Abstract
Adolescence is increasingly viewed as a sensitive period in the development of substance use disorders (SUDs). Neurodevelopmental ‘dual-risk’ theories suggest adolescent vulnerability to problematic substance use is driven by an overactive reward drive mediated by the striatum, and poor cognitive control mediated by the prefrontal cortex. To this end, there has been a growing number of neuroimaging studies examining cognitive and affective neural systems during adolescence for markers of vulnerability to problematic substance use. Here, we perform a coordinate-based meta-analysis on this emerging literature. Twenty-two task-based voxelwise fMRI studies with activation differences associated with substance use vulnerability, representative of approximately 1092 subjects, were identified through a systematic literature search (PubMed, Scopus) and coordinates of activation differences (N = 190) were extracted. Adolescents were defined as ‘at-risk’ for problematic substance use based on a family history of SUD or through prospective prediction of substance use initiation or escalation. Multilevel kernel density analysis was used to identify the most consistent brain regions associated with adolescent substance use vulnerability. Across the included studies, substance use vulnerability was most reliably associated with activation differences in the striatum, where at-risk adolescents had hyper-activation in the dorsal subdivision (putamen). Follow-up analyses suggested striatal differences were driven by tasks sharing a motivational and/or reward component (e.g., monetary incentive) and common across subgroups of substance use risk (family history and prospective prediction studies). Analyses examining the role of psychiatric comorbidity revealed striatal activation differences were significantly more common in samples whose definition of substance use risk included cooccurring externalizing psychopathology. Furthermore, substance use risk meta-analytic results were no longer significant when excluding these studies, although this may reflect limitations in statistical power. No significant activation differences were observed in prefrontal cortex in any analysis. These results suggest striatal dysfunction, rather than prefrontal, may be a more primary neural feature of adolescent vulnerability to problematic substance use, possibly through a dimension of individual variability shared with externalizing psychopathology. However, our systematic literature search confirms this is still an emerging field. More studies, increased data sharing, and further quantitative integration are necessary for a comprehensive understanding of the neuroimaging markers of adolescent substance use risk.
Keywords: meta-Analysis, Substance use risk, Children of alcoholics, Addiction, Substance use disorder, fMRI, Cognitive control, Reward, Adolescence, Striatum
1. Introduction
Substance use typically begins in adolescence (Johnston et al., 2018), a developmental period characterized by increased sensation-seeking and risk-taking behaviors and the continued refinement of cognitive and affective brain systems (Luna et al., 2015). Early and problematic substance use increases the risk for impulsive behaviors (de Wit, 2009) and subsequent substance use disorders (SUDs)(McGue et al., 2001). While an extensive literature has outlined sociodemographic, psychological, and behavioral risk factors for problematic substance use, considerably less is known about reliable neurobiological risk factors. This disparity undermines the construction of models that integrate across psychosocial and neuroscientific domains, which are likely essential for comprehensive substance use prevention (O’Connell et al., 2009).
Neurodevelopmental models suggest a predominance of striatal-reward function over cortically-mediated cognitive control may distinguish adolescence as a sensitive period in the development of problematic substance use (Luna et al., 2015)(Steinberg, 2010)(Casey et al., 2008). Therefore, adolescents at increased risk for problematic substance use, either through a family history of SUD, or early and escalating experimental use, may display an exaggeration of this normative reward-cognitive control imbalance, with hyper-functioning of the striatum and hypo-function of prefrontal and posterior parietal cortical control regions (see (Bickel et al., 2007)(Heitzeg et al., 2015)(Cservenka, 2016)). Importantly, this adolescent vulnerability to future problematic substance use may be neurobiologically distinct from the escalation of problematic substance use to habitual daily use in addiction, where for example, chronic substance use may be maintained by binge, withdrawal, and craving cycles (Koob and Volkow, 2010). To date however, due to methodological constraints and variability amongst initial studies (e.g., small sample sizes and differences in sample characteristics, operational definitions of substance use risk, and neuroimaging tasks), fully testing this neurodevelopmental model of substance use vulnerability has been challenging. To this end, initial neuroimaging studies have been inconsistent in the both the spatial location and direction of effect (hyper-versus hypo-activation) of neural correlates of substance use vulnerability in at-risk adolescents (see (Heitzeg et al., 2015)(Cservenka, 2016)).
A primary challenge posed by the relative inconsistency of the existing literature is that it limits the ability to rule out alternative models of substance use vulnerability. For example, the “reward deficiency hypothesis” suggests hypo-function of the striatal reward system predicts substance use escalation (Blum et al., 2000) and has received support from studies assessing striatal dopamine function in adults with substance use disorders (cf., (Volkow et al., 2004)). The lack of consistency in the literature also prevents further theory development in determining the relative specificity of neuroimaging markers of risk for substance use disorders from those indexing risk for other forms of psychopathology. For example, “externalizing” psychiatric disorders (e.g., attention deficit hyperactivity disorder, conduct disorder, oppositional defiant disorder) have also been associated with increased activation of the striatum (cf., (Alegria et al., 2016)), increased risk for early onset substance use disorders (Carlson et al., 2007), and have been conceptualized as a part of a transdiagnostic dimension of “disinhibition” linking substance use and psychopathology (Krueger et al., 2002)(Tarter et al., 2003)(see (Iacono et al., 2008) for review). Alternative transdiagnostic theories have suggested fronto-striatal reward sensitivity may be associated with a vulnerability to approach-related hypomanic symptoms, and there are both reward hypo- and hyper-sensitivity pathways to substance use disorders (Nusslock and Alloy, 2017).
Despite the apparent inconsistency in the existing neuroimaging literature on substance use risk, there is an increasing number of published fMRI studies that can be quantitatively integrated and variability among studies can be explored. For example, comparisons can be made between studies defining substance use risk through prospective prediction of substance initiation and escalation and those studies defining substance use risk through a family history of SUD, which confers genetic and environmental risks for SUD (Ystrom et al., 2014). The relative impact of defining substance use risk with and without cooccurring externalizing disorders, a common practice in the literature, can also be evaluated.
Here, we use Multi-level Kernel Density Analysis (MKDA), a coordinate-based meta-analysis approach that determines spatial consistency across whole-brain (voxelwise) statistical results, to quantitatively integrate 22 studies from this literature and examine which neural systems most reliably differentiate adolescents at increased risk for problematic substance use.
Drawing from the neurodevelopmental literature, we hypothesized that risk groups would display hyper-activation of striatal reward systems and hypo-activation in cortical control regions (prefrontal cortex and posterior parietal cortex). We further hypothesized that if striatal-reward and prefrontal/posterior parietal-control activation differences are core features of substance use vulnerability, dysfunction in these regions would be consistent across operational definitions of substance use vulnerability and present prior to significant substance use involvement. In addition to these core neurodevelopmental questions, we also sought to disambiguate the role of co-occuring psychopathology on neuroimaging markers of substance use vulnerability. Therefore we examined the potential moderating role of externalizing psychopathology and indentified overlap between our meta-analytic results and a prior meta-analysis of externalizing psychopathology. Taken together, this work aimed to identify central brain systems underlying adolescent substance use vulnerability by integrating existing available data, thereby advancing neurodevelopmental theory and providing critical targets for future research.
2. Methods
2.1. Study selection
A systematic literature search following PRISMA (Moher et al., 2015) guidelines was conducted to identify whole-brain task-based activation studies of substance use vulnerability (records from January 1st, 1990–November 1st, 2018) using Scopus and PubMed databases. Literature searches used substance use vulnerability search stems (‘substance use risk’, ‘alcoholism risk’, ‘substance use initiation’, ‘prospective prediction of substance use’, ‘prediction of substance use’, ‘substance use vulnerability’, ‘family history of substance use disorder’, ‘family history of SUD’, ‘children of alcoholics’) paired with neuroimaging specifiers (‘neuroimaging’, ‘fMRI’, ‘BOLD’, ‘neural correlates’, ‘brain systems’)to create 45 total search terms (e.g., “neuroimaging substance use risk”: see Supplemental S1). Search terms were combined using “OR” Boolean operators. Reference sections from identified studies and relevant reviews (Heitzeg et al., 2015)(Cservenka, 2016) were also screened to ensure completeness of the search. Titles and abstracts of identified articles were then screened by authors BTC and AQ to exclude review papers, articles that did not utilize task-based functional magnetic resonance imaging, and articles that unambiguously did not meet study inclusion criteria (see below).
Following the initial screen, full text versions of remaining articles were reviewed to 1) ensure the study reported task-based activation results, 2) operationalized substance use vulnerability either through a family history of SUD (typically first-degree relatives, see Supplemental S2) or through prospective prediction of substance use initiation or escalation (baseline activation differences predicted future substance use, see Supplement S2), in order to isolate risk for SUD without requiring current problematic substance use (see Supplemental S3), 3) conducted and reported whole-brain/voxelwise analyses (see Voxelwise Reporting) comparing family history groups to controls or predicting substance use initiation or escalation, 4) reported coordinates of activation differences of substance use risk groups from whole-brain analysis in a standard anatomical space (i.e., in Talairach or Montreal Neurological Institute (MNI) coordinate systems) and 5) included a sample with a mean age below 30-years-old. We note that criterion 5 was added after identifying a small number of studies that examined substance use vulnerability in adults and met our other inclusion criteria (Andrews et al., 2011; Smith et al., 2013; Sjoerds et al., 2013). There was a clear gap in the mean sample age among these studies (range: 32–36) and the remainder of studies meeting our inclusion criteria (range: 10–23). We chose the functional cutoff of a mean sample age at 30-years-old to ensure that distributions of ages in the original studies were inclusive of the adolescent period, typically defined as the second decade of life, and the initiation of problematic substance use (e.g., binge drinking), which peaks in the early twenties (Chassin et al., 2002) but is thought to reflect normative adolescent processes (Heitzeg et al., 2015) that are likely influenced by substance use availability (see (Duell et al., 2018)). In cases where criteria 3 or 4 were not met, corresponding authors were contacted via email (July 2018) to determine whether coordinates of activation differences or un-thresholded statistical images could be provided. This did not yield any additional studies to be included. Ad-hoc searches were also performed using NeuroVault (neurovault.org) with basic keywords (‘family history’, ‘prospective’, ‘substance use’, ‘alcohol’) to try and identify relevant un-thresholded statistical images. No relevant maps were identified. Based on the lack of availability of un-thresholded statistical maps, we chose to pursue a coordinate-based meta-analysis using the available published data of peak coordinates.
Within studies that met inclusion criteria, coordinates of activation difference (foci) associated with substance use risk groups were manually recorded by authors BTC and AQ. In order to ensure accuracy of manual recording, coded foci were then doubly screened by BTC. Subsequently, to further ensure coding of the correct coordinate system (e.g., Talairach/ MNI) and orientation (e.g., RAI/LPI), which remain a significant problem in neuroimaging meta-analyses (cf., (Müller et al., 2018)), spheres convolved from coordinates (see below) were visually inspected and compared to figures from original studies. All foci from activation differences of the group(s) of interest (family history of SUD, prospective prediction) were identified and coded, including those from explicit task contrasts (i.e., go – no-go), implicit baseline contrasts (i.e., go – fixation), or parametric modulators. Additional study information coded included the neuroimaging task used, descriptions of voxelwise approach, sample demographic information (sample sizes, number of males/females, mean age of the sample, IQ), and study inclusion/exclusion criteria for substance use and psychopathology.
2.2. Statistical analysis
Multi-level Kernel Density Analysis: MKDA.
Multi-level Kernel Density Analysis (MKDA) (Wager et al., 2007) was used to determine spatial consistency of identified foci associated with substance use vulnerability. We provide a brief overview of MKDA methods, which have been described in detail elsewhere (Wager et al., 2007). For each included study, MKDA procedures first convert all foci to Montreal Neurological Institute (MNI) template space using the Brett transformation (Brett et al., 2001), convolve the transformed foci with a sphere of a user supplied radius (here, 15 mm, based on a recent simulation study (Salimi-Khorshidi et al., 2009)) and trim these spheres to only include grey matter voxels in the SPM MNI template. This creates a study-specific contrast indicator map (CIM). Subsequently, a weighted average (weight = square root of sample size) of CIMs is computed, creating an overall density map that represents the weighted proportion of studies that show activation differences in each voxel. Monte Carlo simulations (here, 5000 iterations) are then performed to compare the observed density map to a null distribution of density maps, where each null density map is constructed by randomly distributing the center coordinates of clusters (groups of voxels) in each CIM, which preserves the study-specific spatial structure. Significant clusters are established by comparing the combination of the height of activation (the proportion of studies showing activation differences in a given voxel) and the extent of contiguous voxels with suprathreshold heights in the observed density map to null distributions of these metrics generated by the Monte Carlo simulation. Importantly, unlike other coordinate-based meta-analytic approaches, the study-specific CIMs and nested Monte Carlo simulations gives MKDA a multi-level framework, where foci are nested within study. To this end, the unit of analysis in MKDA is the weighted proportion of studies that report activation differences in a given spatial location. This improves generalization of MKDA results to new studies and has the advantage of reducing potential bias from a single study that reports many foci or uses a more liberal statistical threshold (Wager et al., 2007). In all analyses, clusters surpassing a family-wise error rate (FWER) threshold of p < .05 with a single voxel alpha of .01 were considered significant.
We note that following best practices (Müller et al., 2018), MKDA is run on studies reporting voxelwise significant differences. Coordinate-based meta-analyses, including MKDA, identify consistency among reported spatial locations (here foci of activation differences of substance use risk) and determine “significance” through comparisons to a null distribution of random spatial locations across the whole brain, where it is assumed each voxel has an equivalent a priori probability of being reported (see above). Therefore, including region of interest (ROI) or ROI-constrained small volume correction (SVC) studies would violate MKDA assumptions and bias results towards the regions used in ROI/SVC studies, potentially leading to circularity concerning brain regions central to study hypotheses. More specifically, ROI studies, which do not use a voxel as the unit of analysis, but rather compute mean values across many voxels within specific brain regions, and SVC studies, which utilize a voxel as the unit of analysis but limit statistical testing to those voxels within specific brain regions, would bias the type 1 error rate (false positives) in our meta-analysis for regions frequently hypothesized in the literature and bias the type 2 error rate (false negatives) for all brain regions that were not included in the original studies.
MKDA Testing Procedures.
Consistent with other recent functional neuroimaging meta-analyses of clinical phenotypes (Alegria et al., 2016)(Miller et al., 2015), the current project used a tiered procedure for examining consistency in activation differences across the corpus of studies. First, MKDA was performed on all foci from all included studies. This ‘all studies’ analysis permitted the examination of the most consistent activation differences associated with adolescent substance use risk, irrespective of the operational definition of the substance use risk and the specific neuroimaging task used. Second, subdomain analyses were performed, where consistency in activation differences were examined according to the general type of neuroimaging tasks employed in the original studies. Broad task classifications (i.e., cognitive control, emotional stimuli, reward, substance use stimuli: see Table 1) were used to provide sufficient power and were defined based on previous work (Alegria et al., 2016)(Miller et al., 2015). We note however, based on reported activations of tasks included in our meta-analysis and prior research using similar tasks, overlap of task-effect activation patterns is expected within the utilized task classifications (e.g., prefrontal cortex activation across cognitive control tasks: see Supplemental S4). Owing to the insufficient number of studies using substance use stimuli (n = 4; Table 1), we did not perform a subdomain analysis on this group. Finally, in order to investigate possible effects of substance use risk definition, we also examined whether meta-analytic effects varied between family history and prospective prediction studies using MKDA’s χ2 (chi-square) test (Wager et al., 2009). We note that while the above subgroup and subdomain analyses are highly theoretically relevant, these analyses rely on a reduced subset of studies and thus have lower statistical power than the ‘all studies’ analysis.
Table 1.
Studies included in voxelwise meta-analysis of substance use risk.
| Study | Task Group | Risk Type | N Foci | Foci Type | N Subjects | N Female | Mean Age | N Risk Subjects |
|---|---|---|---|---|---|---|---|---|
| Acheson et al. (2014a) | C | FH | 14 | Positive | 52 | 35 | 23.78 | 28 |
| Acheson et al. (2014b) | C | FH | 36 | Positive | 104 | 51 | 12.9 | 72 |
| Cservenka et al. (2012) | C | FH | 4 | Negative | 35 | 14 | 14.09 | 19 |
| Heitzeg et al. (2010b) | C | FH | 5 | ANOVA/F-Statistic | 61 | 26 | 19.17 | 20 & 21 |
| Mackiewicz Seghete et al. (2013) | C | FH | 3 | Positive & Negative | 34 | 15 | 14.36 | 18 |
| Norman et al. (2011) | C | PRO | 16 | Negative | 38 | 19 | 13.68 | 21 |
| Tervo-Clemmens et al. (2018) | C | PRO | 11 | ANOVA/F-Statistic | 84 | 44 | 12.74 | 21 |
| Wetherill et al. (2013) | C | PRO | 6 | ANOVA/F-Statistic | 60 | 27 | 13.37 | 20 & 20 |
| Cservenka et al. (2014) | E | FH | 11 | Negative | 36 | 17 | 14.81 | 19 |
| Hulvershorn et al. (2013) | E | FH | 4 | Positive | 37 | 12 | 12.05 | 19 |
| Peraza et al. (2015) | E | FH | 1 | Negative | 29 | 12 | 13.7 | 14 |
| Qiao et al. (2015) | E | FH | 18 | Positive | 18 | 7 | 13.05 | 11 |
| Cservenka and Nagel (2012) | R | FH | 7 | Positive & Negative | 31 | 11 | 14.21 | 18 |
| Hulvershorn et al. (2015) | R | FH | 4 | ANOVA/F-Statistic | 50 | 22 | 12.08 | 23 |
| Morales et al. (2018) | R | PRO | 3 | Positive | 47 | 28 | 15.07 | NA |
| Stice et al. (2013) | R | PRO | 2 | Positive | 105 | NA | 15.3 | 25 |
| Stice and Yokum (2014) | R | FH | 11 | Positive & Negative | 52 | 27 | 14.8 | 26 |
| Ivanov et al. (2012) | R & C | FH | 7 | Positive & Negative | 20 | 2 | 10.54 | 10 |
| Dager et al. (2013) | SUS | FH | 10 | Positive & Negative | 65 | 25 | 19.3 | 10 & 11 |
| Dager et al. (2014) | SUS | PRO | 5 | ANOVA/F-Statistic | 43 | 23 | 18.48 | 14 |
| Kareken et al. (2013) | SUS | FH | 10 | Positive & Negative | 40 | 17 | 23.15 | 22 |
| Nguyen-Louie et al. (2018)a | SUS | FH | 2 | ANOVA/F-Statistic | 51 | 22 | 16.6 | 23 |
| Totals/Weighted Means | 190 | 1092 | 456 | 15.48 | 513 | |||
Note, Task Group: C, cognitive control tasks without reward or emotional manipulations; E, tasks using emotional stimuli and/or manipulations; R, tasks using rewards or motivational manipulations; SUS, tasks using substance use stimuli or substance use manipulations (see Supplemental S7 for task descriptions of all studies). Risk Type: PRO, prospective predictions of substance use initiation or escalation; FH, family history of substance use disorder. N Foci, number of contributed coordinates to meta-analysis. Foci Type: direction of activation difference with respect to risk group (Positive = risk group hyper-activation, Negative = risk group hypo-activation, ANOVA/F-Statistic = activation difference only/no-sign). NA, information not-available/not-reported. “&” denotes two groups/types reported in original study.
This study, where family history positive and family history negative subjects were prospectively followed, was coded as FH because the reported contrast was with respect to family history status (see Supplemental S13).
In order to first identify which brain regions were most consistently associated with substance use risk, we performed MKDA on all foci (cf., (Kraynak et al., 2018; McTeague et al., 2017)), irrespective of the direction of group differences ((e.g., ‘pooled aberrant activation’ (McTeague et al., 2017)), given that the sign of activation differences can be influenced by study-specific contrast procedures (i.e., explicit task contrasts, implicit baseline contrasts, parametric modulators) and/or conditions (i.e., reward > neutral vs. reward > loss) and can be ambiguous as to whether the signed group differences (e.g., risk > no-risk) reflects a difference in the condition of interest (e.g., reward) or the control condition (e.g., neutral) in certain contrasts (e.g., reward > neutral), without simple effects. Moreover, recent work shows common activation patterns across diverse tasks (Shine et al., 2019), suggesting a potential lack of cognitive and affective specificity in signed within-task activation differences. Finally, this approach had the added benefit of permitting the inclusion of foci from voxelwise contrasts that did not carry a sign (e.g., F statistics). Subsequently, we performed secondary analyses to examine the consistency of the direction of activation differences associated with substance use risk (hyper- or hypo-activation). We note that MKDA procedures examine spatial consistency of reported foci, not an aggregate meta-analytic effect size, and thus hyper- and hypo-activation coordinates must be run on separate models and entirely null results (no substance use risk activation differences) cannot be readily included (see (Rottschy et al., 2012)(Müller et al., 2018) for discussion).
Externalizing and Cooccurring Psychopathology.
Given that substance use risk and externalizing psychopathology are thought to share underlying features (Iacono et al., 2008), multiple studies in the corpus included externalizing psychopathology as part of the substance use risk definition (n = 4), or did not exclude subjects with psychiatric disorders (n = 3) (see Supplemental S5 for individual study psychopathology inclusion and exclusion). We performed a series of analyses examining the potential impact of externalizing and cooccurring psychopathology on the results of our substance use risk meta-analysis. First, we used MKDA’s χ2 (chi-square) test (Wager et al., 2009) to examine whether there was a difference in the proportion of studies showing an activation difference when externalizing or other psychopathology was and was not included in the definition of substance use risk. These analyses were constrained to only include voxels that were significant in the primary meta-analyses (see above) and corrected using the false discovery rate. Second, we re-ran the primary meta-analyses after excluding these studies. Finally, we performed a conjunction analysis between the significant meta-analytic clusters from the primary meta-analyses of substance use risk and significant results from a recent meta-analysis of externalizing disorders (disruptive behavior disorders and severe conduct problems; (Alegria et al., 2016)). Published peak coordinates (Alegria et al. 2016) were handled in the same manner as studies in our meta-analysis: convolved with a sphere with a radius of 15 mm and trimmed to only include grey matter voxels in the SPM MNI template.
Sensitivity Analyses.
As in all meta-analyses, there were a number of methodological and conceptual decision points in this project (see (Müller et al., 2018) for discussion). In order to examine the robustness of meta-analytic results to these methodological decision points and investigate variability in the included literature, three sensitivity analyses were performed. Specifically, the primary meta-analyses were sequentially re-run after excluding those studies that 1) had a mean sample age over 20-years-old (n = 2), 2) included subjects with more than experimental substance use in general, or minimal substance use that significantly differed between groups (n = 6, see below), and 5) used targeted voxelwise reporting (n = 8, see below).
2.3. Data and code availability
All data used in this project (coded foci of activation differences) are available in the Supplemental information. The MKDA code used to perform meta-analyses is freely available via CANLAB (https://sites.dartmouth.edu/canlab/).
3. Results
Included Studies.
See Supplemental S6 for flow diagram and detailed information on study selection. The initial database search yielded a total of 2213 articles. Of these, 76 met initial screen criteria and full-text versions were reviewed for eligibility. Twenty-two studies met all study inclusion criteria, yielding a total of 190 foci (Tables 1 and 2, Supplemental Table S7).
Table 2.
Demographic information of included studies.
| Study | Risk Type | N Subjects |
N Female |
Mean Age |
IQ |
||||
|---|---|---|---|---|---|---|---|---|---|
| Risk | Non-Risk | Risk | Non-Risk | Risk | Non-Risk | Risk | Non-Risk | ||
| Acheson et al. (2014a) | FH | 28 | 24 | 19 | 16 | 23.6 | 24 | NA | NA |
| Acheson et al. (2014b) | FH | 72 | 32 | 37 | 14 | 12.9 | 12.9 | 96.5 | 102 |
| Cservenka et al. (2012) | FH | 19 | 16 | 6 | 8 | 14.01 | 14.18 | 110.16 | 111.25 |
| Heitzeg et al. (2010b) | FH | 20 & 21 | 20 | 17 | 9 | 19.15 | 19.2 | 109.51 | 112 |
| Mackiewicz Seghete et al. (2013) | FH | 18 | 16 | 7 | 8 | 14.52 | 14.18 | 112.67 | 112.69 |
| Norman et al. (2011) | PRO | 21 | 17 | 10 | 9 | 13.9 | 13.4 | NA | NA |
| Tervo-Clemmens et al. (2018) | PRO | 21 | 63 | 10 | 34 | 12.67 | 12.77 | 98.45 | 103.79 |
| Wetherill et al. (2013) | PRO | 20 & 20 | 20 | 18 | 9 | 13.4 | 13.3 | NA | NA |
| Cservenka et al. (2014) | FH | 19 | 17 | 10 | 7 | 14.92 | 14.69 | 110.84 | 113.29 |
| Hulvershorn et al. (2013) | FH | 19 | 18 | 5 | 7 | 12.2 | 11.9 | 98.2 | 111.8 |
| Peraza et al. (2015) | FH | 14 | 15 | 6 | 6 | 13.73 | 13.67 | 115.93 | 119.86 |
| Qiao et al. (2015) | FH | 11 | 7 | 4 | 3 | 12.7 | 13.6 | NA | NA |
| Cservenka and Nagel (2012) | FH | 18 | 13 | 6 | 5 | 14.18 | 14.24 | 110.56 | 111.46 |
| Hulvershorn et al. (2015) | FH | 23 | 27 | 13 | 9 | 12.3 | 11.9 | 96.5 | 111.2 |
| Morales et al. (2018) | PRO | NA | NA | NA | NA | NA | NA | NA | NA |
| Stice et al. (2013) | PRO | 25 | 80 | NA | NA | NA | NA | NA | NA |
| Stice and Yokum (2014) | FH | 26 | 26 | 14 | 13 | 14.7 | 14.9 | NA | NA |
| Ivanov et al. (2012) | FH | 10 | 10 | 1 | 1 | 10.33 | 10.75 | 89.62 | 101.18 |
| Dager et al. (2013) | FH | 10 & 11 | 25 & 19 | 12 | 24 | 19.38 | 19.3 | NA | NA |
| Dager et al. (2014) | PRO | 14 | 16 & 13 | 7 | 16 | 18.21 | 18.61 | NA | NA |
| Kareken et al. (2013) | FH | 22 | 18 | 10 | 7 | 22.7 | 23.7 | NA | NA |
| Nguyen-Louie et al. (2018) | FH | 23 | 28 | NA | NA | NA | NA | NA | NA |
| Totals/Weighted Means | 513 | 540 | 212 | 205 | 15.45 | 15.24 | 103.4 | 108.6 | |
Note, Risk Type: PRO, prospective predictions of substance use initiation or escalation; FH, family history of substance use disorder. NA, information not-available/not-reported. “&” denotes two groups/types reported in original study. In cases where two groups/types were presented, demographic information is a weighted mean based on group sizes.
Voxelwise Reporting.
Following established guidelines (Müller et al., 2018), the primary reason for screened studies’ exclusion was not using a voxelwise approach (i.e., studies using only region-of-interest analysis (ROI) or ROI-constrained small volume correction(SVC)) (n = 29), with the proportion of exclusion for this criterion comparable to prior meta-analyses (cf., (McTeague et al., 2017)). See Methods for more discussion.
A more nuanced challenge remains in understanding the impact of “masking” or targeted voxelwise reporting, where for example, studies limit the report of group differences to voxels where there was a nominal main effect of the task (e.g., task > baseline: cf. (Tervo-Clemmens et al., 2018)(Cservenka et al., 2012)(Cservenka and Nagel, 2012)(Cservenka et al., 2014) (Mackiewicz Seghete et al., 2013)(Peraza et al., 2015)(Ivanov et al., 2012) or where whole-brain thresholding is performed, but not all foci of significant differences are reported in tables or figures (Heitzeg et al., 2010a). Here, again following established guidelines (Müller et al., 2018), we carefully examined the impact of these targeted voxelwise reporting studies in our corpus (n = 8). Ultimately, targeted voxelwise reporting studies were shown to not bias primary results (see MKDA: Sensitivity Analyses).
Demographic Information.
Given that we were unable to clarify individual subjects with investigators, it’s possible some subjects were included in more than one of the studies in the corpus. Note however, that none of the studies included identical samples, supporting a relative independence between studies. Furthermore, combining across similar data sets did not change meta-analytic results (Supplemental S8). Provided this information, the included studies represented approximately 1092 subjects (456 female) with a mean sample age of 15.48 years (range: 10.54–23.78) (Table 1). Demographic information, where available, suggested risk groups were relatively equivalent in number of subjects, gender composition, and IQ (Table 2).
Current and Prior Substance Use.
In the included studies, the modal substance use inclusion criteria were generally consistent with a previous history of “experimentation only” (cf.,(Clark et al., 2005)), defined as, for example, less than 10 alcoholic drinks, less than five marijuana uses ((Cservenka et al., 2012); see Supplemental S3 for more information). Five studies exceeded a threshold of substance use experimentation (Kareken et al., 2013)(Acheson et al., 2014a)(Heitzeg et al., 2010b)(Dager et al., 2013)(Dager et al., 2014), but were retained in the corpus of studies because they included relevant comparisons of family history status or prospective substance use transitions (Supplemental S3). An additional study included subjects with minimal substance use but reported a significant difference between comparison groups (Norman et al., 2011). Nevertheless, these six studies were shown to not bias the primary results in a subsequent sensitivity analyses (see MKDA: Sensitivity Analyses).
Publication Bias.
Established procedures for examining publication biasin MKDA (adapted Galbraith plots) indicated that, among the reported studies, there was a positive slope between sample size and the probability of an activation difference in the primary reported cluster, which is opposite to an association driven by publication bias. Furthermore, trim and fill methods indicated the overall effect size estimates from the included studies did not differ from an effect size estimate adjusted for publication bias (Supplemental S9).
MKDA
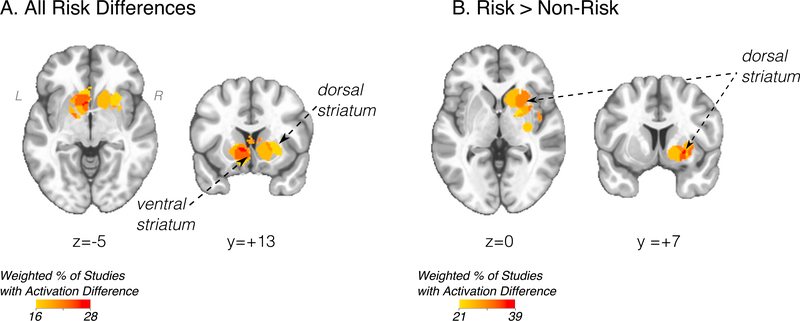
Using all foci from all included studies (‘all studies’ analysis), MKDA revealed the most consistent activation differences associated with substance use risk were located in the striatum, including both dorsal and ventral subdivisions (Fig. 1A). Follow-up analysis examining the sign of activation differences suggested the dorsal striatum (putamen) was significantly hyper-active across studies (Fig. 1B). No other brain regions were significant in either the ‘all studies’ analysis or in analyses examining signed activation differences.
Fig. 1.
A) Primary meta-analysis of all studies and all foci of substance use risk (peak coordinate, LPI MNI -8, 10, 4). B) Increased activation associated with substance use risk (peak coordinate, LPI MNI 26, 8, −4). Statistical maps displayed over Montreal Neurological Institute-152 template in neurological view. All results FWER p < .05 with single voxel p < .01. Scale bar (weighted % of studies with activation difference) refers to the relative proportion of studies with a risk difference, weighted by the square root of the study sample size (see description in Methods section).
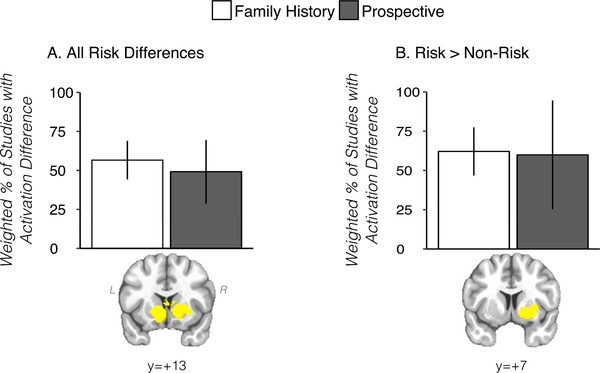
Subgroup analysis using the ‘all studies’ approach (irrespective of sign) revealed no significant (minimum FDR corrected p-value = .848) differences between prospective prediction (n = 6) and family history (n = 16) studies among striatal voxels identified in the sign-invariant ‘all studies’ meta-analysis. To this end, the relative proportions of studies with activation differences in the prospective prediction and family history subgroups were similar in the striatum (Fig. 2A). Prospective prediction and family history subgroups were also approximately equivalent for the risk > non-risk contrast (Fig. 2B), although the limited number of studies (n = 12: family history = 10, prospective prediction = 2) prevent a reliable statistical inference from these data.
Fig. 2.
Results from prospective prediction and family history of SUD studies in striatal clusters defined from primary meta-analysis (‘all studies’, A) and from ‘risk > non-risk’ studies (B). Y-axes (weighted % of studies with activation difference) refers to the relative proportion of studies with a risk difference, weighted by the square root of the study sample size (see description in Methods section). Cluster displayed in yellow to denote the proportion of studies with activation differences was calculated across the cluster.
MKDA: Task Subdomains.
When sub-setting studies based on the type of neuroimaging task used, MKDA indicated that consistent activation differences in the striatum occurred during reward tasks (n = 6), but not cognitive control tasks (n = 9) (Supplemental S10). The former result was qualitatively similar when analyzing a broader affective subdomain by additionally including studies using emotional stimuli (n = 10). No new brain regions were identified when only analyzing the cognitive control tasks (n = 9).
Externalizing and Cooccurring Psychopathology.
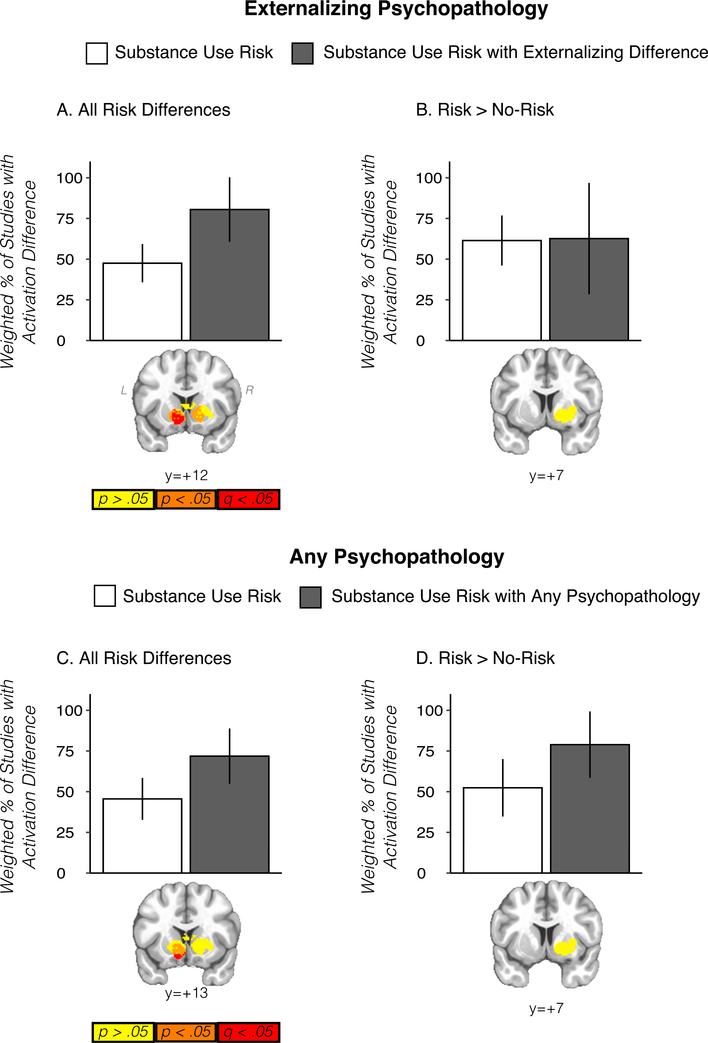
Striatal activation differences in the sign-invariant ‘all studies’ analysis were significantly more common (FDR corrected q < 0.05) among those studies whose substance use risk definition included externalizing psychopathology (n = 4) versus those whose did not (n = 18) (Fig. 3A). These results were unchanged when aggregating the externalizing substance use risk studies (n = 4) with the studies who more broadly did not exclude for psychopathology (n = 3, 7 studies total) (Fig. 3C) or when removing these studies from the analysis completely. The analyses from the ‘risk > non-risk’ contrast were limited in statistical power but also suggested that cooccurring psychopathology may have driven the results of these analyses (Fig. 3B and D). Given the relatively large meta-analytic differences observed as a function of externalizing and cooccurring psychopathology, we re-ran the main meta-analyses while excluding the externalizing studies (n = 4) and while excluding the externalizing studies plus the other psychopathology studies (n = 3, 7 studies total). In both cases, no voxelwise significant (FWER threshold of p < .05 with a single voxel alpha of .01) meta-analytic substance use risk effects were observed when removing studies that included cooccurring psychopathology, although it’s possible this reflects limitations in statistical power (see Discussion section). Nevertheless, these results are consistent with a well-established behavioral literature suggesting externalizing psychopathology is a critical factor in adolescent vulnerability to substance use (Tarter et al., 2003)(see (Iacono et al., 2008) for review). Providing further support for our striatal results as implicated in externalizing psychopathology, a conjunction analysis of the significant voxels in the ‘all studies’ meta-analysis and convolved spheres created from all the significant coordinates of a recent meta-analysis of disruptive behavioral disorders revealed overlapping voxels in the striatum (Supplemental S11). When restricting this analysis to those locations that implicated increased activation, both studies suggested hyper-activation (i.e., risk > non-risk & externalizing > control) in the dorsal striatum, although the implicated voxels did not overlap (Supplemental S11).
Fig. 3.
Top panel displays results from substance use risk studies with and without externalizing disorders in striatal clusters defined from the primary ’all studies’ meta-analysis (A) and from ’risk > non-risk’ studies (B). Bar plots display proportion of studies with activation differences calculated across the cluster, weighted by the square root of the sample size. Striatal images below show that for the primary meta-analysis (A), studies where substance use risk included externalizing disorders were significantly more likely (FDR-corrected) to report activation differences in the striatum. Scale bar below refers to voxel p-value from chi-square analysis comparing activation difference versus no activation difference in substance use risk and substance use risk with externalizing analysis (yellow, p > .05; orange, p < .05, uncorrected, red, FDR-corrected (q) < 0.05 within striatal mask). C and D display the same information for the analysis where studies including externalizing disorders in substance use risk definitions were aggregated with studies not excluding other psychopathology or where externalizing psychopathology was matched across groups. Statistical maps displayed over Montreal Neurological Institute-152 template in neurological view.
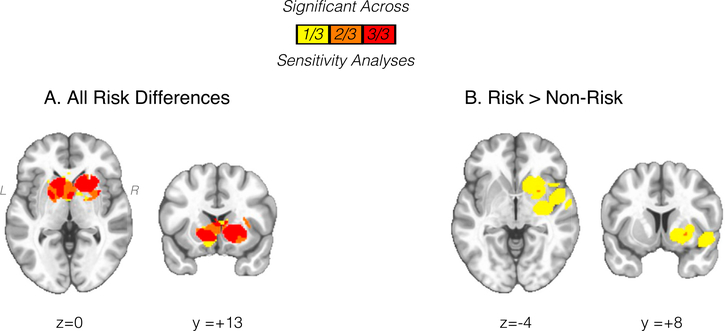
MKDA: Sensitivity Analyses.
Significant striatal activation differences from the ‘all studies’ meta-analysis (n = 22) were observed across all three sensitivity analyses (Fig. 4, Supplemental S12). In contrast, hyper-activation of the striatum from the ‘risk > no-risk’ metanalysis, which overall included fewer studies (n = 12), was observed when excluding studies with a mean sample age over 20-years-old (studies excluded: n = 2, studies included: n = 10), but only marginally (4 significant voxels) observed when excluding studies with subjects differing or having more than experimental substance use (studies excluded: n = 3, studies included: n = 9) and not significant when excluding studies with targeted voxelwise reporting (studies excluded: n = 3, studies included: n = 9)(Fig. 4, Supplemental S12). However, within the limited number of studies, it is difficult to determine whether this result represents true variability driven by methodological differences or rather limited statistical power.
Fig. 4.
Conjunction of significant (FWER p < .05 with single voxel p < .01) clusters across sensitivity analyses (n = 3) for ‘all studies’ (A) and ‘risk > non-risk’ (B). Statistical maps displayed over Montreal Neurological Institute-152 template in neurological view. See Results section in main text and Supplemental S12 for individual sensitivity analyses.
4. Discussion
4.1. Existing neuroimaging studies of adolescent substance use vulnerability
The results from our systematic literature search highlight a growing and diverse literature of neuroimaging studies of adolescent substance use vulnerability. As in many emerging literatures, there is significant heterogeneity in methodology. For example, a challenge faced in the current voxelwise meta-analyses was the high number of region of interest (ROI) or ROI-constrained small volume correction (SVC) studies, which due to potentially biased type 1 and type 2 error rates, limit the ability to make comprehensive inferences regarding vulnerability markers of substance use risk from the entirety of the literature. Accordingly, we suggest the field moves toward sharing of un-thresholded statistical images (cf., (Gorgolewski et al., 2015) (Smith and Delgado, 2017) or full data sets via online repositories (NeuroVault and OpenNeuro), which would permit more sophisticated image-based meta-analyses (Salimi-Khorshidi et al., 2009). The field may also consider including supplementary whole-brain voxelwise results within ROI-based studies, which would likewise support future data integration. Another observation from our meta-analysis and prior literature reviews (e.g., (Heitzeg et al., 2015)) is the relative predominance of studies using reward and cognitive control tasks. While this trend likely reflects dominant reward-cognition theories of adolescent substance use vulnerability, more widespread use of alternative tasks, such as those using emotional or substance use stimuli and/or more complex integrated rewarded-cognitive tasks, may help disambiguate broader neural circuitry associated with substance use vulnerability. Furthermore, our systematic literature search reveals various tasks within the broad domains of reward and cognition. While this task heterogeneity has the advantage of providing potential specificity in the indexed cognitive and affective processes, replication studies and/or multi-task paradigms that provide an opportunity for replication would help in data aggregation efforts to examine the robustness of specific cognitive (response inhibition vs. working memory) and affective (reward anticipation vs. reward consumption) processes implicated in substance use vulnerability.
In addition to and in light of the methodological and reporting patterns across the existing literature, our systematic literature search and meta-analysis support the notion that this is a still-emerging field. Therefore, the results of the current meta-analysis and the broader field should be interpreted within this context. For example, due to sampling variability, early studies with small sample sizes typically have greater error and a larger degree of heterogeneity across studies in effect sizes than later studies with larger samples. Accordingly, as the field continues to develop, and larger studies are available for both prospective prediction and family history analysis (e.g., ABCD study: (Volkow et al., 2017)), our ability to fully disambiguate neuroimaging markers of substance use vulnerability will continue to improve. This point is particularly true for determining whether null results are truly null, or simply require increased statistical power to detect. In addition to larger studies, increased data sharing can help move the field towards better meta-analytic techniques and more comprehensive neural correlates.
4.2. Dual risk model for adolescent substance use
Within the context of the performed meta-analysis, we aimed to test the ‘dual-risk’ theory of adolescent substance use vulnerability. This neurodevelopmental theory suggests vulnerability to problematic substance use is driven, in part, by an exaggeration of adolescents’ enhanced reward drive, mediated by the striatum (Luna et al., 2015)(Steinberg, 2010)(Casey et al., 2008) (see (Shulmanet al., 2016) for review). Consistent with this perspective, our meta-analytic results indicate adolescent substance use risk was most associated with striatal activation differences. Moreover, subdomain analysis suggested activation differences may be specific to motivational context, as striatal differences were observed in studies using reward tasks but not in studies using cognitive control tasks. We were also able to determine substance use risk was associated with increased striatal activation among the corpus of studies, potentially supporting the dual-risk model’s conceptualization of an overactive reward system.
Importantly however, our meta-analytic results suggest nuance in the involvement of the striatum in substance use vulnerability. For example, while striatal activation differences appeared to be driven by tasks with a shared motivational/reward context, the specific affective components indexed by the contrasts were quite diverse (e.g., winning rewards -neutral condition; risky – safe decisions: Supplemental S13). Accordingly, as has been suggested by previous work (Yau et al., 2012), observed striatal effects may represent broader, motivational salience, rather than reward and positive valence-specific processes. Furthermore, when examining signed activation differences, hyper-activation was observed in the dorsal striatum (putamen), which compared to the ventral striatum, may support action selection and habit formation (Tricomi et al., 2009) more than the signaling of primary rewards. As an additional complexity, due to the limited number of studies, our subdomain analyses had lower statistical power than our ‘all studies’ analyses and we were unable to examine meta-analytic differences subdivided by various stages and types of reward processing (e.g., reward anticipation and reward receipt), limiting a comprehensive and computational account of reward function in adolescent substance use risk. Furthermore, striatal activation differences appeared to be critically driven by the cooccurrence of externalizing psychopathology, which prompts a potential distinction between normative adolescent substance use vulnerability and potential trait-level substance use risk factors. Taken together, the current striatal results suggest substance use vulnerability may be characterized by a specific sensitivity to compulsivity and goal-undirected behavior, rather than simply an increased reward drive, but further research into the underlying reward functions of adolescent substance use risk is needed.
Potentially contradicting additional predictions from neurodevelopmental models, our meta-analysis did not identify cognitive control-related prefrontal regions as a reliable neural correlate of substance use vulnerability in any stage of the analysis. However, given the relatively small amount of studies, the null results of this meta-analysis should be interpreted with some degree of caution. Beyond limits of statistical power, it is also possible that prefrontal activation differences were not observed due to performance differences associated with substance use risk, as individual differences in cognitive control tasks have been shown to moderate prefrontal activation (Van Snellenberg et al., 2006). In the current project however, we did not examine the impact of reported cognitive performance differences, owing to the fact that studies did not consistently report performance differences and the available metrics differed in response type (reaction time versus accuracy) and cognitive demands. Similarly, it is possible the task conditions from the included studies did not consistently engage more complex cognitive response processes known to recruit prefrontal activation (cf., (Simmonds et al., 2008)). Another possibility is that prefrontal activation differences may only be reliably observed in samples younger than the ages best represented by this corpus of studies, or in the context of varying developmental trajectories (cf., (Quach et al., 2019)), as recruitment of the prefrontal cortex undergoes normative changes until mid-adolescence, when it reaches adult levels (Simmonds et al., 2017). Nevertheless, provided the current meta-analytic results and the reported inconsistency in previous literature reviews (c.f., (Heitzeg et al., 2015) (Cservenka, 2016)), it is possible that cognitive control-related prefrontal regions may serve a more secondary role in determining adolescent risk for problematic substance use. Supporting this, multiple theoretical perspectives (Luna et al., 2015)(Buckholtz and Meyer-Lindenberg, 2012) and a recent meta-analysis (McTeague et al., 2017) suggest dysfunction of cortically-mediated cognitive control systems acts as a common, transdiagnostic risk factor for psychiatric disorders. To this end, cortical activation differences may serve as non-specific indicators of clinical severity, an explanation which would account for the frequent association of lateral prefrontal and related posterior parietal activation differences in comparisons of early versus late substance use initiation (Tervo-Clemmens et al., 2017)(Becker et al., 2010), where early substance use typically occurs in the context of a broad high-risk profile (Tarter et al., 2003). This idea is supported by longitudinal behavioral research demonstrating that cognitive control deficits are predictive of symptoms of externalizing psychopathology (conduct disorder), while reward responses are specifically associated with substance use (binge drinking) (Castellanos-Ryan et al., 2011).
4.3. Towards an updated model of adolescent substance use risk
In the broadest terms, our results prompt a possible distinction between normative neurodevelopmental models and adolescent markers of vulnerability to problematic substance use. Although adolescence is widely viewed as a period of vulnerability to substance use initiation and escalation, the transition into problematic substance use and addiction is thought to be driven by multiple neurobiological factors (See Koob and Volkow, 2010). Therefore, a comprehensive understanding of adolescent substance use vulnerability should include further work examining the relative specificity of normative developmental processes during adolescence and more developmentally-stable trait-level substance use vulnerability factors, such as family environment and parenting, history of trauma, and familial psychopathology other than substance use disorder.
Defining Substance Use Risk.
Given the diversity of potential risk factors for substance use disorders and their possible distinction between normative adolescent function, operationally defining substance use risk remains a challenge. The current meta-analysis focused on two of the primary ways of operationalizing adolescent vulnerability to substance use by identifying studies that examined participants with a family history of a substance use disorder or those who would later initiate or escalate their substance use. These definitions have the advantage of isolating substance use risk without requiring existing significant current substance use. Nevertheless, as mentioned above, there are a number of other potential substance use risk factors that may provide converegent information regading substance use vulnerability.
A particular focus of the current project and the broader field is that of externalizing psychopathology, which is associated with early substance use initiation (King et al., 2004), relatively early onset of substance use disorders (Carlson et al., 2007), and has been conceptualized as part of a transdiagnostic dimension that includes substance use disorders (Krueger et al., 2002). Given the common co-occurrence of externalizing disorders and problematic substance use, existing studies, including those in the current meta-analysis, have frequently used definitions of substance use risk that include externalizing psychopathology. However, as has been suggested previously (Verdejo-García et al., 2008) and supported by the current project, this may lead to variability in the literature. Specifically, our results suggested striatal activation differences in substance use risk may be moderated by the co-occurrence of externalizing psychopathology. To this end, the lack of externalizing differences might explain, for example, a recent sizeable sample that failed to show striatal activation differences between family history positive and family history negative subjects during a modified monetary incentive delay task (Müller et al., 2015). Nevertheless, while the current results may point towards externalizing psychopathology as a potential moderator or mediator of substance use risk differences, more studies and increased data sharing are required to fully disambiguate these challenges. Notably, increased data sharing would permit more sophisticated image-based meta-analyses (Salimi-Khorshidi et al., 2009) that, unlike coordinate-based meta-analysis, can incorporate entirely null studies (e.g., (Müller et al., 2015)) and provide critical information on overall effect sizes.
Substance Use Risk and Addiction.
In addition to providing clarity on variability among the substance use risk literature, the current meta-analysis may also provide context for the existing addiction literature. For example, while the potential primary role of the striatum in adolescent substance use vulnerability differs from the neurodevelopmental literature, multiple models from the addiction literature have emphasized a particular role of the striatum and reward systems in substance use progression.
A general “impulsivity theory” (cited from (Luijten et al., 2017)) has suggested addiction, and thus potentially addiction vulnerability, is the result of a hyper-functioning striatum and reward system (Bjork et al., 2010)(Bjork et al., 2012) that may manifest as a trait-level behavioral feature, impulsivity. Similarly, allostasis theory predicts increased striatal dopamine functioning and impulsivity mediate early experiences with drugs of abuse (Koob, 2011). These perspectives have the advantage of accounting for the association between impulsive behavioral phenotypes, including disorders of the externalizing spectrum (e.g., oppositional defiance, conduct disorder), and problematic substance use, which were supported by the current project’s results showing the potential moderating or mediating role of externalizing psychopathology on activation difference associated with substance use risk.
Complimentary to theories concerning an early bias towards substance use, other perspectives highlight the dynamic pharmacological interactions between drugs of abuse and brain function. For example, both incentive salience theory (Robinson and Berridge, 1993) and allostasis theory (Koob, 2011) suggest drugs of abuse lead to neuro-adaptations in the striatum, which ultimately lead to substance use escalation. The current meta-analytic results of striatal dysfunction that precede problematic substance use may signal a vulnerability to these later neuroadaptations. An advantage of this perspective, wherein pre-existing individual differences predict the course of substance use escalation, is that it might account for the observation of substance use risk factors (externalizing symptoms: (Chassin et al., 2002), (Brook et al., 2011); family history of SUD: (Waldenet al., 2007)) to predict both early onset and more problematic substance use. Nevertheless, future longitudinal neuroimaging research, equipped to parse pre-existing between-subject differences and within-subject escalation of substance use (e.g., ABCD study: (Volkow et al., 2017)), are required to test these predictions. Such dense longitudinal neuroimaging research can also assist in examining cortical control areas that may serve as secondary markers of clinical severity and substance use risk. Additionally, longitudinal analyses of functional connectivity may help clarify potential interactions between cortical control and striatal regions in problematic substance use and related forms of psychopathology.
Dorsal versus Ventral Striatal Dysfunction.
An intriguing result from the current project is the potential distinction between dorsal and ventral subdivisions of the striatum in substance use risk. For example, striatal hyper-activation in substance use risk was more associated with the dorsal subdivision of the striatum, which has also been implicated in a prior meta-analysis of externalizing psychopathology (Alegria et al., 2016). While we have reviewed theories of addiction in the context of the striatum generally, growing evidence suggests a functional distinction between dorsal and ventral subdivisions of the striatum. For example, work in rodents and humans has suggested that, whereas the reinforcing effects of drugs of abuse are mediated by the ventral striatum, the relative locus of behavioral control transitions to the dorsal striatum during habitual substance use (Everitt and Robbins, 2013)(Vollstädt-Klein et al., 2010). Given the current meta-analysis isolated substance use risk prior to significant substance use involvement, our results may support prior suggestions of a relative predisposition to compulsivity or habit formation (Everitt and Robbins, 2013) that co-occurs with externalizing psychopathology (e.g., trait-level impulsivity). Again however, large longitudinal studies are required to further test these predictions.
4.4. Study limitations
As the first neuroimaging meta-analysis of adolescent substance use vulnerability, this study highlights essential targets for future research and theory development. However, as in any analysis, there are important limitations to consider. First, as mentioned above, due to the large number of studies using only region-of-interest, small volume correction, or multi-step procedures, and our general use of published coordinates, this meta-analysis was limited to a subset of the broader literature. While we took steps to contact authors and examine repositories for unthresholded statistical volumes to try and increase the potential number of included studies, this limitation reflects general analysis and reporting practices. With respect to the current project, the total number of studies included in the primary meta-analysis (n = 22) is comparable to simulation studies (n ≥ 20; (Salimi-Khorshidi et al., 2009)) and other recent meta-analyses of clinical phenotypes. However, due to the limitations raised above, we were limited in our ability to fully explore heterogeneity in this emerging literature. For example, the performed subgroup (e.g., family history and prospective studies) and subdomain (e.g., cognitive control and reward tasks) analyses relied on restricted subsets of studies. Likewise, due to the limited number of studies, we were not able to complete separate analyses on stimulus types within each task domain (i.e., reward anticipation versus reward receipt). Furthermore, we were unable to restrict our analyses to studies using entirely drug naïve youth (see Supplemental S3), which would help disambiguate the current markers of vulnerability to problematic substance use with those associated with substance use initiation. Finally, due to the limited number of studies, and in particular, the limited number of prospective prediction studies, we focused our analyses on commonalities across family history and prospective prediction studies. However, this prevented us from fully exploring heterogeneity within family history studies, as well as differences between family history and prospective prediction studies that can provide critical information on the distinction between risk and resilience to substance use disorders.
Another limitation of the current project and all meta-analyses of clinical phenotypes is that spatial locations of activation differences may be constrained by the types of neuroimaging tasks included in the literature. For example, few studies in our meta-analysis employed tasks using emotional stimuli, which may have limited our indentification of reliable neuroimaging markers of substance use risk in the amygdala and para-limbic systems supporting emotional processing. In addition to this limitation on spatial sensitivity, coordinate-based meta-analyses are limited in spatial specificity by the size of the kernel that is convolved with the reported coordinate. Based on recommendations from simulation (Salimi-Khorshidi et al., 2009), the current project used a sphere with a radius of 15 mm. However, by definition, this procedure limits the certainty of spatial estimates. Finally, the goal of coordinate-based meta-analyses is to determine spatial convergence of reported coordinates, which differs from an “effect-size” meta-analysis typically found in behavioral research. Accordingly, the results from the current meta-analysis should be viewed as a quantitative integration of the included published brain regions implicated in substance use risk, but not for the overall prevalence of such effects. Therefore, while the MKDA procedures we used have been designed to provide results that generalize to new studies, the current results should be interpreted within the context of the included studies and we suggest further quantitative integration in this literature. Ultimately, improved spatial sensitivity and specificity, and meta-analyses of “effect size” (i.e., image based meta-analysis (Salimi-Khorshidi et al., 2009)) will require data sharing of either unthresholded statistical volumes (NeuroVault, neurovault.org) or full data sets (OpenNeuro, openneuro.org).
5. Conclusion
In summary, the current meta-analysis provides preliminary evidence for a more primary role of striatal dysfunction in adolescent vulnerability to substance use, with limited support for prefrontal activation differences hypothesized in ‘dual-risk’ models. We propose hyper-activation of the striatum, possibly through a dimensional predisposition to compulsivity and habit formation shared with externalizing psychopathology, may predict later problematic substance use. However, more studies and increased data sharing are necessary; ary for a comprehensive understanding of the neuroimaging markers of adolescent substance use risk.
Supplementary Material
Acknowledgments
The authors thank Drs. M.A. Sayette, A.G.C. Wright, and J.L. Hanson for helpful feedback on an earlier version of the manuscript, Y.H. Panchal for assistance with data organization and management, and T.E. Kraynak for discussion concerning methodology.
Funding
Mr. Tervo-Clemmens was supported by the National Institute of Mental Health (R03MH113090, R01MH067924), Drs. Calabro and Luna were supported by the National Institute of Mental Health (R03MH113090, R01MH067924, R01MH08024) and the Staunton Farm Foundation.
Footnotes
Declaration of competing interest
The authors report no financial relationships with commercial interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuroimage.2019.116476.
References
- Acheson A, Franklin C, Cohoon AJ, Glahn DC, Fox PT, Lovallo WR, 2014a. Anomalous temporoparietal activity in individuals with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Alcohol Clin. Exp. Res. 38, 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Tagamets MA, Rowland LM, Mathias CW, Wright SN, Hong LE, Kochunov P, Dougherty DM, 2014b. Increased forebrain activations in youths with family histories of alcohol and other substance use disorders performing a Go/ NoGo task. Alcohol Clin. Exp. Res. 38, 2944–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegria AA, Radua J, Rubia K, 2016. Meta-analysis of fMRI studies of disruptive behavior disorders. Am. J. Psychiatry 173, 1119–1130. [DOI] [PubMed] [Google Scholar]
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Stevens MC, O’Malley S, Book GA, Reynolds B, 2011. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol. Psychiatry 69, 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J, 2010. The impact of early-onset cannabis use on functional brain correlates of working memory. Prog. Neuro Psychopharmacol. Biol. Psychiatry 34, 837–845. 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA, 2007. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 90, S85–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Smith AR, Hommer DW, 2010. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. JCPP (J. Child Psychol. Psychiatry) 51, 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW, 2012. Mesolimbic recruitment by nondrug rewards in detoxified alcoholics: effort anticipation, reward anticipation, and reward delivery. Hum. Brain Mapp. 33, 2174–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJ, Comings DE, 2000. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoact. Drugs 32. Suppl, i–iv, 1–112. [DOI] [PubMed] [Google Scholar]
- Brett M, Christoff K, Cusack R, Lancaster J, 2001. Using the Talairach atlas with the MNI template. Neuroimage 13, 85–85. [Google Scholar]
- Brook JS, Zhang C, Brook DW, 2011. Developmental trajectories of marijuana use from adolescence to adulthood: personal predictors. Arch. Pediatr. Adolesc. Med. 165, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A, 2012. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron 74, 990–1004. [DOI] [PubMed] [Google Scholar]
- Carlson SR, McLarnon ME, Iacono WG, 2007. P300 amplitude, externalizing psychopathology, and earlier-versus later-onset substance-use disorder. J. Abnorm. Psychol. 116, 565. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA, 2008. The adolescent brain. Ann. N. Y. Acad. Sci. 1124, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Rubia K, Conrod PJ, 2011. Response inhibition and reward response bias mediate the predictive relationships between impulsivity and sensation seeking and common and unique variance in conduct disorder and substance misuse. Alcohol Clin. Exp. Res. 35, 140–155. [DOI] [PubMed] [Google Scholar]
- Chassin L, Pitts SC, Prost J, 2002. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: predictors and substance abuse outcomes. J. Consult. Clin. Psychol. 70, 67. [PubMed] [Google Scholar]
- Clark DB, Cornelius JR, Kirisci L, Tarter RE, 2005. Childhood risk categories for adolescent substance involvement: a general liability typology. Drug Alcohol Depend. 77, 13–21. [DOI] [PubMed] [Google Scholar]
- Cservenka A, 2016. Neurobiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend. 158, 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Fair DA, Nagel BJ, 2014. Emotional processing and brain activity in youth at high risk for alcoholism. Alcohol Clin. Exp. Res. 38, 1912–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Herting MM, Nagel BJ, 2012. Atypical frontal lobe activity during verbal working memory in youth with a family history of alcoholism. Drug Alcohol Depend. 123, 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Nagel BJ, 2012. Risky decision-making: an FMRI study of youth at high risk for alcoholism. Alcohol Clin. Exp. Res. 36, 604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, Anderson BM, Rosen R, Khadka S, Sawyer B, Jiantonio-Kelly RE, Austad CS, Raskin SA, Tennen H, Wood RM, 2014. Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction 109, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, Anderson BM, Stevens MC, Pulido C, Rosen R, Jiantonio-Kelly RE, Sisante J-F, Raskin SA, Tennen H, Austad CS, 2013. Influence of alcohol use and family history of alcoholism on neural response to alcohol cues in college drinkers. Alcohol Clin. Exp. Res. 37, E161–E171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, 2009. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol. 14, 22–31. 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duell N, Steinberg L, Icenogle G, Chein J, Chaudhary N, Di Giunta L, Dodge KA, Fanti KA, Lansford JE, Oburu P, 2018. Age patterns in risk taking across the world. J. Youth Adolesc. 47, 1052–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2013. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci. Biobehav. Rev. 37, 1946–1954. [DOI] [PubMed] [Google Scholar]
- Gorgolewski KJ, Varoquaux G, Rivera G, Schwarz Y, Ghosh SS, Maumet C, Sochat VV, Nichols TE, Poldrack RA, Poline J-B, 2015. NeuroVault. org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front. Neuroinf. 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Cope LM, Martz ME, Hardee JE, 2015. Neuroimaging risk markers for substance abuse: recent findings on inhibitory control and reward system functioning. Curr. Addict. Rep. 2, 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau W-YW, Zucker RA, Zubieta J-K, 2010a. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol. Psychiatry 68, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau W-YW, Zucker RA, Zubieta J-K, 2010b. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol. Psychiatry 68, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn LA, Finn P, Hummer TA, Leibenluft E, Ball B, Gichina V, Anand A, 2013. Cortical activation deficits during facial emotion processing in youth at high risk for the development of substance use disorders. Drug Alcohol Depend. 131, 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn LA, Hummer TA, Fukunaga R, Leibenluft E, Finn P, Cyders MA, Anand A, Overhage L, Dir A, Brown J, 2015. Neural activation during risky decision-making in youth at high risk for substance use disorders. Psychiatry Res. Neuroimaging 233, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M, 2008. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu. Rev. Clin. Psychol. 4, 325–348. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Liu X, Shulz K, Fan J, London E, Friston K, Halperin JM, Newcorn JH, 2012. Parental substance abuse and function of the motivation and behavioral inhibition systems in drug-naïve youth. Psychiatry Res. Neuroimaging 201, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME, 2018. Monitoring the Future National Survey Results on Drug Use, 1975–2017: Overview, Key Findings on Adolescent Drug Use.
- Kareken DA, Dzemidzic M, Wetherill L, Eiler W, Oberlin BG, Harezlak J, Wang Y, O’Connor SJ, 2013. Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology 228, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M, 2004. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction 99, 1548–1559. [DOI] [PubMed] [Google Scholar]
- Koob GF, 2011. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. In: Behavioral Neurobiology of Alcohol Addiction. Springer, pp. 3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraynak TE, Marsland AL, Wager TD, Gianaros PJ, 2018. Functional neuroanatomy of peripheral inflammatory physiology: a meta-analysis of human neuroimaging studies. Neurosci. Biobehav. Rev. 94, 76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M, 2002. Etiologic connections among substance dependence, antisocial behavior and personality: modeling the externalizing spectrum. J. Abnorm. Psychol. 111, 411. [PubMed] [Google Scholar]
- Luijten M, Schellekens AF, Kühn S, Machielse MWJ, Sescousse G, 2017. Disruption of reward processing in Addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatr. 74, 387–398. 10.1001/jamapsychiatry.2016.3084. [DOI] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R, 2015. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci. 38, 151–170. 10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz Seghete KL, Cservenka A, Herting MM, Nagel BJ, 2013. Atypical spatial working memory and task-general brain activity in adolescents with a family history of alcoholism. Alcohol Clin. Exp. Res. 37, 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I, 2001. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin. Exp. Res. 25, 1156–1165. [PubMed] [Google Scholar]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A, 2017. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am. J. Psychiatry 174, 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CH, Hamilton JP, Sacchet MD, Gotlib IH, 2015. Meta-analysis of functional neuroimaging of major depressive disorder in youth. JAMA Psychiatr. 72, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, Jones SA, Ehlers A, Lavine JB, Nagel BJ, 2018. Ventral striatal response during decision making involving risk and reward is associated with future binge drinking in adolescents. Neuropsychopharmacology 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller KU, Gan G, Banaschewski T, Barker GJ, Bokde AL, Büchel C, Conrod P, Fauth-Bühler M, Flor H, Gallinat J, 2015. No differences in ventral striatum responsivity between adolescents with a positive family history of alcoholism and controls. Addict. Biol. 20, 534–545. [DOI] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Laird AR, Fox PT, Radua J, Mataix-Cols D, Tench CR, Yarkoni T, Nichols TE, Turkeltaub PE, 2018. Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 84, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Courtney KE, Squeglia LM, Bagot K, Eberson S, Migliorini R, Alcaraz AR, Tapert SF, Pulido C, 2018. Prospective changes in neural alcohol cue reactivity in at-risk adolescents. Brain Imag. Behav. 12, 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF, 2011. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 119, 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Alloy LB, 2017. Reward processing and mood-related symptoms: an RDoC and translational neuroscience perspective. J. Affect. Disord. 216, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell ME, Boat T, Warner KE, 2009. Committee on the Prevention of Mental Disorders and Substance Abuse Among Children, Youth, and Young Adults: Research Advances and Promising Interventions. Preventing Mental, Emotional, and Behavioral Disorders Among Young People: Progress and Possibilities.
- Peraza J, Cservenka A, Herting MM, Nagel BJ, 2015. Atypical parietal lobe activity to subliminal faces in youth with a family history of alcoholism. Am. J. Drug Alcohol Abuse 41, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Wang Z, Geronazzo-Alman L, Amsel L, Duarte C, Lee S, Musa G, Long J, He X, Doan T, 2015. Brain activity classifies adolescents with and without a familial history of substance use disorders. Front. Hum. Neurosci. 9, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach A, Tervo-Clemmens B, Foran W, Calabro FJ, Chung T, Clark DB, Luna B, 2019. Neurocognitive Development of Inhibitory Control and Substance Use Vulnerability bioRxiv 781286. [DOI] [PMC free article] [PubMed]
- Robinson TE, Berridge KC, 1993. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Rev. 18, 247–291. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB, 2012. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage 60, 830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE, 2009. Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage 45, 810–823. [DOI] [PubMed] [Google Scholar]
- Shine JM, Breakspear M, Bell PT, Martens KE, Shine R, Koyejo O, Sporns O, Poldrack RA, 2019. Human cognition involves the dynamic integration of neural activity and neuromodulatory systems. Nat. Neurosci. 1. [DOI] [PubMed] [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, Steinberg L, 2016. The dual systems model: review, reappraisal, and reaffirmation. Dev. Cogn. Neurosci. 17, 103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Luna B, 2017. Protracted development of executive and mnemonic brain systems underlying working memory in adolescence: a longitudinal fMRI study. Neuroimage 157, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH, 2008. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 46, 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, van Tol M-J, Van den Brink W, Van der Wee NJ, Aleman A, Beekman AT, Penninx BW, Veltman DJ, 2013. Family history of alcohol dependence modulates functional neurophysiology in mood/anxiety disorders. Psychol. Med. 43, 1487–1497. [DOI] [PubMed] [Google Scholar]
- Smith DG, Jones PS, Bullmore ET, Robbins TW, Ersche KD, 2013. Cognitive control dysfunction and abnormal frontal cortex activation in stimulant drug users and their biological siblings. Transl. Psychiatry 3, e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Delgado MR, 2017. Meta-analysis of psychophysiological interactions: revisiting cluster-level thresholding and sample sizes. Hum. Brain Mapp. 38, 588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, 2010. A dual systems model of adolescent risk-taking. Dev. Psychobiol. 52, 216–224. 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, 2014. Brain reward region responsivity of adolescents with and without parental substance use disorders. Psychol. Addict. Behav. 28, 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, 2013. Elevated reward region responsivity predicts future substance use onset but not overweight/obesity onset. Biol. Psychiatry 73, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D, 2003. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am. J. Psychiatry 160, 1078–1085. [DOI] [PubMed] [Google Scholar]
- Tervo-Clemmens B, Simmonds D, Calabro F, Montez DF, Lekht J, Day NL, Richardson GA, Luna B, 2018. Early cannabis use and neurocognitive risk: a prospective functional neuroimaging study. Biol. Psychiatry: Cogn. Neurosci. Neuroimag. [DOI] [PMC free article] [PubMed]
- Tervo-Clemmens B, Simmonds D, Calabro FJ, Day NL, Richardson GA, Luna B, 2017. Adolescent Cannabis Use and Brain Systems Supporting Adult Working Memory Encoding, Maintenance, and Retrieval. NeuroImage 169, 496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi E, Balleine BW, O’Doherty JP, 2009. A specific role for posterior dorsolateral striatum in human habit learning. Eur. J. Neurosci. 29, 2225–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snellenberg JX, Torres IJ, Thornton AE, 2006. Functional Neuroimaging of Working Memory in Schizophrenia: Task Performance as a Moderating Variable. American Psychological Association. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L, 2008. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci. Biobehav. Rev. 32, 777–810. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Swanson JM, 2004. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry 9, 557. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, Perez-Stable EJ, Riley WT, Bloch MH, Conway K, 2017. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev. Cogn. Neurosci. 32, 4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, Hermann D, Mann K, 2010. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction 105, 1741–1749. [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, Kaplan L, 2007. Meta-analysis of functional neuroimaging data: current and future directions. Soc. Cogn. Affect. Neurosci. 2, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist MA, Nichols TE, Kober H, Van Snellenberg JX, 2009. Evaluating the consistency and specificity of neuroimaging data using meta-analysis. Neuroimage 45, S210–S221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden B, Iacono WG, McGue M, 2007. Trajectories of change in adolescent substance use and symptomatology: impact of paternal and maternal substance use disorders. Psychol. Addict. Behav. 21, 35. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Castro N, Squeglia LM, Tapert SF, 2013. Atypical neural activity during inhibitory processing in substance-naive youth who later experience alcohol-induced blackouts. Drug Alcohol Depend. 128, 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau W-YW, Zubieta J-K, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM, 2012. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. J. Neurosci. 32, 2544–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystrom E, Reichborn-Kjennerud T, Neale MC, Kendler KS, 2014. Genetic and environmental risk factors for illicit substance use and use disorders: joint analysis of self and co-twin ratings. Behav. Genet. 44, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this project (coded foci of activation differences) are available in the Supplemental information. The MKDA code used to perform meta-analyses is freely available via CANLAB (https://sites.dartmouth.edu/canlab/).