Abstract
Purpose of review:
The purpose of this review is to summarize the evidence supporting a role of short chain fatty acids (SCFAs) as messengers facilitating crosstalk between the host and gut microbiota and discuss the effects of altered SCFA signaling in obesity and hypertension.
Recent findings:
Recent evidence suggests there to be a significant contribution of gut microbiota-derived SCFAs to microbe:host communication and host metabolism. SCFA production within the intestine modulates intestinal pH, microbial composition, and intestinal barrier integrity. SCFA signaling through host receptors, such as PPARγ and GPCRs modulate host health and disease physiology. Alterations in SCFA signaling, and downstream effects on inflammation are implicated in the development of obesity and hypertension.
Summary:
SCFAs are crucial components of the holobiont relationship; in the proper environment, they support normal gut, immune, and metabolic function. Dysregulation of microbial SCFA signaling affects downstream host metabolism, with implications in obesity and hypertension.
Keywords: gut microbiome, short chain fatty acids, diet, obesity, hypertension
Introduction:
The human gut microbiome has coevolved as a vital determinant of host health, and has been linked to multiple diseases, including obesity and hypertension (HTN). Gut microbiota are indispensable to digestion and biosynthesize nutrients essential to host health. Microbiota enhance and develop the host immune response, confer resistance to infection, and are emerging as key drivers of host metabolism. Yet large gaps remain in our understanding of the bidirectional microbiota:host relationship, physiology, and related-disease etiology. The composition, health, and functionality of the gut microbiota is dependent on the host, and in return, the gut microbiota’s functions as an endocrine organ can have diverse and clinically significant effects on host health. This bidirectional relationship, comprising the holobiont [1], is of great interest for its significance to human health and disease.
A consensus definition of a ‘healthy’ gut microbiota remains elusive due to substantial inter- and intra-individual variability, with complex interplay of the gut-host macroenvironment. Current evidence, primarily derived from observational studies, suggests that characteristics of a ‘healthy’ gut microbiota include greater diversity and microbial richness, greater abundance and functionality of short-chain fatty acid (SCFA)-producing bacteria, and a relatively stable-community of obligate anaerobes in larger abundance as opposed to facultative anaerobes [2–6]. Communication between microbiota and their host is facilitated by signaling messengers, such as metabolites and small molecules. The SCFAs, acetate, propionate, and butyrate, have received particular attention for their role in microbiota:host communication, with distinctive effects on both local intestinal and systemic host-physiology. Within this review we summarize the evidence supporting a role of SCFAs as messengers facilitating crosstalk between the host and gut microbiota, and discuss the effects of altered SCFA signaling on obesity and hypertension.
Host diet regulates availability of SCFA precursors and exerts selective pressure on microbial community composition
There are three primary SCFAs which have been studied for their relevance in human health: acetate, propionate, and butyrate. These are present at differing concentrations in the body, and are thought to have distinct effects on host metabolism. However, as the specific individual and combinatorial effects have not been fully elucidated, and the SCFAs are often studied together, we primarily describe mechanisms that have been attributed to SCFAs in general. The primary source of SCFAs in humans is gut microbial synthesis from dietary precursors, with some SCFAs obtained directly from dietary intake of microbial-fermented foods [7] and de novo synthesis by the liver or other metabolic organs [8]. Prebiotics or microbe-accessible carbohydrates, including plant-derived oligosaccharides, polysaccharides, resistant starch, and inulin, are selectively fermented by commensal SCFA-producing bacteria (Fig 1.A.a), including those within the Clostridium cluster IV and XIVa, and more specifically: Clostridium Leptum, Coprococcus spp., Faecalibacterium prausnitzii, Eubacterium spp., Anaerostipes, and Roseburia spp [9,10]. Some bacteria, such as those within the Lactobacillus and Bifidobacteria genus, which don’t produce SCFAs themselves, contribute to the SCFA pool indirectly via metabolic cross-feeding – in which their breakdown of dietary fiber provide the necessary substrate (i.e. oligosaccharides, lactate, and acetate) for other SCFA-producing bacteria [9,11]. Diet shapes the gut microbiota by selecting for microbes which preferentially digest specific nutrients or dietary substrates. Alterations in diet can lead to rapid and significant modifications in gut microbial composition and diversity in as little as 2–3 days [12], though these changes tend to be transient with extensive interindividual variability [13–16]. Plant-based diets, rich in complex carbohydrates, are associated with increased abundance of SCFA-producing bacteria [17–20], while greater intake of saturated fat, animal products, and simple sugars associates with an increase in facultative anaerobic bacteria and diminished SCFA abundance due to both a reduction in SCFA-producing bacteria and enhanced excretion of SCFAs (Fig 1.B.a) [2,12,21–25]. Interestingly, although very-low-carbohydrate diets may reduce SCFA-substrate availability, they may contribute to the SCFA-pool and mimic SCFA-functionality by increasing production of ketones, which can be utilized as a fuel source for colonic epithelial cells (CECs) and participate in similar signaling pathways [26]. However, diet alone is not a consistent predictor of SCFA production or abundance. The considerable interindividual variability observed in the response to dietary interventions may be due to differences in gut microbial composition. Individuals with lower microbial richness and diversity are shown to have a more robust response to a variety of interventions [16,27,28]. Still, some evidence suggests variability in the change in bacterial composition and metabolite production in response to dietary intervention, regardless of baseline microbial richness and diversity [28–30]. Whether this is related to host genetics, or other factors, is unknown, and more research is needed to define the determinants of response to dietary interventions. However, baseline gut microbial composition has been successfully used to predict a subject’s response to an intervention, establishing feasibility for precision nutrition approaches aimed at altering SCFA production [31–36••].
FIGURE 1. Gut microbiota-derived short chain fatty acids modulate host health with implications in obesity and hypertension.
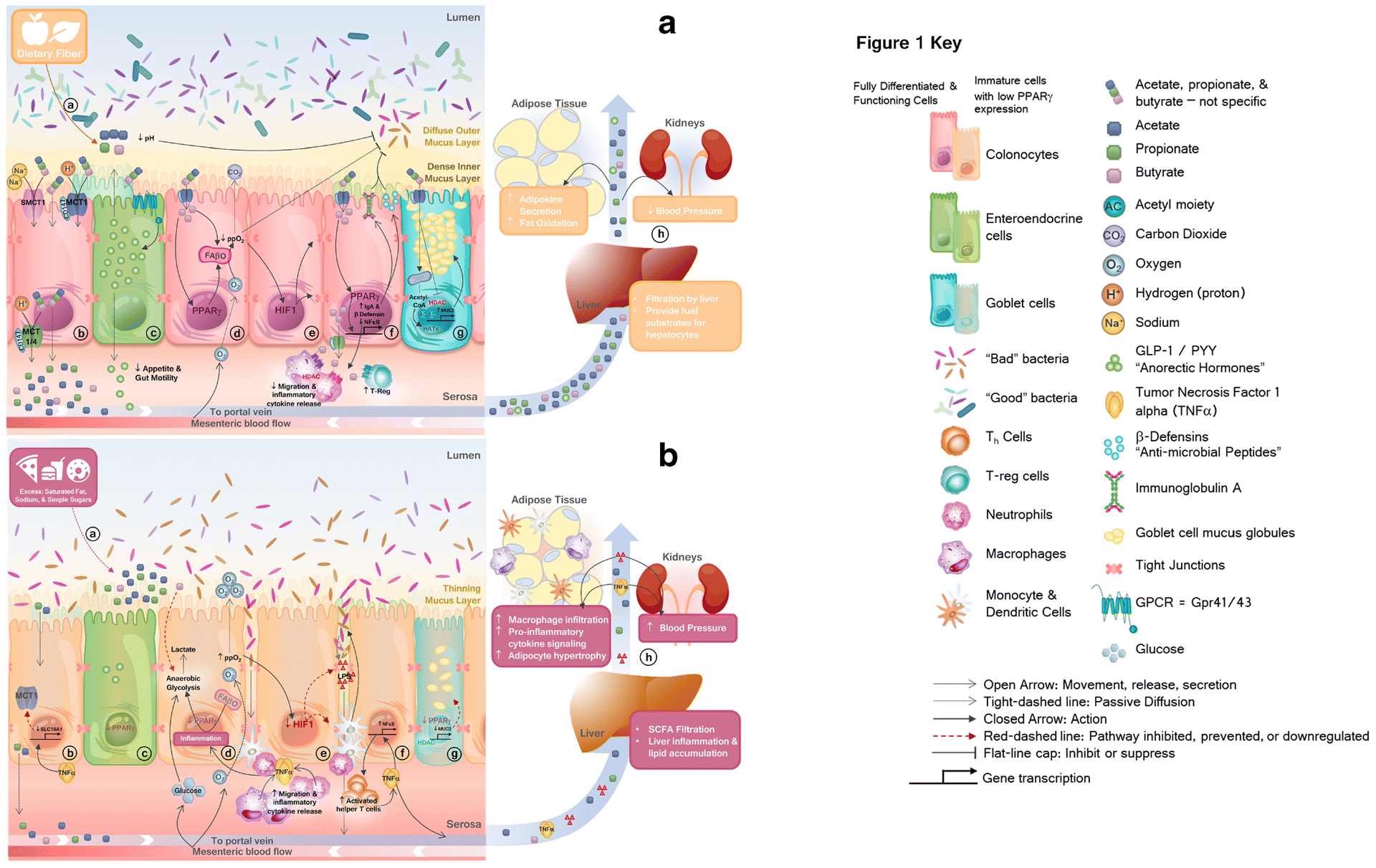
A.
a) Dietary fibers provide substrate for commensal bacteria to produce short chain fatty acids (SCFAs), acetate, propionate, and butyrate. Bacterial fermentation of dietary fibers reduces luminal pH. b) Protonated-SCFAs can passively diffuse through the membrane, while anionic-SCFAs require carrier mediated transport (by MCT1, MCT4, SMCT1 and SMCT2) c) SCFAs bind to and activate Gpr41/43, causing secretion of anorectic hormones, peptide YY (PYY) and glucagon-like peptide (GLP)-1, d) Within fully differentiated colonocytes, SCFAs activate peroxisome proliferator activated receptor gamma (PPARγ) which activates mitochondrial fatty acid beta-oxidation (FAβO), reducing luminal availability of oxygen. e) Within colonocytes, hypoxic conditions activate hypoxia-inducible factor 1 (HIF1), which upregulates expression and function of tight junction proteins, maintaining gut barrier integrity. f) Butyrate activation of PPARγ upregulates anti-inflammatory immunoglobulin A (IgA) and beta-defensin (β-defensin) expression and inhibits nuclear factor kappa b (NFκB) signaling. Butyrate enhances activation of regulatory T-cells and suppresses migration, activation, or release of pro-inflammatory mediators from resident immune cells via histone deacetylase (HDAC) inhibition and GPCR-signaling. g) Fully differentiated and functional goblet cells with sufficient PPARγ expression allows butyrate-mediated histone acetyltransferase (HAT) or HDAC-inhibition-mediated upregulation of mucin, or MUC2, maintaining a healthy mucus layer. h) SCFAs which are not used in colonocyte metabolic processes, are sent to the periphery via portal circulation where they elicit beneficial systemic effects.
B.
a) Excess dietary saturated fat, sodium, and simple sugars provide nutrient advantage for facultative anaerobic bacteria. b) Inflammation downregulates SCFA-transporters, reducing colonocyte uptake and systemic circulation of SCFA. c) Reduced SCFA signaling and activation of PPARγ within intestinal crypts dysregulates normal growth and proper differentiation of cells. d) Diminished abundance and uptake of SCFAs limit substrate available to fuel colonocytes, and may prompt a metabolic switch from high oxygen-consuming mitochondrial FAβO to anaerobic glycolysis, forcing cells to pull exogenous carbon-sources from blood-supply (i.e. glucose) resulting in lactate production. e) Loss of cellular and luminal physiological hypoxia reduces HIF1 expression, resulting in loss of tight junction and adhesion molecule expression and function. This allows bacterial translocation, triggering the innate immune response. f) Release of endotoxin lipopolysaccharide (LPS) induces pro-inflammatory NFκB signaling. g) Immature goblet cells lacking proper PPARγ and SCFA-signaling produce less mucin, leading to a reduced mucus layer. Nutrient selection, reduced bacterial competition, and increased luminal oxygen availability provide a selective advantage for pathogenic overgrowth. h) Local intestinal inflammation leads to organ-specific and systemic inflammation, increasing cardiometabolic risk.
SCFAs are metabolically active, and interact with host signaling at local and systemic levels
SCFAs are key players in the holobiont relationship. Their production, absorption, and distribution into systemic circulation are critical determinants of their functionality as secondary messengers. They are primarily produced in the cecum and ascending colon where they alter gut pH, indirectly regulate gastrointestinal (GI)-motility and blood flow, influence nutrient bioavailability, foster normal immune-function, and promote GI-health and stability [37,38]. SCFAs not utilized for fuel by colonocytes or metabolized by other microbes are either absorbed or excreted. Due to their relatively short-carbon-chain length (less than 6-carbons), both dietary and gut microbiota derived SCFA do not require micellarization to be absorbed nor re-esterification once inside cells [39]. Absorption occurs through several mechanisms within the colon depending on the SCFA-hydronation state. Protonated-SCFA are absorbed in the colon-epithelium via simple diffusion down a chemical gradient; whereas nonionic forms operate under carrier-mediated transport [40]. These transporters include monocarboxylate transporters (MCT1 and MCT4) which require an ancillary chaperone protein, CD147, for translocation to the cell-surface, and sodium-coupled-MCTs (SMCT1 and SMCT2) (Fig 1.A.b) [40]. MCT1, SMCT1, and SMCT2 transporters are expressed on the apical membranes of intestinal epithelium, whereas, MCT1 and MCT4 are expressed basolaterally (Fig 1.A.b) [40]. Transporter expression is regulated by SCFAs and by inflammation, and SCFA uptake may be altered in the setting of obesity and certain disease states [6,41–43].
After absorption, SCFAs are taken up directly into the portal vein en route to the liver, where they can be used as an energy source, incorporated into endogenous molecules (i.e. cholesterol, fatty acids, glucose), or function as signaling molecules (Fig 1.A.h) [44]. Excretion of remaining SCFA from the body occurs in the stool, urine, and breath [44]. As a consequence of rapid and efficient splanich-extraction, relatively little SCFAs make it into circulation [44–46], which is especially the case for butyrate. After meeting approximately 70–90% of the CECs’ energy needs, filtration of butyrate by the liver is upwards of 100%, with little reaching systemic circulation [44–46]. In spite of this, circulating SCFAs are still shown to play some role in cardiometabolic health [47], suggesting concentration- and receptor-dependent effects. Furthermore, evidence suggests differential effects of individual SCFAs, with potentially opposing or synergistic activity [48]. Additional studies are needed to better clarify this relationship.
SCFAs modulate gut barrier health
Butyrate has beneficial effects on various organ-systems, including the brain, skin, immune system, and most notably, the GI-tract. Within the gut, butyrate exerts beneficial effects by suppressing colonic inflammation, regulating cell cycle, and improving gut-barrier integrity (Fig 1.A.) [9]. The structural integrity of the gut and the mucus layers which protect it are paramount for maintaining symbiotic homeostasis. Butyrate boosts mucus production via epigenetic regulation of mucin (Muc2) expression in goblet cells by either promoting histone acetyl transferase (HAT) activity at low concentrations or inhibiting histone deacetylase (HDAC) activity at higher concentrations (Fig 1.A.g) [49,50]. Enhanced mucus-production within the dense inner-layer along the apical membrane of CECs protects the gut-lining from infiltration and exposure to both pathogen and commensal bacteria alike, tamping down the innate immune response, and reducing overall inflammatory-tone [15]. Enhanced production of mucus within the ‘outer-layer’ provides bacteria with a semi-stable home and for some, a glycoprotein substrate for fermentation [51]. The reduction of luminal pH as a byproduct of dietary-fiber fermentation and commensal SCFA production adds an additional layer of protection from pathogenic bacterial infiltration and colonization [52].
Host G-protein coupled receptors mediate SCFA secondary signaling actions
SCFAs are potent signaling molecules which bind to G-protein coupled receptors (GPCRs), including Gpr41 (FFAR3), Gpr43 (FFAR2), Olf78 (OR51E2), Gpr91, Gpr109A (HCAR2), and Gpr164 [53–55]. The GPCRs are widely expressed in many cell- and tissue-types and demonstrate varying affinity for SCFA-ligand activity [54]. Through HDAC regulation and GPCR signaling, SCFAs stimulate the production of anti-inflammatory mediators, enhance the differentiation and activation of regulatory T-cells in both the intestines and peripheral organs, and can attenuate the migration and subsequent inflammatory response in macrophages and neutrophils (Fig 1.A.f) [56]. Interestingly, this pathway may have multi-generational effects on metabolic disease. Gut microbiota-derived SCFAs cross the placental-barrier during pregnancy, and modulate metabolic and sensory neural development in murine offspring via Gpr43 and Gpr41, respectively [57••]. This represents an additional “hidden” mechanism whereby gut microbial SCFA signaling modulates disease risk and may confound our ability to identify consistent and reproducible associations between gut microbiota and hosts within a single generation.
Butyrate - PPARγ signaling maintains healthy intestinal function
In the healthy gut, butyrate and to a lesser extent propionate and acetate, act as ligands which bind to and selectively activate peroxisome proliferator-activated receptor gamma (PPARγ) [58,59]. This signaling in mature CECs transcriptionally regulates and activates mitochondrial β-oxidation (Fig 1.A.d) [59]. Metabolism of both short- and long-chain fatty acids enhances oxygen consumption via oxidative phosphorylation. The subsequent reduction of oxygen available to freely diffuse across epithelial membranes procures an optimal partial pressure of <1% oxygen, maintaining physiological hypoxia [60]. Additionally, butyrate-mediated oxygen-depletion in CECs helps to stabilize hypoxia-inducible factor 1 (HIF1) which in turn upregulates expression of genes critical to gut-barrier function, such as occludin, zonula occludens, and junctional adhesion molecules (Fig 1.A.e) [61,62].
Conversely, reduced abundance and bioavailability of butyrate may be a common link in many of the disorders of the gut and creates a vicious feed-forward cycle. Whereas normal PPARγ-butyrate signaling would downregulate production of pro-inflammatory cytokines via inhibition of the nuclear factor-κB pathway (Fig 1.A.f), diminished production or impaired uptake of butyrate and inadequate expression or activation of PPARγ induces inflammation (Fig 1.B.f) [63–65]. Inflammation and lack of microbially-derived substrate drives metabolic reprogramming in CECs, forcing them to pull glucose from the bloodstream as their primary fuel source, utilizing glycolysis in lieu of fatty acid β-oxidation (Fig 1.B.d) [38]. This switch to anaerobic respiration significantly reduces oxygen consumption, prompting loss of luminal hypoxia and creating a selective advantage for pathogenic facultative anaerobes, such as Proteobacteria [38]. Within the Proteobacteria phylum, the Enterobacteriaceae are a family of rapidly dividing, aerotolerant, and simple-sugar-oxidizing bacteria including several notorious pathogens, such as E. Coli, Salmonella, Enterobacter, Shigella, and Yersinia, of which are well documented to induce pro-inflammatory responses and are now considered to be a signature of gut microbiota dysbiosis [24,64,66]. Left unchecked, these pathogens will exhaust exogenous-nutrient availability and turn to metabolizing, and therefore diminishing, the protective mucus layers. This allows bacterial interaction with the mucosal barrier, inciting immune-cell activation and pro-inflammatory cytokine release (Fig 1.B.e–f). Ultimately, this impairs gut-barrier integrity allowing leakage of luminal contents into systemic circulation – further exacerbating inflammatory conditions and inducing metabolic endotoxemia (Fig 1.B.f, h) [67].
The inflammatory cascade resulting from direct exposure of bacteria to the mucosa or intestinal damage stimulates colonic-crypt stem cell proliferation [68]. However, butyrate deficiency impairs PPARγ-mediated growth and differentiation; therefore, these transitionally suspended cells ascend from the crypts to the luminal surface without fully developing into mature colonocytes or goblet cells [38,61].This cell-developmental stunting results in reduced PPARγ expression and impaired cellular-functionality in colonocytes as well as reduced mucus production by goblet cells – initiating a positive feedback loop for further detrimental effects (Fig 1.B.c–g). PPARγ is also a critical mediator of the host-innate immune system within the colon, maintaining expression of the major microbial ‘defense’ system, β-defensin [69], and preventing diminution of the anti-inflammatory immunoglobulin A in response to acute stress (Fig 1.A.f) [70]. Therefore, activation of PPARγ is required to activate aerobic metabolism and maintain luminal hypoxia, properly differentiate CECs, and to support immune system and gut-barrier function.
Microbial-derived SCFAs are an important energy source, with relevance for energy homeostasis and obesity
The gut microbiome in the obese state is typically characterized by inflammation, dysbiosis, and in many reports, a relative increase in Firmicutes to Bacteroidetes [71–75]. However, data regarding the Firmicutes to Bacteroidetes ratio are inconsistent, and measurement at the phylum level likely vastly over-simplifies the relationship, given the huge variability in function at the species and strain level [76,77]. Evidence is currently lacking in humans to determine whether this ratio is a driving force for obesity, a reflection of host genetics or diet, or caused by disease itself [15]. In support of a causal role for gut microbiota in obesity, germ-free mice tend to gain less weight than wild-type mice on the same diet [78], possibly from the reduced production of SCFAs, while fecal material transfer (FMT) from obese mice and humans result in significant weight gain [3,78,79]. Furthermore, obesity-related alterations in the gut microbial composition has been implicated in contributing to weight regain greater than baseline during yoyo dieting [35]. SCFAs have significant local and systemic effects on host metabolism, energetics, and appetite control, making them a compelling factor in the study of obesity pathophysiology [80]. Gut microbiota derived SCFAs are primarily used to meet the energy needs of cells lining the mucosa and provide an additional source of calories to the host. The ‘in vs out’ theory of energy flux is thus an overly simplified model which disregards the metabolic complexities of the holobiont [81]. Unlike closed thermodynamic systems, humans are complex and dynamic metabolic systems, where gut microbiota contribute up to 10% of the host’s total daily energy needs with variable energy extracting efficiency [3]. Whether this enhanced harvesting is a function of gut microbiota composition independent of energy intake, or if composition changes occur to compensate for excessive energy intake requires further examination.
Beyond providing energy directly, SCFAs stimulate enteroendocrine L-cells to produce satiety hormones such as peptide YY (PYY) and glucagon-like peptide (GLP-1) locally (Fig 1.A.c), while in circulation they epigenetically regulate expression of adipokines such as leptin, adiponectin, and resistin (Fig 1.A.h) [82–86]. In addition, SCFAs may indirectly contribute to obesity through modulation of intestinal and systemic inflammation, promoting or exacerbating cardiometabolic dysfunction (Fig 1.A.h & 1.B.h) [15]. Obese individuals have been reported to have a greater abundance of fecal-SCFAs, yet relatively low plasma-SCFA levels (Fig 1.B.h) [3,6,87,88]. This may suggest defects in absorption into the colonocyte and systemic circulation [47]. Alteration of epithelial cell gene expression networks within the gut coordinating nutrient metabolism and inflammation, as well as SCFA and cytokine-mediated downregulation of SCFA-transporters may at least partially explain the reduced uptake and increased fecal excretion (Fig 1.B.b) [43,63]. Thus, in the setting of inflammation and obesity, SCFAs produced in the intestine may be less able to mediate their systemic-second messenger effects. Interestingly, several human clinical trials demonstrated SCFA-supplementation to enhance resting and total energy expenditure as well as augment fat-oxidation (Fig 1.A.h) [89,90]. Although administration location and method or SCFA type may have differential effects on metabolic outcome, most studies demonstrate beneficial effects of SCFAs in human obesity studies [89–92]. Taken together, these data suggest that SCFAs may modulate the development of obesity via caloric availability and appetite regulation; however, there may be paradoxical effects in the setting of established obesity. The increased fecal levels of gut microbiota-derived SCFAs in obese individuals may reflect overall greater substrate availability, reduced absorptive capacity, or a state of “SCFA-resistance”. Further studies are needed to resolve these questions.
The unique interplay of the gut microbiota and hypertension
The GI-tract is the gatekeeper for many of the primary modifiable risk factors contributing to HTN, including an excess intake of sodium, alcohol, lipids, and simple carbohydrates. Conversely, fiber is a well-known cardioprotective-agent shown to lower arterial blood pressure [93], which also has direct interaction with the GI-tract. These interactions provide a compelling link, bridging the effects of the gut microbiota and HTN. As with several other diseases, the mechanisms linking the gut microbiota to HTN include dysbiosis, inflammation, intestinal permeability, and reduced production of SCFAs, especially butyrate [94–96]. A recent large multi-ethnic cohort describes an association of altered gut microbial composition and blood pressure while demonstrating large discrepancies among different ethnic groups [97••]. Studies using metabolomic and metagenomic sequencing of stool samples from either prehypertensive or hypertensive subjects observed diminished microbial diversity and richness, reduced butyrate producers and abundance, and prominent intestinal inflammation and permeability versus their normotensive counterparts [94–96]. Similar observations were recently made in pregnant women diagnosed with preeclampsia [98•]. Broad-spectrum antibiotic use is also implicated in contributing to gut dysbiosis and either directly causing or exacerbating the hypertensive response, possibly in an individual or genetic-dependent manner [99,100]. The SCFA-paradox seen in obesity is recapitulated in recent HTN-studies. SCFA-producing bacteria and plasma-SCFA levels were inversely proportional to blood pressure while fecal SCFA content was positively associated with blood pressure [97, 101••]. Furthermore, mice humanized with stool samples from hypertensive-subjects displayed donor-symptomology, establishing a gut microbiota-transferrable augmentation in blood pressure, and a possible direct effect in this model [95]. Similar studies conducted in rodent-analogous models obtained comparable results [102–105].
The intestinal microbiome was recently demonstrated to play a role in circadian rhythm coordination of water retention and diurnal variation in blood pressure [106,107]. Temporal dynamics and sympathetic nervous system activity of the gut microbiota and its metabolites have also been implicated in obstructive sleep apnea-induced HTN [102,106–110]. This was primarily contributed to gut dysbiosis, inflammation, and permeability [102,106–110] and can be exacerbated by excessive dietary salt intake (Fig 1.B.h) [107,109]. In some cases, commensal gut bacteria-derived peptides confer anti-inflammatory benefits while also modulating host hypertensive hormones, such as angiotensin converting enzyme and renin [111]. The neuro-gut-immune axis and its interactions with hormone-related compounds in regards to blood pressure regulation has been recently reviewed [112].
SCFAs have therapeutic potential for hypertension
Normal production and function of SCFAs have been considered beneficial in most diseases, including HTN. SCFAs signal through GPCRs in the kidney, heart, gut, immune cells, and vasculature to modulate blood pressure primarily through regulation of vascular tone and inflammation (Fig 1.A.h) [113–115]. Biologically, this mechanism may be used to enhance blood flow and exchange of nutrients after digestion and absorption; however, this has alternatively elicited advantageous effects on blood pressure regulation [116]. Prebiotic and probiotic supplementation associated with enhanced SCFA-production reduce blood pressure in both genetic and diet-induced HTN experimental models [110,117–119]. Manipulation of SCFA abundance, production, and function either via modulation of the gut microbiota through dietary supplementation with prebiotics, targeted microbiota therapy with antibiotics and probiotics, or via direct supplementation with SCFA are promising strategies still requiring further study. Experimentally, supplementation with SCFA was shown to reduce blood pressure, as demonstrated by using specialized oral formulations [94,98,115,117,120,121], as well as intraperitoneal [94] and intragastric infusions [110]. The composition of SCFA oral supplementation for therapeutic use must be carefully considered; controlled-release systems such as pH-sensitive polymers or coatings, time-release capsules, or covalent connections with colonic bacterial-degrading compounds are required to promote bioactive signaling and avoid utilization by the host as energy substrates in the proximal small intestine [44]. While additional experimental studies and well-designed clinical trials are required to obtain a better understanding of SCFA-kinetics in health and disease states, SCFAs remain a promising target for future management of hypertension and inflammatory cardiometabolic disease.
Bridging the Gap:
The bidirectional relationship of the gut microbiota and human health is still a nascent area of research with many questions that remain to be addressed. Although relatively well studied, much of the individual SCFAs direct, opposing, synergistic, and off-target effects in humans remain largely unknown. Discrepancies in SCFA quantification methodologies, as well as large interindividual variation, exacerbate difficulties in cross-study comparability. Due to the low relative concentrations and dynamic nature of SCFAs in circulation, it remains unclear whether measurement of SCFAs in serum or plasma, as is commonly implemented in epidemiological studies, is informative for health status. Fecal or tissue measurements may be required to better understand the physiological effects of SCFAs in clinical and population studies, but these introduce logistical challenges. Rigorous and standardized methodologies are needed to better assess dietary intake, define microbiome composition, and quantify individual SCFAs at relevant sites of action. Multidisciplinary collaborative approaches may be needed to better understand and predict gut microbiota functionality, and move towards mechanistic understanding [122].
Conclusions:
In conclusion, SCFAs are bioactive microbiota-derived signaling molecules, which play diverse roles in human health, and represent a key mechanism whereby microbiota communicate with their hosts. Understanding the mechanisms of SCFA signaling, and their association with obesity, hypertension and cardiometabolic disease risk is an active research area, which promises to shed light on disease pathophysiology and may open new therapeutic avenues for disease prevention and treatment.
Acknowledgments
This work was supported by funding from the Layton Family Fund, and by R01DK117144. We would like to thank Katie Meyer for her review and suggestions for the manuscript.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.van de Guchte M, Blottière HM, Doré J. Humans as holobionts: implications for prevention and therapy. Microbiome. 2018;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barone M, Turroni S, Rampelli S, Soverini M, D’Amico F, Biagi E, et al. Gut microbiome response to a modern Paleolithic diet in a Western lifestyle context. Loor JJ, editor. PLOS ONE. 2019;14:e0220619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- 4.Zinöcker M, Lindseth I. The Western Diet–Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients. 2018;10:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity. 2010;18:190–5. [DOI] [PubMed] [Google Scholar]
- 6.de la Cuesta-Zuluaga J, Mueller N, Álvarez-Quintero R, Velásquez-Mejía E, Sierra J, Corrales-Agudelo V, et al. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients. 2018;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfe BE, Dutton RJ. Fermented Foods as Experimentally Tractable Microbial Ecosystems. Cell. 2015;161:49–55. [DOI] [PubMed] [Google Scholar]
- 8.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. [DOI] [PubMed] [Google Scholar]
- 10.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front Microbiol [Internet]. 2016. [cited 2020 Sep 4];7. Available from: http://journal.frontiersin.org/Article/10.3389/fmicb.2016.00979/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, et al. Two Routes of Metabolic Cross-Feeding between Bifidobacterium adolescentis and Butyrate-Producing Anaerobes from the Human Gut. Appl Environ Microbiol. 2006;72:3593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science. 2011;334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meijnikman AS, Gerdes VE, Nieuwdorp M, Herrema H. Evaluating Causality of Gut Microbiota in Obesity and Diabetes in Humans. Endocr Rev. 2018;39:133–53. [DOI] [PubMed] [Google Scholar]
- 16.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominika Ś, Arjan N, Karyn RP, Henryk K. The study on the impact of glycated pea proteins on human intestinal bacteria. Int J Food Microbiol. 2011;145:267–72. [DOI] [PubMed] [Google Scholar]
- 18.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayengbam S, Lambert JE, Parnell JA, Tunnicliffe JM, Nicolucci AC, Han J, et al. Impact of dietary fiber supplementation on modulating microbiota–host–metabolic axes in obesity. J Nutr Biochem. 2019;64:228–36. [DOI] [PubMed] [Google Scholar]
- 20.Reimer RA, Soto-Vaca A, Nicolucci AC, Mayengbam S, Park H, Madsen KL, et al. Effect of chicory inulin-type fructan–containing snack bars on the human gut microbiota in low dietary fiber consumers in a randomized crossover trial. Am J Clin Nutr. 2020;111:1286–96. [DOI] [PubMed] [Google Scholar]
- 21.Fava F, Gitau R, Griffin BA, Gibson GR, Tuohy KM, Lovegrove JA. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int J Obes. 2013;37:216–23. [DOI] [PubMed] [Google Scholar]
- 22.Wan Y, Tong W, Zhou R, Li J, Yuan J, Wang F, et al. Habitual animal fat consumption in shaping gut microbiota and microbial metabolites. Food Funct. 2019;10:7973–82. [DOI] [PubMed] [Google Scholar]
- 23.Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci. 2010;107:14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saffouri GB, Shields-Cutler RR, Chen J, Yang Y, Lekatz HR, Hale VL, et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019;10:2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki K, Sasaki D, Hannya A, Tsubota J, Kondo A. In vitro human colonic microbiota utilises D-β-hydroxybutyrate to increase butyrogenesis. Sci Rep. 2020;10:8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ANR MicroObes consortium, ANR MicroObes consortium members, Cotillard A, Kennedy SP, Kong LC, Prifti E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–8. [DOI] [PubMed] [Google Scholar]
- 28.Balamurugan R, Pugazhendhi S, Balachander GM, Dharmalingam T, Mortimer EK, Gopalsamy GL, et al. Effect of Native and Acetylated Dietary Resistant Starches on Intestinal Fermentative Capacity of Normal and Stunted Children in Southern India. Int J Environ Res Public Health. 2019;16:3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, et al. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015;22:971–82. [DOI] [PubMed] [Google Scholar]
- 30.Miller LM, Lampe JW, Newton KM, Gundersen G, Fuller S, Reed SD, et al. Being overweight or obese is associated with harboring a gut microbial community not capable of metabolizing the soy isoflavone daidzein to O- desmethylangolensin in peri- and post- menopausal women. Maturitas. 2017;99:37–42. [DOI] [PubMed] [Google Scholar]
- 31.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell. 2015;163:1079–94. [DOI] [PubMed] [Google Scholar]
- 32.Bennet SMP, Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut. 2018;67:872–81. [DOI] [PubMed] [Google Scholar]
- 33.Korpela K, Flint HJ, Johnstone AM, Lappi J, Poutanen K, Dewulf E, et al. Gut Microbiota Signatures Predict Host and Microbiota Responses to Dietary Interventions in Obese Individuals. Bereswill S, editor. PLoS ONE. 2014;9:e90702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Chang Y, Zhang K, Chen H, Tao S, Zhang Z. Implication of the gut microbiome composition of type 2 diabetic patients from northern China. Sci Rep. 2020;10:5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thaiss CA, Itav S, Rothschild D, Meijer MT, Levy M, Moresi C, et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016;540:544–51. [DOI] [PubMed] [Google Scholar]
- 36.••.Deehan EC, Yang C, Perez-Muñoz ME, Nguyen NK, Cheng CC, Triador L, et al. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe. 2020;27:389–404.e6. [DOI] [PubMed] [Google Scholar]; This study uses specialized structures of resistant starches to precisely, predictably, and dose-dependently modulate human gut microbial composition and metabolites produced, demonstrating a targeted and personalized nutritional approach to altering the gut microbiome.
- 37.Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020;11:411–55. [DOI] [PubMed] [Google Scholar]
- 38.Litvak Y, Byndloss MX, Bäumler AJ. Colonocyte metabolism shapes the gut microbiota. Science. 2018;362:eaat9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. 2016;57:943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sivaprakasam S, Bhutia YD, Yang S, Ganapathy V. Short-Chain Fatty Acid Transporters: Role in Colonic Homeostasis. In: Terjung R, editor. Compr Physiol [Internet]. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2017. [cited 2020 Aug 27]. p. 299–314. Available from: http://doi.wiley.com/10.1002/cphy.c170014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villodre Tudela C, Boudry C, Stumpff F, Aschenbach JR, Vahjen W, Zentek J, et al. Down-regulation of monocarboxylate transporter 1 ( MCT1 ) gene expression in the colon of piglets is linked to bacterial protein fermentation and pro-inflammatory cytokine-mediated signalling. Br J Nutr. 2015;113:610–7. [DOI] [PubMed] [Google Scholar]
- 42.Thibault R, De Coppet P, Daly K, Bourreille A, Cuff M, Bonnet C, et al. Down-Regulation of the Monocarboxylate Transporter 1 Is Involved in Butyrate Deficiency During Intestinal Inflammation. Gastroenterology. 2007;133:1916–27. [DOI] [PubMed] [Google Scholar]
- 43.Ferrer-Picón E, Dotti I, Corraliza AM, Mayorgas A, Esteller M, Perales JC, et al. Intestinal Inflammation Modulates the Epithelial Response to Butyrate in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020;26:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study: Short-chain fatty acid systemic availability and metabolism in humans. J Physiol. 2017;595:541–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bloemen JG, Venema K, van de Poll MC, Olde Damink SW, Buurman WA, Dejong CH. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin Nutr. 2009;28:657–61. [DOI] [PubMed] [Google Scholar]
- 46.van der Beek CM, Bloemen JG, van den Broek MA, Lenaerts K, Venema K, Buurman WA, et al. Hepatic Uptake of Rectally Administered Butyrate Prevents an Increase in Systemic Butyrate Concentrations in Humans. J Nutr. 2015;145:2019–24. [DOI] [PubMed] [Google Scholar]
- 47.Müller M, Hernández MAG, Goossens GH, Reijnders D, Holst JJ, Jocken JWE, et al. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci Rep. 2019;9:12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, et al. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature. 2016;534:213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. 2009;420:211–9. [DOI] [PubMed] [Google Scholar]
- 50.Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, et al. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol-Gastrointest Liver Physiol. 2004;287:G1168–74. [DOI] [PubMed] [Google Scholar]
- 51.Hansson GC. Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol. 2012;15:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers. 2015;3:e982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S, Kim J-H, Park BO, Kwak YS. Perspectives on the therapeutic potential of short-chain fatty acid receptors. BMB Rep. 2014;47:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, et al. GPR109A Is a G-protein-Coupled Receptor for the Bacterial Fermentation Product Butyrate and Functions as a Tumor Suppressor in Colon. Cancer Res. 2009;69:2826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X-F, Chen X, Tang X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin Sci. 2020;134:657–76. [DOI] [PubMed] [Google Scholar]
- 56.Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MAR. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol. 2016;5:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.••.Kimura I, Miyamoto J, Ohue-Kitano R, Watanabe K, Yamada T, Onuki M, et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science. 2020;367:eaaw8429. [DOI] [PubMed] [Google Scholar]; Describes the multi-generational effects of SCFA signaling on offspring metabolic and neural development.
- 58.Wächtershäuser A, Loitsch SM, Stein J. PPAR-γ Is Selectively Upregulated in Caco-2 Cells by Butyrate. Biochem Biophys Res Commun. 2000;272:380–5. [DOI] [PubMed] [Google Scholar]
- 59.Alex S, Lange K, Amolo T, Grinstead JS, Haakonsson AK, Szalowska E, et al. Short-Chain Fatty Acids Stimulate Angiopoietin-Like 4 Synthesis in Human Colon Adenocarcinoma Cells by Activating Peroxisome Proliferator-Activated Receptor. Mol Cell Biol. 2013;33:1303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeitouni NE, Chotikatum S, von Köckritz-Blickwede M, Naim HY. The impact of hypoxia on intestinal epithelial cell functions: consequences for invasion by bacterial pathogens. Mol Cell Pediatr. 2016;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bach Knudsen K, Lærke H, Hedemann M, Nielsen T, Ingerslev A, Gundelund Nielsen D, et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients. 2018;10:1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015;17:662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shulzhenko N, Morgun A, Hsiao W, Battle M, Yao M, Gavrilova O, et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med. 2011;17:1585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwab M, Reynders V, Loitsch S, Steinhilber D, Stein J, Schröder O. Involvement of different nuclear hormone receptors in butyrate-mediated inhibition of inducible NFκB signalling. Mol Immunol. 2007;44:3625–32. [DOI] [PubMed] [Google Scholar]
- 66.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, et al. Host-Mediated Inflammation Disrupts the Intestinal Microbiota and Promotes the Overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–29. [DOI] [PubMed] [Google Scholar]
- 67.Satokari R High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients. 2020;12:1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rousseaux C, El-Jamal N, Fumery M, Dubuquoy C, Romano O, Chatelain D, et al. The 5-aminosalicylic acid antineoplastic effect in the intestine is mediated by PPARγ. Carcinogenesis. 2013;34:2580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peyrin-Biroulet L, Beisner J, Wang G, Nuding S, Oommen ST, Kelly D, et al. Peroxisome proliferator-activated receptor gamma activation is required for maintenance of innate antimicrobial immunity in the colon. Proc Natl Acad Sci. 2010;107:8772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ponferrada Á, Caso JR, Alou L, Colón A, Sevillano D, Moro MA, et al. The Role of PPARγ on Restoration of Colonic Homeostasis After Experimental Stress-Induced Inflammation and Dysfunction. Gastroenterology. 2007;132:1791–803. [DOI] [PubMed] [Google Scholar]
- 71.Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci. 2005;102:11070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mathur R, Barlow GM. Obesity and the microbiome. Expert Rev Gastroenterol Hepatol. 2015;9:1087–99. [DOI] [PubMed] [Google Scholar]
- 74.Miranda VPN, dos Santos Amorim PR, Bastos RR, de Faria ER, de Castro Moreira ME, do Carmo Castro Franceschini S, et al. Abundance of Gut Microbiota, Concentration of Short-Chain Fatty Acids, and Inflammatory Markers Associated with Elevated Body Fat, Overweight, and Obesity in Female Adolescents. Mediators Inflamm. 2019;2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Crovesy L, Masterson D, Rosado EL. Profile of the gut microbiota of adults with obesity: a systematic review. Eur J Clin Nutr. 2020;74:1251–62. [DOI] [PubMed] [Google Scholar]
- 76.Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588:4223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ley RE. Prevotella in the gut: choose carefully. Nat Rev Gastroenterol Hepatol. 2016;13:69–70. [DOI] [PubMed] [Google Scholar]
- 78.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci. 2007;104:979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science. 2013;341:1241214–1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Byrne CS, Chambers ES, Morrison DJ, Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes. 2015;39:1331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arencibia-Albite F Serious analytical inconsistencies challenge the validity of the energy balance theory. Heliyon. 2020;6:e04204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Lahham SH, Roelofsen H, Priebe M, Weening D, Dijkstra M, Hoek A, et al. Regulation of adipokine production in human adipose tissue by propionic acid. Eur J Clin Invest. 2010;40:401–7. [DOI] [PubMed] [Google Scholar]
- 83.Yao H, Fan C, Fan X, Lu Y, Wang Y, Wang R, et al. Effects of gut microbiota on leptin expression and body weight are lessened by high-fat diet in mice. Br J Nutr. 2020;124:396–406. [DOI] [PubMed] [Google Scholar]
- 84.Yao H, Fan C, Lu Y, Fan X, Xia L, Li P, et al. Alteration of gut microbiota affects expression of adiponectin and resistin through modifying DNA methylation in high-fat diet-induced obese mice. Genes Nutr. 2020;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Larraufie P, Martin-Gallausiaux C, Lapaque N, Dore J, Gribble FM, Reimann F, et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes. 2012;61:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TMS, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4:e121–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim KN, Yao Y, Ju SY. Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and Meta-Analysis. Nutrients. 2019;11:2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chambers ES, Byrne CS, Aspey K, Chen Y, Khan S, Morrison DJ, et al. Acute oral sodium propionate supplementation raises resting energy expenditure and lipid oxidation in fasted humans. Diabetes Obes Metab. 2018;20:1034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Canfora EE, van der Beek CM, Jocken JWE, Goossens GH, Holst JJ, Olde Damink SWM, et al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep. 2017;7:2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van der Beek CM, Canfora EE, Lenaerts K, Troost FJ, Olde Damink SWM, Holst JJ, et al. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci. 2016;130:2073–82. [DOI] [PubMed] [Google Scholar]
- 92.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577–91. [DOI] [PubMed] [Google Scholar]
- 93.Aleixandre A, Miguel M. Dietary fiber and blood pressure control. Food Funct. 2016;7:1864–71. [DOI] [PubMed] [Google Scholar]
- 94.Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci. 2018;132:701–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M. Increased Systolic and Diastolic Blood Pressure Is Associated With Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension. 2016;68:974–81. [DOI] [PubMed] [Google Scholar]
- 97.••.Verhaar BJH, Collard D, Prodan A, Levels JHM, Zwinderman AH, Bäckhed F, et al. Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: the HELIUS study. Eur Heart J. 2020;ehaa704. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrated a population and ethnic-specific association of the fecal microbiota composition in a direct association with blood pressure as SCFA-producing bacteria and plasma-SCFA levels were inversely proportional to blood pressure while fecal SCFA content were positively associated with blood pressure.
- 98.•.Chang Y, Chen Y, Zhou Q, Wang C, Chen L, Di W, et al. Short-chain fatty acids accompanying changes in the gut microbiome contribute to the development of hypertension in patients with preeclampsia. Clin Sci. 2020;134:289–302. [DOI] [PubMed] [Google Scholar]; This study observed diminished microbial diversity and richness, reduced butyrate producers and abundance, and prominent intestinal inflammation and permeability in a population of pregnant women diagnosed with preeclampsia.
- 99.Galla S, Chakraborty S, Cheng X, Yeo J, Mell B, Zhang H, et al. Disparate effects of antibiotics on hypertension. Physiol Genomics. 2018;50:837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jose PA, Raj D. Gut microbiota in hypertension: Curr Opin Nephrol Hypertens. 2015;24:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.••.Calderón-Pérez L, Gosalbes MJ, Yuste S, Valls RM, Pedret A, Llauradó E, et al. Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study. Sci Rep. 2020;10:6436. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study characterized fecal SCFA and microbial profiles which were specific to and regarded as a signature for individuals with HTN prior to drug therapy.
- 102.Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, et al. Role of the Gut Microbiome in Obstructive Sleep Apnea–Induced Hypertension. Hypertension. 2016;67:469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Toral M, Robles-Vera I, Visitación N, Romero M, Sánchez M, Gómez-Guzmán M, et al. Role of the immune system in vascular function and blood pressure control induced by faecal microbiota transplantation in rats. Acta Physiol. 2019;e13285. [DOI] [PubMed] [Google Scholar]
- 104.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yan X, Jin J, Su X, Yin X, Gao J, Wang X, et al. Intestinal Flora Modulates Blood Pressure by Regulating the Synthesis of Intestinal-Derived Corticosterone in High Salt-Induced Hypertension. Circ Res. 2020;126:839–53. [DOI] [PubMed] [Google Scholar]
- 106.Huart J, Leenders J, Taminiau B, Descy J, Saint-Remy A, Daube G, et al. Gut Microbiota and Fecal Levels of Short-Chain Fatty Acids Differ Upon 24-Hour Blood Pressure Levels in Men. Hypertension. 2019;74:1005–13. [DOI] [PubMed] [Google Scholar]
- 107.Chakraborty S, Mandal J, Cheng X, Galla S, Hindupur A, Saha P, et al. Diurnal Timing Dependent Alterations in Gut Microbial Composition Are Synchronously Linked to Salt-Sensitive Hypertension and Renal Damage. Hypertension. 2020;76:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mashaqi S, Gozal D. Obstructive Sleep Apnea and Systemic Hypertension: Gut Dysbiosis as the Mediator? J Clin Sleep Med. 2019;15:1517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu J, Li T, Wu H, Shi H, Bai J, Zhao W, et al. Lactobacillus rhamnosus GG strain mitigated the development of obstructive sleep apnea-induced hypertension in a high salt diet via regulating TMAO level and CD4+ T cell induced-type I inflammation. Biomed Pharmacother. 2019;112:108580. [DOI] [PubMed] [Google Scholar]
- 110.Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, et al. Prebiotics, Probiotics, and Acetate Supplementation Prevent Hypertension in a Model of Obstructive Sleep Apnea. Hypertension. 2018;72:1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dave LA, Hayes M, Montoya CA, Rutherfurd SM, Moughan PJ. Human gut endogenous proteins as a potential source of angiotensin-I-converting enzyme (ACE-I)-, renin inhibitory and antioxidant peptides. Peptides. 2016;76:30–44. [DOI] [PubMed] [Google Scholar]
- 112.Richards EM, Pepine CJ, Raizada MK, Kim S. The Gut, Its Microbiome, and Hypertension. Curr Hypertens Rep. 2017;19:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci. 2013;110:4410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miyamoto J, Kasubuchi M, Nakajima A, Irie J, Itoh H, Kimura I. The role of short-chain fatty acid on blood pressure regulation: Curr Opin Nephrol Hypertens. 2016;25:379–83. [DOI] [PubMed] [Google Scholar]
- 115.Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation. 2019;139:1407–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Felizardo RJF, Watanabe IKM, Dardi P, Rossoni LV, Câmara NOS. The interplay among gut microbiota, hypertension and kidney diseases: The role of short-chain fatty acids. Pharmacol Res. 2019;141:366–77. [DOI] [PubMed] [Google Scholar]
- 117.Marques FZ, Nelson E, Chu P-Y, Horlock D, Fiedler A, Ziemann M, et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation. 2017;135:964–77. [DOI] [PubMed] [Google Scholar]
- 118.Hsu C-N, Lin Y-J, Hou C-Y, Tain Y-L. Maternal Administration of Probiotic or Prebiotic Prevents Male Adult Rat Offspring against Developmental Programming of Hypertension Induced by High Fructose Consumption in Pregnancy and Lactation. Nutrients. 2018;10:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hsu C-N, Hou C-Y, Chan JYH, Lee C-T, Tain Y-L. Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients. 2019;11:2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Na L, Chu X, Jiang S, Li C, Li G, He Y, et al. Vinegar decreases blood pressure by down-regulating AT1R expression via the AMPK/PGC-1α/PPARγ pathway in spontaneously hypertensive rats. Eur J Nutr. 2016;55:1245–53. [DOI] [PubMed] [Google Scholar]
- 121.Hsu C, Chang-Chien G, Lin S, Hou C, Tain Y. Targeting on Gut Microbial Metabolite Trimethylamine- N -Oxide and Short-Chain Fatty Acid to Prevent Maternal High-Fructose-Diet-Induced Developmental Programming of Hypertension in Adult Male Offspring. Mol Nutr Food Res. 2019;63:1900073. [DOI] [PubMed] [Google Scholar]
- 122.Vital M, Howe AC, Tiedje JM. Revealing the Bacterial Butyrate Synthesis Pathways by Analyzing (Meta)genomic Data. Moran MA, editor. mBio. 2014;5:e00889–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


