Abstract
Skeletal muscles have the intrinsic ability to regenerate after minor injury, but under certain circumstances such as severe trauma from accidents, chronic diseases or battlefield injuries the regeneration process is limited. Skeletal muscle regenerative engineering has emerged as a promising approach to address this clinical issue. The regenerative engineering approach involves the convergence of advanced materials science, stem cell science, physical forces, insights from developmental biology, and clinical translation. This article reviews recent studies showing the potential of the convergences of technologies involving biomaterials, stem cells and bioactive factors in concert with clinical translation, in promoting skeletal muscle regeneration. Several types of biomaterials such as electrospun nanofibers, hydrogels, patterned scaffolds, decellularized tissues, and conductive matrices are being investigated. Detailed discussions are given on how these biomaterials can interact with cells and modulate their behavior through physical, chemical and mechanical cues. In addition, the application of physical forces such as mechanical and electrical stimulation are reviewed as strategies that can further enhance muscle contractility and functionality. The review also discusses established animal models to evaluate regeneration in two clinically relevant muscle injuries; volumetric muscle loss (VML) and muscle atrophy upon rotator cuff injury. Regenerative engineering approaches using advanced biomaterials, cells, and physical forces, developmental cues along with insights from immunology, genetics and other aspects of clinical translation hold significant potential to develop promising strategies to support skeletal muscle regeneration.
Keywords: skeletal muscle regeneration, biomaterials, animal models, cell therapy, small molecules
Lay Summary
Skeletal muscle has robust regeneration properties, but in extreme conditions, the regeneration ability is hindered. It remains a common clinical problem that could lead to long-term disability. The available treatments such as muscle flap transposition present various limitations. To address these limitations, promising strategies based on regenerative engineering are being developed. This review article discusses the different approaches to tissue regeneration using the regenerative engineering paradigm. A specific discussion involves biomaterials and their interactions with cells and bioactive molecules. In addition, the advantages of physical and mechanical stimulation in muscle regeneration are discussed.
1. Introduction
Skeletal muscle is a critical component of the human body, consisting of 40– 45% of an adult’s body mass[1]. It is mainly responsible for generating forces which facilitate voluntary movement and locomotion[2]. When moderate injury occurs, skeletal muscle has the robust ability to regenerate after minor injury through the activation of muscle progenitor cells, also termed as satellite cells[3]. These satellite cells play a vital role in muscle regeneration as they not only participate during myogenesis but can also undergo a self-renewal process to maintain an undifferentiated population in the tissue niche[4]. The mechanism is that in response to injury, dormant satellite cells are activated. They will undergo proliferation and subsequently differentiate into myoblasts and further fuse to form multinucleated myofibers. These myofibers will then integrate into the functional muscle tissue[5].
However, there is a limit to regeneration by satellite cell activation. Such mechanisms fail for example when large volumes of muscle are lost due to trauma or chronic disease. The current standard approach for treating volumetric muscle loss involves either tissue debridement or muscle transposition. Both procedures present a host of issues such as donor site morbidity and incomplete recovery of pre-injury muscle strength and functionality[2]. Regenerative Engineering is a transdisciplinary approach that uses the Convergence of Advanced Materials Sciences, Stem Cell Sciences, Physics, Developmental Biology and Clinical Translation for the regeneration of complex tissues and organ systems. This emerging field has the potential to address muscle loss in moderate injuries with minimal to no scar formation and may also offer a regeneration strategy in the case of large volume muscle loss [6–10].
In this paper, we discuss cell therapy treatment for muscle repair and regeneration, as well as compare and contrast the benefits of employing different types of biomaterials. Biomaterials have the ability to protect loaded cells from direct exposure to the immune environment, provide three-dimensional structure, physicochemical properties, and mechanical cues to modulate regeneration process[5]. In addition, the review explores the incorporation of small molecules and drug release concepts for muscle regeneration strategies, and discusses the impact of physical forces such as mechanical and electrical stimulation on regeneration. Translational studies involving volumetric muscle loss model and rotator cuff muscle injury will be discussed. These studies examine the potential of regenerative engineering strategies to result in clinically meaningful results.
2. Cell Therapy
Cell therapy approaches for degenerative muscle diseases are based on the delivery of cells that can contribute to skeletal muscle regeneration and repair. The cells used for transplantation can either be genetically corrected autologous cells from the patient or allogeneic from healthy donors [11]. In this section, we review the various cells that have been investigated in a pre-clinical or clinical setting for the treatment of muscular dystrophies.
Muscular dystrophies are genetic myopathies, characterized by progressive muscle wasting and degeneration. The most common and severe form is Duchenne muscular dystrophy (DMD), which is caused by mutations in the dystrophin gene. Dystrophin is a structural component of myocytes and is responsible for the maintenance of muscle fiber integrity, mediation of cytoplasmic signaling cascades, and muscle functions [11]. The lack of functional dystrophin causes fragility of muscle fibers during contraction. Damaged fibers may be repaired by proliferation and fusion with activated proliferating progenitor cells called myoblasts. However, the repaired fibers still lack dystrophin, leaving them vulnerable to injury through successive contractions. This progressive and continuous faulty loop of degeneration and repair causes the myoblasts to rapidly senescence [12]. DMD patients lose mobility between 10 and 12 years of age and die from respiratory and/or cardiac failure by the third decade of life [13]. Unfortunately, there is still no cure for DMD. The most widely used animal model for DMD is the mdx mouse. The mdx mouse carries a null mutation of the dystrophin gene resulting in the absence of the protein and fragility and necrosis of the muscle. Due to the ease of breeding, genetic uniformity, cost-efficiency, and convenience for laboratory experiments the mdx mouse model is extensively used to study DMD [14].
2.1. Satellite cells/Myoblasts
Myoblasts were the first candidate cell types that were considered to treat muscular dystrophies. Myoblasts are satellite cell derived progenitors with the ability to generate skeletal muscle. Studies performed in mdx mice demonstrated that intramuscular injection of myoblasts resulted in their fusion with host cells to form new or hybrid fibers, thus restoring dystrophin expression[15]. These encouraging results led to a number of clinical trials in the 1990s, however, failed to show significant clinical benefit. The major factors contributing to this unfavorable outcome were poor cell survival, limited cell migration and immune rejection of the transplanted cells, which drastically reduced their engraftment [16]. Over the years, numerous studies have addressed these challenges and worked towards overcoming them. Increasing the number of transplanted myoblasts[17], improving their survival [18], migration and engraftment [19], and immunosuppression [20] have been some of the approaches utilized to improve the therapeutic potential of myoblast transplantation. A recent phase I/IIa clinical trial found that local injection of autologous myoblasts in the pharyngeal muscles of patients with oculo-pharyngeal muscular dystrophy (OPMD) is safe and efficient [21]. Myoblast cell therapy could be an effective treatment for localized and less extended muscular dystrophies. Therefore, myoblast cell therapy holds great promise as an effective treatment for localized and less extended muscular dystrophies.
2.2. Muscle derived stem cells (MDSCs)
Several other cell populations, other than satellite cells/myoblasts, from the muscle were subsequently investigated. Muscle derived stem cells are postnatal stem cells identified and distinguished from satellite cells by their relatively low level of commitment to the myogenic lineage [22,23]. These cells are isolated from the skeletal muscle by the preplate technique, which enriches the population of slowly adhering MDSCs by eliminating the populations of more adherent cell types [24]. These cells offer several advantages over myoblasts such as high proliferative and self-renewal capability, multi-potency, and immune-privileged behavior resulting in an overall improved transplantation efficacy compared to myoblasts/satellite cells [23,25]. Notably, a population of poorly adherent MDSCs (known as MuStem cells) has been shown to contribute to myofiber regeneration, satellite cell replenishment, and long-term dystrophin expression in golden retriever muscular dystrophy dogs (the clinically relevant large model of DMD). When systemically injected, these cells contributed to partial remodeling of the skeletal muscle and enhanced regeneration resulting in a persistent stabilization of the dog’s clinical status and major improvement in locomotion features [26,27]. There have been reports identifying the human analog of these muscle derived cells [28,29]. However further studies will be needed before they can be evaluated for their therapeutic potential.
2.3. CD133+ cells
CD133 is a transmembrane protein expressed on a subpopulation of circulating human hematopoietic/endothelial progenitors. Torrente et al. showed that a subpopulation of CD133+ circulating cells express early myogenic markers. When injected systemically or intramuscularly in scid/mdx mice, they were found to contribute to muscle regeneration, replenishment of the satellite cell pool, and recovery of force [30]. Furthermore, their local injection appears to accelerate skeletal muscle regeneration through increased vasculogenesis [31]. CD133+ cells have also been isolated from human skeletal muscle. When injected intramuscularly in immunodeficient mice, these cells were more efficient in regenerating skeletal muscle than human myoblasts mainly due to their greater migratory potential and contribution to the satellite cell pool [32]. In addition, a double-blinded phase I clinical trial assessing the safety of autologous transplantation of human muscle derived CD133+ stem cells in DMD patients found no local or systemic side effects [33].
2.4. Mesoangioblasts
Mesoangioblasts are vessel-associated stem cells that can differentiate into several mesoderm cell types including skeletal muscle [34]. In inflammatory conditions, such as in DMD, they have been shown to cross the vessel wall and colonize dystrophic muscles. This makes them cell candidates to be delivered systemically. Intra-arterial delivery of murine and canine mesoangioblasts has been shown to produce morphological and functional improvements in models of muscular dystrophy [35–38]. The human equivalent of mesoangioblasts express markers of pericytes, a subpopulation of muscle-resident cells. Human mesoangioblasts have also been shown to cross the vessel wall and participate in muscle regeneration in dystrophic muscles, restoring dystrophin expression [36]. Genetically corrected mesoangioblasts/pericytes isolated from DMD models have shown to produce dystrophin-positive muscle fibers in vivo [39,40]. Based on these pre-clinical studies, a phase I/II clinical trial for intra-arterial delivery of HLA-identical allogeneic mesoangioblasts to patients with DMD under immunosuppression was performed. The study proved to be relatively safe; however, minimal efficacy was observed and the outcome was inconclusive in terms of muscle function. Further studies are needed to determine the clinical benefit of mesoangioblasts for DMD patients [41].
2.5. Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells isolated from several sources such as the bone marrow [42–47], synovial membrane [48,49], and umbilical cord [50], and adipose tissue have been identified for their myogenic potential. However, the ability of bone marrow MSCs to contribute to muscle regeneration and improve muscle function in vivo has not been conclusively established and requires further studies [43,45].
On the other hand, adipose derived mesenchymal stem cells (ADSCs) isolated from the adipose tissue are considered as one of the most promising and progressing stem cell populations for tissue regeneration. ADSCs offer several advantages for cell therapy. They can be easily isolated from an accessible source of adipose tissue with little patient discomfort or donor site morbidity and expanded in large quantities [53] and have been shown to have multipotent differentiation capacity [54], immunomodulatory properties, and anti-inflammatory and paracrine effects [55]. Rodriguez et al. were the first to report on the use of multipotent adipose-derived stem cells (hMADs) for skeletal muscle regeneration in vivo. When injected into mdx mice, hMADs were able to restore dystrophin expression but failed to form myotubes [56]. Their myogenic potential can be improved by forced expression of MyoD; however, the clinical safety of lentiviral vectors has yet to be fully proven [57]. Adipose tissue derived cells have also been shown to promote regeneration in other types of muscular disorders such as hind-limb ischemia, limb girdle muscle dystrophy (LGMD) or collagen VI-related muscle disorder [58–60]. Furthermore, human adipose derived stromal cells have been shown to migrate, engraft and differentiate into muscle cells expressing dystrophin when administered intravenously into GRMD dogs, without immunosuppression [61]. The immuno-privileged behavior of adipose tissue derived cells could be a significant clinical advantage for allogeneic cell therapy. The unique traits of MSCs such as anti-inflammatory and immunomodulatory properties[51], availability from various tissues, and trophic effects that indirectly have a positive influence on repair and regeneration[52], makes them potential candidates for muscle cell therapy.
2.6. Embryonic stem (ES) cells and induced pluripotent cells (iPSCs)
Pluripotent ES cells have the ability to differentiate into cells of all three germ layers and generate large numbers of specific cell populations, possibly overcoming the major obstacle of obtaining sufficient numbers of cells for cell therapy. There have been a number of studies focused on deriving muscle precursor cells from ES cells that would be suitable for [62–68]; however, given the ethical concerns surrounding the use of ES cells, attention has shifted towards using iPSCs. Studies have reported on the successful generation of human and mice iPSC-derived myogenic progenitors[2,69]. Darabi et al. demonstrated that the myogenic progenitors generated from human iPSCs can be obtained in large quantities and efficiently engraft into the dystrophic muscle of mdx mice, restoring dystrophin expression for over 11 months [66]. Furthermore, transplantation of patient-derived genetically corrected iPSCs offers the possibility of autologous cell therapy, reducing concerns of immune rejection and donor availability. Tedesco et al. transplanted genetically corrected iPSC-derived progenitors from patients with LGMD into mice of the same disease and found functional amelioration of the dystrophic phenotype and restoration of the depleted progenitors[70]. Despite these promising results, there are safety and efficacy issues that still need to be fully addressed before these cells can be used clinically. The development and application of standardized protocols to safely generate iPSCs and iPSC-derived myogenic progenitors, together with screening for genome stability, tumorigenic potential, transgene expression, and immunological studies will be necessary to further advance the use of these cells.
2.7. Challenges and Future Directions for Cell-Based Muscle Therapies
Considerable progress has been made in the field of cell therapy for muscular disorders. A number of cells have been investigated for pre-clinical and clinical use offering invaluable insight into the biology and reparative mechanisms of skeletal muscle. Nonetheless, further studies are required to better understand the inflammatory, fibrotic, and immune responses. The skeletal muscle is the largest organ in the body that is systemically affected in degenerative muscular disorders. A successful cell-based treatment would require systemically delivering a large number of cells that are capable of targeting and migrating towards the compromised muscle groups as opposed to local intramuscular injections. Multiple cell types are being explored for cell therapy with each holding great therapeutic promise. The optimal cell candidate would need to be highly proliferative, and have high survival, homing, and engraftment abilities. Therefore, it is instrumental to optimize cell expansion and transplantation conditions, and determine the mechanisms of migration and homing to ensure efficient regeneration. Moreover, although this review focused on cell therapy for the treatment of degenerative muscular diseases, the use of combinatory therapeutic strategies such as gene therapy and/or pharmacotherapy may be pivotal to augmenting the regenerative outcome.
3. Delivery of Bioactive Factors in Skeletal Muscle Regenerative Engineering
In skeletal muscle regeneration, signaling molecules play an important role in guiding the differentiation and proliferation of satellite cells into myoblasts, and eventually to multinucleated myofibers [71]. A common strategy in musculoskeletal regenerative engineering is the delivery of bioactive factors to stimulate the development of engineered tissue [11]. These signaling molecules can be incorporated within biomaterial-based scaffold systems to enhance the proliferation and differentiation of stem cells and progenitor cells. Based on the properties of each factor and molecule, different encapsulation techniques may be used to modulate the release profile and maximize their desired therapeutic effect [72]. Two standard approaches are physical encapsulation, where the molecules are embedded within the biomaterial matrix during the scaffold fabrication process, and chemical immobilization, where the bioactive factors are adsorbed or covalently bonded onto the surface of the structure [73,74].
3.1. Growth Factors
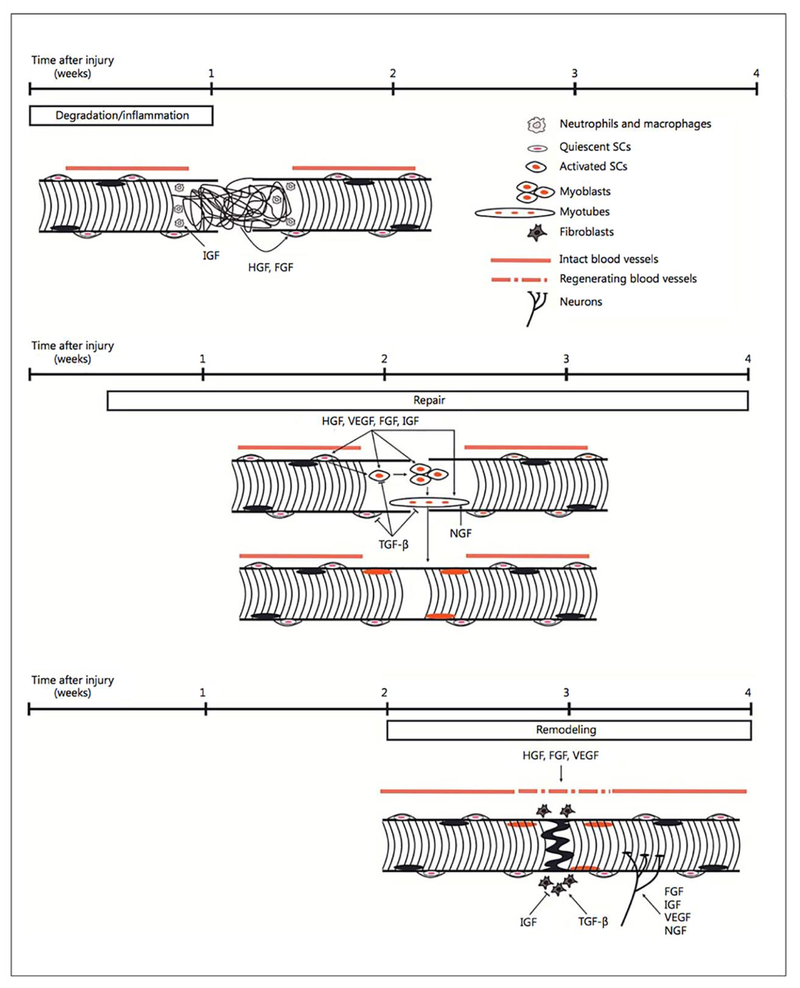
Growth factors are signaling proteins that through interaction with certain binding receptors, activate signal transduction pathways [73,8]. Major growth factors present throughout the skeletal muscle regeneration process include hepatocyte growth factor (HGF), insulin-like growth factor (IGF), fibroblast growth factor (FGF), transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VEGF) [75–77]. (Fig. 1) illustrates the contribution of these growth factors during each stage of muscle regeneration [75].
Figure 1.
Schematic representation of growth factor contribution to the three stages of muscle regeneration. The black and orange ovals represent the nuclei of the pre-existing and regenerated skeletal muscle fibers respectively. Adapted from Ref [75] with permission from Elsevier.
Studies have shown the capability of incorporating growth factors within engineered skeletal muscle constructs to enhance regeneration both in vitro and in vivo. TGF-β1 plays an important role in myogenesis through regulating satellite cell proliferation and differentiation, as well as promoting endothelial cell activity [77,78]. Weist et al. investigated the effect of TGF-β1 in improving the functionality of 3D skeletal muscle constructs. The muscle construct was derived from an adult rat tail tendon seeded with satellite cells [79]. At a TGF-β1 concentration of 2.0 ng/mL, a significant increase in the differentiation, contractility, and extracellular matrix deposition by the cells was observed. Studies have also shown that HGF and FGF-2 signaling play an important role in stimulating satellite cell activation and proliferation, and when delivered in unison, the therapeutic effects are significantly improved [80–82]. Hill et al. were able to develop an HGF and FGF-2 dual delivery system using G4RGDSP peptide modified alginate polymer scaffolds in order to study their effects on myoblast migration and survival in vitro [18]. HGF and FGF-2 (250 ng) were added to the alginate solutions and subsequently gelled to form the scaffold. The percent viability and migration of the myoblasts at day 5 within the peptide-modified scaffolds were significantly higher than the control and the HGF/FGF-2 alone groups. Similarly, VEGF has been studied as a potential factor to stimulate neoangiogenesis and vascularization[83,84]. Analogous to HGF, IGF-1 can be utilized to trigger satellite cell differentiation and proliferation through the PI3K/Akt and Ras/Raf-1/ERK MAP kinase signaling pathways [85,86]. Borselli et al. explored the synergetic capability of delivering VEGF and IGF-1 to promote the repair of injured muscle within the ischemic hindlimbs of mice [87]. The mice were injected with 50 μL of alginate gel containing 3 μg of VEGF and/or IGF-1 either alone or in combination. Subsequent data analysis illustrated that the sustained delivery of both growth factors restored the functionality of the skeletal muscle, with a reduction in ischemia.
3.2. Small Molecules
The capability of harnessing the therapeutic potential of small molecules for musculoskeletal regenerative engineering applications has also raised interest [13]. Small molecules are organic compounds with a molecular weight less than 1000 Da. They are able to rapidly diffuse through the cellular membrane and regulate certain biological processes [88]. The inherent properties of these small molecules have resulted in improved stability, low production cost, and lower levels of immunogenic response compared to their growth factor counterparts [89,90].
Several small molecules have gained a significant level of interest within the realm of skeletal muscle regeneration. Sphingosine 1-phosphate (S1P) is a sphingolipid biomolecule responsible for the modulation of several functions in muscle progenitor cells varying from the differentiation of myoblasts to the motility and division of satellite cells [91,92]. Danieli-Betto et al. studied the in vivo activity of S1P in skeletal muscle repair through injecting a 50 μM concentration of S1P into the damaged soleus muscle of rats and mice [93]. A significant increase in the cross-sectional area of the myofibers and the expression of MyoD and myogenin, were observed in the treated muscle tissues [94]. Retinoic acid (RA), a derivative of vitamin A, was studied to understand their ability to differentiate stem cells to a myogenic lineage. Kennedy et al. treated P19 and mouse embryonic stem cells with RA. [95,96] Significant increase in the expression of MyoD, myogenin, Meox1, and Pax3 was observed in the treated embryonic stem cells, implying the potential of RA as a therapeutic option in skeletal muscle regeneration. Lee et al. used a combination approach to screen the skeletal muscle regeneration potential of a range of small molecules [97,98]. Two small molecules, skeletal muscle inducer 1 and 2 (SMI 1 and 2), has been identified due to their ability to enhance Pax3 expression through the upregulation of the Wnt and suppression of the Shh and Smad 2/3 signaling pathways.
3.3. Challenges in the Usage of Growth Factors and Small Molecules for Skeletal Muscle Regeneration
In spite of pre-clinical and clinical success with growth factor mediated regeneration, certain limitations still exist. These include their high production cost, immunogenicity, supraphysiological dosage requirement, elevated market cost, and instability during the drug delivery formulation process [99,100]. In order to overcome these issues, alternative approaches geared toward the reduction of growth factor usage have been investigated. Of these options, small molecules have gained considerable interest due to their favorable intrinsic physical properties. However, due to the potential of non-specific side effects, the route of administration and dosage amount must be taken into account to maximize their pharmacological effect. Therefore, modulating the spatial control and release kinetics of these bioactive factors from advanced biomaterial delivery systems is key in their potential therapeutic capabilities.
4. Biomaterial-based strategies for skeletal muscle regeneration
Biomaterial-based strategies have progressed tremendously over the past few decades. The ultimate biomaterial needs to be biocompatible, biodegradable, have appropriate mechanical stability, long term in vivo functionality as well as to provide a conductive structure for cellular proliferation and differentiation [101]. A combination of both biomaterials and cells can potentially improve the therapeutic effects of cells for muscle regeneration [2].
4.1. Electrospun nanofiber scaffolds
It is highly desirable to engineer scaffolds that mimic the natural extracellular matrix (ECM) as they can provide structural support as well as regulate a variety of important cell functions such as assembling cells into various tissues and organs, regulating growth, and cell–cell communication[102]. Laurencin and his colleagues were the first to describe the use of electron nanofibers for tissue regeneration purposes [103]. Their work has continued to demonstrate the promise of the electrospinning technique and electrospun fibers for regeneration [104–112]. Electrospun nanofibrous scaffolds are beneficial because of their similarity to native muscle fibers [105]. For example, Choi et al. used aligned co-electrospun poly(ε-caprolactone) (PCL) and collagen nanofibers to evaluate their potential for skeletal muscle regeneration. The study showed the ability of the scaffolds to support alignment and myotube formation of skeletal muscle cells, implying the substrate’s potential to treat patients with large muscle losses [113]. This was further demonstrated by Aviss et al. using aligned poly(lactic-co-glycolic acid) (PLGA) nanofiber scaffolds seeded with C2C12 murine myoblasts. The myoblasts not only showed aligned morphology but also had significantly higher expression of muscle differentiation markers such as fast myosin heavy chain compared with random oriented fibers [114]. In addition to substrate alignment, electrical cues have also shown to promote myotube formation. Electrically conductive random and align matrices were prepared by adding different weight percentages of polyaniline (PANi) into PCL. The study showed that substrate alignment along with electrical stimulation can favorably affect skeletal muscle regeneration [115,116].
4.2. Hydrogels
Hydrogels are prepared from both natural and synthetic polymers with high water content [117]. They have been extensively used in the biomedical field [118,119]. Rossi et al. used an in situ photo-cross-linkable hyaluronan-based hydrogel to deliver satellite cells or muscle progenitor cells to treat partially ablated tibialis anterior (TA) mice. Results showed a major improvement when the injured tibialis was treated with freshly isolated satellite cells encapsulated in the hydrogels. Alginates cannot specifically interact with mammalian cells. Rowley et al. studied the effect of hydrogel chemistry. Alginates with different monomeric ratios were used to covalently bond RGD-containing cell adhesion ligands. RGD has been extensively incorporated in synthetic non-biological systems without cell adhesion motifs to increase cell adhesion. The study showed a significant effect of the alginate monomeric ratio or the density of RGD ligands attached at the surface of substrate on myoblast proliferation and differentiation [101].
Using alginate scaffolds, Borselli et al. showed that transplanted myoblasts combined with localized delivery of VEGF and IGF-1 could enhance muscle regeneration and angiogenesis [120]. Nichol et al. demonstrated that gelatin methacrylate (GelMA) was beneficial in creating cell-responsive microtissues, such as endothelialized microvasculature. The hydration and mechanical properties of GelMA can be tuned via methacrylation degree and gel concentration making it a suitable hydrogel for various applications[121]. Another study investigated the role of biomaterials as mechanically competent matrices for skeletal muscle. The matrix was prepared by encapsulating different proteins (collagen I/fibrin/Matrigel) with neonatal rat skeletal muscle cells to form bundles. This resulted in an increased contractile force derived mainly from cell-matrix interactions. In summary, biomaterials play a pivotal role in modulating cellular response for effective skeletal muscle regeneration [122,123].
4.3. Patterned scaffolds
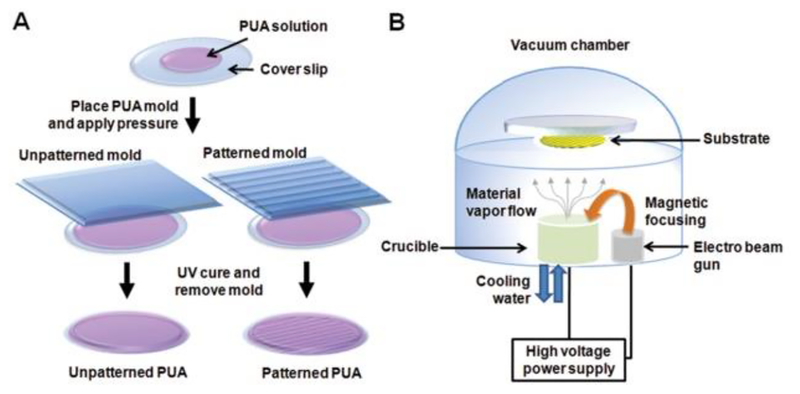
In addition to the different types of materials, cell behavior can also be altered via substrate surface features [124]. Patterning is a popular technique to modify biomaterial surface properties. It generally involves the use of an elastomeric master such as PDMS, which is easy to mold or emboss and can be used directly as a substrate for biomedical applications [5]. A unique study by Yang et al. showed that muscle derived cells cultured on gelatin-coated nanopatterned PLGA substrates showed enhanced myogenic maturity in vivo compared to cells grown on flat PLGA substrates after 4 weeks of transplantation [125]. The group further demonstrated that by combining electrical and topographical cues, nanopatterned electroconductive substrates created by capillary force lithography can mimic highly aligned collagen bundles from the ECM of skeletal muscle tissue. The combination of both topographical and electrical cues can enhance myogenic differentiation and maturation [126]. Various types of materials such as polystyrene [127], agarose [128], and methacrylated gelatin [129] have been fabricated with these topographical cues and have been shown to influence skeletal muscle formation. For example, Yang et al. used electron beam deposition (Fig. 2) to achieve a thin layer of either gold or titanium on nanopatterned substrates to create electroconductive substrates with thin layers of either gold or alumina. It was found that biomimetic nanotopography combined with these coatings promotes myogenic differentiation and maturation [126]. Together, they all play an important role in the formation of functional myotubes in vitro [130,131]. Patel et al. used acid catalyzed sol-gel process to produce silica coating on CNT-grafted hierarchical substrates. The generated CNT carpets were deposited on both interconnected microporous carbon foams as well as aligned carbon fiber mats. The results showed that the foam structure did not support myotube formation whereas the aligned carbon mats supported myocyte fusion to form multinucleated myotubes. The study showed the specific effects of nanostructure in modulating cell behavior [132].
Figure 2.
A schematic illustration of the fabrication of A) unpatterned and patterned PUA substrates and B) electron beam evaporation deposition of Au and Ti onto PUA substrates [126]. Adapted from Ref [126] with permission from Elsevier.
4.4. Decellularized tissues
Biologic scaffolds that are composed of ECM have been used to reinforce or replace damaged tissues in clinical applications [133]. Decellularized ECM provides a promising alternative to synthetic scaffolds and a foundation for regenerative efforts[134]. Immobilization of ECM molecules on synthetic matrices has also shown to favorably modulate cellular performance [135]. Decellularized tissues are able to maintain their ECM structure as well as their vascular network and have mechanical properties resembling native tissue [2]. In a study done by Wolf et al., a decellularized skeletal muscle ECM scaffold was shown to provide bioactive compounds such as growth factors, glycosaminoglycans, and basement membrane structural proteins that are typically missing from non-muscle ECM derived from the small intestine (SIS). The decellularized skeletal muscle ECM was shown to support myogenic cell proliferation in vitro. In vivo implantation in a rat abdominal wall injury model showed a constructive remodeling response along with scaffold degradation, even though it showed no statistical difference in remodeling outcome compared with SIS-induced ECM [136]. Another study by Ward et al. tested the hypothesis that muscle functional efficacy can be retained by using lesser amounts of autologous muscle grafts via a collagen hydrogel. The study showed that using approximately 50% of the minced graft, suspended in a collagen hydrogel, can improve muscle functionality similar to the 100% minced graft repair. More studies are required to identify optimal carrier materials for efficient regeneration [137].
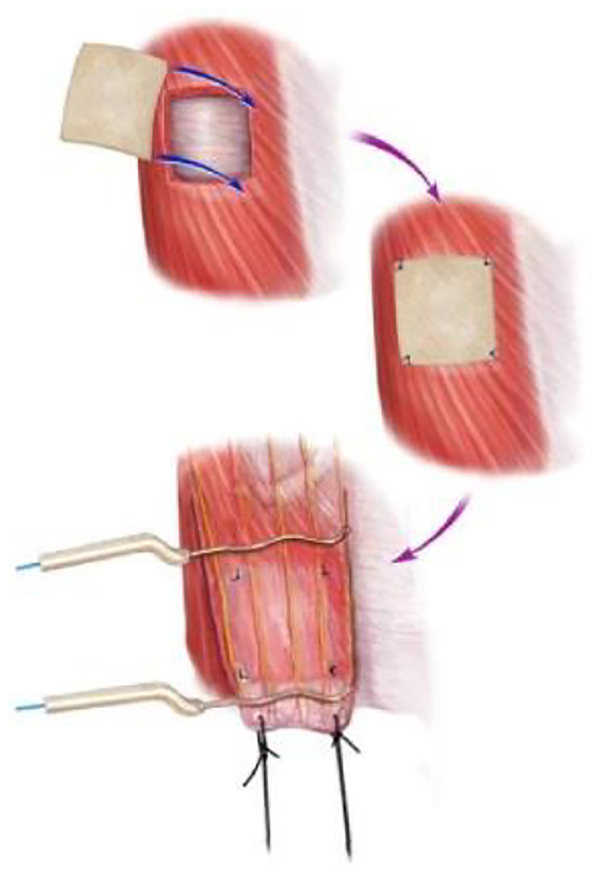
Valentin et al. tested four types of grafts in a rat injury model: porcine small intestinal submucosa (SIS)-ECM, carbodiimide-crosslinked porcine SIS-ECM, autologous tissue, and a polypropylene mesh. The graft (1.5 cm × 1.5 cm) was placed on the defect in the ventral lateral abdominal wall musculature (Fig. 3). The three biological scaffolds supported functional skeletal muscle reconstruction. The regenerated tissue did not mimic the native tissue however there was abundant vascularization and innervation. The regenerated muscle also showed the presence of types I and II muscle fibers [133].
Figure 3.
Representative image of a tissue flap. Top: the musculoskeletal defect was created by excising the external and internal oblique layers of the abdominal wall, leaving the transversalus fascia intact. Middle: the test article was implanted in the defect site and secured with Prolene sutures at each of the four corners. Bottom: twenty-six weeks post-implantation, a flap of tissue was created which contained the site of test article placement, identified by the preplaced Prolene sutures. The tissue flap maintained the integrity of the muscular arteries and the thoracic spinal nerve branches that supplied the site of tissue remodeling. The dense connective tissue at the insertion site at the linea alba was connected to the force transducer with silk suture and positioned such that the direction of the contractile function testing was aligned parallel to the rib origin. Platinum electrodes were placed across the flap proximal and distal to the scaffold placement site [133]. Adapted from Ref [133] with permission from Elsevier.
The creation of the very first tissue engineered muscle repair (TEMR) construct showed a significant improvement in enhancing myotube numbers and functionality. The TEMR construct was implanted in a two-month-old murine latissimus dorsi muscle injury model [128,129]. The bladder acellular matrix (BAM) was used as a matrix to culture muscle derived cells for 10 days allowing for cell growth and differentiation. The construct was then preconditioned in a bioreactor with a 10% uniaxial mechanical as described [138]. Another study investigated the role of preconditioning muscle derived cells with a uniaxial mechanical strain on decellularized ECM with TEMR-positive responders. The study demonstrated that the combination approach significantly increased functional recovery in skeletal muscle compared with the construct alone in VML injury. The study showed the greater regenerative capacity of using stem or progenitor cells in combination with a scaffold [140].
4.5. Electrically conductive materials
Electrically conductive biomaterials are considered as one of the most promising new generations of materials that can provide direct delivery of electrical, electrochemical or electromechanical stimulation from a substrate to cells [135,141]. With the development of biocompatible electrically conducting materials, several biomedical applications have been identified [135,142,143]. Polypyrrole [144–146], PANi [145,147], polythiophene and their derivatives [148,149] have been evaluated in bone, neural or skin regeneration. For example, Gilmore et al. studied the benefits of modified polypyrrole on skeletal muscle growth and differentiation. Polypyrrole was doped with the major components of the ECM such as hyaluronic acid (HA) and chondroitin sulfate A as well as non-biological active molecules such as dodecyl benzene sulphonic acid, and para-toluene sulphonic acid (pTS). The modified polymers supported moderate myoblast differentiation and myofiber formation. The various dopants affected the various stages in skeletal muscle myogenesis differently. It was found that the physical properties of the polypyrrole/ HA or polypyrrole/pTS films were better suited for facilitating the different stages in skeletal muscle myogenesis [150]. As mentioned in 4.1, electrical stimulation through the incorporation of PANi into poly(L-lactide-co-ε-caprolactone) (PLCL) electrospun fibers improved myoblast differentiation [115,116]. The presence of the conducting polymer significantly increased myotube maturity and the expression of myogenic genes such as myogenin, troponin T, and MHC [115,116].
Ahadian et al. incorporated different concentrations of multi-walled carbon nanotubes (MWCNTs) into gelatin fibers. Incorporation of MWCNTs improved the mechanical properties demonstrated by an increase in the Young’s modulus from 509 ± 37 kPa to 1077 ± 266 kPa and 1170 ± 168 kPa for gelatin nanofibers containing 0 mg/ml, 0.5 mg/ml, and 5 mg/ml MWCNTs, respectively. The stiffer matrices were subsequently shown to improve myotube formation and maturation due to the increased mechanotransduction in these substrates [151].
5. External stimulation to enhance muscle regeneration
Biophysical stimulation such as mechanical and electrical forces play an important role in modulating muscle cell growth and differentiation [152,153].
5.1. Mechanical stimulation to enhance myoblast differentiation
Mechanical stimulation can potentially be a very effective approach to support muscle regeneration [1]. Du et al. demonstrated the unique advantage of using appropriate mechanical forces to precondition primary human muscle cell scaffold constructs in vitro. The constructs were subjected to cyclic strain in a computer-controlled bioreactor system. The retrieved constructs generated tetanic and twitch contractile responses with a specific force of 1% and 10%, unlike the native tissue constructs, demonstrating the advantage of exogenous mechanical forces [154]. Vandenburgh et al. used a cell stimulator device to dynamically stretch embryonic skeletal muscle cells by maintaining the cells in a horizontal position during mechanical stretching for up to 400% of substratum length. The dynamic stretch increased cell proliferation and orientation. More importantly, it stimulated the myoblasts to fuse into robust and abundant arrays of myotubes, which were two to four times longer than those grown under static culture conditions [155].
Ultrasound has also been used as a physical force to enhance skeletal muscle regeneration. The EPI® technique is an ultrasound guided technique that can generate a galvanic current transmitted through an acupuncture needle. Abat et al. used the EPI technique to treat tendon muscle lesions in a rat model and observed a significant increase in the expression of anti-inflammatory and angiogenic proteins [157]. This shows the potential of mechanical stimulation in treating muscle injuries and enhancing regeneration.
5.2. Electrical stimulation of myoblast differentiation
Electrical signals play an essential role in the development, function, and repair of tissues and organs [158–160]. Various groups have investigated applying electrical stimulation to enhance cellular functions [160]. Overall the ability to enhance myotube formation and maturity has been demonstrated upon electrical stimulation [161,162]. Fujita et al. demonstrated robust and evident contractile activity of C2C12 cells when they were subjected to electrical stimulation (40 V/60 mm, 2 ms, 1 Hz) [161]. The study showed the efficacy of electrical stimulation to produce contraction-inducible myokines that could favorably modulate metabolic and immune responses, and angiogenesis [162]. In addition, other studies have also reported the ability of electrical stimulation to support muscle cell elongation and secretion of bioactive factors such as IGF-1 [163,164].
Langelaan et al. compared the efficacy of C2C12 cells with primary cell culture under electrical stimulation by utilizing a collagen type I/Matrigel™ hydrogel. Under electrical stimulation, both 2D and 3D culture accelerated sarcomere assembly formation. The primary cell source was preferred as they were more susceptible to the electrical stimulus [165]. Pedrotty et al. evaluated the effect of electrical current flux on the mitogenic activity of skeletal muscle derived cells in vitro. The cells were seeded on 3-D polyglycolic acid mesh scaffolds and were subjected to cardiac-like electrical current flux treatments, or conditioned media obtained from mature cardiomyocytes. The study showed that both electrical stimulation and cardiac-derived soluble factors could stimulate cell proliferation [166].
Burch et al. compared gene expression in electrically stimulated muscle cell culture with non-electrically simulated muscle cells. The electrically stimulated C2C12 cells showed qualitative transcriptional adaptations similar to those in trained muscle, but muscle gene expression differed from those with acute effects of exercise. It is therefore important to study the molecular mechanisms that regulate exercise adaptation in muscle [167]. Furthermore, Trumble et al. have shown that long-term stimulation of rabbit muscles could change muscle fiber types and remodel the ECM indicated by the presence of collagen type I and fibrillin [168]. Furthermore, the ability of chronic low-frequency stimulation to induce the formation of slow muscle fibers and enhance glucose uptake in skeletal muscle has also been demonstrated [149,169].
6. Volumetric Muscle Loss (VML)
Although skeletal muscle has the intrinsic ability to regenerate, massive defects such as volumetric muscle loss (VML) cannot be regenerated [170]. In a cohort of battlefield-injured soldiers, muscle conditions were found to account for more than 65% of the disabilities. Furthermore, 92% of the muscle conditions were identified as VML [171].
The current standard approach to treat muscle volumetric loss is to use functional free muscle transfer and advanced bracing [172]. Although it looks promising, the procedure is very complex and successful outcomes depend on the surgical team. Moreover, using free muscle transfer may not be viable in patients with combat extremity injuries [172]. Advances in biomaterial scaffolds, and stem cells suggest a promising solution. These materials can be surgically implanted at the site of VML, encouraging local muscle regeneration and improving function in clinical settings [173]. To this end, different animal models and various biomaterial scaffolds have been utilized to study VML [170,174].
6.1. VML animal model
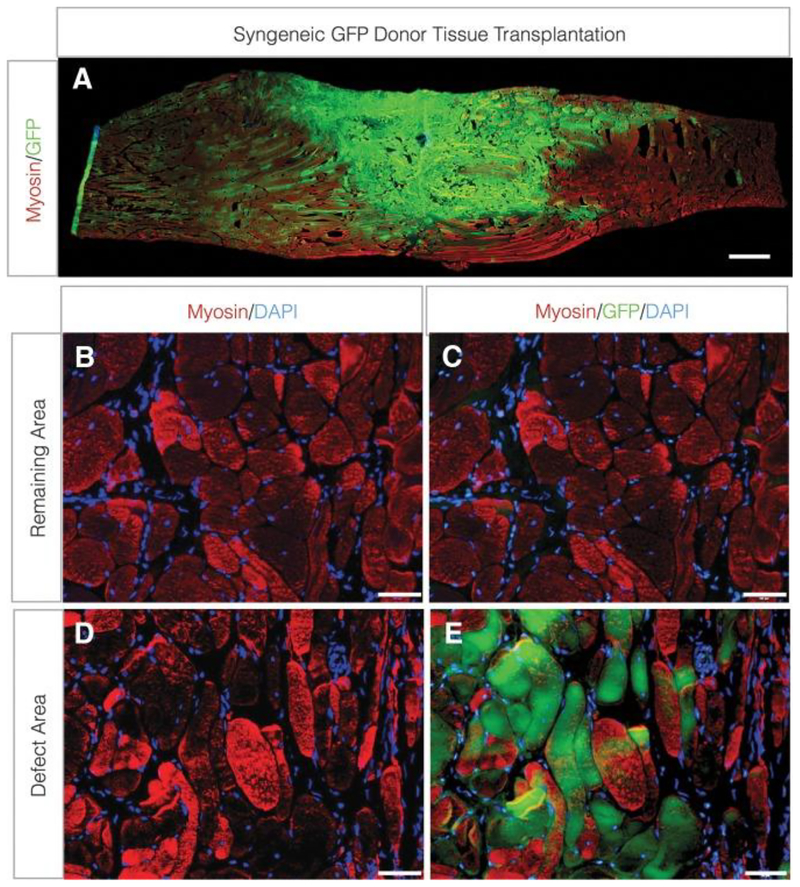
To mimic VML in animal models, the defect is achieved using an aseptic technique. In a murine model, the VML injury was created by surgically removing approximately 50% of the lattissimus dorsi muscle area [178]. Briefly, after a longitudinal incision along the midline of the back, the trapezius muscle that covers the lattissimus dorsi muscle was lifted. Then the medial half of the muscle was removed indicated by suture markers using a scissor [138]. In a rat model, the defect dimension (10 mm*7mm*3mm) was first labeled and removed using a scalpel from the medial and lateral margins of the muscle [175]. A surgical defect was created in the middle third of the TA muscle and a large portion of the estimated TA muscle weight was removed [173–176]. Corona et al. used three different BAM collagen scaffolds seeded with muscle derived cells (section 4.2) to create a TEMR construct in a mouse model. Based on the histological and molecular results, the TEMR constructs helped promote muscle regeneration at different rates and magnitudes. Functional recovery via regeneration of functional muscle fibers occurred either at the interface of the construct and the native tissue or within the BAM scaffold independent of the native tissue [177]. The same group further evaluated the efficacy of syngeneic muscle-derived ECM (mECM) seeded with bone marrow-derived MSCs in treating rat TA muscle in a VML injury. The study showed recovery of one-third of the original functional deficit using mECM two months post-surgery. Moreover, the presence of mECM decreased further muscle damage in the remaining muscle (Fig. 5). The study demonstrated the potential of biological ECM scaffolds in preventing prolonged overload of injured muscle [129].
Figure 5.
Contribution of donor-derived myogenic cells to de novo fiber regeneration after VML injury. TA muscle minced grafts derived from donor GFP-Lewis rats were transplanted to the site of VML injury at a 100% tissue replacement.
(A) Longitudinal (scale bar = 1.0 cm) and (B–E) cross-sections (scale bars = 50 lm) from the remaining muscle mass (B, C) and defect area (D, E) were probed for sarcomeric myosin and GFP co-localization. TA, tibialis anterior; VML, volumetric muscle loss [137] Adapted from Ref [129] with permission from Elsevier.
Badylak et al. used a canine model to demonstrate the efficacy of ECM-based scaffolds in treating VML in a large animal model. The study showed that an ECM-based scaffold helped promote functional restoration of the distal gastrocnemius musculotendinous junction after complete resection of the tissue [179]. Another study evaluated the efficacy of non-crosslinked ECM derived from porcine SIS in a canine model of complex quadriceps muscle injury. Although the initial remodeling appeared promising, at later stages there was fibrosis, dense collagenous tissue and a small portion of nonfunctional muscle. Further studies are required to understand the efficacy of these biological matrices in treating VML [178]. Sicari et al. evaluated ECM scaffolds using a mouse VML model and showed the ability of the matrix in supporting skeletal muscle formation as well as innervation. This was followed by a clinical study using this matrix in five patients with VML. Six months post-implantation, all patients showed signs of new muscle formation and vascular structures at the implantation site. Three of the five patients showed 20% or greater improvement in limb strength during physical therapy. The two patients without functional changes did report improvements in nonfunctional tasks, such as balance, as well as an improvement in quality of life. Because of the widespread availability and known safety of cell-free ECM-based materials, they hold promise as a candidate material in treating VML [173]. Despite the promising outcomes of ECM-based scaffolds, there are contradicting results in their usage [179]. This may be due to the differences in scaffold fabrication as well as surgical models (TA muscle vs. lateral gastrocnemius) [178].
7. Muscle atrophy in rotator cuff injury
Rotator cuff tear is one of the most frequent orthopedic conditions, and repair of rotator cuff tears is a common procedure [180,181]. With aging, the rotator cuff becomes more prone to degenerative tears. It was estimated that 40% of individuals aged over 60 suffer from rotator cuff issues. The repair failure rate ranges from 20% to 70%, thus resulting in a significant clinical challenge [182].
Tears that are greater than 4 cm are called massive rotator cuff tears (RCTs) which usually lead to atrophy and fatty infiltration in the supraspinatus and infraspinatus muscles [183]. This leads to poor recovery outcome due to the inelasticity and poor function of the muscle-tendon unit which can lead to shoulder dysfunction [184,185]. Gladstone et al. demonstrated that lower skeletal muscle quality, especially in the infraspinatus muscle region, negatively affects the outcome. It has also been shown that a successful rotator repair can significantly decrease the likelihood of fatty infiltration and muscle atrophy [186].
Overall, chronic RCTs lead to pain and suffering in patients [183]. So far studies have been focused on developing improved repair techniques [187] using biologic factors [188,189] or biomaterials [190,191] to improve tendon to bone healing. Implantation of aligned electrospun PCL scaffolds led to better cellular infiltration [192]. Thangarajah et al. used demineralized bone matrix (DBM) derived from cortical bone to treat female Wistar rats that underwent unilateral detachment of the supraspinatus tendon three weeks post-surgery. Compared to treatment with the commercially available GraftJacket, DBM led to a shorter gap between the tendon and bone. There was also a more organized structure with less abnormal collagen fibers. However, the use of DBM scaffolds did not improve the healing of the enthesis [191]. Peach et al. created hybrid polymer matrices by modifying the surface of PCL nanofibers with polyphosphazene poly[(ethyl alanato)1(p-methyl phenoxy)1] phosphazene to improve matrix hydrophilicity for rotator cuff augmentation. Loading the scaffolds with bone-derived MSCs accelerated tendon remodeling in an acute rat rotator cuff injury model [193]. Zheng et al. used 3D aligned collagen/silk scaffolds in a rabbit tendon tear model. The aligned matrices improved cellular infiltration and enhanced tenogenic differentiation [194].
To study the progression of muscle atrophy, rats, rabbits and sheep models have been used. These models allow for a better understanding of the timely changes that take place in the supraspinatus muscle region after tendon detachment [194,195]. Tendon detachment leads to lack of loading and significant loss of muscle weight. Using a rat rotator cuff model, Barton et al. observed consistent fiber shift throughout the study, rapid loss of muscle volume as well as scar tissue formation in the muscle bed [196]. In another study on acute and chronic rotator cuff repair, it was shown that failure to repair the tendon at the time of tear will lead to continuous progression of fatty degeneration. This is commonly seen in some patients that receive rotator cuff repair [197]. Rowshan et al. used a rabbit denervation rotator cuff injury model and found that denervation worsens muscle atrophy and fatty degeneration. Six weeks post-surgery, the complete tear and complete tear with nerve transection groups had significantly lower muscle wet mass and increase in fat content [198].
In larger animal models, signs of muscle atrophy and fatty infiltration after injury are more obvious. In a sheep rotator cuff injury model, these symptoms occurred six months after the injury. The repaired group showed increased expression of muscle atrophy, fatty infiltration, and fibrosis related genes. This demonstrates the clinical importance of addressing fatty infiltration and fibrosis upon rotator cuff tear [199]. However, biomaterial-based strategies for improving muscle atrophy issue in rotator cuff injuries have not been well studied. Tang et al. investigated the potential effects of electrospun nanofibers containing the conducting polymer PEDOT: PSS to stimulate muscle regeneration, thereby suppressing fatty expansion [200].
8. Concluding remarks
Skeletal muscle injury, especially large muscle injuries and chronic degenerative diseases, remain a clinical concern. Muscle conditions can also be secondary but have a vital influence on the surgical outcome in cases such as in rotator cuff injury [196]. Various strategies are being investigated to treat muscle injury. This review discussed the use of regenerative engineering technologies for tackling the problem of skeletal muscle injury repair and regeneration. A variety of biomaterial scaffolds such as electrospun nanofibers, hydrogels, and decellularized tissues for muscle regeneration. Understanding stem cell science, and physical forces and there interactions with cells are important. The biomaterial scaffolds not only serve as vehicles to deliver cells, growth factors or small molecules but also act as substrates to control cellular functions to enhance muscle regeneration. Moreover, the muscle contractility and functionality can be significantly enhanced by using physical forces such as mechanical and electrical stimulation. It is critically important to focus future research efforts on developing novel biomaterials and understanding cellular responses towards these materials to develop translational approaches to skeletal muscle regeneration.
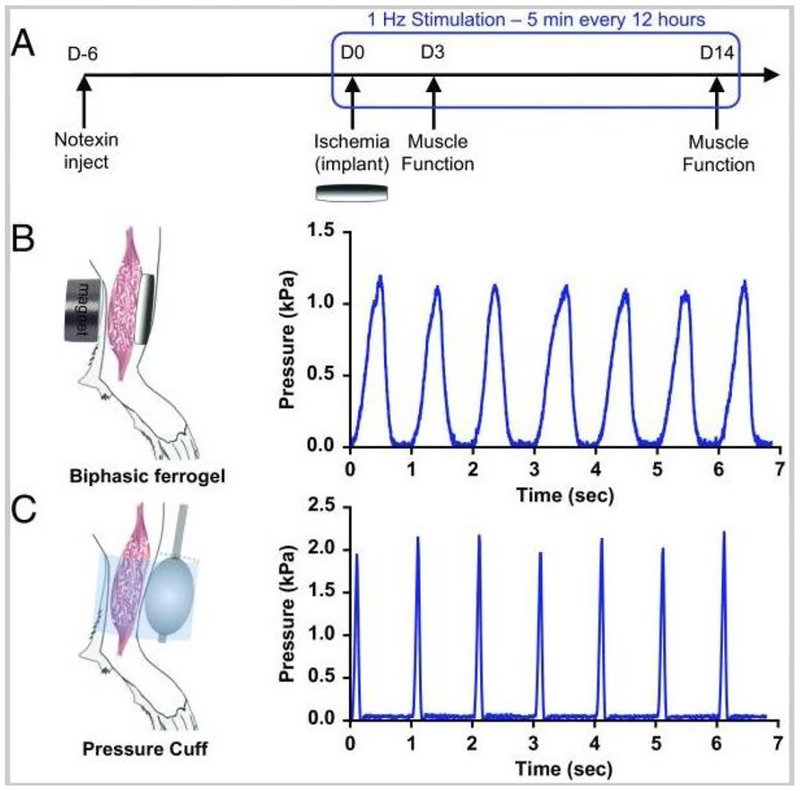
Figure 4.
Biphasic ferrogels and pressure cuffs generate cyclic mechanical compressions. (A) Experimental design showing injury, implant, and stimulation profile. (B) Schematic of biphasic ferrogel implant in mouse hind limb depicting the orientation of ferrogel relative to the skin, muscle tissue, and magnet (Left). Pressure profile of biphasic ferrogel undergoing repeated magnetic stimulations (Right). (C) Schematic of pressure cuff on mouse hind limb depicting the orientation of balloon and polycarbonate cuff relative to skin and muscle tissue (Left). Pressure profile of balloon cuff undergoing repeated inflations and deflations (Right). Adapted from Ref [156] with permission from Elsevier.
Acknowledgments
The authors would like to acknowledge the NSF EFRI 1332329 and NIH DP1AR068147 and NIH RO1 AR063698 for funding this work (C.T.L.). Dr. Laurencin is a recipient of the National Medal of Technology and Innovation.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- [1].Laumonier T, Menetrey J, Muscle injuries and strategies for improving their repair, J. Exp. Orthop 3 (2016) 15. doi: 10.1186/s40634-016-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Qazi TH, Mooney DJ, Pumberger M, Geißler S, Duda GN, Biomaterials Biomaterials based strategies for skeletal muscle tissue engineering : Existing technologies and future trends, Biomaterials. 53 (2015) 502–521. doi: 10.1016/j.biomaterials.2015.02.110. [DOI] [PubMed] [Google Scholar]
- [3].Brack AS, Rando TA, Tissue-specific stem cells: Lessons from the skeletal muscle satellite cell, Cell Stem Cell. 10 (2012) 504–514. doi: 10.1016/j.stem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shi X, Muscle stem cells in development, regeneration, and disease, Genes Dev. 20 (2006) 1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- [5].Ostrovidov S, Hosseini V, Ahadian S, Fujie T, Parthiban SP, Ramalingam M, Bae H, Kaji H, Khademhosseini A, Skeletal muscle tissue engineering: methods to form skeletal myotubes and their applications., Tissue Eng. Part B. Rev 20 (2014) 403–36. doi: 10.1089/ten.TEB.2013.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Laurencin CT, Khan Y, Regenerative engineering, Sci. Transl. Med 4 (2012) 1–3. doi: 10.1126/scitranslmed.3004467. [DOI] [PubMed] [Google Scholar]
- [7].Laurencin CT, Khan Y. Regenerative Engineering 1st ed. CRC Press; 2013 [Google Scholar]
- [8].Lo KWH, Jiang T, Gagnon KA, Nelson C, Laurencin CT, Small-molecule based musculoskeletal regenerative engineering, Trends Biotechnol 32 (2014) 74–81. doi: 10.1016/j.tibtech.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Laurencin CT, Nair LS, Regenerative engineering: approaches to limb regeneration and other grand challenges, Regen Eng Transl Med 1 (2015) 1–3. doi: 10.1007/s40883-015-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Laurencin CT, Nair LS, The quest toward limb regeneration: a regenerative engineering approach. Regen Biomater (2016) 123–125. doi: 10.1093/rb/rbw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Negroni E, Gidaro T, Bigot A, Butler-Browne GS, Mouly V, Trollet C, Invited review: Stem cells and muscle diseases: Advances in cell therapy strategies, Neuropathol. Appl. Neurobiol 41 (2015) 270–287. doi: 10.1111/nan.12198. [DOI] [PubMed] [Google Scholar]
- [12].B. P, J.P. T, Past, present and future of myoblast transplantation in the treatment of Duchenne muscular dystrophy, Pediatr. Transplant 14 (2010) 813–819. [DOI] [PubMed] [Google Scholar]
- [13].Rinaldi F, Perlingeiro RCR, Stem cells for skeletal muscle regeneration: therapeutic potential and roadblocks, Transl. Res 163 (2014) 409–417. doi: 10.1016/j.trsl.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Grounds MD, Radley HG, Lynch GS, Nagaraju K, De Luca A, Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of Duchenne muscular dystrophy, Neurobiol. Dis 31 (2008) 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM, Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts, Nature 337 (1989) 176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- [16].Péault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, Gussoni E, Kunkel LM, Huard J, Stem and Progenitor Cells in Skeletal Muscle Development, Maintenance, and Therapy, Mol. Ther 15 (2007) 867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- [17].Skuk D, Roy B, Goulet M, Tremblay JP, Successful myoblast transplantation in primates depends on appropriate cell delivery and induction of regeneration in the host muscle, Exp. Neurol 155 (1999) 22–30. [DOI] [PubMed] [Google Scholar]
- [18].Hill E, Boontheekul T, Mooney DJ, Designing scaffolds to enhance transplanted myoblast survival and migration, Tissue Eng 12 (2006) 1295–1304. doi: 10.1089/ten.2006.12.1295. [DOI] [PubMed] [Google Scholar]
- [19].Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM, Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture, Science (80-. ) 329 (2010) 1078–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kinoshita I, Roy R, Dugre FJ, Gravel C, Roy B, Goulet M, Asselin I, Tremblay JP, Myoblast transplantation in monkeys: control of immune response by FK506, J. Neuropathol. Exp. Neurol 55 (1996) 687–697. [DOI] [PubMed] [Google Scholar]
- [21].Périé S, Trollet C, Mouly V, Vanneaux V, Mamchaoui K, Bouazza B, Marolleau JP, Laforêt P, Chapon F, Eymard B, Butler-Browne G, Larghero J, St Guily JL, Autologous myoblast transplantation for oculopharyngeal muscular dystrophy: a phase I/IIa clinical study., Mol. Ther 22 (2014) 219–25. doi: 10.1038/mt.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, Usas A, Gates C, Robbins P, Wernig A, Huard J, Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing, J. Cell Biol 150 (2000) 1085–1099. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J, Identification of a novel population of muscle stem cells in mice, J. Cell Biol 157 (2002) 851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, Péault B, Cummins J, Huard J, Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique, Nat. Protoc 3 (2008) 1501–1509. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- [25].Deasy BM, Gharaibeh BM, Pollett JB, Jones MM, Lucas MA, Kanda Y, Huard J, Long-term self-renewal of postnatal muscle-derived stem cells., Mol. Biol. Cell 16 (2005) 3323–33. doi: 10.1091/mbc.E05-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rouger K, Larcher T, Dubreil L, Deschamps JY, Le Guiner C, Jouvion G, Delorme B, Lieubeau B, Carlus M, Fornasari B, Theret M, Orlando P, Ledevin M, Zuber C, Leroux I, Deleau S, Guigand L, Testault I, Le Rumeur E, Fiszman M, Cherel Y, Systemic Delivery of Allogenic Muscle Stem (MuStem) Cells Induces Long-Term Muscle Repair and Clinical Efficacy in Duchenne Muscular Dystrophy Dogs, Am J Pathol (2011). doi: 10.1016/j.ajpath.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lardenois A, Jagot S, Lagarrigue M, Gu??vel B, Ledevin M, Larcher T, Dubreil L, Pineau C, Rouger K, Gu??vel L, Quantitative proteome profiling of dystrophic dog skeletal muscle reveals a stabilized muscular architecture and protection against oxidative stress after systemic delivery of MuStem cells, Proteomics 16 (2016) 2028–2042. doi: 10.1002/pmic.201600002. [DOI] [PubMed] [Google Scholar]
- [28].Chirieleison SM, Feduska JM, Schugar RC, Askew Y, Deasy BM, Human Muscle-Derived Cell Populations Isolated by Differential Adhesion Rates: Phenotype and Contribution to Skeletal Muscle Regeneration in Mdx/SCID Mice, Tissue Eng. Part A 18 (2012) 232–241. doi: 10.1089/ten.tea.2010.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].J. L, N. J, I. L, C. S, C. Z, M. C, B. L, Y. P, A. M, A. H, O. A, K. R, Immunomodulatory properties of human MuStem cells: Assessing their impact on adaptive and innate immunity., ESGCT FSGT (2015). doi: 10.1089/hum.2015.29008.abstracts. [DOI] [Google Scholar]
- [30].Torrente Y, Belicchi M, Sampaolesi M, Pisati F, Meregalli M, D’Antona G, Tonlorenzi R, Porretti L, Gavina M, Mamchaoui K, Pellegrino MA, Furling D, Mouly V, Butler-Browne GS, Bottinelli R, Cossu G, Bresolin N, Human circulating AC133+ stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle, J. Clin. Invest 114 (2004) 182–195. doi: 10.1172/JCI200420325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shi M, Ishikawa M, Kamei N, Nakasa T, Adachi N, Deie M, Asahara T, Ochi M, Acceleration of skeletal muscle regeneration in a rat skeletal muscle injury model by local injection of human peripheral blood-derived CD133-positive cells., Stem Cells 27 (2009) 949–60. doi: 10.1002/stem.4. [DOI] [PubMed] [Google Scholar]
- [32].Negroni E, Riederer I, Chaouch S, Belicchi M, Razini P, Di Santo J, Torrente Y, Butler-Browne GS, Mouly V, In Vivo Myogenic Potential of Human CD133+ Muscle-derived Stem Cells: A Quantitative Study, Mol. Ther 17 (2009) 1771–1778. doi: 10.1038/mt.2009.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Torrente Y, Belicchi M, C M, G D, F C, F P, M G, R G, R T, G F, C L, L P, R L, M S, L V, N G, F T, V S, P B, A P, L F, M G, O P, C R, V M, GS B-B, GP C, P B, M M, SM G, N S, A P, MG D, A T, R B, G C, P R, N. B, Autologous Transplantation of CD133+ Stem Cells in Duchenne Muscle Patients, Cell Transplant 16 (2007) 563–577. [DOI] [PubMed] [Google Scholar]
- [34].Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G, The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues., Development 129 (2002) 2773–2783. doi: 10.1098/rstb.2000.0631. [DOI] [PubMed] [Google Scholar]
- [35].Sampaolesi M, Cell Therapy of -Sarcoglycan Null Dystrophic Mice Through Intra-Arterial Delivery of Mesoangioblasts, Science (80-. ) 301 (2003) 487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- [36].Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud J-L, Galvez BG, Barthélémy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G, Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs, Nature 444 (2006) 574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- [37].Berry SE, Liu J, Chaney EJ, Kaufman SJ, Multipotential mesoangioblast stem cell therapy in the mdx/utrn−/−mouse model for Duchenne muscular dystrophy, Regen. Med 2 (2007) 275–288. [DOI] [PubMed] [Google Scholar]
- [38].Díaz-Manera J, Touvier T, Dellavalle A, Tonlorenzi R, Tedesco FS, Messina G, Meregalli M, Navarro C, Perani L, Bonfanti C, Illa I, Torrente Y, Cossu G, Partial dysferlin reconstitution by adult murine mesoangioblasts is sufficient for full functional recovery in a murine model of dysferlinopathy, Cell Death Dis 1 (2010) e61. doi: 10.1038/cddis.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dellavalle A, Sampaolesi M, Tonlorenzi1 R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G, Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells, Nat. Cell Biol 9 (2007) 255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- [40].Tedesco FS, Hoshiya H, D’Antona G, Gerli MFM, Messina G, Antonini S, Tonlorenzi R, Benedetti S, Berghella L, Torrente Y, Kazuki Y, Bottinelli R, Oshimura M, Cossu G, Stem cell-mediated transfer of a human artificial chromosome ameliorates muscular dystrophy., Sci. Transl. Med 3 (2011) 96ra78. doi: 10.1126/scitranslmed.3002342. [DOI] [PubMed] [Google Scholar]
- [41].Cossu G, Previtali SC, Napolitano S, Cicalese MP, Tedesco FS, Nicastro F, Noviello M, Roostalu U, Natali Sora MG, Scarlato M, De Pellegrin M, Godi C, Giuliani S, Ciotti F, Tonlorenzi R, Lorenzetti I, Rivellini C, Benedetti S, Gatti R, Marktel S, Mazzi B, Tettamanti A, Ragazzi M, Imro MA, Marano G, Ambrosi A, Fiori R, Sormani MP, Bonini C, Venturini M, Politi LS, Torrente Y, Ciceri F, Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy., EMBO Mol. Med 7 (2015) 1513–28. doi: 10.15252/emmm.201505636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dezawa M, Bone Marrow Stromal Cells Generate Muscle Cells and Repair Muscle Degeneration, Science (80-. ) 309 (2005) 314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- [43].Wernig G, Janzen V, Schäfer R, Zweyer M, Knauf U, Hoegemeier O, Mundegar RR, Garbe S, Stier S, Franz T, Wernig M, Wernig A, The vast majority of bone-marrow-derived cells integrated into mdx muscle fibers are silent despite long-term engraftment., Proc. Natl. Acad. Sci. U. S. A 102 (2005) 11852–7. doi: 10.1073/pnas.0502507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Matziolis G, Winkler T, Schaser K, Wiemann M, Krocker D, Tuischer J, Perka C, Duda GN, Autologous Bone Marrow-Derived Cells Enhance Muscle Strength Following Skeletal Muscle Crush Injury in Rats, Tissue Eng 12 (2006) 361–367. doi: 10.1089/ten.2006.12.361. [DOI] [PubMed] [Google Scholar]
- [45].Gang EJ, Darabi R, Bosnakovski D, Xu Z, Kamm KE, Kyba M, Perlingeiro RCR, Engraftment of mesenchymal stem cells into dystrophin-deficient mice is not accompanied by functional recovery, Exp. Cell Res 315 (2009) 2624–2636. doi: 10.1016/j.yexcr.2009.05.009. [DOI] [PubMed] [Google Scholar]
- [46].von Roth P, Duda GN, Radojewski P, Preininger B, Strohschein K, Röhner E, Perka C, Winkler T, Intra-Arterial MSC Transplantation Restores Functional Capacity After Skeletal Muscle Trauma., Open Orthop. J 6 (2012) 352–6. doi: 10.2174/1874325001206010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Winkler T, Ph D, Von Roth P, Matziolis G, Mehta M, Eng MS, Perka C, Duda GN, Ph D, Dose – Response Relationship of Mesenchymal Stem Cell Transplantation and Functional Regeneration after Severe Skeletal Muscle Injury in Rats, Eng. Part A 14 (2008) 1–6. [DOI] [PubMed] [Google Scholar]
- [48].De Bari C, Dell’Accio F, Vandenabeele F, Vermeesch JR, Raymackers J-M, Luyten FP, Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane, J. Cell Biol 160 (2003) 909–918. doi: 10.1083/jcb.200212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Meng J, The contribution of human synovial stem cells to skeletal muscle regeneration, Neuromuscul. Disord 20 (2010) 6–15. [DOI] [PubMed] [Google Scholar]
- [50].Gang EJ, Skeletal Myogenic Differentiation of Mesenchymal Stem Cells Isolated from Human Umbilical Cord Blood, Stem Cells 22 (2004) 617–624. doi: 10.1634/stemcells.22-4-617. [DOI] [PubMed] [Google Scholar]
- [51].Shabbir A, Zisa D, Leiker M, Johnston C, Lin H, Lee T, Muscular Dystrophy Therapy by Nonautologous Mesenchymal Stem Cells: Muscle Regeneration Without Immunosuppression and Inflammation, Transplantation 87 (2009) 1275–1282. doi: 10.1097/TP.0b013e3181a1719b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Caplan A, Dennis J, Mesenchymal stem cells as trophic mediators, J. Cell. Biochem (2006). [DOI] [PubMed] [Google Scholar]
- [53].Mizuno H, Tobita M, Uysal A, Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine, Stem Cells (2012). [DOI] [PubMed] [Google Scholar]
- [54].Zuk PA, Human Adipose Tissue Is a Source of Multipotent Stem Cells, Mol. Biol. Cell 13 (2002) 4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zuk P, Adipose-Derived Stem Cells in Tissue Regeneration: A Review, Int. Sch. Res. Not 2013 (2013) e713959. doi: 10.1155/2013/713959. [DOI] [Google Scholar]
- [56].Rodriguez A-M, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne J-Y, Wdziekonski B, Villageois A, Bagnis C, Breittmayer J-P, Groux H, Ailhaud G, Dani C, Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse, J. Exp. Med 201 (2005) 1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Goudenege S, Pisani DF, Wdziekonski B, Di Santo JP, Bagnis C, Dani C, Dechesne CA, Enhancement of Myogenic and Muscle Repair Capacities of Human Adipose–derived Stem Cells With Forced Expression of MyoD, Mol. Ther 17 (2009) 1064–1072. doi: 10.1038/mt.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Di Rocco G, Iachininoto MG, Tritarelli A, Straino S, Zacheo A, Germani A, Crea F, Capogrossi MC, Myogenic potential of adipose-tissue-derived cells, J. Cell Sci 119 (2006) 2945–2952. doi: 10.1242/jcs.03029. [DOI] [PubMed] [Google Scholar]
- [59].Vieira NM, Bueno CR, Brandalise V, Moraes LV, Zucconi E, Secco M, Suzuki MF, Camargo MM, Bartolini P, Brum PC, Vainzof M, Zatz M, SJL Dystrophic Mice Express a Significant Amount of Human Muscle Proteins Following Systemic Delivery of Human Adipose-Derived Stromal Cells Without Immunosuppression, Stem Cells 26 (2008) 2391–2398. doi: 10.1634/stemcells.2008-0043. [DOI] [PubMed] [Google Scholar]
- [60].Alexeev V, Arita M, Donahue A, Bonaldo P, Chu M-L, Igoucheva O, Human adipose-derived stem cell transplantation as a potential therapy for collagen VI-related congenital muscular dystrophy., Stem Cell Res. Ther 5 (2014) 21. doi: 10.1186/scrt411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Vieira NM, Valadares M, Zucconi E, Secco M, Bueno Junior CR, Brandalise V, Assoni A, Gomes J, Landini V, Andrade T, Caetano HVA, Vainzof M, Zatz M, Human adipose-derived mesenchymal stromal cells injected systemically Into GRMD dogs without immunosuppression are able to reach the host muscle and express human dystrophin, Cell Transplant 21 (2012) 1407–1417. doi: 10.3727/096368911X. [DOI] [PubMed] [Google Scholar]
- [62].Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L, Derivation of engraftable skeletal myoblasts from human embryonic stem cells, Nat. Med 13 (2007) 642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- [63].Darabi R, Gehlbach K, Bachoo RM, Kamath S, Osawa M, Kamm KE, Kyba M, Perlingeiro RCR, Functional skeletal muscle regeneration from differentiating embryonic stem cells, Nat. Med 14 (2008) 134–143. doi: 10.1038/nm1705. [DOI] [PubMed] [Google Scholar]
- [64].Sakurai H, Okawa Y, Inami Y, Nishio N, Isobe K, Paraxial Mesodermal Progenitors Derived from Mouse Embryonic Stem Cells Contribute to Muscle Regeneration via Differentiation into Muscle Satellite Cells, Stem Cells 26 (2008) 1865–1873. doi: 10.1634/stemcells.2008-0173. [DOI] [PubMed] [Google Scholar]
- [65].Chang H, Yoshimoto M, Umeda K, Iwasa, Mizuno Y, Fukada S.-i., Yamamoto H, Motohashi N, Miyagoe-Suzuki Y, Takeda S, Heike T, Nakahata T, Generation of transplantable, functional satellite-like cells from mouse embryonic stem cells, FASEB J 23 (2009) 1907–1919. doi: 10.1096/fj.08-123661. [DOI] [PubMed] [Google Scholar]
- [66].Filareto A, Darabi R, Perlingeiro RCR, Engraftment of ES-Derived Myogenic Progenitors in a Severe Mouse Model of Muscular Dystrophy., J. Stem Cell Res. Ther 10 (2012) 1–5. doi: 10.4172/2157-7633.S10-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Darabi R, Santos F, Filareto A, Pan W, Koene R, Assessment of the myogenic stem cell compartment following transplantation of Pax3/Pax7-induced embryonic stem cell-derived progenitors, Stem (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M, Perlingeiro RCR, Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice, Cell Stem Cell 10 (2012) 610–619. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Quattrocelli M, Palazzolo G, Floris G, Schöffski P, Anastasia L, Orlacchio A, Vandendriessche T, Chuah MKL, Cossu G, Verfaillie C, Sampaolesi M, Intrinsic cell memory reinforces myogenic commitment of pericyte-derived iPSCs, J. Pathol 223 (2011) 593–603. doi: 10.1002/path.2845. [DOI] [PubMed] [Google Scholar]
- [70].Tedesco FS, Gerli MFM, Perani L, Benedetti S, Ungaro F, Cassano M, Antonini S, Tagliafico E, Artusi V, Longa E, Tonlorenzi R, Ragazzi M, Calderazzi G, Hoshiya H, Cappellari O, Mora M, Schoser B, Schneiderat P, Oshimura M, Bottinelli R, Sampaolesi M, Torrente Y, Broccoli V, Cossu G, Transplantation of Genetically Corrected Human iPSC-Derived Progenitors in Mice with Limb-Girdle Muscular Dystrophy, Sci. Transl. Med 4 (2012) 140ra89–140ra89. doi: 10.1126/scitranslmed.3003541. [DOI] [PubMed] [Google Scholar]
- [71].Yoshida T, Galvez S, Tiwari S, Rezk BM, Semprun-Prieto L, Higashi Y, Sukhanov S, Yablonka-Reuveni Z, Delafontaine P, Angiotensin II inhibits satellite cell proliferation and prevents skeletal muscle regeneration, J. Biol. Chem 288 (2013) 23823–23832. doi: 10.1074/jbc.M112.449074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Majewski RL, Zhang W, Ma X, Cui Z, Ren W, Markel DC, Bioencapsulation technologies in tissue engineering, J Appl Biomater Funct Mater 14 (2016) e395–e403. doi: 10.5301/jabfm.5000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lee K, Silva EA, Mooney DJ, Growth factor delivery-based tissue engineering: general approaches and a review of recent developments, J. R. Soc. Interface 8 (2011) 153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tallawi M, Rosellini E, Barbani N, Cascone MG, Rai R, Saint-Pierre G, Boccaccini AR, Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: a review, J R Soc Interface 12 (2015) 20150254. doi: 10.1098/rsif.2015.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Passipieri JA, Christ GJ, The Potential of Combination Therapeutics for More Complete Repair of Volumetric Muscle Loss Injuries: The Role of Exogenous Growth Factors and/or Progenitor Cells in Implantable Skeletal Muscle Tissue Engineering Technologies, Cells Tissues Organs 202 (2016) 202–213. doi: 10.1159/000447323. [DOI] [PubMed] [Google Scholar]
- [76].Syverud BC, VanDusen KW, Larkin LM, Growth factors for skeletal muscle tissue engineering, Cells Tissues Organs. 202 (2016) 169–179. doi: 10.1159/000444671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Longo UG, Loppini M, Berton A, Spiezia F, Maffulli N, Denaro V, Tissue Engineered Strategies for Skeletal Muscle Injury, Stem Cells Int. 2012 (2012). doi: 10.1155/2012/175038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Delaney K, I. of Z. 02–096 W.P. of Warsaw Faculty of Biology Department of Cytology, P. Kasprzycka, I. of Z. 02–096 W.P. of Warsaw Faculty of Biology Department of Cytology, M.A. Ciemerych, I. of Z. 02–096 W.P. of Warsaw Faculty of Biology Department of Cytology, M. Zimowska, I. of Z. 02–096 W.P. of Warsaw Faculty of Biology Department of Cytology, The role of TGF-β1 during skeletal muscle regeneration, Cell Biol. Int (2017). doi: 10.1002/cbin.10725. [DOI] [PubMed] [Google Scholar]
- [79].Weist MR, Wellington MS, Bermudez JE, Kostrominova TY, Mendias CL, Arruda EM, Larkin LM, TGF-β1 enhances contractility in engineered skeletal muscle, J. Tissue Eng. Regen. Med 7 (2013) 562–571. doi: 10.1002/term.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sheehan SM, Allen RE, Skeletal muscle satellite cell proliferation in response to members of the fibroblast growth factor family and hepatocyte growth factor, J Cell Physiol 181 (1999) 499–506. doi:. [DOI] [PubMed] [Google Scholar]
- [81].Pawlikowski B, Vogler TO, Gadek K, Olwin BB, Regulation of skeletal muscle stem cells by fibroblast growth factors, Dev Dyn (2017). doi: 10.1002/dvdy.24495. [DOI] [PubMed] [Google Scholar]
- [82].Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE, HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells, Dev Biol 194 (1998) 114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- [83].Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M, Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo, Mol Ther 10 (2004) 844–854. doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- [84].Wagner PD, The critical role of VEGF in skeletal muscle angiogenesis and blood flow, Biochem Soc Trans 39 (2011) 1556–1559. doi: 10.1042/bst20110646. [DOI] [PubMed] [Google Scholar]
- [85].Witt R, Weigand A, Boos AM, Cai A, Dippold D, Boccaccini AR, Schubert DW, Hardt M, Lange C, Arkudas A, Horch RE, Beier JP, Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering, BMC Cell Biol 18 (2017) 15. doi: 10.1186/s12860-017-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hammers DW, Sarathy A, Pham CB, Drinnan CT, Farrar RP, Suggs LJ, Controlled release of IGF-I from a biodegradable matrix improves functional recovery of skeletal muscle from ischemia/reperfusion, Biotechnol Bioeng 109 (2012) 1051–1059. doi: 10.1002/bit.24382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Borselli C, Storrie H, Benesch-lee F, Shvartsman D, Cezar C, Lichtman JW, Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors, 107 (2010). doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].James R, Laurencin C, Musculoskeletal Regenerative Engineering: Biomaterials, Structures, and Small Molecules, Adv. Biomater Volume 201 (2014) 1–12. [Google Scholar]
- [89].Aravamudhan A, M Ramos D, Nip J, Subramanian A, James R, D Harmon M, Yu X, G Kumbar S, Osteoinductive small molecules: growth factor alternatives for bone tissue engineering, Curr. Pharm. Des 19 (2013) 3420–3428. [DOI] [PubMed] [Google Scholar]
- [90].Carbone EJ, Rajpura K, Jiang T, Laurencin CT, Lo KW-H, Regulation of bone regeneration with approved small molecule compounds, Adv. Regen. Biol 1 (2014). [Google Scholar]
- [91].Bernacchioni C, Cencetti F, Blescia S, Donati C, Bruni P, Sphingosine kinase/sphingosine 1-phosphate axis: a new player for insulin-like growth factor-1-induced myoblast differentiation, in: Skelet Muscle, 2012: p. 15. doi: 10.1186/2044-5040-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Maceyka M, Harikumar KB, Milstien S, Spiegel S, Sphingosine-1-phosphate signaling and its role in disease, Trends Cell Biol 22 (2012) 50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Danieli-Betto D, Peron S, Germinario E, Zanin M, Sorci G, Franzoso S, Sandona D, Betto R, Sphingosine 1-phosphate signaling is involved in skeletal muscle regeneration, Am J Physiol Cell Physiol 298 (2010) C550–8. doi: 10.1152/ajpcell.00072.2009. [DOI] [PubMed] [Google Scholar]
- [94] 2nd.Smith CK, Janney MJ, Allen RE, Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells, J Cell Physiol 159 (1994) 379–385. doi: 10.1002/jcp.1041590222. [DOI] [PubMed] [Google Scholar]
- [95].Kennedy KAM, Porter T, Mehta V, Ryan SD, Price F, Peshdary V, Karamboulas C, Savage J, Drysdale TA, Li S-C, Bennett SAL, Skerjanc IS, Retinoic acid enhances skeletal muscle progenitor formation and bypasses inhibition by bone morphogenetic protein 4 but not dominant negative β-catenin, BMC Biol 7 (2009) 67. doi: 10.1186/1741-7007-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Blomhoff R, Blomhoff HK, Overview of retinoid metabolism and function, J Neurobiol 66 (2006) 606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- [97].Lee H, Haller C, Manneville C, Doll T, Fruh I, Keller CG, Richards SM, Ibig-Rehm Y, Patoor M, Goette M, Bouchez LC, Mueller M, Identification of Small Molecules Which Induce Skeletal Muscle Differentiation in Embryonic Stem Cells via Activation of the Wnt and Inhibition of Smad2/3 and Sonic Hedgehog Pathways, Stem Cells 34 (2016) 299–310. doi: 10.1002/stem.2228. [DOI] [PubMed] [Google Scholar]
- [98].Darabi R, Gehlbach K, Bachoo RM, Kamath S, Osawa M, Kamm KE, Kyba M, Perlingeiro RC, Functional skeletal muscle regeneration from differentiating embryonic stem cells, Nat Med 14 (2008) 134–143. doi: 10.1038/nm1705. [DOI] [PubMed] [Google Scholar]
- [99].Lo KWH, Ashe KM, Kan HM, Laurencin CT, The role of small molecules in musculoskeletal regeneration, Regen. Med 7 (2012) 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wu F, Jin T, Polymer-Based Sustained-Release Dosage Forms for Protein Drugs, Challenges, and Recent Advances, in: AAPS PharmSciTech, 2008: pp. 1218–1229. doi: 10.1208/s12249-008-9148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Rowley JA, Mooney DJ, Alginate type and RGD density control myoblast phenotype, J. Biomed. Mater. Res 60 (2002) 217–223. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- [102].Fu C, Ziegler F, Vibration prone multi-purpose buildings and towers effectively damped by tuned liquid column-gas dampers, Asian J. Civ. Eng 10 (2009) 21–56. doi: 10.2217/nnm.10.12.Engineering. [DOI] [Google Scholar]
- [103].Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK, Electrospun nanofibrous structure: a novel scaffold for tissue engineering, J Biomed Mater Res 60 (2002) 613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- [104].Nair LS, Bhattacharyya S, Laurencin CT, Development of novel tissue engineering scaffolds via electrospinning, Expert Opin Biol Ther 4 (2004) 659–668. doi: 10.1517/14712598.4.5.659. [DOI] [PubMed] [Google Scholar]
- [105].Laurencin CT, Kumbar SG, Nukavarapu SP, James R, Hogan MV, Recent patents on electrospun biomedical nanostructures: an overview, Recent Pat Biomed Eng 1 (2008) 68–78. doi: 10.2174/1874764710801010068. [DOI] [Google Scholar]
- [106].Jiang T, Carbone EJ, Lo KW-H, Laurencin CT, Electrospinning of polymer nanofibers for tissue regeneration, Prog. Polym. Sci 46 (2015) 1–24. doi: 10.1016/j.progpolymsci.2014.12.001. [DOI] [Google Scholar]
- [107].Nair LS, Bhattacharyya S, Bender JD, Greish YE, Brown PW, Allcock HR, Laurencin CT, Fabrication and optimization of methylphenoxy substituted polyphosphazene nanofibers for biomedical applications, Biomacromolecules 5 (2004) 2212–2220. doi: 10.1021/bm049759j. [DOI] [PubMed] [Google Scholar]
- [108].Kumbar SG, Nukavarapu SP, James R, Nair LS, Laurencin CT. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 29 (2008) 4100–4107. doi: 10.1016/j.biomaterials.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Katti DS, Robinson KW, Ko FK, Laurencin CT, Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters, J Biomed Mater Res Part B Appl Biomater 70 (2004) 286–296. doi: 10.1002/jbm.b.30041. [DOI] [PubMed] [Google Scholar]
- [110].Merrell JG, McLaughlin SW, Tie L, Laurencin CT, Chen AF, Nair LS, Curcumin-loaded poly(epsilon-caprolactone) nanofibres: diabetic wound dressing with anti-oxidant and anti-inflammatory properties, Clin Exp Pharmacol Physiol 36 (2009) 1149–1156. doi: 10.1111/j.1440-1681.2009.05216.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bhattcharyya S, Nair LS, Singh A, Krogman NR, Greish YE, Brown PW, Allcock HR, Laurencin CT, Electrospinning of Poly[bis(ethyl alanato) phosphazene] Nanofibers, J Biomed Nanotechno 2 (2006) 36–45. doi: 10.1166/jbn.2006.008 [DOI] [Google Scholar]
- [112].Deng M, Kumbar SG, Nair LS, Weikel AL, Allcock HR, Laurencin CT, Biomimetic Structures: Biological Implications of Dipeptide- Substituted Polyphosphazene–Polyester Blend Nanofiber Matrices for Load- Bearing Bone Regeneration, Adv Funct Mater 21 (2011) 2641–2651. doi: 10.1002/adfm.201100275. [DOI] [Google Scholar]
- [113].Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ, The influence of electrospun aligned poly(??-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes, Biomaterials 29 (2008) 2899–2906. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- [114].Aviss KJ, Gough JE, Downes S, Aligned electrospun polymer fibres for skeletal muscle regeneration, Eur. Cells Mater 19 (2010) 193–204. doi:vol019a19 [pii]. [DOI] [PubMed] [Google Scholar]
- [115].Chen M-C, Sun Y-C, Chen Y-H, Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering., Acta Biomater 9 (2013) 5562–72. doi: 10.1016/j.actbio.2012.10.024. [DOI] [PubMed] [Google Scholar]
- [116].Jun I, Jeong S, Shin H, The stimulation of myoblast differentiation by electrically conductive sub-micron fibers., Biomaterials 30 (2009) 2038–47. doi: 10.1016/j.biomaterials.2008.12.063. [DOI] [PubMed] [Google Scholar]
- [117].Bian W, Bursac N, Tissue engineering of functional skeletal muscle: challenges and recent advances., IEEE Eng. Med. Biol. Mag 27 (2008) 109–13. doi: 10.1109/MEMB.2008.928460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].a Chikar J, Hendricks JL, Richardson-Burns SM, Raphael Y, Pfingst BE, Martin DC, The use of a dual PEDOT and RGD-functionalized alginate hydrogel coating to provide sustained drug delivery and improved cochlear implant function., Biomaterials 33 (2012) 1982–90. doi: 10.1016/j.biomaterials.2011.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Juhas M, Bursac N, Engineering skeletal muscle repair., Curr. Opin. Biotechnol 24 (2013) 880–6. doi: 10.1016/j.copbio.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ, The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration., Biomaterials 32 (2011) 8905–14. doi: 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A, Cell-laden microengineered gelatin methacrylate hydrogels, Biomaterials 31 (2010) 5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Hinds S, Bian W, Dennis RG, Bursac N, The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle, Biomaterials 32 (2011) 3575–3583. doi: 10.1016/j.biomaterials.2011.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Bian W, Bursac N, Engineered skeletal muscle tissue networks with controllable architecture, Biomaterials 30 (2009) 1401–1412. doi: 10.1016/j.biomaterials.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].CURTIS AS, the Mechanism of Adhesion of Cells To Glass. a Study By Interference Reflection Microscopy., J. Cell Biol 20 (1964) 199–215. doi: 10.1083/jcb.20.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Yang HS, Ieronimakis N, Tsui JH, Kim HN, Suh KY, Reyes M, Kim DH, Nanopatterned muscle cell patches for enhanced myogenesis and dystrophin expression in a mouse model of muscular dystrophy, Biomaterials 35 (2014) 1478–1486. doi: 10.1016/j.biomaterials.2013.10.067. [DOI] [PubMed] [Google Scholar]
- [126].Yang HS, Lee B, Tsui JH, Macadangdang J, Jang S-YY, Im SG, Kim D-HH, Electroconductive Nanopatterned Substrates for Enhanced Myogenic Differentiation and Maturation, Adv. Healthc. Mater 5 (2015) n/a–n/a. doi: 10.1002/adhm.201500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Li K-C, Hwang S-M, Chien H-H, Yuan C-C, Lu H-E, Ma K-J, Tseng C-P, Submicron-grooved culture surface extends myotube length by forming parallel and elongated motif, Micro Nano Lett 8 (2013) 440–444. doi: 10.1049/mnl.2013.0153. [DOI] [Google Scholar]
- [128].Patz TM, Doraiswamy A, Narayan RJ, Modi R, Chrisey DB, Two-dimensional differential adherence and alignment of C2C12 myoblasts, Mater. Sci. Eng. B Solid-State Mater. Adv. Technol 123 (2005) 242–247. doi: 10.1016/j.mseb.2005.08.088. [DOI] [Google Scholar]
- [129].Hosseini V, Ahadian S, Ostrovidov S, Camci-Unal G, Chen S, Kaji H, Ramalingam M, Khademhosseini A, Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate., Tissue Eng. Part A 18 (2012) 2453–65. doi: 10.1089/ten.TEA.2012.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].El-Mohri H, Wu Y, Mohanty S, Ghosh G, Impact of matrix stiffness on fibroblast function, Mater. Sci. Eng. C 74 (2017) 146–151. doi: 10.1016/j.msec.2017.02.001. [DOI] [PubMed] [Google Scholar]
- [131].Chen S, Nakamoto T, Kawazoe N, Chen G, Engineering multi-layered skeletal muscle tissue by using 3D microgrooved collagen scaffolds, Biomaterials 73 (2015) 23–31. doi: 10.1016/j.biomaterials.2015.09.010. [DOI] [PubMed] [Google Scholar]
- [132].Patel A, Mukundan S, Wang W, Karumuri A, Sant V, Mukhopadhyay SM, Sant S, Carbon-based hierarchical scaffolds for myoblast differentiation: Synergy between nano-functionalization and alignment, Acta Biomater 32 (2016) 77–88. doi: 10.1016/j.actbio.2016.01.004. [DOI] [PubMed] [Google Scholar]
- [133].Valentin JE, Turner NJ, Gilbert TW, Badylak SF, Functional skeletal muscle formation with a biologic scaffold, Biomaterials 31 (2010) 7475–7484. doi: 10.1016/j.biomaterials.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Song JJ, Ott HC, Organ engineering based on decellularized matrix scaffolds, Trends Mol. Med 17 (2011) 424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- [135].Yu X, Tang X, Gohil SV, Laurencin CT, Biomaterials for Bone Regenerative Engineering, Adv. Healthc. Mater 4 (2015) 1268–1285. doi: 10.1002/adhm.201400760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wolf MT, Daly KA, Reing JE, Badylak SF, Biologic scaffold composed of skeletal muscle extracellular matrix, Biomaterials 33 (2012) 2916–2925. doi: 10.1016/j.biomaterials.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ward CL, Ji L, Corona BT , An Autologous Muscle Tissue Expansion Approach for the Treatment of Volumetric Muscle Loss., Biores. Open Access 4 (2015) 198–208. doi: 10.1089/biores.2015.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Machingal MA, Corona BT, Walters TJ, Kesireddy V, Koval CN, Dannahower A, Zhao W, Yoo JJ, Christ GJ, A Tissue-Engineered Muscle Repair Construct for Functional Restoration of an Irrecoverable Muscle Injury in a Murine Model, Tissue Eng. Part A 17 (2011) 2291–2303. doi: 10.1089/ten.tea.2010.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Corona BT, Machingal MA, Criswell T, Vadhavkar M, Dannahower AC, Bergman C, Zhao W, Christ GJ, Further Development of a Tissue Engineered Muscle Repair Construct In Vitro for Enhanced Functional Recovery Following Implantation In Vivo in a Murine Model of Volumetric Muscle Loss Injury, Tissue Eng. Part A 18 (2012) 1213–1228. doi: 10.1089/ten.tea.2011.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Corona BT, Ward CL, Baker HB, Walters TJ, Christ GJ, Implantation of In Vitro Tissue Engineered Muscle Repair Constructs and Bladder Acellular Matrices Partially Restore In Vivo Skeletal Muscle Function in a Rat Model of Volumetric Muscle Loss Injury, Tissue Eng. Part A 20 (2013) 131219054609007. doi: 10.1089/ten.tea.2012.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Balint R, Cassidy NJ, Cartmell SH, Conductive polymers: Towards a smart biomaterial for tissue engineering., Acta Biomater 10 (2014) 2341–2353. doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- [142].Rivers TJ, Hudson TW, Schmidt CE, Synthesis of a Novel, Biodegradable Electrically Conducting Polymer for Biomedical Applications, Adv. Funct. Mater 12 (2002) 33. doi:. [DOI] [Google Scholar]
- [143].Guo B, Glavas L, Albertsson A-C, Biodegradable and electrically conducting polymers for biomedical applications, Prog. Polym. Sci 38 (2013) 1263–1286. doi: 10.1016/j.progpolymsci.2013.06.003. [DOI] [Google Scholar]
- [144].Mao C, Zhu A, Wu Q, Chen X, Kim J, Shen J, New biocompatible polypyrrole-based films with good blood compatibility and high electrical conductivity., Colloids Surf. B. Biointerfaces 67 (2008) 41–5. doi: 10.1016/j.colsurfb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- [145].Lee JY, a Bashur C, Goldstein AS, Schmidt CE, Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications., Biomaterials 30 (2009) 4325–35. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Sajesh KM, Jayakumar R, V Nair S, Chennazhi KP, Biocompatible conducting chitosan/polypyrrole-alginate composite scaffold for bone tissue engineering., Int. J. Biol. Macromol 62 (2013) 465–71. doi: 10.1016/j.ijbiomac.2013.09.028. [DOI] [PubMed] [Google Scholar]
- [147].Qazi TH, Rai R, Boccaccini AR, Biomaterials Tissue engineering of electrically responsive tissues using polyaniline based polymers : A review, Biomaterials (2014) 1–19. doi: 10.1016/j.biomaterials.2014.07.020. [DOI] [PubMed] [Google Scholar]
- [148].Peramo A, Urbanchek MG, a Spanninga S, Povlich LK, Cederna P, Martin DC, In situ polymerization of a conductive polymer in acellular muscle tissue constructs., Tissue Eng. Part A 14 (2008) 423–32. doi: 10.1089/tea.2007.0123. [DOI] [PubMed] [Google Scholar]
- [149].Hadjizadeh A, Doillon CJ, Directional migration of endothelial cells towards angiogenesis using polymer fibres in a 3D co-culture system., J. Tissue Eng. Regen. Med 4 (2010) 524–531. doi: 10.1002/term. [DOI] [PubMed] [Google Scholar]
- [150].Gilmore KJ, Kita M, Han Y, Gelmi A, Higgins MJ, Moulton SE, Clark GM, Kapsa R, Wallace GG, Biomaterials Skeletal muscle cell proliferation and differentiation on polypyrrole substrates doped with extracellular matrix components, Biomaterials 30 (2009) 5292–5304. doi: 10.1016/j.biomaterials.2009.06.059. [DOI] [PubMed] [Google Scholar]
- [151].Ostrovidov S, Shi X, Zhang L, Liang X, Kim SB, Fujie T, Ramalingam M, Chen M, Nakajima K, Al-Hazmi F, Bae H, Memic A, Khademhosseini A, Myotube formation on gelatin nanofibers - Multi-walled carbon nanotubes hybrid scaffolds, Biomaterials 35 (2014) 6268–6277. doi: 10.1016/j.biomaterials.2014.04.021. [DOI] [PubMed] [Google Scholar]
- [152].Hyoungshin Park RL, Rajat Bhalla1, Rajiv Saigal, Milica Radisic, Nicki Watson, 4* and Gordana Vunjak-Novakovic1, Effects of electrical stimulation in C2C12muscle constructs, J. Tissue Eng. Regen. Med 2 (2008) 279–287. doi: 10.1002/term.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Lutolf MP, Hubbell JA, Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering, Nat. Biotechnol 23 (2005) 47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- [154].Moon du G, Christ G, Stitzel JD, Atala A, Yoo JJ, Cyclic mechanical preconditioning improves engineered muscle contraction, Tissue Eng Part A 14 (2008) 473–482. doi: 10.1089/tea.2007.0104. [DOI] [PubMed] [Google Scholar]
- [155].Vandenburgh HH, Karlisch P, Longitudinal growth of skeletal myotubes in vitro in a new horizontal mechanical cell stimulator., In Vitro Cell. Dev. Biol 25 (1989) 607–616. [DOI] [PubMed] [Google Scholar]
- [156].Cezar CA, Roche ET, Vandenburgh HH, Duda GN, Walsh CJ, Mooney DJ, Biologic-free mechanically induced muscle regeneration., Proc. Natl. Acad. Sci. U. S. A 113 (2016) 1534–9. doi: 10.1073/pnas.1517517113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Abat F, Valles SL, Gelber PE, Polidori F, Stitik TP, García-Herreros S, Monllau JC, Sanchez-Ibánez JM, Molecular repair mechanisms using the Intratissue Percutaneous Electrolysis technique in patellar tendonitis, Rev. Española Cirugía Ortopédica Y Traumatol. (English Ed 58 (2014) 201–205. doi: 10.1016/j.recote.2014.05.005. [DOI] [PubMed] [Google Scholar]
- [158].R.-C. KY P. Langelaan Marloes L., M. Boonen Kristel J., P. MJand B. FPT J.van derSchaft* DaisyW., Advanced maturation by electrical stimulation: Differences in response between C2C12 and primary muscle progenitor cells, J. Tissue Eng. Regen. Med 4 (2010) 524–531. doi: 10.1002/term. [DOI] [PubMed] [Google Scholar]
- [159].Pette D, Vrbova G, What does chronic electrical stimulation teach us about muscle plasticity?, Muscle Nerve 22 (1999) 666–677. [DOI] [PubMed] [Google Scholar]
- [160].Xia Y, Buja LM, Scarpulla RC, McMillin JB, Electrical stimulation of neonatal cardiomyocytes results in the sequential activation of nuclear genes governing mitochondrial proliferation and differentiation., Proc. Natl. Acad. Sci. U. S. A 94 (1997) 11399–11404. doi: 10.1073/pnas.94.21.11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Fujita H, Nedachi T, Kanzaki M, Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes, Exp. Cell Res 313 (2007) 1853–1865. doi: 10.1016/j.yexcr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- [162].Nedachi T, Fujita H, Kanzaki M, Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle., Am. J. Physiol. Endocrinol. Metab 295 (2008) E1191–E1204. doi: 10.1152/ajpendo.90280.2008. [DOI] [PubMed] [Google Scholar]
- [163].Kanno S, Oda N, Abe M, Saito S, Hori K, Handa Y, Tabayashi K, Sato Y, Establishment of a simple and practical procedure applicable to therapeutic angiogenesis., Circulation 99 (1999) 2682–2687. doi: 10.1161/01.CIR.99.20.2682. [DOI] [PubMed] [Google Scholar]
- [164].Brutsaert TD, Gavin TP, Fu Z, Breen EC, Tang K, Mathieu-Costello O, Wagner PD, Regional differences in expression of VEGF mRNA in rat gastrocnemius following 1 hr exercise or electrical stimulation., BMC Physiol 2 (2002) 8. doi: 10.1186/1472-6793-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].van der Schaft DWJ, van Spreeuwel ACC, Boonen KJM, Langelaan MLP, Bouten CVC, Baaijens FPT, Engineering skeletal muscle tissues from murine myoblast progenitor cells and application of electrical stimulation., J. Vis. Exp (2013) e4267. doi: 10.3791/4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Pedrotty DM, Koh J, Davis BH, a Taylor D, Wolf P, Niklason LE, Engineering skeletal myoblasts: roles of three-dimensional culture and electrical stimulation, Am J Physiol Hear. Circ Physiol 288 (2005) H1620–6. doi: 10.1152/ajpheart.00610.2003. [DOI] [PubMed] [Google Scholar]
- [167].Burch N, Arnold AS, Item F, Summermatter S, Santos GBS, Christe M, Boutellier U, Toigo M, Handschin C, Electric pulse stimulation of cultured murine muscle cells reproduces gene expression changes of trained mouse muscle, PLoS One 5 (2010). doi: 10.1371/journal.pone.0010970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Trumble DR, Changping D, a Magovern J, Effects of Long-Term Stimulation on Skeletal Muscle PhenotypeExpression and Collagen/Fibrillin Distribution, Basic Appl Myol 11 (2) (2001) 91–98. [Google Scholar]
- [169].Hamada T, Sasaki H, Hayashi T, Moritani T, Nakao K, Enhancement of whole body glucose uptake during and after human skeletal muscle low-frequency electrical stimulation., J. Appl. Physiol 94 (2003) 2107–12. doi: 10.1152/japplphysiol.00486.2002. [DOI] [PubMed] [Google Scholar]
- [170].Wolf MT, Dearth CL, Sonnenberg SB, Loboa EG, Badylak SF, Naturally derived and synthetic scaffolds for skeletal muscle reconstruction, Adv. Drug Deliv. Rev 84 (2015) 208–221. doi: 10.1016/j.addr.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].R. Corona BT, JC Rivera, Owens JG, Wenke JC, CR, Volumetric muscle loss leads to permanent disability following extremity trauma., J Rehabil Res Dev 52 (2015) 785–792. doi: 10.1682/JRRD.2014.07.0165. [DOI] [PubMed] [Google Scholar]
- [172].Grogan BF, Hsu MAJJR, Volumetric Muscle Loss, 19 (2011). [DOI] [PubMed] [Google Scholar]
- [173].Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M, Turner NJ, Weber DJ, Simpson TW, Wyse A, Brown EHP, Dziki JL, Fisher LE, Brown S, Badylak SF, An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss., Sci. Transl. Med 6 (2014) 234ra58. doi: 10.1126/scitranslmed.3008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Corona BT, Wu X, Ward CL, McDaniel JS, Rathbone CR, Walters TJ, The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM, Biomaterials 34 (2013) 3324–3335. doi: 10.1016/j.biomaterials.2013.01.061. [DOI] [PubMed] [Google Scholar]
- [175].Wu X, Corona BT, Chen X, Walters TJ, A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies, Biores Open Access 1 (2012) 280–290. doi: 10.1089/biores.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Garg K, Ward CL, Hurtgen BJ, Wilken JM, Stinner DJ, Wenke JC, Owens JG, Corona BT, Volumetric muscle loss: Persistent functional deficits beyond frank loss of tissue, J. Orthop. Res 33 (2015) 40–46. doi: 10.1002/jor.22730. [DOI] [PubMed] [Google Scholar]
- [177].Corona BT, Machingal MA, Criswell T, Vadhavkar M, Dannahower AC, Bergman C, Zhao W, Christ GJ, Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury, Tissue Eng Part A 18 (2012) 1213–1228. doi: 10.1089/ten.TEA.2011.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Turner NJ, Badylak JS, Weber DJ, Badylak SF, Biologic scaffold remodeling in a dog model of complex musculoskeletal injury, J. Surg. Res 176 (2012) 490–502. doi: 10.1016/j.jss.2011.11.1029. [DOI] [PubMed] [Google Scholar]
- [179].Turner NJ, Yates AJ, Weber DJ, Qureshi IR, Stolz DB, Gilbert TW, Badylak SF, Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction., Tissue Eng. Part A 16 (2010) 3309–3317. doi: 10.1089/ten.tea.2010.0169. [DOI] [PubMed] [Google Scholar]
- [180].Cofield RH, Parvizi J, Hoffmeyer PJ, Lanzer WL, Ilstrup DM, Rowland CM, Surgical Repair of Chronic Rotator Cuff Tears., J. Bone Jt. Surgery, Am. Vol 83 (2001) 71. doi: 10.2106/00004623-200101000-00010. [DOI] [PubMed] [Google Scholar]
- [181].Narayanan G, Nair LS, Laurencin CT, Regenerative Engineering of the Rotator Cuff of the Shoulder, ACS Biomater. Sci. Eng 4 (2018) 751–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Ricchetti ET, Aurora A, Iannotti JP, a Derwin K, Scaffold devices for rotator cuff repair., J. Shoulder Elbow Surg 21 (2012) 251–65. doi: 10.1016/j.jse.2011.10.003. [DOI] [PubMed] [Google Scholar]
- [183].Liu X, Manzano G, Kim HT, Feeley BT, A rat model of massive rotator cuff tears, J. Orthop. Res 29 (2011) 588–595. doi: 10.1002/jor.21266. [DOI] [PubMed] [Google Scholar]
- [184].Cofield RH, Parvizi J, Hoffmeyer P, Lanzer WL, Ilstrup D, Rowland CM, Surgical Repair of Chronic Rotator Cuff Tears: A Prospective Long-Term Study, 2001. doi: 10.2106/00004623-200101000-00010. [DOI] [PubMed] [Google Scholar]
- [185].Feeley BT, Rotator Cuff Muscle Atrophy and Fatty Infiltration Progress in understanding the underlying mechanisms, Am. Acad. Orthopadic Surg (2014). [Google Scholar]
- [186].Gladstone JN, Bishop JY, Lo IKY, Flatow EL, American Journal of Sports Fatty Infiltration and Atrophy of the Rotator Cuff Do Not Improve After Rotator Cuff Repair and, Sport. Med (2007) 719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- [187].Dines JS, Bedi A, ElAttrache NS, Dines DM, Single-row versus double-row rotator cuff repair: techniques and outcomes., J. Am. Acad. Orthop. Surg 18 (2010) 83–93. [DOI] [PubMed] [Google Scholar]
- [188].Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL, Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors., J. Bone Joint Surg. Am 89 (2007) 2485–2497. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- [189].Prabhath A, Vernekar VN, Sanchez E, Laurencin CT, Growth factor delivery strategies for rotator cuff repair and regeneration, Int. J. Pharm 544 (2018) 358–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Perry SM, Gupta RR, Van Kleunen J, Ramsey ML, Soslowsky LJ, Glaser DL, Use of small intestine submucosa in a rat model of acute and chronic rotator cuff tear, J. Shoulder Elb. Surg 16 (2007) 179–183. doi: 10.1016/j.jse.2007.03.009. [DOI] [PubMed] [Google Scholar]
- [191].Thangarajah T, Henshaw F, Sanghani-Kerai A, Lambert SM, Blunn GW, Pendegrass CJ, The effectiveness of demineralized cortical bone matrix in a chronic rotator cuff tear model, J. Shoulder Elb. Surg 26 (2017) 619–626. doi: 10.1016/j.jse.2017.01.003. [DOI] [PubMed] [Google Scholar]
- [192].Beason DP, Connizzo BK, Dourte LM, Mauck RL, Soslowsky LJ, Steinberg DR, Bernstein J, Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model, J. Shoulder Elb. Surg 21 (2012) 245–250. doi: 10.1016/j.jse.2011.10.021. [DOI] [PubMed] [Google Scholar]
- [193].Sean Peach M, Ramos DM, James R, Morozowich NL, Mazzocca AD, Doty SB, Allcock HR, Kumbar S, Laurencin CT, Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering, 2017. doi: 10.1371/journal.pone.0174789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Yin Z, Heng BC, Feng G, Le H, Tang C, Huang J, Alignment of collagen fiber in knitted silk scaffold for functional massive rotator cuff repair, Acta Biomater 51 (2017) 317–329. doi: 10.1016/j.actbio.2017.01.041. [DOI] [PubMed] [Google Scholar]
- [195].Hast MW, a Zuskov LJ Soslowsky, The role of animal models in tendon research., Bone Joint Res 3 (2014) 193–202. doi: 10.1302/2046-3758.36.2000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Barton ER, a Gimbel J, Williams GR, Soslowsky LJ, Rat supraspinatus muscle atrophy after tendon detachment., J. Orthop. Res 23 (2005) 259–65. doi: 10.1016/j.orthres.2004.08.018. [DOI] [PubMed] [Google Scholar]
- [197].Davis ME, Stafford PL, Jergenson MJ, Bedi A, Mendias CL, Muscle Fibers are Injured at the Time of Acute and Chronic Rotator Cuff Repair, Clin. Orthop. Relat. Res 473 (2015) 226–232. doi: 10.1007/s11999-014-3860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Rowshan K, Hadley S, Pham K, Caiozzo V, Lee TQ, Gupta R, Development of fatty atrophy after neurologic and rotator cuff injuries in an animal model of rotator cuff pathology., J. Bone Joint Surg. Am 92 (2010) 2270–8. doi: 10.2106/JBJS.I.00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Easley JT, Feeley B, Luan T, Liu X, Ravishankar B, Puttlitz C, Feeley BT, Muscle atrophy and fatty infiltration after an acute rotator cuff repair in a sheep model, Muscles. Ligaments Tendons J 5 (2015) 106–112. doi: 10.11138/mltj/2015.5.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Tang X, Khan Y, Laurencin CT, Biomimetic electroconductive scaffolds for muscle regenerative engineering, Adv. Mater. Lett 8 (2017) 587–591. doi: 10.5185/amlett.2017.7106. [DOI] [Google Scholar]