Abstract
Parry Romberg Syndrome (PRS), also known as idiopathic progressive hemifacial atrophy, is a rare neurocutaneous disorder characterized by loss of skin and subcutaneous fat of face, muscles, and bones causing unilateral atrophy. Most patients require only soft tissue augmentation although syndrome has varying grades of severity. In the majority of reported cases, it has been treated with surgical flap or autologous fat transplantation. However, these treatments need complicated surgical skills which take a lot of time and cost. Herein we report the first case of PRS augmented by hyaluronic acid (HA) filler in a 42-year-old female patient to suggest that HA filler could be a safe, simple, and even rational economic alternative to surgical treatment.
Keywords: Facial hemiatrophy, Filler, Hyaluronic acid
INTRODUCTION
Parry-Romberg Syndrome (PRS) was first described by Parry in 1825 and by Romberg in 19461. It is a rare neurocutaneous disorder that is characterized by loss of skin, subcutaneous fat, and muscles of the face resulting in unilateral atrophy. It can even involve bone, teeth, hair and eyes2. Usually it starts in the second division of the trigeminal nerve, followed by the first and third division3. Until now, the two prominent treatments for PRS were autologous fat graft and surgical flap4,5,6. However, these treatments need complicated surgical skills which take a lot of time and cost. The objective of this study is to report that hyaluronic acid (HA) filler can be used as a safe, simple, and even rational economic alternative for the treatment of PRS.
We received the patient's consent form about publishing all photographic materials.
CASE REPORT
A 42-year-old female patient came to dermatology clinic for the correction of facial hemiatrophy and hyperpigmentation on the right side of the face (Fig. 1A, 2A). She suffered from progressive symptoms since 20 years ago. She complained of intermittent unilateral migraine and trigerminal neuralgia, but there were no bony or vascular abnormalities on imaging study. She showed normal teeth arrangement and visual acuity, and had no other systemic or congenital disorder. She had been relieving her neurologic pain with painkiller for over ten years. She also received Nd-Yag laser toning (1064 nm, 1.5 J/cm2) for 8 times to treat hyperpigmented lesion. However, she did not receive surgical flap or fat graft for facial atrophy.
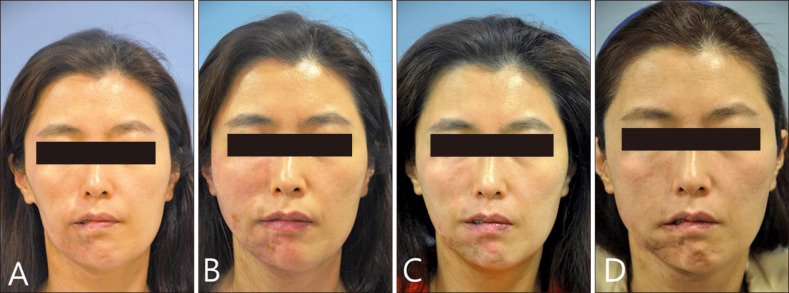
Fig. 1. Frontal views of the patient. At first visit, the patient shows overall atrophy of subcutaneous fat and hyperpigmentation on the right face (A). Immediately after injection by fanning technique. Total 7 ml of filler was injected (B). One month after the first filler injection, additional 1.5 ml hyaluronic acid (HA) filler was injected on the chin (C). One year after first injection, additional 3 ml HA filler was injected on the chin (D).
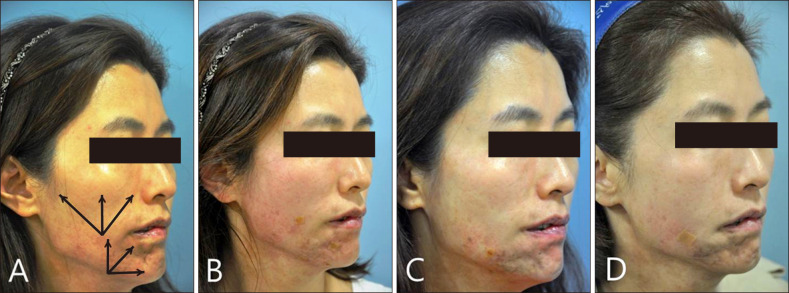
Fig. 2. Lateral views of the patient. At first visit, we planned to inject fillers in the direction of the arrows with fanning technique (A). After subcision with the cannula, total 7 ml of filler was injected via two holes on the cheek and chin (B). One month after the first filler injection (C). One year after first injection (D).
We choose a mixed form of filler (Restylane SubQ® [Q-Med, Uppsala, Sweden]: Restylane Vital® [Q-Med]=2:1), composed of HA, for soft tissue augmentation. And we made two puncture sites with 22 gauge needle on the right cheek and mandibular area. After local anesthesia, HA Filler was injected via 23 gauge cannula pulling the needle back. A total of 7 ml of filler was used: 4 ml of HA filler was injected into the cheek, and the remaining 3 ml was injected into the mandibular area (Fig. 1B, 2B). Because the adhesion between facial skin and subcutaneous fat was too tight to inject HA filler, we made a split space by subcision with a cannula before HA filler injection. A part of depressed area would need to be recontoured by second filler injection, however the patient was satisfied with cosmetic result and did not want to undergo another painful subcision. So we finished injecting further HA filler. It only took about 30 minutes for the procedure overall. One month after the first injection, additional 1.5 ml of HA filler was injected using fanning technique on the chin (Fig. 1C, 2C). After 1 year later, additional therapy was done with 3 ml of HA filler, significant improvement of contour of left chin was seen (Fig. 1D, 2D). Two months later, she did not show any signs of atrophy, and she was satisfied with the procedure. Also, there were no adverse events such as bruising, tenderness, overcorrection, chewing discomfort, Tyndall effect, the occlusion of the vessel or foreign body reaction.
DISCUSSION
PRS or idiopathic hemifacial atrophy could be an extreme form of localized scleroderma7. PRS usually occurs on the trigerminal area with hyperpigmentation. However, because the PRS is rare, the etiology and pathogenesis are poorly understood. Besides, the patho-physiologic relevance between PRS and localized scleroderma remains unclear. However Tollefson and Witman8 reported that 28% of 54 patients with en coup de sabre and PRS have both diseases simultaneously. Prevalence of seizure and epilepsy are especially high in both. This significant overlap may explain the similarity of neurologic mechanism in scleroderma and PRS.
There are numerous PRS treatment options available from medical treatments to surgical techniques. However, medications such as topical steroid or calcipotriol, oral steroid, methotrexate, D-penicillamine and antimalarial drug are effective only at the progressive phase and may not be adequate to correct depression8. Most patients require only soft tissue augmentation although syndrome has varying grades of severity9. In the majority of reported cases, PRS has been treated with surgical flap or autologous fat transplantation3. However, there is no established surgical technique for treatment of PRS among free flap, latissimus dorsi flap, anteriolateral thigh adipofascial flap, pedicle flap, liposuction and fat graft. It would depend on the experience and preference of the physician. Furthermore, these treatments need complicated surgical skills, lots of instruments, several hours of surgery and long time for recovery. The physician should also consider the possibility of surgical complications such as graft failure, hematoma, infection, scar and the quality of life after flap or graft. Moreover, our patient was too thin (body weight, 45 kg) to obtain autologous donor fat. If the patient also had severe bony abnormality, we would definitely have considered surgical flap including bone augmentation and multiple stage treatment10. But the patient has no bony abnormality, thus finding the simplest and safest method is best for her.
These days, numerous fillers are widely used in cosmetic fields such as the correction of nasolabial fold or tear trough, rhinoplasty, forehead augmentation and other depressed scar. These fillers can also be applied for depression of PRS. Until now, two PRS cases treated by fillers have been reported and one was with calcium hydroxylapatite (CaHA) and the other was with permanent polyacrylamide hydrogel (PAAG) filler7,11. CaHA has a dramatic volumizing effect. After injection, the gel carrier is promptly spread out into adjacent tissue, and the remaining CaHA stimulates secondary collagen generation7. A nonabsorbable volumetric PAAG filler is composed of 97.5% water and 2.5% cross-linked PAAG. Like CaHA, PAAG induces formation of internal scaffold vessel bearing connective tissue11. It has longer lasting effect than HA. However, if the complications like unintended recontour or compression of adjacent vessel or nerve occur, there are no eliminators available for CaHA or PAAG whereas hyaluronidase can be used to break down HA in case of side effects.
HA product is an absorbable, non-permanent filler and have replaced other implant materials as the standard filler12. Because it shows a good safety profile and can be hydrolyzed by hyaluronidase, it had been used for facial fat atrophy in HIV-positive patients13,14. HA has identical chemical composition in all species and tissues15. Furthermore Restylane® (Q-Med) is an non-animal stabilized hyaluronic acid produced by fermentation of streptococcal bacteria15. Thus it has less antigenic effect and is thought to induce less hypersensitivity reaction than animal source products13. Because Restylane SubQ® (Q-Med) has larger gel particle size (approximately 1,000 particles/ml) than Restylane® (Q-Med), it is more suitable for contouring the cheeks and the chin15. Also for the fine volumizing effect in upper dermal layer, we mixed Restylane SubQ® (Q-Med) with Restylane Vital® (Q-Med). Generally, Restylane Vital® (Q-Med), which has the smallest particle size among all of the Restylane® (Q-Med) fillers, has been used for facial hydrofilling or hydrolifting in Korea16.
In conclusion, HA filler has been widely used by dermatologists, which means that many dermatologists would prefer HA filler rather than flap, graft or other filler material. So, we suggest that HA filler will be the simplest and safest way for the correction of atrophy in PRS patient.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Hunstad JP, Shifrin DA, Kortesis BG. Successful treatment of Parry-Romberg syndrome with autologous fat grafting: 14-year follow-up and review. Ann Plast Surg. 2011;67:423–425. doi: 10.1097/SAP.0b013e31820b3aa8. [DOI] [PubMed] [Google Scholar]
- 2.Balaji SM. Subdermal fat grafting for Parry-Romberg syndrome. Ann Maxillofac Surg. 2014;4:55–59. doi: 10.4103/2231-0746.133081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agostini T, Spinelli G, Marino G, Perello R. Esthetic restoration in progressive hemifacial atrophy (Romberg disease): structural fat grafting versus local/free flaps. J Craniofac Surg. 2014;25:783–787. doi: 10.1097/SCS.0000000000000831. [DOI] [PubMed] [Google Scholar]
- 4.Alencar JC, Andrade SH, Pessoa SG, Dias IS. [Autologous fat transplantation for the treatment of progressive hemifacial atrophy (Parry-Romberg syndrome: case report and review of medical literatute)] An Bras Dermatol. 2011;86(4 Suppl 1):S85–S88. doi: 10.1590/s0365-05962011000700022. Portuguese. [DOI] [PubMed] [Google Scholar]
- 5.Chang Q, Li J, Dong Z, Liu L, Lu F. Quantitative volumetric analysis of progressive hemifacial atrophy corrected using stromal vascular fraction-supplemented autologous fat grafts. Dermatol Surg. 2013;39:1465–1473. doi: 10.1111/dsu.12310. [DOI] [PubMed] [Google Scholar]
- 6.Myung Y, Lee YH, Chang H. Surgical correction of progressive hemifacial atrophy with onlay bone graft combined with soft tissue augmentation. J Craniofac Surg. 2012;23:1841–1844. doi: 10.1097/SCS.0b013e318264b0ab. [DOI] [PubMed] [Google Scholar]
- 7.Cox SE, Soderberg JM. Idiopathic hemifacial atrophy treated with serial injections of calcium hydroxylapatite. Dermatol Surg. 2010;36:542–545. doi: 10.1111/j.1524-4725.2010.01499.x. [DOI] [PubMed] [Google Scholar]
- 8.Tollefson MM, Witman PM. En coup de sabre morphea and Parry-Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol. 2007;56:257–263. doi: 10.1016/j.jaad.2006.10.959. [DOI] [PubMed] [Google Scholar]
- 9.Chai G, Tan A, Yao CA, Magee WP, 3rd, Junjun P, Zhu M, et al. Treating Parry-Romberg syndrome using three-dimensional scanning and printing and the anterolateral thigh dermal adipofascial flap. J Craniofac Surg. 2015;26:1826–1829. doi: 10.1097/SCS.0000000000001903. [DOI] [PubMed] [Google Scholar]
- 10.Yu-Feng L, Lai G, Zhi-Yong Z. Combined treatments of facial contour deformities resulting from Parry-Romberg syndrome. J Reconstr Microsurg. 2008;24:333–342. doi: 10.1055/s-2008-1080536. [DOI] [PubMed] [Google Scholar]
- 11.Al-Niaimi F, Taylor JA, Lyon CC. Idiopathic hemifacial atrophy treated with permanent polyacrylamide subdermal filler. Dermatol Surg. 2012;38:143–145. doi: 10.1111/j.1524-4725.2011.02241.x. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Park KY, Yeo IK, Cho SY, Ah YC, Koh HJ, et al. Investigation of the degradation-retarding effect caused by the low swelling capacity of a novel hyaluronic Acid filler developed by solid-phase crosslinking technology. Ann Dermatol. 2014;26:357–362. doi: 10.5021/ad.2014.26.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bugge H, Negaard A, Skeie L, Bergersen B. Hyaluronic acid treatment of facial fat atrophy in HIV-positive patients. HIV Med. 2007;8:475–482. doi: 10.1111/j.1468-1293.2007.00494.x. [DOI] [PubMed] [Google Scholar]
- 14.Becker M, Balague N, Montet X, Calmy A, Salomon D, Toutous-Trellu L. Hyaluronic Acid filler in HIV-associated facial lipoatrophy: evaluation of tissue distribution and morphology with MRI. Dermatology. 2015;230:367–374. doi: 10.1159/000379747. [DOI] [PubMed] [Google Scholar]
- 15.Verpaele A, Strand A. Restylane SubQ, a non-animal stabilized hyaluronic acid gel for soft tissue augmentation of the mid- and lower face. Aesthet Surg J. 2006;26(1s):S10–S17. doi: 10.1016/j.asj.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Lee BM, Han DG, Choi WS. Rejuvenating effects of facial hydrofilling using restylane vital. Arch Plast Surg. 2015;42:282–287. doi: 10.5999/aps.2015.42.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]