Abstract
Background
Although many therapeutic agents have been developed, only a few drugs are known to target multiple pathogenic factors in the treatment of acne.
Objective
The purpose of this study was to identify a new drug candidate, platycodin D, which is a substance extracted from the root of Platycodon grandiflorum.
Methods
Using western blotting and Cell Counting Kit-8 assay, we studied the effects of platycodin D on SEB-1 sebocytes, fibroblasts, and keratinocytes. We investigated its effects in view of lipogenesis, collagen production, anti-inflammatory activity, and dyskeratinization.
Results
In SEB-1 sebocytes, platycodin D showed a sebosuppressive effect by downregulating ERK and insulin- like growth factor-1R/PI3K/Akt/sterol-regulatory element binding protein-1 signaling pathways. In addition, adiponectin, one of the adipokines responsible for sebum production, was decreased in platycodin D-treated SEB-1 sebocytes. In fibroblasts, platycodin D increased collagen production and reduced inflammation by inhibiting nuclear factor kappa B and matrix metalloproteinases. Platycodin D also showed anti-inflammatory effects on keratinocytes. It also suppressed keratin 16 expression induced by lipopolysaccharide. Furthermore, platycodin D showed no cytotoxicity on both SEB-1 sebocytes and fibroblasts.
Conclusion
Our data demonstrate the clinical feasibility of platycodin D for acne treatment and the prevention of acne scarring by sebosuppressive and anti-inflammatory effects, as well as through an increase in collagen levels.
Keywords: Acne vulgaris, Acne scar, Collagen, Inflammation, Platycodin D, Sebum
INTRODUCTION
Platycodon grandiflorum is a flowering plant native to East Asia. While its root has traditionally served culinary purposes, it also has uses in oriental medicine, namely, in decreasing sputum production, as well as cholesterol and blood sugar levels. Recent studies have revealed that platycodin D, a substance extracted from the root of Platycodon grandiflorum, has pharmacological properties, such as anti-cancer effect1,2. Platycodin D also inhibits lipogenesis in 3T3-L1 apidocytes by modulating KLF-2 and PPAR-γ23. Based on the similarities of adipocytes and sebocytes, we investigated the effects of platycodin D on the two major factors of acne pathogenesis: sebum production and inflammation.
In this research, we investigated the sebosuppressive and anti-inflammatory effects of platycodin D by identifying the related upstream and downstream molecules. Specifically, we hypothesized that sterol-regulatory element binding protein (SREBP), a major transcriptional factor responsible for the regulation of cholesterol and fatty acids, is crucial in the mechanism of the anti-lipogenic effects of platycodin D. To identify upstream regulators responsible for platycodin D-mediated suppression of the SREBP pathway, we focused on the insulin-like growth factor (IGF)-1R/PI3K/Akt pathway because platycodin D modulates the PI3K/Akt pathway in various cell lines4,5. To examine the anti-inflammatory effects, we investigated the correlation between the dose of platycodin D and levels of several transcription factors that regulate the inflammatory response.
MATERIALS AND METHODS
SEB-1 sebocyte culture
Immortalized SEB-1 sebocytes were generated by transfection of secondary sebocytes with SV40 large T antigens6. Generated SEB-1 sebocytes were cultured and maintained in standard culture medium with DMEM (Invitrogen, Carlsbad, CA, USA), 5.5 mM glucose/Ham's F-12 3:1 (Invitrogen), fetal bovine serum 2.5% (HyClone, Logan, UT, USA), adenine 1.8×10−4 M (Sigma, St Louis, MO, USA), hydrocortisone 0.4 µg ml−1 (Sigma), insulin 10 ng ml−1 (Sigma), epidermal growth factor 3 ng ml−1 (Austral Biologicals, San Ramon, CA, USA), and cholera toxin 1.2×10−10 M (Sigma) at 37℃ in a 5% CO2 incubator.
Fibroblast and HaCaT cell culture
The Detroit 551 cells (human embryonic skin fibroblast cell line, ATCC CCL-110) were maintained in Eagle's minimum essential medium and Dulbecco's modified Eagle's medium (DMEM) (Sigma) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, San Diego, CA, USA), at 37℃ and in a humidified atmosphere containing 5% CO2. The HaCaT cells were cultured as monolayers in a standard culture medium (DMEM) supplemented with 5% fetal calf serum, 2 mM glutamine, and 100 IU/ml penicillin at 37℃ in a humidified atmosphere containing 5% CO2.
Western blot
Protein was extracted using cell lysis buffer (Cell Signaling Technology, Beverly, MA, USA). Protein content in the lysate was determined using the BCA Protein Assay (Pierce, Rockford, IL, USA). Equal amounts of protein were run on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and then transferred to a polyvinylidene difluoride membrane. The following antibodies were used: matrix metalloproteinase (MMP)-1, SREBP-1, and Keratin 16 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), nuclear factor kappa B (NF-kB) p65, interleukin (IL)-1α, IL-8, IL-6, collagen1, adiponectin, and phosphorylated insulin- like growth factor 1 receptor (Abcam, Cambridge, MA, USA), phospho ERK, Akt, and PI3K (Cell Signaling Technology), MMP-7, and MMP-12 (Thermo, Pittsburgh, PA, USA). Secondary anti-rabbit immunoglobulin G (IgG) and anti-mouse IgG antibody (Cell Signaling Technology) were used to detect primary antibodies. Films of blots were analyzed and quantified using a densitometric program (TINA, Raytest Isotopenmebgerate, Straubenhardt, Germany). All experiments were repeated a minimum of four times.
CCK8 assay of sebocyte, fibroblast and keratinocyte cell viability
SEB-1 cells were seeded in each well of a 96-well tissue culture plate at a density of 1×104 cells per well. Fibroblasts and HaCaT keratinocytes were seeded in each well of a 96-well tissue culture plate with confluence of 90% per well. The medium was replaced with new medium containing each concentration of platycodin D (Cayman Chemical, Ann Arbor, MI, USA). The cells were incubated for 24, 48, and 72 hours. For the Cell Counting Kit-8 (CCK8) assay, 10 µl of CCK8 solution (Dojindo Molecular Technologies, Rockville, MD, USA) was added to the wells and reacted for 3 hours at 37℃. Absorbance at 450 nm was measured by a spectrophotometer. All experiments were repeated a minimum of four times.
Statistical analysis
We compared different groups from the in vitro experiments using Kruskal-Wallis test with SPSS software (version 23, IBM Co., Armonk, NY, USA).
RESULTS
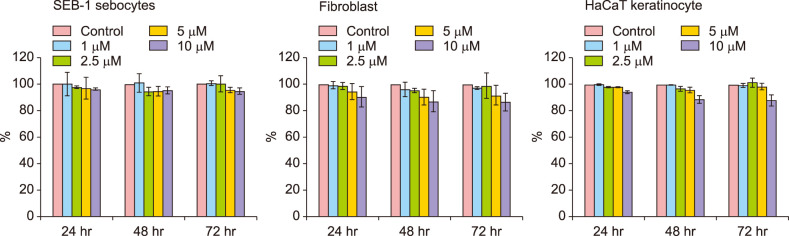
Platycodin D exhibited no cytotoxicity on sebocytes, fibroblasts, and keratinocytes
When treated with platycodin D, no toxicity was observed on SEB-1 sebocytes, fibroblasts and keratinocytes. Although the number of sebocytes and fibroblasts decreased slightly as the concentration of platycodin D used increased, the correlation was statistically insignificant (p>0.05) (Fig. 1).
Fig. 1. Cell Counting Kit-8 assay results. Platycodin D exhibited no cytotoxicity on sebocytes, fibroblasts and keratinocytes. There was no significant decrease in cell counts. All experiments were repeated a minimum of four times.
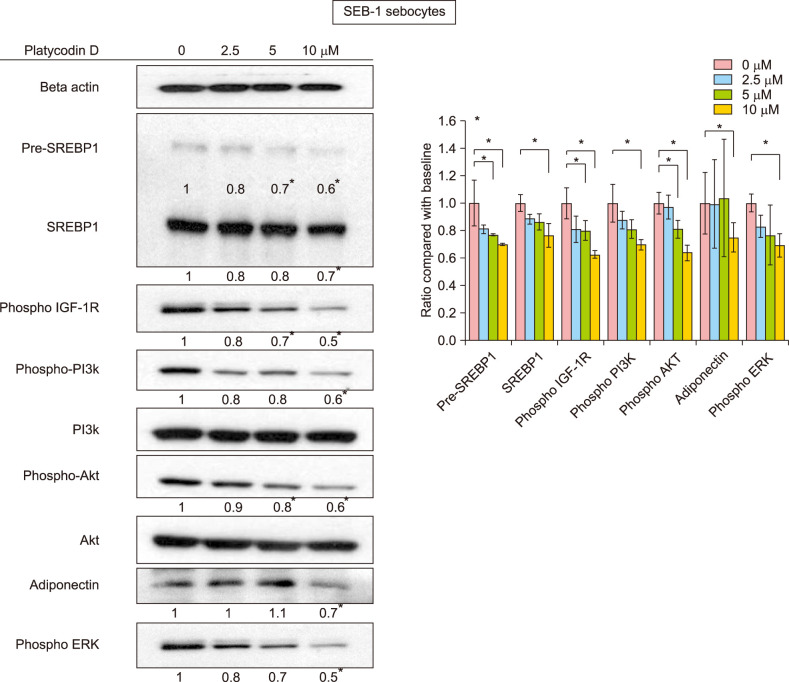
Platycodin D downregulated lipogenesis through various pathways, including the IGF-1R/PI3K/Akt/SREBP pathway
To examine the sebosuppressive effect of platycodin D, we focused on the IGF-1R/PI3K/Akt pathways and SREBP-1, which is the master regulator of lipid metabolism in sebocytes7. Platycodin D significantly decreased the expression of precursors and the mature forms of SREBP-1 proteins, as evidenced from western blot results (p<0.05) (Fig. 2). Specifically, platycodin D downregulated the IGF-1R/PI3K/Akt pathway in a dose-dependent manner after 6 hours, and SREBP-1 protein levels were subsequently decreased (p<0.05). In addition, platycodin D decreased adiponectin (p<0.05), which was recently discovered to regulate lipid production in human sebocytes8. ERK was also significantly decreased by platycodin D treatment (p<0.05).
Fig. 2. Insulin-like growth factor (IGF)-1R, PI3K, Akt, SREBP-1, adiponectin, and ERK levels in platycodin D-treated SEB-1 sebocytes. All precursor and mature forms of SREBP-1, phosphorylated PI3K, phosphorylated Akt, adiponectin, and phosphorylated ERK were decreased by platycodin D. *p<0.05 between control and each concentration of platycodin D-treated groups. All experiments were repeated a minimum of four times.
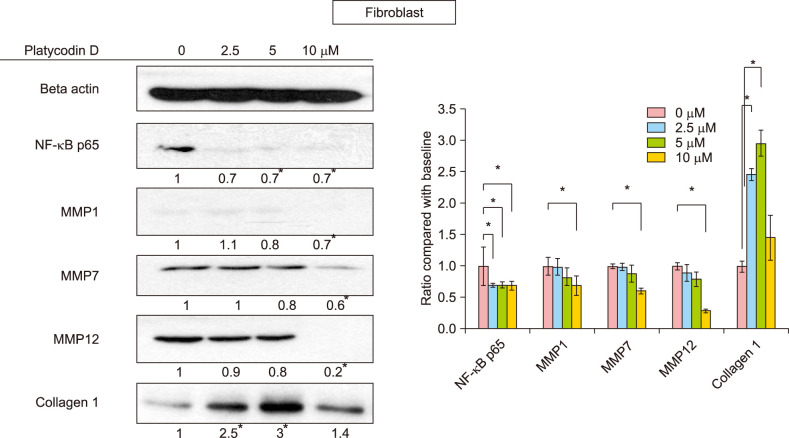
Platycodin D increases collagen levels and modulates inflammation in fibroblasts by inhibiting NF-κB and MMP
Platycodin D increased the level of collagen 1, and the maximum increase in collagen 1 levels was observed at 5 µM platycodin D (p<0.05). As inflammation plays an important role in the pathogenesis of acne, we examined several transcriptional factors for the regulation of the inflammatory response. Among them, NF-κB p65 was decreased in a dose-dependent manner after platycodin D treatment in fibroblasts (p<0.05). In addition, MMP-1 and MMP-7, which are known as collagenases, and MMP-12, which is an elastase, were decreased in platycodin D-treated fibroblasts (p<0.05) (Fig. 3).
Fig. 3. NF-κB p65, matrix metalloproteinase (MMP)-1, 7, 12, and collagen 1 levels in platycodin D-treated fibroblasts. NF-κB p65 levels significantly decreased after treatment with 2.5 µM platycodin D. MMP-1, 7, and 12 were significantly decreased in 10 µM of platycodin D. Collagen 1 was increased by platycodin D treatment, and the expression peaked at 5 µM platycodin D. *p<0.05 between control and each concentration of platycodin D-treated groups. All experiments were repeated a minimum of four times.
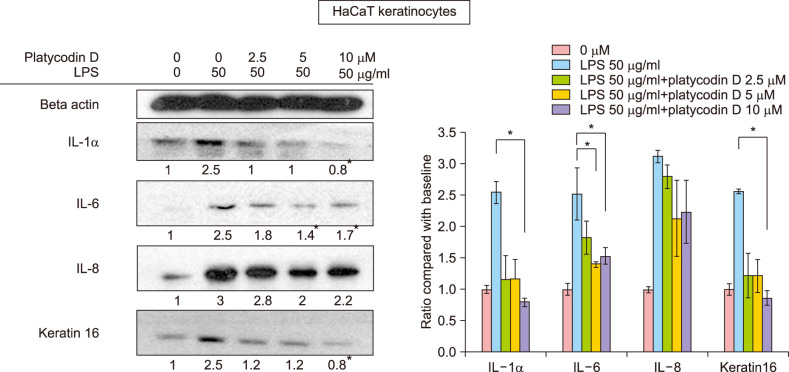
Platycodin D suppresses the increase in the levels of cytokines and keratin 16 induced by lipopolysaccharide (LPS)
HaCaT keratinocytes were stimulated by LPS, resulting in the production of IL-1α, IL-6, and IL-8. Platycodin D treatment suppressed the increase of IL-6 levels significantly at 5 µM and 10 µM (p<0.05) (Fig. 4). IL-8 also showed a tendency to decrease with platycodin D treatment but it was not statistically significant. IL-1α and keratin 16 decreased significantly below the baseline values following treatment with 10 µM of platycodin D (p<0.05).
Fig. 4. Interleukin (IL)-1α, IL-6, IL-8, and keratin 16 levels in platycodin D-treated HaCaT keratinocytes. IL-1α, IL-6, and keratin 16 were significantly decreased by platycodin D treatment. *p<0.05 between lipopolysaccharide (LPS) treatment only and each concentration of platycodin D-treated groups. All experiments were repeated a minimum of four times.
DISCUSSION
Acne is one of the most commonly occurring skin diseases. Its pathogenesis is characterized by four factors: excessive sebum production, inflammation, follicular hyperkeratinization and overgrowth of Propionibacterium acnes. Despite abundant research on the treatment of acne, very few drugs can target the multiple pathogenic factors listed above. In this study, we demonstrated that platycodin D can downregulate lipogenesis, as well as modulate inflammation.
This study was performed in line with recent research seeking new treatment options from natural products9,10. It had been elucidated that activation of the IGF-1R/PI3K/Akt pathway is related with lipogenesis in SEB-1 sebocytes7,9,10. Platycodin D showed sebosuppressive effects through the IGF-1R/PI3K/Akt/SREBP-1 pathway in SEB-1 sebocytes in our study. Platycodin D downregulated the IGF-1R pathway, and subsequently inhibited the PI3K/Akt pathway, which led to the reduction of SREBP-1, for both precursor and mature forms. This indicated that, as a result, cholesterol and fatty acid metabolism in sebocytes may have been decreased11. Although we were not able to perform fatty acid analysis, changes in the concentration of free fatty acids such as palmitoic acid and linoleic acid may also lead to alleviation of inflammation9.
It should be noted that inhibition of the IGF-1R/PI3K/ Akt/SREBP-1 pathway may not be the only mechanism of platycodin D-induced inhibition of lipogenesis. Jung et al.8 discovered that adiponectin regulates lipogenesis in human sebocytes via the APPL-AMPK/Akt/SREBP-1 pathway. In our study, adiponectin expression was decreased in platycodin D-treated SEB-1 sebocytes. This suggests the involvement of the adiponectin pathway in the platycodin D-induced inhibition of lipogenesis. The activation of ERK in sebocytes was known to be associated with IGF-1 stimulation of sebocytes, resulting in the activation of SREBP-15. In our study, ERK was also significantly decreased by platycodin D treatment.
We also found that platycodin D modulates inflammation through inhibition of the NF-κB pathway. Many previous studies showed that NF-κB significantly increased after P. acnes treatment, suggesting that NF-κB induces pro-inflammatory cytokine production10,12. Decreased expression of NF-κB will lead to decreased production of pro-inflammatory cytokines including IL-1α, IL-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α. Previous studies have shown that adiponectin not only suppresses the secretion of pro-inflammatory cytokines like TNF-α, but also induces expression of anti-inflammatory cytokines such as IL-10 in keratinocytes. They also revealed that adiponectin plays some roles in cutaneous wound healing processes13. However, since adiponectin expression was decreased by platycodin D treatment in our experiment, it may not have a significant effect on the inflammatory process of acne. Aydin et al have studied adiponectin levels in severe acne patients14 but additional researches will be necessary to investigate the role of adiponectin in inflammation of acne. Acne inflammation, which involves the activation of NF-kB and MMPs, is closely associated with acne scar formation. Platycodin D decreased expression of MMP-1, 7, 12. As MMPs are known to play important roles in the formation of acne scarring, decreased levels of MMPs can reduce the probability of acne scar formation15,16. Previous studies showed that P. acnes augment the expression of MMP-1, 2, 9, 13 in various cell lines17,18. In our experiment, we discovered that MMP-7, a collagenase, and MMP-12, an elastase, were decreased by platycodin D treatment. As elastic fiber is important for maintaining the tension of the skin, a decrease in the expression of an elastase may contribute to the treatment of acne scars. Furthermore, collagen 1 was increased by platycodin D. All of these results for fibroblasts suggest that platycodin D may prevent the formation of acne scars.
Platycodin D suppressed the increase of IL-1α, IL-6, and IL-8 in keratinocytes stimulated by LPS. Although the suppressive effect was not strong enough to restore IL-6 and IL-8 to baseline values, it has some anti-inflammatory effects. In addition, IL-1α is a strong inducer of hypercornification of the hair follicle infundibulum and keratin 16 is a marker of abnormal differentiation in keratinocytes10. The decrease of IL-1α and keratin 16 by platycodin D may suggest its ability to correct epidermal hyperkeratinization/dyskeratinization, which is one of the pathogenetic factors of acne.
Concerning safety issues, we demonstrated that platycodin D has no significant cytotoxic effects on sebocytes, fibroblasts and keratinocytes. Because Platycodon grandiflorum has been used as a food material in East Asia, including Korea, the nontoxicity of platycodin D was also empirically tested.
In conclusion, platycodin D can affect the key pathogenic factors of acne, including at least hyperseborrhea and inflammation. In addition, it increases collagen and inhibits MMPs. These results suggest that platycodin D may be used as an effective modality of acne treatment and acne scar prevention.
ACKNOWLEDGMENT
We greatly appreciate permission of use of SEB-1 by Prof. Diane M Thiboutot.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Li T, Chen X, Dai XY, Wei B, Weng QJ, Chen X, et al. Novel Hsp90 inhibitor platycodin D disrupts Hsp90/Cdc37 complex and enhances the anticancer effect of mTOR inhibitor. Toxicol Appl Pharmacol. 2017;330:65–73. doi: 10.1016/j.taap.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Wang B, Gao Y, Zheng G, Ren X, Sun B, Zhu K, et al. Platycodin D inhibits interleukin-13-induced the expression of inflammatory cytokines and mucus in nasal epithelial cells. Biomed Pharmacother. 2016;84:1108–1112. doi: 10.1016/j.biopha.2016.10.052. [DOI] [PubMed] [Google Scholar]
- 3.Lee EJ, Kang M, Kim YS. Platycodin D inhibits lipogenesis through AMPKα-PPARγ2 in 3T3-L1 cells and modulates fat accumulation in obese mice. Planta Med. 2012;78:1536–1542. doi: 10.1055/s-0032-1315147. [DOI] [PubMed] [Google Scholar]
- 4.Xu C, Sun G, Yuan G, Wang R, Sun X. Effects of platycodin D on proliferation, apoptosis and PI3K/Akt signal pathway of human glioma U251 cells. Molecules. 2014;19:21411–21423. doi: 10.3390/molecules191221411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin H, Du X, Zhang Y, Wang R. Platycodin D, a triterpenoid saponin from platycodon grandiflorum, induces G2/M arrest and apoptosis in human hepatoma HepG2 cells by modulating the PI3K/Akt pathway. Tumour Biol. 2014;35:1267–1274. doi: 10.1007/s13277-013-1169-1. [DOI] [PubMed] [Google Scholar]
- 6.Thiboutot D, Jabara S, McAllister JM, Sivarajah A, Gilliland K, Cong Z, et al. Human skin is a steroidogenic tissue: steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1) J Invest Dermatol. 2003;120:905–914. doi: 10.1046/j.1523-1747.2003.12244.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith TM, Gilliland K, Clawson GA, Thiboutot D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286–1293. doi: 10.1038/sj.jid.5701155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung YR, Lee JH, Sohn KC, Lee Y, Seo YJ, Kim CD, et al. Adiponectin signaling regulates lipid production in human sebocytes. PLoS One. 2017;12:e0169824. doi: 10.1371/journal.pone.0169824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon JY, Kwon HH, Min SU, Thiboutot DM, Suh DH. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J Invest Dermatol. 2013;133:429–440. doi: 10.1038/jid.2012.292. [DOI] [PubMed] [Google Scholar]
- 10.Kwon HH, Yoon JY, Park SY, Min S, Kim YI, Park JY, et al. Activity-guided purification identifies lupeol, a pentacyclic triterpene, as a therapeutic agent multiple pathogenic factors of acne. J Invest Dermatol. 2015;135:1491–1500. doi: 10.1038/jid.2015.29. [DOI] [PubMed] [Google Scholar]
- 11.Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y, et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem. 1999;274:35832–35839. doi: 10.1074/jbc.274.50.35832. [DOI] [PubMed] [Google Scholar]
- 12.Lee WR, Kim KH, An HJ, Kim JY, Chang YC, Chung H, et al. The protective effects of melittin on propionibacterium acnes-induced inflammatory responses in vitro and in vivo. J Invest Dermatol. 2014;134:1922–1930. doi: 10.1038/jid.2014.75. [DOI] [PubMed] [Google Scholar]
- 13.Kovács D, Lovászi M, Póliska S, Oláh A, Bíró T, Veres I, et al. Sebocytes differentially express and secrete adipokines. Exp Dermatol. 2016;25:194–199. doi: 10.1111/exd.12879. [DOI] [PubMed] [Google Scholar]
- 14.Aydin K, Çetinözman F, Elcin G, Aksoy DY, Ucar F, Yildiz BO. Suppressed adiponectin levels and increased adiponectin response to oral glucose load in lean women with severe acne normalizes after isotretinoin treatment. Dermatology. 2017;233:314–319. doi: 10.1159/000484168. [DOI] [PubMed] [Google Scholar]
- 15.Papakonstantinou E, Aletras AJ, Glass E, Tsogas P, Dionyssopoulos A, Adjaye J, et al. Matrix metalloproteinases of epithelial origin in facial sebum of patients with acne and their regulation by isotretinoin. J Invest Dermatol. 2005;125:673–684. doi: 10.1111/j.0022-202X.2005.23848.x. [DOI] [PubMed] [Google Scholar]
- 16.Sato T, Shirane T, Noguchi N, Sasatsu M, Ito A. Novel anti-acne actions of nadifloxacin and clindamycin that inhibit the production of sebum, prostaglandin E(2) and promatrix metalloproteinase-2 in hamster sebocytes. J Dermatol. 2012;39:774–780. doi: 10.1111/j.1346-8138.2012.01525.x. [DOI] [PubMed] [Google Scholar]
- 17.Jalian HR, Liu PT, Kanchanapoomi M, Phan JN, Legaspi AJ, Kim J. All-trans retinoic acid shifts propionibacterium acnes-induced matrix degradation expression profile toward matrix preservation in human monocytes. J Invest Dermatol. 2008;128:2777–2782. doi: 10.1038/jid.2008.155. [DOI] [PubMed] [Google Scholar]
- 18.Choi JY, Piao MS, Lee JB, Oh JS, Kim IG, Lee SC. Propionibacterium acnes stimulates pro-matrix metalloproteinase-2 expression through tumor necrosis factor-alpha in human dermal fibroblasts. J Invest Dermatol. 2008;128:846–854. doi: 10.1038/sj.jid.5701188. [DOI] [PubMed] [Google Scholar]