Vincristine-induced peripheral neuropathy is driven by innate immune system activation. This could be prevented by repurposing the IL-1 receptor antagonist anakinra as a co-treatment strategy.
Abstract
Vincristine-induced peripheral neuropathy (VIPN) is a prevalent and painful complication in cancer patients that lacks effective treatments. In this issue of JEM, Starobova et al. (2021. J. Exp. Med. https://doi.org/10.1084/jem.20201452) report that VIPN is driven by innate immune system activation, a discovery that unlocks immunotherapies as potential treatments.
Chemotherapy-induced peripheral neuropathy (CIPN) is a prevalent dose-limiting side effect in cancer patients and lacks effective treatments (Starobova and Vetter, 2017). CIPN can be triggered by platinum drugs and mitotic, proteasome, and immune checkpoint inhibitors through multifactorial mechanisms. Pathophysiology includes axon degeneration, altered neuronal excitability, oxidative stress, apoptotic signaling, calcium dysregulation, and immune involvement. Although chemotherapeutics have long been known to exert immunosuppressive effects, chemotherapy-induced neuro-inflammation is increasingly recognized, including in CIPN, through both the innate and adaptive arms of the immune system. However, the precise mechanisms of this immune activation aspect in CIPN are unclear.
Insights from Masha G. Savelieff and Eva L. Feldman.
In this issue of JEM, Starobova et al. (2021) report that vincristine-induced peripheral neuropathy (VIPN) is driven by innate immune system activation through the NLRP3 inflammasome (NLR family pyrin domain containing 3). Vincristine, a microtubule-disrupting chemotherapeutic, causes a painful, length-dependent sensory neuropathy with frequent motor involvement. In the VIPN mouse model, this manifests as mechanical allodynia and gait instability. Motivated by recent literature on vincristine-associated neuro-inflammation (Starobova and Vetter, 2017), the investigators assessed macrophage infiltration into the nervous system of VIPN mice. They found macrophage recruitment into the peripheral sciatic nerve and dorsal root ganglia (DRG), but not the central nervous system, specifically the spinal cord, of VIPN mice. To verify immune involvement, the authors depleted macrophages from VIPN mice, which rescued mechanical allodynia, whereas granulocyte depletion had no effect.
To investigate the molecular mechanisms, Starobova et al. focused on the NLRP3 inflammasome as their candidate pathway, because it is almost uniquely expressed in macrophages and plays a role in sterile inflammation (Mangan et al., 2018). To pinpoint NLRP3 signaling, VIPN was induced in WT, Nlrp3−/−, and NLRP3 inhibitor (MCC950)–treated WT (WTMCC950) mice. Vincristine-induced mechanical allodynia and gait were rescued in Nlrp3−/− and WTMCC950 mice, indicating NLRP3 involvement in VIPN. Furthermore, NLRP3 activation elicits pro-inflammatory IL-1β production from macrophages. This NLRP3-driven activity was leveraged in a series of in vitro experiments, which concluded that vincristine functioned as an NLRP3 activation cue and not as a priming signal. However, vincristine did not activate NLRP3 through danger-associated molecular patterns, which are usual NLPR3 activation cues. Furthermore, lipopolysaccharide-stimulated IL-1β production in response to vincristine was blunted in Nlrp3−/− macrophages, reinforcing a role for NLRP3 signaling in VIPN.
NLRP3 activation can occur through canonical caspase 1 (casp1)– and gasdermin D (GSDMD)–mediated or noncanonical caspase-11 (casp11)–mediated activation (Mangan et al., 2018). Therefore, to elucidate vincristine-triggered pathways, Starobova et al. conducted a comprehensive study in mice with KO of canonical and noncanonical NLRP3 signaling proteins, Gsdmd−/−, Casp11−/−, and double KO of both pathways Ice−/− (Casp1−/−/Casp11null/null). Vincristine-induced mechanical allodynia and gait were rescued in Gsdmd−/− and Ice−/− but not Casp11−/− mice, suggesting VIPN is mediated by canonical NLPR3 activation. To confirm that downstream IL-1β production contributes to VIPN, experiments were repeated in Il1b−/− and Il1R1−/− (IL-1R [IL-1 receptor]) mice, which blocked the development of both mechanical allodynia and gait defects. Recognizing IL-1β/IL-1R participates in VIPN, Starobova et al. leveraged anakinra, an IL-1R antagonist, as a possible approach for preventing VIPN. Indeed, aligned with the investigators’ hypothesis, anakinra attenuated VIPN-triggered mechanical allodynia and gait disturbances. Critically, anakinra did not interfere with vincristine efficacy in three xenograft medulloblastoma mouse models but retained the ability to prevent mechanical allodynia (Starobova et al., 2021).
These findings concluded an elegant study by Starobova et al., which provided mechanistic insight and potential treatment approaches for VIPN. Like all well-conducted studies, Starobova et al. opened several new avenues of investigation. First, the authors found that KO of their candidate IL-1β–sensitized nociceptor, NaV1.9, did not fully block vincristine-induced allodynia and had little effect on gait. Therefore, the targets downstream of NLRP3-mediated IL-1β release that generate sensory neuropathy in VIPN require continued investigation. Moreover, the mechanism of vincristine NLRP3 priming in vivo also remains unknown. Second, in a test of additional chemotherapeutics, the authors found anakinra also prevented ixabepilone- but not oxaliplatin- or cisplatin-induced mechanical allodynia, suggesting targeting the NLRP3–IL-1β axis is effective specifically against microtubule-directed agents. This is substantiated by another report, which found that paclitaxel, another microtubule-directed chemotherapeutic, also primes NLRP3 (Son et al., 2019). Further investigating this interesting shared aspect of NLRP3 activation by microtubule-directed agents could shed light on the priming or activation mechanisms. Collectively, these findings also lead to a third major area of investigation—determining whether other CIPNs are immune mediated and through what mechanisms to identify possible therapeutics. Finally, the journey to translate from bench to bedside may be challenging, since vincristine induces neuro-inflammation in the nerve milieu but may be immunosuppressive systemically. Thus, although anakinra is FDA approved, which would facilitate translation, it may pose a medical risk by blocking immunity in immunocompromised cancer patient populations and will require evaluating safety and future efficacy studies further down the line. The same challenges will be faced if direct NLRP3 inhibitors, currently in the drug development pipeline (Mangan et al., 2018), are considered as future therapeutic options for treating VIPN.
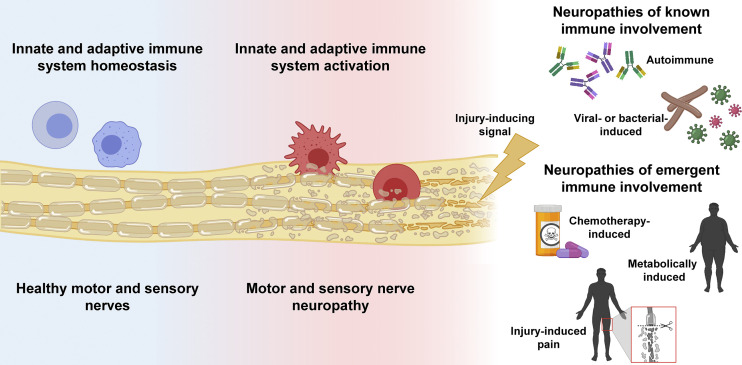
In the past, only specific types of neuropathies, such as acute or chronic inflammatory demyelinating polyneuropathy (AIDP, CIDP; Bourque et al., 2015), along with infectious neuropathies like leprosy, varicella zoster, and human immunodeficiency virus (Sindic, 2013), were categorized as immune-mediated neuropathies (see figure). These neuropathies are treated with therapies targeted at specific immune cell types, immune-mediated disease mechanisms, or the infectious agent. For example, AIDP and CIDP respond well to intravenous immunoglobulin therapy, and the FDA recently approved rituximab, a B cell depleting therapy, for treating the CIDP subtype associated with IgG4 autoantibodies. Leprosy and varicella are treated with antibiotics and antivirals, respectively, although these drugs target the infectious with the goal to prevent neuropathy. The critical importance of studies like that by Starobova et al. is that they are broadening the role of the immune system in the pathogenesis of neuropathy. This is crucial, because neuropathy is the most common neurodegenerative disease among all neurological disorders. Moreover, the burden of neuropathy is massive (Callaghan et al., 2020). Currently, over half a billion individuals with diabetes and obesity worldwide are at risk of developing debilitating neuropathy. In young individuals, like pediatric cancer patients, neuropathy commits them to a lifetime of morbidity and complications. Defining the role of the immune system in neuropathies previously thought to be solely of toxic or metabolic etiologies unlocks the door to whole new avenues of investigation and potential novel and much-needed treatments (see figure).
Overview of neuropathies with known and emergent immune involvement. Homeostatic innate and adaptive immune function, blue. Injury-inducing signal(s) lead(s) to immune involvement and infiltration into the nerve, causing motor and sensory neuropathy, red. Created, in part, with BioRender.com.
Our recent research supports the work of Starobova et al. that neuropathies of diverse etiologies may have an underlying immune component. In the high-fat-diet mouse model of prediabetes and metabolic neuropathy, transcriptomic analyses of affected peripheral nerves identified involvement of TLR pathways (Elzinga et al., 2019). TLR2 and TLR4 KO delayed neuropathy onset, suggesting a role for inflammation in the early stages of neuropathy. We also identified immune system dysregulation in the transcriptome of peripheral nerves from a type 2 diabetes (T2D) mouse model with neuropathy, the db/db mouse (Hinder et al., 2018), and in a combined epigenomic-transcriptomic analysis of human sural nerves from T2D patients with progressive neuropathy (Guo et al., 2020). When we compared gene expression in peripheral nerves from type 1 diabetes (T1D) and T2D patients with neuropathy to T1D and T2D neuropathy mouse models, we identified inflammation as a highly conserved pathway in both humans and mice (McGregor et al., 2018). These findings are supported by a relatively old and small histological study of femoral or sural nerve biopsies from T2D patients (n = 15) with progressive, painful, asymmetric neuropathy, which revealed the presence of polymorphonuclear leukocytic vasculitis in around a quarter of the nerve biopsies (Kelkar et al., 2000). Clearly, while relatively unexplored, the role of immunity in the pathogenesis of metabolically acquired neuropathies, especially those related to obesity, prediabetes, and T2D, opens up an entirely new field of investigation and therapeutic opportunities.
Emerging studies also reveal a significant role for immunity in neuropathic pain secondary to injury. Neuropathic pain in nerve injury is associated with both innate and adaptive immunity, frequently as a double-edged sword, with pain-inducing and pain-resolving roles in population-specific manners (Liu et al., 2021). In a partial sciatic nerve ligation mouse model, initial CD4+ T cell influx into the injury site is followed by regulatory T cell infiltration, which promotes pain resolution by inhibiting the inflammatory CD4+ type 1 helper T cell response (Davoli-Ferreira et al., 2020). In a sciatic nerve crush mouse model, natural killer cells are recruited to the injury site and eliminate damaged sensory neurons to lower post-injury hypersensitivity (Davies et al., 2019). These studies suggest that specific immune populations may possibly be leveraged to modulate peripheral nerve pain.
30 million Americans suffer from peripheral neuropathy secondary to multiple etiologies. From this overview, and based on the important findings reported by Starobova et al. in this JEM issue, there is a growing appreciation the immune system may play a critical part in neuropathy pathogenesis (see figure). Understanding these immune-mediated mechanisms across the spectrum of peripheral neuropathy can identify new immune targets, which can be leveraged for treatment with repurposed preexisting immunotherapies or in the future with newly developed ones. We await the day that peripheral neuropathies of diverse etiologies, from drug- to metabolically induced, advance from a series of untreatable to treatable diseases.
Acknowledgments
The authors thank Dr. Benjamin J. Murdock, University of Michigan, for immunology discussions.
Funding is provided by National Institutes of Health grants R01 DK107956 and R24 DK082841.
The authors declare no competing interests.
References
- Bourque, P.R., et al. 2015. Clin. Chim. Acta. 10.1016/j.cca.2015.02.039 [DOI] [Google Scholar]
- Callaghan, B.C., et al. 2020. JAMA. 10.1001/jama.2020.0700 [DOI] [Google Scholar]
- Davies, A.J., et al. 2019. Cell. 10.1016/j.cell.2018.12.022 [DOI] [Google Scholar]
- Davoli-Ferreira, M., et al. 2020. Pain. 10.1097/j.pain.0000000000001879 [DOI] [Google Scholar]
- Elzinga, S., et al. 2019. Exp. Neurol. 10.1016/j.expneurol.2019.112967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, K., et al. 2020. Clin. Epigenetics. 10.1186/s13148-020-00913-6 [DOI] [Google Scholar]
- Hinder, L.M., et al. 2018. Exp. Neurol. 10.1016/j.expneurol.2018.03.011 [DOI] [Google Scholar]
- Kelkar, P., et al. 2000. Neurology. 10.1212/WNL.55.1.83 [DOI] [Google Scholar]
- Liu, J.A., et al. 2021. Int. J. Mol. Sci. 10.3390/ijms22031448 [DOI] [Google Scholar]
- Mangan, M.S.J., et al. 2018. Nat. Rev. Drug Discov. 10.1038/nrd.2018.97 [DOI] [PubMed] [Google Scholar]
- McGregor, B.A., et al. 2018. Sci. Rep. 10.1038/s41598-018-36098-5 [DOI] [Google Scholar]
- Sindic, C.J. 2013. Curr. Opin. Neurol. 10.1097/WCO.0b013e328364c036 [DOI] [PubMed] [Google Scholar]
- Son, S., et al. 2019. Front. Immunol. 10.3389/fimmu.2019.01108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starobova, H., and Vetter I.. 2017. Front. Mol. Neurosci. 10.3389/fnmol.2017.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starobova, H., et al. 2021. J. Exp. Med. 10.1084/jem.20201452 [DOI] [Google Scholar]