Abstract
Objective
Identify predictors of clinical deterioration in a virtual hospital (VH) setting for COVID-19.
Design
Real-world prospective observational study.
Setting
VH remote assessment service in West Hertfordshire NHS Trust, UK.
Participants
Patients with suspected COVID-19 illness enrolled directly from the community (postaccident and emergency (A&E) or medical intake assessment) or postinpatient admission.
Main outcome measure
Death or (re-)admission to inpatient hospital care during VH follow-up and for 2 weeks post-VH discharge.
Results
900 patients with a clinical diagnosis of COVID-19 (455 referred from A&E or medical intake and 445 postinpatient) were included in the analysis. 76 (8.4%) of these experienced clinical deterioration (15 deaths in admitted patients, 3 deaths in patients not admitted and 58 additional inpatient admissions). Predictors of clinical deterioration were increase in age (OR 1.04 (95% CI 1.02 to 1.06) per year of age), history of cancer (OR 2.87 (95% CI 1.41 to 5.82)), history of mental health problems (OR 1.76 (95% CI 1.02 to 3.04)), severely impaired renal function (OR for eGFR <30=9.09 (95% CI 2.01 to 41.09)) and having a positive SARS-CoV-2 PCR result (OR 2.0 (95% CI 1.11 to 3.60)).
Conclusions
These predictors may help direct intensity of monitoring for patients with suspected or confirmed COVID-19 who are being remotely monitored by primary or secondary care services. Further research is needed to confirm our findings and identify the reasons for increased risk of clinical deterioration associated with cancer and mental health problems.
Keywords: COVID-19, epidemiology, general medicine (see internal medicine), infectious diseases
Strengths and limitations of this study.
The study uses anonymised data from all patients registered for the virtual hospital (VH) between 17 March 2020 and 17 May 2020, and therefore selection bias is not an issue.
At the time of this study, this was the only service providing remote follow-up for patients with suspected COVID-19 in the area, and therefore our findings are likely to be relevant to primary care patients receiving remote follow-up.
We were able to collect reliable data on a wide range of clinical and demographic features, and reliably follow all patients for the primary outcome for at least 2 weeks following their discharge from the VH.
We were not able to extract detailed symptom or clinical examination data on all participants, and had to use laboratory result data from initial presentation (including in those who had an inpatient admission).
Our study is likely underpowered to detect all predictors, especially in the analysis of our two subgroups.
Background
The COVID-19 pandemic has created unprecedented challenges to healthcare services. Concerns about hospital services being overwhelmed led National Health Service (NHS) institutions to develop novel approaches to caring for patients with suspected COVID-19. These include virtual hospitals (VH) where patients who have come to the attention of hospital services and need close monitoring, but do not necessarily need inpatient care, are followed remotely by hospital-based clinicians.1 VH are particularly valuable during periods of high disease prevalence, when inpatient hospital services are struggling to cope. During these periods, patients are likely to come from two main routes—those that are becoming increasingly unwell in the community, including patients who have presented at accident and emergency (A&E) and patients referred to the hospital by general practitioners, and those who have had an inpatient admission and are being offered a supported early discharge.
COVID-19 infection is often mild, self-limiting or asymptomatic, but up to 20% of symptomatic individuals may have severe illness.2 Identifying those likely to have a worse prognosis is therefore extremely important. Several studies have reported prognostic factors in hospitalised patients, but there have been no studies looking at prognosis in those managed out of hospital via remote patient monitoring services in virtual ward/VH settings, who have less severe clinical presentations but may be at risk of deterioration. Understanding factors associated with prognosis in these patients is important in designing services and deciding on admission and escalation criteria, monitoring protocols and discharge criteria. These data are likely to be particularly valuable in informing subsequent waves of COVID-19 and are likely to be relevant to primary care services providing enhanced surveillance of patients with suspected COVID-19 in the community. We therefore set out to identify predictors of clinical deterioration in a cohort of patients admitted to a VH at one general hospital in England.
Methods
This is a retrospective observational study using data collected as part of routine clinical care by clinicians working in West Hertfordshire Hospitals Trust, which serves a population of over 500 000 living in west Hertfordshire, a mix of rural and towns, and also serves residents in north London, Buckinghamshire and Bedfordshire. In response to the emerging pandemic, clinicians at Watford General Hospital set up a VH in March 2020. The aim was to reduce pressure on inpatient capacity by providing remote clinical assessment to patients at home in place of hospital admission, or to facilitate early discharge from hospital. Watford General Hospital has approximately 600 beds and is the main site for urgent hospital services in West Hertfordshire Hospitals Trust.
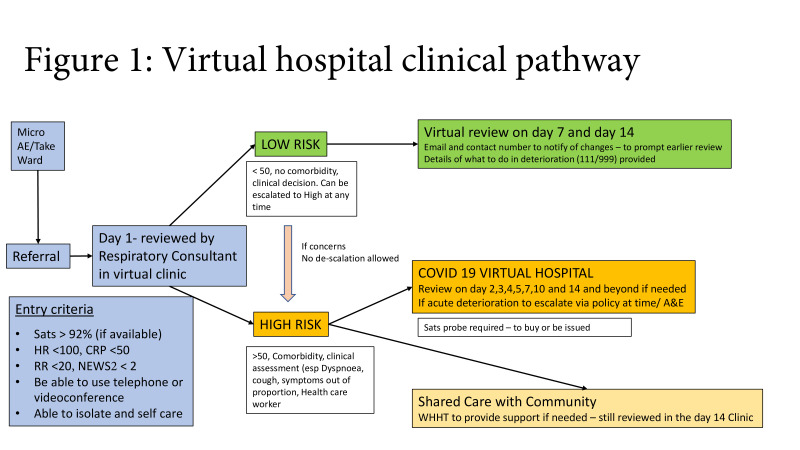
Patients with suspected or confirmed COVID-19 were managed in the VH if they met the inclusion criteria: oxygen saturation >92% on air (or >88% if known to have long-term saturations <92%), resting respiratory rate <20, National Early Warning Score 2 (NEWS2) <2, C reactive protein (CRP) <50, resting HR <100, were able to self-isolate and self-care and had access to a telephone or webcam). Patients were triaged into high-risk or low-risk pathways for follow-up. Patients were either referred directly from A&E or medical intake (referred to the hospital for assessment but not admitted) (community patients) or were stepped down following a hospital admission (figure 1).
Figure 1.
Virtual hospital clinical pathway. A&E, accident and emergency; CRP, C reactive protein; RR, respiratory rate; NEWS2, National Early Warning Score 2; WHHT, West Hertfordshire Hospitals Trust.
Data collection
Participants are patients enrolled in the VH between 17 March and 17 May 2020. Data were recorded as part of routine clinical care with an approved clinical pathway, so participants did not provide informed consent. Data were pseudonymised by staff at West Hertfordshire Hospitals by removing all personal identifying data such as names, date of birth, address. Participants were identified with a unique identifying number, with the key held at West Hertfordshire Hospitals. Pseudonymised data were transferred securely to researchers at the University of Southampton, who analysed the data.
Participants came from one of two routes: (1) patients referred to the VH from A&E or medical intake (community) or (2) patients who were discharged (early) directly to the VH (postinpatient). At baseline, a general or respiratory consultant working in the VH assessed, examined and investigated patients as part of their clinical care, and documented data in their medical record. Data supporting the management of the VH were extracted from participants’ medical records, and these data were used for this study. Therefore, data were not collected in a protocolised way but reflect the recording of healthcare data in a busy clinical setting.
Baseline data were collected for the day the patient was admitted to the VH (discharge date for postinpatients), and include: age (calculated from date of birth), gender, smoking status, type of domicile (home, residential home, nursing home, mental health unit, sheltered accommodation, other), comorbid conditions (diabetes, asthma, chronic obstructive pulmonary disease (COPD), other respiratory, cardiovascular disease, chronic kidney disease (CKD), cancer (if recorded in general practitioner (GP) or hospital record), connective tissue disorder, mental health problem), frailty (defined as having a Rockwood score >3 at time of presentation), medications assessed as potentially relevant to COVID-19 prognosis at the time of VH setup (ACE inhibitors, angiotensin II receptor blockers (AR2b), non-steroidal anti-inflammatory drugs, immunosuppressants, oral diabetic medications, insulin, anticoagulants (including direct oral anticoagulants), long-acting beta-agonist inhalers, long-acting muscarinic antagonist inhalers, inhaled corticosteroid inhalers, beta-blockers, proton pump inhibitors, antidepressants, azithromycin and hydroxychloroquine), symptoms (presence or absence of shortness of breath, cough, fever, chest pain, diarrhoea, headache, myalgia, fatigue). Baseline examination and investigation data extracted for the study include: oxygen saturation, chest X-ray (CXR) result (normal or abnormal), blood tests (white cell count (WCC), lymphocytes, oeosinophils, platelets, CRP, creatinine, ferritin, D-dimer, troponin). Investigation results used in the analysis were those obtained during the initial assessment in A&E or medical admissions. Oxygen saturation levels were categorised as ≤91, 92–93, 94–95, ≥96. Clinicians running the VH attempted to obtain nasal/throat swabs for SARS-CoV-2 testing from all patients. However, during the early phase of the pandemic there was insufficient testing capacity and patients who were not admitted were not tested. SARS-CoV-2 testing was done by PCR at Public Health England-approved laboratories. Participants were then classified as: COVID-19 positive, negative, inconclusive or not tested.
Patients referred to the VH were followed up through periodic phone calls to check on their status. High-risk patients were followed up by a respiratory consultant on days 2–5, 7, 10, 14 and beyond if needed, whereas low-risk patients were followed up by a consultant physician or GP on days 7 and 14. Both high-risk and low-risk patients were included in the study. Decisions about discharge from the VH were made by the clinician responsible for the patient based on overall clinical assessment and were not protocolised. In addition, participants’ hospital records were screened for overnight re-admission to the hospital and/or death within the 2 weeks following their initial discharge from the VH. Admission was defined as any COVID-related (including complications such as pneumonia or dehydration) admission to a hospital ward or any stay in the assessment unit that continued past midnight.
Data analysis
Following data cleaning, standard statistical approaches (proportions, mean and SD) were used to describe the study population, split by route of admission to the VH (from the community or postinpatient discharge).
Our primary study end point was ‘clinical deterioration’, defined as death or overnight hospital (re-)admission during the follow-up period (until 2 weeks after discharge from VH). The relationship between potential baseline predictors and outcome were explored using univariable and then multivariable logistic regression models. Potential predictors included in the model were: gender, age, comorbid conditions, medications, symptoms, oxygen saturation, CXR result, COVID-19 testing and laboratory test results (WCC, lymphocytes, oeosinophils, platelets, CRP, creatinine). All variables were included in a multivariable logistic regression model regardless of the statistical significance of their univariate associations. Backward selection was used with variables retained if p<0.20 (based on log-likelihood). Sensitivity analyses were carried out using a threshold of p<0.10 and using only hospital admissions as an outcome. All adjusted associations are reported as ORs with 95% CIs.
We fitted an initial model controlling for the two routes of admission, and we also fitted separate models for these subgroups.
Multiple imputation using chained equations was used to impute the values of any missing predictors or outcome variables.
Sample size calculation
Our sample size calculation was based on the minimum required sample size for a multivariable prediction model as set out in the study by Riley et al3 and based on the assumption that 10% of patients experience the outcome and allowed for up to 10 parameters in the final model, with r2 of 20% (based on previous literature). Using these parameters and the Stata pmsampsize function,4 we calculated a minimum required sample size of 398 patients. Assuming that approximately half of the patients would enter the VH through each of the two routes of admission and allowing for loss to follow-up and missing data, we aimed to include 900 patients.
Patient involvement
This was an unfunded study set up to analyse existing routinely collected data during a pandemic. Patients were not involved in the design, conduct or reporting of the study.
Results
Data from the first 900 patients treated in VH were made available for analysis. This included 455 patients who were admitted directly from the community and 445 patients who entered the VH postinpatient admissions. Participants were followed in the VH for a median of 21 days (range 15–46), with very little difference between the community (median 21, range 15–43 days) and postinpatient (median 21, range 15–46 days) groups.
The demographic features, comorbid illnesses and current medications of the community and postinpatient discharge groups are described in table 1. The population admitted to the VH directly from the community included a greater proportion of females, had a younger average age, more never-smokers and fewer ex-smokers, fewer nursing home residents, fewer patients with physical comorbidities and slightly more with comorbid mental health problems than the postinpatient group. Baseline symptoms, oxygen saturation levels and results of investigations are described in table 2. A slightly larger proportion of the community group reported shortness of breath, cough, chest pain, headache, myalgia and fatigue, than in the postinpatient group. However, reporting of fever and diarrhoea occurred in a slightly smaller proportion of the community group compared with the postinpatient group. Normal oxygen saturation levels were much more prevalent in the community group compared with the postinpatient group (86.5% vs 58.6%) and a smaller proportion of the community group had an abnormal CXR result compared with the post-inpatient group (48.9% vs 77.5%).
Table 1.
Patient characteristics
| Post-inpatient (n=445) | Community (n=455) | |
| Female | 202/444 (45.5%) | 275/455 (60.4%) |
| Mean age (SD) | 61.0 (17.38) | 48.9 (14.01) |
| BAME | 114/438 (26.0%) | 153/448 (34.1%) |
| Smoking | ||
| No | 35/190 (18.4%) | 51/163 (31.3%) |
| Yes | 10/190 (5.3%) | 8/163 (4.9%) |
| Ex-smoker | 145/190 (76.3%) | 104/163 (63.8%) |
| Residence prior to admission | ||
| Home | 392/428 (91.6%) | 414/427 (96.7%) |
| Residential home | 3/428 (0.7%) | 0/427 (0.0%) |
| Nursing home | 30/428 (7.0%) | 6/427 (1.4%) |
| Mental unit | 2/428 (0.5%) | 4/427 (0.9%) |
| Sheltered accommodation | 1/428 (0.2%) | 2/427 (0.5%) |
| Other | 0/428 (0.0%) | 1/427 (0.2%) |
| Comorbid conditions | ||
| Diabetes | 110/424 (25.9%) | 52/423 (12.3%) |
| Frail | 89/430 (20.7%) | 9/426 (2.1%) |
| Mental health | 133/429 (31.0%) | 142/424 (33.5%) |
| CKD | 49/426 (11.5%) | 11/424 (2.6%) |
| CTD | 74/426 (17.4%) | 52/424 (12.3%) |
| CVD | 44/425 (10.4%) | 13/424 (3.1%) |
| Cancer | 47/428 (11.0%) | 27/424 (6.4%) |
| Asthma | 94/431 (21.8%) | 124/427 (29.0%) |
| COPD | 52/429 (12.1%) | 18/425 (4.2%) |
| Other respiratory | 30/430 (7.0%) | 17/425 (4.0%) |
| Number of comorbid conditions | ||
| None | 100/445 (22.5%) | 159/455 (35.0%) |
| 1 | 109 (24.5%) | 125 (27.5%) |
| 2 | 87 (19.6%) | 97 (21.3%) |
| 3 | 58 (13.0%) | 57 (12.5%) |
| 4 | 48 (10.8%) | 9 (2.0%) |
| 5+ | 43 (9.7%) | 8 (1.8%) |
| Medications | ||
| ACEi | 72/425 (16.9%) | 47/411 (11.4%) |
| AR2b | 44/424 (10.4%) | 24/410 (5.9%) |
| Sildenafil | 12/424 (2.8%) | 4/410 (1.0%) |
| NSAID | 60/425 (14.1%) | 43/410 (10.5%) |
| Immunosuppressants | 22/425 (5.2%) | 16/410 (3.9%) |
| LABA | 67/424 (15.8%) | 39/410 (9.5%) |
| ICS | 85/424 (20.1%) | 68/410 (16.6%) |
| LAMA | 30/424 (7.1%) | 10/410 (2.4%) |
| DOAC or other anticoagulant | 77/424 (18.2%) | 22/411 (5.4%) |
| HQ | 5/424 (1.2%) | 2/410 (0.5%) |
| Oral diabetes medication | 71/424 (16.8%) | 33/410 (8.1%) |
| Insulin | 20/424 (4.7%) | 9/410 (2.2%) |
| Azithromycin | 3/424 (0.7%) | 0/410 (0.0%) |
| Beta-blockers | 78/425 (18.4%) | 36/410 (8.8%) |
| PPI | 154/425 (36.2%) | 88/410 (21.5%) |
| Antidepressants | 76/424 (17.9%) | 79/410 (19.3%) |
ACEi, ACE inhibitor; AR2b, angiotensin II receptor blocker; BAME, Black, Asian, Minority Ethnic; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CTD, connective tissue disorder; CVD, cardiovascular disease; DOAC, direct oral anticoagulant; HQ, hydroxychloroquine; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor.
Table 2.
Illness presentation
| Postinpatient | Community | |
| Median (IQR) duration of symptoms prior to contact with VH | 7 (4, 11.5) n=188 | 7 (5, 14) n=307 |
| Length of stay prior to admission to VH (postinpatient group) | ||
| Median (IQR) | 4 (3, 8) | |
| 3 days or less—N (%) | 159/415 (38.3%) | |
| 4–5 days—N (%) | 91/415 (21.9%) | |
| 6+ days | 165/415 (39.8%) | |
| ITU admission during hospital stay prior to VH (postinpatient group) | 11 (2.5%) | |
| Shortness of breath | 295/438 (67.4%) | 319/449 (71.1%) |
| Cough | 301/438 (68.7%) | 341/450 (75.8%) |
| Fever | 284/438 (64.8%) | 281/449 (62.6%) |
| Chest pain | 57/438 (13.0%) | 118/449 (26.3%) |
| Diarrhoea | 72/438 (16.4%) | 61/449 (13.6%) |
| Headache | 46/438 (10.5%) | 82/449 (18.3%) |
| Myalgia | 88/438 (20.1%) | 129/449 (28.7%) |
| Fatigue | 128/438 (29.2%) | 137/449 (30.4%) |
| COVID test result | ||
| Positive | 271/445 (60.9%) | 143/455 (31.4%) |
| Negative | 156/445 (35.1%) | 193/455 (42.4%) |
| Not done | 9/445 (2.0%) | 95/455 (20.9%) |
| Not valid/Pending | 9/445 (2.0%) | 24/455 (5.3%) |
| Abnormal CXR | 303 (77.5%) | 208 (48.9%) |
| Oxygen saturation | ||
| ≤91 | 36/309 (11.7%) | 5/377 (1.3%) |
| 92–93 | 31/309 (10.0%) | 13/377 (3.5%) |
| 94–95 | 61/309 (19.7%) | 33/377 (8.8%) |
| ≥96 | 181/309 (58.6%) | 326/377 (86.5%) |
| Baseline blood tests median values and categories | ||
| Platelets | 268.5 (189.5, 364) | 241 (188, 300) |
| <150 | 37 (9.6%) | 41 (11.0%) |
| 150–450 | 300 (78.1%) | 324 (86.9%) |
| >450 | 47 (12.2%) | 8 (2.1%) |
| WCC | 6.85 (5.35, 9) | 6.8 (5.3, 8.9) |
| <4 | 30 (7.8%) | 26 (7.0%) |
| 4–11 | 307 (80.0%) | 300 (80.4%) |
| 11 | 47 (12.2%) | 47 (12.6%) |
| Lymphocytes | 1.15 (0.8, 1.62) | 1.47 (1.03, 2.12) |
| <0.8 | 95 (24.7%) | 53 (14.2%) |
| 0.8–5.0 | 288 (75.0%) | 319 (85.5%) |
| >5.0 | 1 (0.3%) | 1 (0.3%) |
| Eosinophils | 0.06 (0.01, 0.14) | 0.06 (0.01, 0.18) |
| <0.5 | 374 (97.4%) | 354 (94.9%) |
| 0.5–1.0 | 8 (2.1%) | 16 (4.3%) |
| >1.0 | 2 (0.5%) | 3 (0.8%) |
| CRP | 43.75 (16.4, 74)1 | 8.25 (0.00, 42.35) |
| Normal | 44 (12.0%) | 157 (43.6%) |
| 5–19 | 58 (15.8%) | 67 (18.6%) |
| 20–100 | 205 (55.7%) | 108 (30.0%) |
| >100 | 61 (16.6%) | 28 (7.8%) |
| eGFR (CKD stage) | 89.8 (71.2, 108.0) | 88.8 (77.2, 98.9) |
| ≥90 (normal) | 188 (49.5%) | 170 (46.0%) |
| 60–89 (stage 2) | 139 (36.6%) | 174 (47.0%) |
| 45–59 (stage 3a) | 28 (7.4%) | 21 (5.7%) |
| 30–44 (stage 3b) | 19 (5.0%) | 2 (0.5%) |
| 15–29 (stage 4) | 5 (1.3%) | 3 (0.8%) |
| <15 (stage 5) | 1 (0.3%) | 0 (0.0%) |
| Risk status | ||
| High risk | 388 (88.8%) | 408 (89.9%) |
| Low risk | 49 (11.2%) | 46 (10.1%) |
CKD, chronic kidney disease; CRP, C reactive protein; CXR, chest X-ray; eGFR, estimated glomerular filtration rate; ITU, intensive therapy unit; VH, virtual hospital; WCC, white cell count.
Seven hundred sixty-three (84.8%) of the cohort had a valid COVID-19 PCR test result available, with 33 (3.7%) having an invalid test result and 104 (11.6%) not having a test performed (20.9% of the community group and 2.0% of the postinpatient group). Of those who had a valid test result, 143/336 (42.6%) of the community group had a test that was positive for COVID-19, and 271/427 (63.5%) of the postinpatient group had a positive test.
Predictors of clinical deterioration
Seventy-six (8.4%) participants experienced a clinical deterioration. Fifty-eight participants had a hospital admission that they survived, 15 patients had a hospital admission and did not survive and 3 deaths occurred in patients that did not have a hospital admission (table 3). Univariable and multivariable models identifying predictors of clinical deterioration, controlling for route of admission, are shown in table 4.
Table 3.
Experienced clinical deterioration
| Postinpatient (n=445) | Community (n=455) | |
| Experienced clinical deterioration | 52/420 (12.4%) | 24/439 (5.5%) |
| Hospital admission (survived) | 36/420 (8.6%) | 22/439 (5.0%) |
| Hospital admission (died) | 13/419 (3.1%) | 2/439 (0.05%) |
| Death without hospital admission | 3/419 (0.1%) | 0/439 (0.0%) |
Table 4.
Features associated with clinical deterioration
| Univariate OR (95% CI) | Adjusted OR (95% CI) with all variables in the model | Adjusted OR (95% CI) retaining only those with p<0.20 (backward selection) | |
| Community | 0.40 (0.24 to 0.66) | 0.68 (0.34 to 1.37) | |
| Low-risk status | 0.43 (0.15 to 1.20) | 0.35 (0.10 to 1.29) | |
| Male | 1.08 (0.67 to 1.74) | 0.53 (0.23 to 1.22) | |
| Age | 1.05 (1.04 to 1.07) | 1.03 (1.00 to 1.06) | 1.04 (1.02 to 1.06) |
| BAME | 0.66 (0.38 to 1.16) | 1.05 (0.50 to 2.18) | |
| Comorbid conditions | |||
| Diabetes | 2.95 (1.78 to 4.89) | 2.12 (1.07 to 4.19) | 1.71 (0.95 to 3.10) |
| Mental health | 1.64 (1.00 to 2.68) | 1.71 (0.92 to 3.19) | 1.76 (1.02 to 3.04) |
| CKD | 2.04 (0.97 to 4.29) | 0.27 (0.08 to 0.94) | 0.41 (0.15 to 1.14) |
| CTD | 0.82 (0.38 to 1.78) | 0.40 (0.13 to 1.26) | 0.46 (0.19 to 1.09) |
| CVD | 2.04 (0.96 to 4.35) | 0.77 (032 to 1.85) | |
| Cancer | 3.74 (2.03 to 6.88) | 3.33 (1.32 to 8.40) | 2.87 (1.41 to 5.82) |
| COPD | 2.62 (1.31 to 5.23) | 1.86 (0.46 to 7.43) | |
| Asthma | 0.69 (0.38 to 1.25) | 0.67 (0.19 to 2.48) | |
| Other respiratory | 0.91 (0.35 to 2.37) | 0.40 (0.08 to 1.90) | |
| Number of comorbid conditions | |||
| None | Ref | Ref | |
| 1 | 1.45 (0.68 to 3.08) | 2.88 (0.96 to 8.66) | |
| 2 | 1.44 (0.64 to 3.24) | 2.01 (0.50 to 8.17) | |
| 3 | 3.40 (1.58 to 7.30) | 3.92 (0.71 to 21.59) | |
| 4 | 2.99 (1.13 to 7.92) | 1.56 (0.18 to 13.35) | |
| 5+ | 5.55 (2.30 to 13.33) | 3.65 (0.28 to 48.41) | |
| Medications | |||
| ACEI | 1.55 (0.84 to 2.80) | 0.91 (0.39 to 2.09) | |
| AR2b | 1.09 (0.48 to 2.50) | 0.62 (0.21 to 1.81) | |
| Immunosuppressant | 0.83 (0.25 to 2.75) | 0.94 (0.23 to 3.86) | |
| NSAID | 1.79 (0.96 to 3.34) | 1.17 (0.52 to 2.63) | |
| ICS | 1.08 (0.58 to 1.99) | 0.90 (0.33 to 2.46) | |
| DOAC or other anticoagulant | 2.91 (1.62 to 5.19) | 1.10 (0.49 to 2.50) | |
| Shortness of breath | 0.80 (0.49 to 1.30) | 1.07 (0.56 to 2.04) | |
| Cough | 0.85 (0.51 to 1.39) | 1.17 (0.61 to 2.26) | |
| Fever | 1.09 (0.66 to 1.78) | 1.23 (0.62 to 2.46) | |
| Chest pain | 0.66 (0.34 to 1.27) | 1.31 (0.59 to 4.42) | |
| Diarrhoea | 0.67 (0.32 to 1.43) | 0.57 (0.23 to 1.38) | 0.55 (0.24 to 1.25) |
| Headache | 0.59 (0.26 to 1.31) | 1.63 (0.60 to 4.42) | |
| Myalgia | 0.64 (0.35 to 1.17) | 0.89 (0.42 to 1.89) | |
| Fatigue | 0.86 (0.51 to 1.47) | 0.69 (0.35 to 1.33) | |
| Normal CXR | 0.49 (0.28 to 0.86) | 1.10 (0.52 to 2.32) | |
| Oxygen saturation | |||
| ≤91 | 1.88 (0.72 to 4.62) | 0.61 (0.17 to 2.22) | |
| 92–93 | 1.36 (0.49 to 3.50) | 0.82 (0.24 to 2.84) | |
| 94–95 | 1.41 (0.69 to 2.87) | 0.83 (0.34 to 2.02) | |
| ≥96 | Ref | Ref | |
| SARS-CoV-2 test results | |||
| Positive | 2.14 (1.27 to 3.63) | 1.92 (0.93 to 3.94) | 2.00 (1.11 to 3.60) |
| Negative | Ref | Ref | Ref |
| Invalid/Pending | 0.93 (0.21 to 4.15) | 1.10 (0.19 to 6.47) | 1.21 (0.24 to 5.99) |
| No swab | 0.28 (0.07 to 1.23) | 0.41 (0.08 to 2.06) | 0.38 (0.08 to 1.76) |
| Platelets | |||
| <150 | 1.40 (0.71 to 2.79) | 0.88 (0.38 to 2.06) | |
| 150–450 | Ref | Ref | |
| >450 | 1.03 (0.95 to 2.71) | 1.06 (0.34 to 3.33) | |
| WCC | |||
| <4 | 1.58 (0.75 to 3.33) | 1.35 (0.50 to 3.68) | |
| 4–11 | Ref | Ref | |
| >11 | 0.95 (0.45 to 2.02) | 0.70 (0.27 to 1.85) | |
| Lymocytes | |||
| <0.8 | 2.86 (1.72 to 4.74) | 1.40 (0.71 to 2.76) | |
| 0.8–5.0 | Ref | Ref | |
| >5.0 | 13.41 (0.82 to 217.82)* | 3.78 (0.05 to 297.56) | |
| Oeosinophils | |||
| <0.5 | Ref | Ref | Ref |
| 0.5–1.0 | NA | NA | NA |
| >1.0 | NA† | NA | NA |
| CRP | |||
| <5 | Ref | Ref | |
| 5–19 | 1.22 (0.49 to 3.03) | 0.74 (0.25 to 2.24) | |
| 20–100 | 2.11 (1.06 to 4.19) | 0.92 (0.36 to 2.40) | |
| >100 | 3.06 (1.33 to 7.03) | 1.43 (0.45 to 4.52) | |
| eGFR (CKD stage) | |||
| ≥90 (normal) | Ref | Ref | Ref |
| 60–89 (stage 2) | 0.99 (0.55 to 1.80) | 1.14 (0.47 to 2.74) | 0.78 (0.41 to 1.48) |
| 45–59 (stage 3a) | 1.12 (0.49 to 2.53) | 1.80 (0.51 to 6.35) | 0.98 (0.41 to 2.34) |
| 30–44 (stage 3b) | 3.90 (1.70 to 8.98) | 4.96 (1.28 to 19.28) | 2.38 (0.88 to 6.46) |
| <30 (stage 4/5) | 10.65 (3.38 to 33.59) | 26.19 (3.96 to 173.10) | 9.09 (2.01 to 41.09) |
*There was only one person in this group.
†No one in this group had the outcome.
ACEi, ACE inhibitor; AR2b, angiotensin II receptor blocker; BAME, Black, Asian, Minority Ethnic; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C reactive protein; CTD, connective tissue disorder; CVD, cardiovascular disease; CXR, chest X-ray; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; ICS, inhaled corticosteroid; N/A, not available; NSAID, non-steroidal anti-inflammatory drug; Ref, reference; WCC, white cell count.
Univariate analyses found that factors associated with increased odds of clinical deterioration were: postinpatient (compared with community-referred); increasing age; comorbid diabetes, COPD, cancer and mental health; increasing number of comorbid conditions; anticoagulant medication; abnormal CXR; positive COVID-19 test result; lower lymphocyte count (<0.8) and lower eGFR (<45). The backward stepwise multivariable regression model controlling for route of admission to VH found that factors associated with an increase in the odds of clinical deterioration were: increasing age (OR 1.04 (95% CI 1.02 to 1.06) per year), comorbid cancer (OR 2.87 (95% CI 1.41 to 5.82)), comorbid mental health problems (OR 1.76 (95% CI 1.02 to 3.04)), eGFR consistent with CKD stage 4 or 5 (OR 9.09 (95% CI 2.01 to 41.09) compared with eGFR ≥90) and having a positive SARS-CoV-2 PCR result (OR 2.00 (95% CI 1.11 to 3.60) compared with negative test result). The area under the receiver operating characteristic for the model including these values, after bootstrapping, is 0.76 (95% CI 0.70 to 0.83). To shed more light on the results of the regression analyses, we reviewed the medical records of participants to further classify the ‘cancer’ and ‘mental health’ comorbid condition categories. This demonstrated that the ‘cancer’ category included cutaneous (20%), breast (20%), haematological (11%), prostate (11%), renal (7%), lung (5%) and other (26%); and the ‘mental health’ category included anxiety (17.9%), depression (29.3%), mixed anxiety and depression (21.7%), alcohol abuse/dependency (6.1%), dementia (8.4%) and other (15.6%).
We conducted sensitivity analyses excluding death and using only (re-)admission to hospital as an outcome. Excluding only the 3 patients that died without having a hospital admission did not lead to any change in the results, however excluding all 18 patients that died resulted in age no longer being statistically significantly associated with clinical deterioration, but no other change in significant predictors.
The results of multivariable models for the community and postinpatient groups separately are shown in table 5. In the group referred from the community, only diabetes was found to be a significant predictor of clinical deterioration (OR 14.82 (95% CI 1.14 to 192.34)). In the postinpatient group, cancer (OR 4.81 (95% CI 1.42 to 16.33)), number of comorbid conditions and eGFR consistent with stage 4 or 5 CKD (OR 34.77 (95% CI 2.62 to 459.77)) were significantly associated with increased odds of clinical deterioration and having an ‘other respiratory condition’ was significantly associated with a reduced odds of clinical deterioration (OR 0.14 (95% CI 0.03 to 0.76)). The most common conditions coded as ‘other respiratory condition’ were history of tuberculosis (40%), pulmonary embolism (15%), community acquired pneumonia (9%), asbestosis (6%), sarcoidosis (6%), pulmonary fibrosis (4%), pneumothorax (4%), lung carcinoma (4%).
Table 5.
Association with clinical deterioration in the community and inpatient subgroups
| Inpatient | Community | |||||
| Experienced clinical deterioration (n=52) | Univariate OR (95% CI) | Adjusted OR (95% CI) with all variables in the model | Experienced clinical deterioration (n=24) | Univariate OR (95% CI) | Adjusted OR (95% CI) with all variables in the model | |
| Low-risk status | 4/52 (7.7%) | 0.60 (0.21 to 1.74) | 0.38 (0.08 to 1.80) | 0/24 (0.0%) | N/A | N/A |
| Male | 23/52 (44.2%) | 0.65 (0.36 to 1.17) | 0.34 (0.12 to 0.97) | 14/24 (58.3%) | 2.17 (0.94 to 5.00) | 2.36 (0.15 to 37.41) |
| Age | 69.8 (21.99) | 1.04 (1.02 to 1.06) | 1.01 (0.98 to 1.05) | 62.1 (13.17) | 1.07 (1.04 to 1.11) | 1.09 (0.98 to 1.20) |
| BAME | 0.57 (0.27 to 1.21) | 0.74 (0.25 to 2.15) | 1.00 (0.42 to 2.42) | 2.49 (0.35 to 17.90) | ||
| Comorbid conditions | ||||||
| Diabetes | 19/51 (37.3%) | 1.86 (1.00 to 3.45) | 1.32 (0.42 to 4.14) | 8/20 (40.0%) | 4.90 (1.94 to 12.37) | 14.82 (1.14 to 192.34) |
| Mental health | 22/52 (42.3%) | 1.84 (1.01 to 3.34) | 0.62 (0.20 to 1.94) | 9/21 (42.9%) | 1.56 (0.64 to 3.79) | 4.55 (0.28 to 75.00) |
| CKD | 7/52 (13.5%) | 1.22 (0.52 to 2.87) | 0.23 (0.05 to 1.01) | 2/21 (9.5%) | 4.14 (0.84 to 20.31) | 0.51 (0.003 to 77.02) |
| CTD | 6/50 (12.0%) | 0.67 (0.27 to 1.63) | 0.23 (0.05 to 1.17) | 2/21 (9.5%) | 0.89 (0.20 to 3.91) | 0.11 (0.002 to 5.22) |
| CVD | 29/51 (56.9%) | 1.56 (0.87 to 2.81) | 0.32 (0.09 to 1.16) | 10/19 (52.6%) | 2.83 (1.17 to 6.87) | 1.78 (0.11 to 29.55) |
| Cancer | 12/52 (23.1%) | 2.67 (1.28 to 5.58) | 3.24 (0.93 to 11.20) | 5/21 (23.8%) | 5.21 (1.76 to 15.40) | 9.94 (0.69 to 142.15) |
| COPD | 9/52 (17.3%) | 1.92 (0.87 to 4.21) | 0.71 (0.15 to 3.31) | 2/21 (9.5%) | 3.67 (0.86 to 15.75) | 8.46 (0.15 to 475.34) |
| Asthma | 11/52 (21.2%) | 0.93 (0.46 to 1.89) | 0.47 (0.12 to 1.82) | 3/22 (13.6%) | 0.43 (0.13 to 1.48) | 0.24 (0.01 to 8.03) |
| Other respiratory | 2/52 (3.9%) | 0.51 (0.15 to 1.72) | 0.09 (0.01 to 0.70) | 2/21 (9.5%) | 1.86 (0.40 to 8.62) | 1.69 (0.04 to 72.72) |
| Number of comorbid conditions | ||||||
| None | 5/52 (9.6%) | Ref | Ref | 8/24 (33.3%) | Ref | Ref |
| 1 | 11/52 (21.2%) | 2.27 (0.78 to 6.81) | 8.97 (1.32 to 60.85) | 5/24 (20.8%) | 0.79 (0.25 to 2.47) | 0.85 (0.10 to 7.52) |
| 2 | 9/52 (17.3%) | 2.43 (0.78 to 7.54) | 10.61 (1.02 to 110.14) | 3/24 (12.5%) | 0.62 (0.16 to 2.39) | 0.12 (0.01 to 2.27) |
| 3 | 12/52 (23.1%) | 5.24 (1.74 to 15.75) | 33.76 (2.03 to 561.65) | 5/24 (20.8%) | 1.82 (0.57 to 5.81) | 0.52 (0.01 to 23.80) |
| 4 | 6/52 (11.5%) | 3.26 (0.96 to 11.11) | 17.53 (0.63 to 489.02) | 1/24 (4.2%) | 2.31 (0.26 to 20.81) | 0.01 (0.00 to 13.14) |
| 5+ | 9/52 (17.3%) | 5.31 (1.66 to 16.98) | 78.72 (1.44 to 4295.20) | 2/24 (8.3%) | 6.17 (1.08 to 35.53) | 0.19 (0.0002 to 167.12) |
| Medications | ||||||
| ACEI | 13/50 (26.0%) | 1.73 (0.87 to 3.43) | 1.98 (0.64 to 6.15) | 2/22 (9.1%) | 0.71 (0.16 to 3.14) | 0.02 (0.0004 to 0.81) |
| AR2b | 4/50 (8.0%) | 0.63 (0.22 to 1.85) | 0.68 (0.16 to 2.85) | 3/22 (13.6%) | 2.39 (0.66 to 8.70) | 0.89 (0.03 to 23.24) |
| Immunosuppressant | 1/50 (2.0%) | 0.28 (0.04 to 2.14) | 0.20 (0.02 to 2.16) | 2/22 (9.1%) | 2.33 (0.50 to 10.92) | 9.80 (0.11 to 843.23) |
| NSAID | 8/50 (16.0%) | 1.23 (0.54 to 2.80) | 0.95 (0.28 to 3.19) | 6/22 (27.3%) | 3.29 (1.23 to 8.81) | 1.73 (0.16 to 18.91) |
| ICS | 11/50 (22.0%) | 1.27 (0.62 to 2.57) | 0.93 (0.27 to 3.17) | 1/22 (4.6%) | 0.49 (0.10 to 2.48) | 1.22 (0.04 to 40.98) |
| DOAC or other anticoagulant | 15/50 (30.0%) | 2.01 (1.03 to 3.91) | 1.09 (0.38 to 3.15) | 4/22 (18.2%) | 3.87 (1.19 to 12.54) | 0.50 (0.02 to 10.25) |
| Shortness of breath | 32/52 (61.5%) | 0.76 (0.42 to 1.38) | 1.07 (0.46 to 2.53) | 17/24 (70.8%) | 1.02 (0.41 to 2.53) | 3.66 (0.61 to 22.08) |
| Cough | 35/52 (67.3%) | 0.97 (0.52 to 1.80) | 1.40 (0.57 to 3.43) | 17/24 (70.8%) | 0.77 (0.31 to 1.92) | 0.71 (0.12 to 4.38) |
| Fever | 31/52 (59.6%) | 0.76 (0.42 to 1.37) | 1.11 (0.45 to 2.76) | 19/24 (79.2%) | 2.29 (0.84 to 6.21) | 3.72 (0.33 to 41.95) |
| Chest pain | 8/52 (15.4%) | 1.19 (0.53 to 2.68) | 1.84 (0.59 to 5.72) | 3/24 (12.5%) | 0.41 (0.12 to 1.40) | 0.58 (0.08 to 4.44) |
| Diarrhoea | 4/52 (7.7%) | 0.42 (0.15 to 1.17) | 0.28 (0.07 to 1.05) | 4/24 (16.7%) | 1.28 (0.42 to 3.88) | 1.50 (0.18 to 12.31) |
| Headache | 3/52 (5.8%) | 0.47 (0.14 to 1.56) | 0.89 (0.16 to 4.80) | 4/24 (16.7%) | 0.95 (0.31 to 2.88) | 4.05 (0.55 to 30.08) |
| Myalgia | 7/52 (13.5%) | 0.55 (0.24 to 1.26) | 0.91 (0.30 to 2.79) | 7/24 (29.2%) | 0.99 (0.40 to 2.46) | 0.71 (0.13 to 4.01) |
| Fatigue | 12/52 (23.1%) | 0.73 (0.36 to 1.45) | 0.55 (0.22 to 1.42) | 8/24 (33.3%) | 1.19 (0.49 to 2.85) | 0.77 (0.13 to 4.61) |
| Normal CXR | 12/49 (24.5%) | 1.09 (0.54 to 2.19) | 1.69 (0.57 to 4.97) | 5/24 (20.8%) | 0.24 (0.09 to 0.65) | 1.00 (0.15 to 6.70) |
| Oxygen saturation | ||||||
| ≤91 | 4 (7.8%) | 0.94 (0.33 to 2.72) | 0.57 (0.12 to 2.70) | 1/24 (4.2%) | 5.13 (0.57 to 46.24) | 0.96 (0.0003 to 2730.82) |
| 92–93 | 4 (7.8%) | 0.82 (0.29 to 2.35) | 1.22 (0.28 to 5.33) | 1/24 (4.2%) | 2.11 (0.31 to 14.49) | 0.54 (0.01 to 52.40) |
| 94–95 | 8 (15.4%) | 0.93 (0.39 to 2.21) | 0.77 (0.25 to 2.39) | 2/24 (8.3%) | 1.59 (0.37 to 6.89) | 0.66 (0.03 to 14.57) |
| ≥96 | 36 (69.2%) | Ref | Ref | 20/24 (83.3%) | Ref | Ref |
| SARS-CoV-2 test results | ||||||
| Positive | 37/52 (71.2%) | 1.67 (0.86 to 3.22) | 2.01 (0.75 to 5.39) | 14/24 (58.3%) | 2.50 (1.02 to 6.15) | 4.08 (0.43 to 38.43) |
| Negative | 13/52 (25.0%) | Ref | Ref | 8/24 (33.3%) | Ref | Ref |
| Invalid/Pending | 1/52 (1.9%) | 1.24 (0.14 to 10.63) | 0.42 (0.02 to 10.63) | 1/24 (4.2%) | 0.98 (0.12 to 8.19) | 2.13 (0.08 to 55.79) |
| No swab | 1/52 (1.9%) | 1.24 (0.14 to 10.63) | 5.58 (0.35 to 88.35) | 1/24 (4.2%) | 0.24 (0.03 to 1.94) | 0.13 (0.01 to 3.93) |
| Platelets | ||||||
| <150 | 6/50 (12.0%) | 1.25 (0.49 to 3.16) | 0.77 (0.23 to 2.62) | 5/24 (20.8%) | 2.00 (0.70 to 5.72) | 1.74 (0.23 to 13.22) |
| 150–450 | 40/50 (80.0%) | Ref | Ref | 18/24 (75.0%) | Ref | Ref |
| >450 | 4/50 (8.0%) | 0.64 (0.22 to 1.86) | 0.89 (0.22 to 3.59) | 1/24 (4.2%) | 1.91 (0.22 to 16.19) | 7.47 (0.18 to 301.89) |
| WCC | ||||||
| <4 | 8/50 (16.0%) | 2.47 (1.02 to 5.98) | 2.43 (0.64 to 9.22) | 1/24 (4.2%) | 0.41 (0.05 to 3.16) | 0.21 (0.01 to 5.81) |
| 4–11 | 34/50 (68.0%) | Ref | Ref | 22/24 (91.7%) | Ref | Ref |
| >11 | 8/50 (16.0%) | 1.42 (0.61 to 3.31) | 1.18 (0.35 to 4.01) | 1/24 (4.2%) | 0.27 (0.03 to 2.04) | 0.02 (0.0002 to 2.40) |
| Lymphocytes | ||||||
| <0.8 | 20/50 (40.0%) | 2.31 (1.24 to 4.30) | 1.57 (0.63 to 3.93) | 8/24 (33.3%) | 3.37 (1.36 to 8.32) | 1.00 (0.11 to 9.13) |
| 0.8–5.0 | 29/50 (58.0%) | Ref | Ref | 16/24 (66.7%) | Ref | Ref |
| >5.0 | 1/50 (2.0%) | N/A | N/A | 0/24 (0.0%) | N/A | N/A |
| Eosinophils | 0.02 (0.0003 to 1.36) | 0.14 (0.01 to 1.52) | ||||
| <0.5 | 50/50 (100.0%) | N/A | N/A | 24/24 (100%) | N/A | N/A |
| 0.5–1.0 | 0/50 (100.0%) | N/A | N/A | 0/24 (0.0%) | N/A | N/A |
| >1.0 | 0/50 (100.0%) | N/A | N/A | 0/24 (0.0%) | N/A | N/A |
| CRP | ||||||
| <5 | 6/47 (12.8%) | Ref | Ref | 5/21 (23.8%) | Ref | Ref |
| 5–19 | 5/47 (10.6%) | 0.67 (0.19 to 2.34) | 0.46 (0.10 to 2.22) | 3/21 (14.3%) | 1.46 (0.34 to 6.36) | 0.96 (0.07 to 13.06) |
| 20–100 | 25/47 (53.2%) | 0.96 (0.38 to 2.43) | 0.55 (0.14 to 2.21) | 10/21 (47.6%) | 2.78 (0.93 to 8.30) | 0.65 (0.05 to 8.61) |
| >100 | 11/47 (23.4%) | 1.42 (0.49 to 4.13) | 1.23 (0.25 to 6.12) | 3/21 (14.3%) | 3.52 (0.79 to 15.74) | 1.03 (0.03 to 31.33) |
| eGFR (CKD stage) | ||||||
| ≥90 (normal) | 20/49 (40.8%) | Ref | Ref | 7/22 (31.8%) | Ref | Ref |
| 60–89 (stage 2) | 15/49 (30.6%) | 0.81 (0.40 to 1.63) | 1.31 (0.44 to 3.89) | 10/22 (45.5%) | 1.00 (0.30 to 3.29) | 0.39 (0.02 to 9.63) |
| 45–59 (stage 3a) | 5/49 (10.2%) | 0.55 (0.17 to 1.78) | 0.86 (0.14 to 5.34) | 3/22 (13.6%) | 2.62 (0.79 to 8.65) | 2.12 (0.07 to 61.95) |
| 30–44 (stage 3b) | 7/49 (14.3%) | 2.25 (0.81 to 6.22) | 5.51 (0.91 to 33.49) | 1/22 (4.6%) | 7.68 (1.79 to 32.81) | 10.37 (0.07 to 1616.48) |
| <30 (stage 4/5) | 2/49 (4.1%) | 5.69 (1.42 to 22.82) | 32.57 (2.28 to 465.30) | 1/22 (4.6%) | 24.93 (3.14 to 197.81) | 43.36 (0.06 to 32 422.92) |
ACEi, ACE inhibitor; AR2b, angiotensin II receptor blocker; BAME, Black, Asian, Minority Ethnic; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C reactive protein; CTD, connective tissue disorder; CVD, cardiovascular disease; CXR, chest X-ray; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; ICS, inhaled corticosteroid; N/A, not available; NSAID, non-steroidal anti-inflammatory drug; Ref, reference; WCC, white cell count.
Discussion
Summary of results
In this observational study of 900 patients admitted to a VH for remote follow-up of suspected COVID-19 illness, we found that 8.1% of the population were (re-)admitted only 2.0% died during follow-up, giving an overall rate of clinical deterioration of 8.4%. Increasing age, comorbid cancer, comorbid mental health, impaired renal function (lower eGFR) and a positive COVID-19 test result were all independently associated with an increased odds of clinical deterioration in the combined population, having diabetes was associated with clinical deterioration in the community group and history of cancer, eGFR consistent with CKD stage 4 or 5 and not having ‘other respiratory conditions’ were associated with clinical deterioration in the postinpatient group.
Strengths and weaknesses
A strength of this study is the real-world nature of the clinical data used. This was a novel service set up rapidly during a time of crisis, and we included all of the first 900 patients registered with the VH service. It is reasonably safe to assume that the population included in this study includes the vast majority of those that required monitoring in the community during this period as there were no other services providing remote monitoring of patients that had required a face-to-face assessment in the area at that time. This means that we are unlikely to have the selection bias that characterises many applied research studies. Indeed, by including both patients recruited directly from the community and those who were postinpatient admission, we have been able to look at predictors in this population suitable for remote follow-up overall, and within each subpopulation. For most of the recruitment period, there were no general practice hubs assessing patients with suspected COVID-19 in the West Hertfordshire area, and therefore our sample likely includes the majority of patients with suspected COVID-19 that were managed in the community and needed a clinical assessment. A review of the baseline characteristics of these groups demonstrates that we were able to include populations that are likely to be representative of those being followed in the community directly, and those being followed postinpatient admission. A potential limitation of including all patients admitted to the VH is that by including both community referrals and postinpatients we have introduced significant heterogeneity into our population. However, we would argue that this is representative of many VH services that have been set up, and it is important for clinicians managing services like this to understand whether there are predictors of clinical deterioration that are common to the whole population, as well as whether there are different predictors for the two main subgroups (community referred and postinpatient). By conducting a whole population analysis that controls for subgroup, and separate subgroup analyses, we have been able to identify common and separate predictors. We were able to collect reliable data on a wide range of clinical and demographic features, and reliably follow all patients for the primary outcome for at least 2 weeks following their discharge from the VH through a review of their hospital records. Another strength is that clinicians were able to validate data collected at baseline during their regular follow-up phone calls. The ‘real-world’ nature of our study also poses several limitations. The hospital experienced significant demand from COVID-19 during the time course of this study, and this may have affected the findings. We were not able to extract specific symptom data (such as duration and severity) or data on clinical examination findings (except oxygen saturation) in a consistent and reliable way and there were significant amounts of missing data for some of these variables (eg, oxygen saturation). We were also not able to collect detailed data on treatments received during hospitalisations prior to admission to the VH, but have included data on length of stay and whether the patient was admitted to intensive therapy unit or not. For ease of data collection, we used baseline laboratory results from the initial assessment in A&E or the medical admission unit, and therefore for patients that had a hospital admission these were sometimes not the most recent results. Because COVID-19 tests were initially only available to inpatients, 20% of the community group did not have a test. We were also unable to collect data on body mass index (BMI) on enough patients to warrant inclusion in our models. It is possible that some patients travelled out of area and were lost to follow-up. However, this seems unlikely given the travel restrictions at the time. Our study is likely underpowered to detect all predictors, especially in the analysis of our two subgroups. A further weakness of our study is that we did not have a sufficiently large sample to be able to split our sample into development and validation sets. Therefore, our findings need to be validated using an external data set.
Comparison with other published studies
The most recent version of a ‘living systematic review’ of prediction models for diagnosis and prognosis of COVID-19 included 51 studies describing 66 prediction models.5 Of the studies included in the review, 32 used data from China, 2 from Italy, 1 from Singapore, 1 from the USA, 10 international data, 2 simulated data and 3 where the origin of the data was not clear. The majority of the prognostic studies were based on hospitalised patients, and there were no studies of prognosis in VH settings.
Age has consistently been shown to be a risk factor for poor prognosis in hospitalised5–11 and non-hospitalised12 populations. A large, well-conducted study using data from 575 hospitals in China used data from 1590 patients to develop a clinical score for predicting ‘critical illness’ in patients admitted with COVID-19, and validated their score in 710 patients.11 Consistent with our findings, they reported age, CXR abnormality and history of cancer as predictive of clinical deterioration. They also identified haemoptysis, dyspnoea, unconsciousness, number of comorbidities, neutrophil-to-lymphocyte ratio, lactate dehydrogenase (LDH) and direct bilirubin (BR) as predictors. We did not have accurate data on symptoms, and did not have enough data on neutrophil count, LDH or BR to assess these predictors in our model. Another study found that cancer was a risk factor for intubation but not mortality in 5688 patients admitted to one hospital in New York City with COVID-19.13 Although there has been much debate about the effect of COVID-19 on mental health, the association between mental health and clinical deterioration from COVID-19 has not, to the best of our knowledge, been reported in other case series, which have been predominantly based around inpatient cohorts. It is possible that those with mental health problems were admitted more frequently because of perceived vulnerability on the part of the clinicians undertaking review assessments, rather than an actual increased risk of physical deterioration. It is also possible that the association between mental health and obesity found in this study was confounded by obesity,14 as we were not able to document BMI consistently in this study. However, there may be a variety of reasons why those with mental health disorders are more vulnerable, including reduced levels of activity, impaired socioeconomic status and reduced healthcare usage for other medical problems. Patients with mental disorders have been noted to have poorer outcomes from other comorbidities, including mortality.15 Dementia was a key mental health problem in this cohort, and those with dementia do appear to be at high risk. A study of death certificates in England found that 25.7% of COVID-19 deaths were in patients with dementia, compared with 23.8% of all deaths.16 Dementia is clearly associated with other risk factors for poor outcome, but the hypothesis that dementia is associated with a direct causal effect on prognosis warrants further exploration. Close proximity of carers, increased risk of falls and risk of ‘happy hypoxia’ are possible mechanisms. Other mental illnesses are unlikely to be mentioned on a death certificate, and we have not been able to identify other studies exploring the association between mental health problems and the need for hospital admission for COVID-19.
Given the lack of COVID-19 testing availability during the first few months of the outbreak, diagnostics were only available for patients admitted or those judged to be most at risk. In this cohort, a positive PCR was independently correlated with an increased risk of clinical deterioration. This may reflect that testing was initially confined to those patients deemed to be most unwell or may be because patients who did not have COVID-19, or who had a low viral load which was not detected, had a better prognosis.
The inverse association between being coded as having an ‘other respiratory condition’ and experiencing a clinical deterioration is difficult to explain. Patients with a history of an assortment of previous and ongoing chronic and acute conditions were lumped together in this category and it is therefore very difficult to interpret the results. Some of the included conditions are associated with immune dysfunction, but it seems unlikely that there is a biological mechanism through which such an assortment of acute and chronic conditions would have a protective effect on clinical deterioration. Therefore, this is more likely to represent a chance finding or unmeasured confounding.
Implications for policy, practice and research
COVID-19 has changed the face of modern society,17 the impact felt from the home to the workplace. The health service has embraced virtual working and remote patient care at scale, in a way never before attempted or achieved. Changes have been rushed through at great pace and now is the time to reflect, analyse and consider. Same Day Emergency Care (and other out-of-hospital care pathways) are increasingly being used to manage an ever wider range of conditions, ranging from frailty to pneumothorax.18 Recent advice from NHS England has advocated the use of oxygen saturation probes in the safe management of COVID-19 as part of remote patient monitoring services.19 COVID-19 is a novel disease entity, and unlike many of the other pathologies managed within ambulatory care settings, the natural course of the disease is not yet fully understood. Primary and secondary care practitioners require interim guidance as well as knowledge of clinical practice outside of their own region to guide patient care pending the outcomes of large-scale high-quality research projects.
The relatively low incidence of death and readmission in the multimorbid patients in this study suggest that the clinicians managing this service were able to select and monitor patients in a way that was safe. Comparing outcomes with other approaches to managing these patients, ideally in a randomised trial, would provide more reassurance in this regard.
Our results suggest that in addition to well-known risk factors such as age, clinicians working at the primary-secondary care interface should be aware that patients with coexisting cancer, severely impaired renal function and mental illness are all at greater risk of hospital admission and/or death, and therefore warrant more careful follow-up. Further research is urgently needed to validate these findings and understand the reasons for the apparent worse prognosis in these patients. There is also a need to assess the most cost-effective approaches to monitoring and supporting patients in the community with suspected/confirmed COVID-19 who do not (yet) require hospital admission.
Conclusions
This observational study of a real-world remote monitoring VH service, set up rapidly during the onset of the worst pandemic seen in decades, has demonstrated that it was possible to set up a service providing a safety net in order to both provide a safe alternative to hospital admission and support early discharge. We have demonstrated that service resulted in a low incidence of deaths (2.0%) and readmissions (8.1%) overall, and in both of these populations. When planning and commissioning services in primary and secondary care to manage patients with COVID-19 during this ongoing pandemic, we would suggest that the risk factors for deterioration identified in this cohort, namely age, significant renal impairment (CKD stage 4–5), history of cancer and history of mental health problems, may be of use in helping to identify those requiring more intensive follow-up and monitoring.
Supplementary Material
Acknowledgments
The authors would like to thank Dr David Evans and Mr Alex Newland Smith, who provided a lot of assistance in collecting the data.
Footnotes
Twitter: @nickafrancis
Contributors: NF, BS, MK, RV, MW, AB and MM contributed to the conception and design of the study. MK, RV, CO and AB contributed to data collection. BS led the data analysis of the data. NF, BS, MK, RV, CO, MW, AB and MM contributed to data interpretation. NF wrote the first draft of the paper and all authors contributed to revising the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: Regulatory and ethical approval for the study were provided by the Health Research Authority and Chelsea Research Ethics Committee (REC reference: 20/HRA/2342). The data used in this study were collected as part of routine healthcare during a pandemic. Participants did not provide consent to participate. Data were extracted from medical records by clinicians providing care for the patients and anonymised data were provided to the research team at the University of Southampton. No identifiable data left the hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. The data that support the findings of this study are available from West Hertfordshire Hospitals NHS Trust but restrictions apply to the availability of these data, which were used under licence for the current study, and so are not publicly available. Data are however available from the authors on reasonable request and with permission of West Hertfordshire Hospitals NHS Trust.
References
- 1.Thornton J. The "virtual wards" supporting patients with covid-19 in the community. BMJ 2020;369:m2119. 10.1136/bmj.m2119 [DOI] [PubMed] [Google Scholar]
- 2.To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020;20:565-574. 10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley RD, Snell KI, Ensor J, et al. Minimum sample size for developing a multivariable prediction model: PART II - binary and time-to-event outcomes. Stat Med 2019;38:1276–96. 10.1002/sim.7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ensor J. PMSAMPSIZE: Stata module to calculate the minimum sample size required for developing a multivariable prediction model. Statistical Software Components S458569 2018.
- 5.Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ 2020;369:m1328. 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong J, Ou J, Qiu X, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis 2020;71:833–40. 10.1093/cid/ciaa443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Yu X, Zhao H, et al. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care 2020;24:108. 10.1186/s13054-020-2833-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie J, Hungerford D, Chen H, et al. Development and external validation of a prognostic multivariable model on admission for hospitalized patients with COVID-19. medRxiv 2020;10. 10.2139/ssrn.3562456 [DOI] [Google Scholar]
- 9.Shang W, Dong J, Ren Y, et al. The value of clinical parameters in predicting the severity of COVID-19. J Med Virol 2020;92:2188–92. 10.1002/jmv.26031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun L, Song F, Shi N, et al. Combination of four clinical indicators predicts the severe/critical symptom of patients infected COVID-19. J Clin Virol 2020;128:104431. 10.1016/j.jcv.2020.104431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med 2020;180:1081. 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeCaprio D, Gartner J, Burgess T. Building a COVID-19 vulnerability index. arXiv 2020. [Google Scholar]
- 13.Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? an experience in New York City. Ann Oncol 2020;31:1088–9. 10.1016/j.annonc.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: a national Institute of mental health meeting report. Am J Prev Med 2009;36:341–50. 10.1016/j.amepre.2008.11.020 [DOI] [PubMed] [Google Scholar]
- 15.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 2015;72:334–41. 10.1001/jamapsychiatry.2014.2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Public Health England . Disparities in the risk and outcomes from COVID-19 2020.
- 17.Yong E. How the pandemic defeated America: the Atlantic, 2020. Available: https://www.theatlantic.com/magazine/archive/2020/09/coronavirus-american-failure/614191/
- 18.NHS Improvement . Same day emergency care: NHS improvement, 2019. Available: https://improvement.nhs.uk/resources/same-day-emergency-care/
- 19.NHS England . Pulse oximetry to detect early deterioration of patients with COVID-19 in primary and community care settings: NHS England, 2020. Available: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/06/C0445-remote-monitoring-in-primary-care-v1.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.