider P., et al. Fractalkine is expressed in early and advanced atherosclerotic lesions and supports monocyte recruitment via CX3CR1. PLoS ONE. 2012;7(8):e43572. doi: 10.1371/journal.pone.0043572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanki T., Imai T., Nagasaka K., Urasaki Y., Nonomura Y., Taniguchi K., et al. Migration of CX3CR1-positive T cells producing type 1 cytokines and cytotoxic molecules into the synovium of patients with rheumatoid arthritis. Arthritis Rheum. 2002;46(11):2878–2883. doi: 10.1002/art.10622. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Yang W, Ying R, Shi Y, Jiang H, Cai D, et al. Influence of aspirin on the CX3CL1/CX3CR1 signaling pathway in acute pulmonary embolism. Int J Mol Med 2017,39:1580-1588. [DOI] [PubMed]
- 33.Schafer A, Schulz C, Eigenthaler M, Fraccarollo D, Kobsar A, Gawaz M, et al. Novel role of the membrane-bound chemokine fractalkine in platelet activation and adhesion. Blood 2004,103:407-412. [DOI] [PubMed]
- 34.Hatakeyama M., Imaizumi T., Tamo W., Yamashita K., Yoshida H., Fukuda I., et al. Heparin inhibits IFN-gamma-induced fractalkine/CX3CL1 expression in human endothelial cells. Inflammation. 2004;28:7–13. doi: 10.1023/b:ifla.0000014706.49598.78. [DOI] [PubMed] [Google Scholar]
- 35.Ruze A, Zhao Y, Li H, Gulireba X, Li J, Lei D, et al. Low shear stress upregulates the expression of fractalkine through the activation of mitogen-activated protein kinases in endothelial cells. Blood Coagul Fibrinolysis 2018,29:361-368. [DOI] [PMC free article] [PubMed]
- 36.Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., Uhl S., Hoagland D., Møller R., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong M, Jiang Y, Xia D, Xiong Y, Zheng Q, Chen F, et al. Elevated expression of serum endothelial cell adhesion molecules in COVID-19 patients. J Infect Dis 2020,222:894-898. [DOI] [PMC free article] [PubMed]
- 38.Ridderstad Wollberg A., Ericsson-Dahlstrand A., Jureus A., Ekerot P., Simon S., Nilsson M., et al. Pharmacological inhibition of the chemokine receptor CX3CR1 attenuates disease in a chronic-relapsing rat model for multiple sclerosis. Proc Natl Acad Sci USA. 2014;111(14):5409–5414. doi: 10.1073/pnas.1316510111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh D.-J., Dursun B., He Z., Lu L., Hoke T.S., Ljubanovic D., et al. Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Physiol Renal Physiol. 2008;294(1):F264–F271. doi: 10.1152/ajprenal.00204.2007. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann Markus, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271. doi: 10.1016/j.cell.2020.02.052. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]