Abstract
Background
Bullous pemphigoid (BP) is an autoimmune subepidermal blistering disease characterized by tissue-bound and circulating autoantibodies directed against BP180 and/or BP230 antigens. Various inflammatory cells are involved in the development of blister in BP.
Objective
The aim of this study was to evaluate the correlation between peripheral leukocyte counts and BP severity.
Methods
We retrospectively included 60 patients with BP, who had not been treated with systemic steroid at the time of blood sampling. The patients were classified into two groups, those with admission history (admission group) and those without admission history (non-admission group). Disease severity was evaluated using three parameters: admission history, initial steroid dosage, and modified version of a pemphigus scoring system. We evaluated the correlation between peripheral leukocyte counts and disease severity measured by the three parameters.
Results
The admission group showed a significant increase in disease severity measured by initial steroid dosage and severity score compared with the non-admission group. Additionally, the admission group had increased total leukocyte, eosinophil, and neutrophil counts. In the correlation study, the peripheral eosinophil and neutrophil counts showed positive correlation with BP severity evaluated by both initial steroid dosage and the pemphigus scoring system.
Conclusion
Peripheral eosinophil and neutrophil counts can be used as a marker in predicting disease severity in patients with BP.
Keywords: Bullous pemphigoid, Eosinophil, Leukocyte, Neutrophil
INTRODUCTION
Bullous pemphigoid (BP) is the most common autoimmune subepidermal blistering disease affecting the elderly. BP is characterized by tissue-bound and circulating autoantibodies directed against the structural components of hemidesmosomes, BP180 and BP230, in the dermoepidermal junction1. According to an international guideline, corticosteroid is the first-line treatment for BP2. Since BP is a chronic disease frequently affecting elderly persons, long-term immunosuppressive treatment is one of the major causes of disease-related morbidity and mortality. To avoid inadequate immunosuppressive treatment, the precise prediction factors that correlate with disease severity could be useful in clinical decision making3.
The classic form of BP is characterized by tense large blisters and pruritic urticarial patches. Previous studies demonstrated that wide spectra of inflammatory cells and cytokines were involved in the pathogenesis of BP4 and a significant increase in peripheral eosinophil has been reported in patients with BP compared with healthy controls5. Herein, we evaluated the correlation between peripheral leukocyte count and severity of BP in the patients, measured by admission history, initial steroid dosage, and disease activity score.
MATERIALS AND METHODS
Patients
Medical records of 85 patients who were first diagnosed with BP between January 2012 and December 2016 at the Department of Dermatology, Gangnam Severance Hospital, were reviewed. BP was diagnosed by characteristic clinical findings, histological evidence of subepidermal blisters with inflammatory cell infiltration, linear deposition of immunoglobulin G (IgG) and/or C3 along the basement membrane zone (BMZ) by direct immunofluorescence, and IgG deposition on the epidermal side of salt split skin by indirect immunofluorescence.
Sixty patients (34 men, 26 women; mean age 74.7±12.8, range 30~94) who had not been treated with corticosteroid at the time of blood sampling were enrolled in this study. Among the 60 patients, clinical photographs of the whole body were available for 44 (23 men, 21 women; mean age 77.7±10.0, range 45~94) and involvement of body surface area could be assessed. This study was approved by the Institutional Review Board of Gangnam Severance Hospital, Yonsei University (IRB no. 320150186).
Clinical assessment
The results of the total white blood cell (WBC) and differential leukocyte (eosinophil, neutrophil, lymphocyte, and monocyte) counts at the time of diagnosis were examined in 60 patients with BP. To evaluate disease severity, we examined the initial corticosteroid (methylprednisolone) dosage and calculated the severity scores of 44 patients using the modified version of the pemphigus scoring system designed by Herbst and Bystryn (Table 1)6.
Table 1. Modified version of pemphigus scoring system used to evaluate disease activity score of BP.
| Extent of disease (0~7) | Intensity of therapy (1~6) | |
|---|---|---|
| Number of following body areas where the BP skin lesion involved (0~6): scalp, face/neck, chest/abdomen, back, arm, leg | MPD | <8 mg: +1 |
| 8~24 mg: +2 | ||
| >24 mg: +3 | ||
| Dapsone, minocycline, nicotinamide: +1 | ||
| BP lesion involved in oral mucosa: +1 | MPD pulse therapy, IVIG: +2 | |
BP: bullous pemphigoid, MPD: methylprednisolone, IVIG: intravenous immunoglobulin. Disease activity score: 1~4 points=mild, 5~8 points=moderate, 9~13 points=severe.
First, 60 patients were classified into two groups, one group with admission history (admission group) and the other group without admission history (non-admission group). Disease severity, total WBC, and differential leukocyte counts were compared in the two groups. Second, correlation of initial steroid dosage with differential leukocyte count was evaluated in 60 patients. Third, correlation between severity score and leukocyte count was measured in 44 patients in whom the modified version of the pemphigus scoring system could be assessed.
Statistical methods
Comparison of the admission and non-admission groups was evaluated using Mann-Whitney U-test. Correlations between differential leukocytes with initial steroid dosage and severity score were performed by Spearman correlation test. All statistical analyses were performed by GraphPad Prism 5 program (GraphPad Software, La Jolla, CA, USA), and a p-value<0.05 was considered to be significant.
RESULTS
Difference between the admission and non-admission groups
Among the 60 patients enrolled in this study, 19 had admission history (12 men, 7 women; mean age 77.1±12.2, range 45~93) and 41 had no history of admission (22 men, 19 women; mean age 73.6±13.0, range 30~94). In 44 patients who were assessed using the modified version of the pemphigus scoring system, 16 were included in the admission group and 28 were included in the non-admission group.
As expected, the admission group was prescribed significantly higher doses of systemic corticosteroid than the non-admission group (mean±standard deviation [SD]: 39.34±13.87 vs. 15.17±13.23, p<0.0001) (Fig. 1A). In the calculated severity scores, the admission group showed significantly a higher severity score than the non-admission group (mean±SD: 9.50±1.21 vs. 5.61±2.01, p<0.0001) (Fig. 1B).
Fig. 1. Difference in (A) initial methylprednisolone (MPD) dosage and (B) severity score calculated using the modified version of pemphigus scoring system between the admission and non-admission groups.
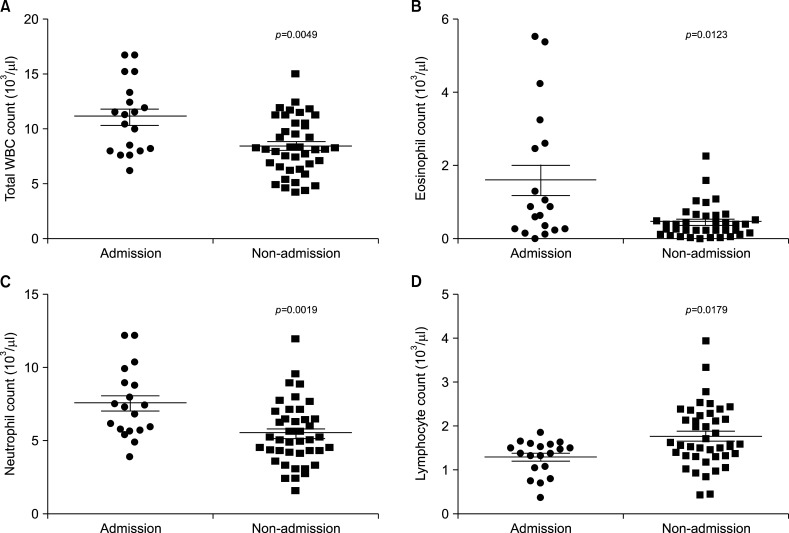
In the comparison of the total WBC count and differential leukocyte counts, the admission group showed significantly increased total WBC (mean±SD: 11.14±3.24 vs. 8.48±2.56, p=0.0049), eosinophil (mean±SD: 1.61±1.80 vs. 0.46±0.44, p=0.0123), and neutrophil counts (mean±SD: 7.56±2.35 vs. 5.49±2.12, p=0.0019) compared with the non-admission group. Lymphocyte counts were adversely decreased in the admission group compared with that of the non-admission group (mean±SD: 1.30±0.39 vs. 1.77±0.72, p=0.0179) (Fig. 2).
Fig. 2. Difference in (A) total white blood cell (WBC), (B) eosinophil, (C) neutrophil, and (D) lymphocyte counts between the admission and non-admission groups.
Correlation between initial steroid dosage and circulating leukocyte counts
In the enrolled 60 patients, the initial steroid dosage prescribed to the patients at the time of the diagnosis was retrospectively reviewed. Positive correlations were found between total WBC (rs=0.40, p=0.0016), eosinophil (rs=0.49, p<0.0001), and neutrophil (rs=0.44, p=0.004) counts and initial steroid dosage (Fig. 3A~C). Lymphocyte (rs=−0.16, p=0.21) (Fig. 3D) and monocyte counts (rs= −0.18, p=0.17) failed to show significant correlation with initial steroid dosage.
Fig. 3. Correlation between the peripheral (A) total WBC, (B) eosinophil, (C) neutrophil, and (D) lymphocyte counts and the initial steroid dosage in 60 patients with BP. WBC: white blood cell, BP: bullous pemphigoid, MPD: methylprednisolone.
Correlation between severity score and circulating leukocyte counts
In 44 patients with clinical photographs, severity score was calculated with the modified version of the pemphigus scoring system. Assessing the correlation between the total and differential leukocyte counts and the severity score, positive correlations was found in eosinophil (rs=0.40, p=0.0075) and neutrophil counts (rs=0.36, p=0.017) (Fig. 4B, C). Total WBC (rs=0.27, p=0.077) (Fig. 4A), lymphocyte (rs=−0.20, p=0.20) (Fig. 4D), and monocyte counts (rs=−0.14, p=0.35) failed to show significantly correlation with the severity assessed in this study.
Fig. 4. Correlation between the peripheral (A) total WBC, (B) eosinophil, (C) neutrophil, and (D) lymphocyte count and the severity scores in 44 patients with BP. WBC: white blood cell, BP: bullous pemphigoid.
DISCUSSION
he pathomechanism of blister formation in BP may be summarized in four steps: complement activation, recruitment of inflammatory cells, release of proteolytic enzymes, and direct interference with the adhesive proteins in BMZ7. Consistent with pathogenesis of BP, lesional skin of BP shows subepidermal blisters with inflammatory infiltration consisting of lymphocytes, histiocytes, neutrophils, and eosinophils. In the mouse model of BP, neutrophils line up along the BMZ and release proteolytic enzymes such as neutrophil elastase, cathepsine G, collagenase, and matrix metalloproteinases, leading to the breakdown of dermal-epidermal cohesion8. Eosinophils are found in BP skin lesions and produce matrix metalloproteinases, elastase, and gelatinase, contributing to tissue damage7.
Urticarial lesions of BP show eosinophil-dominated lesions similar to arthropod bite reaction, parasitic infestation, and hypereosinophilic syndrome9. Interestingly, in addition to lesional skin, approximately 50% of patients with BP have blood eosinophilia10, and peripheral eosinophil counts are reported to positively correlate with BP180 enzyme-linked immunosorbent assay (ELISA) scores11, supporting the significance of these cells in BP pathogenesis5. Recently, elevated number of eosinophils in the serum of patients with BP was shown to be correlated with interleukin 31 serum levels, which mediates pruritus and promotes inflammation12. Although neutrophil is not a dominant feature in BP pathology, purified IgG from patients with BP is able to recruit neutrophils to the BMZ and induce subepidermal separation in ex vivo study, speculating the role of neutrophils in blister formation13.
There are several cytokines and molecules reported in association with BP activity and severity. Increased serum levels of interleukin (IL)-4, IL-5, and CD30 were shown in BP patients, and IL-5 concentration in the blister fluid correlated with disease severity14. IL-5-preactivated eosinophils release mediators involved in blister formation15 and elevation in eosinophil granule proteins, such as eosinophil cationic protein (ECP), have been shown in the sera of patients with active BP16. Serum ECP level is a marker of eosinophil activation and shows positive correlation with peripheral eosinophil counts in various skin diseases17. In a recent report, serum concentration of ECP demonstrated clinical relevance with disease severity and outcomes in BP patients, highlighting importance of eosinophil activation in BP pathogenesis16.
In this report, we aimed to find the correlation between various leukocytes and BP severity evaluated by initial steroid dosage and disease activity score. Severity evaluated by initial steroid dosage and disease activity score showed significant positive correlation with peripheral eosinophil and neutrophil counts. Lymphocyte count failed to show any correlation with disease severity and expected to have less significance in pathogenesis of blister formation in BP. Such results showed that the number of neutrophils and eosinophils in the peripheral blood can be used as a marker for predicting disease activity in initial workup and during treatment.
In conclusion, this study shows that circulating eosinophil and neutrophil counts, acquirable from simple and inexpensive laboratory study, correlate with baseline BP severity and could be helpful in estimating pre-treatment severity and deciding initial steroid dosage. This is the first study to show correlations of various leukocytes with BP severity using severity scoring system. To improve our understanding of the role of circulating leukocytes in patients with BP, further study evaluating the correlation between differential leukocyte counts and disease severity during the treatment is needed.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- 2.Feliciani C, Joly P, Jonkman MF, Zambruno G, Zillikens D, Ioannides D, et al. Management of bullous pemphigoid: the European dermatology forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172:867–877. doi: 10.1111/bjd.13717. [DOI] [PubMed] [Google Scholar]
- 3.Bağcı IS, Horváth ON, Ruzicka T, Sárdy M. Bullous pemphigoid. Autoimmun Rev. 2017;16:445–455. doi: 10.1016/j.autrev.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Bieber K, Ernst AL, Tukaj S, Holtsche MM, Schmidt E, Zillikens D, et al. Analysis of serum markers of cellular immune activation in patients with bullous pemphigoid. Exp Dermatol. 2017;26:1248–1252. doi: 10.1111/exd.13382. [DOI] [PubMed] [Google Scholar]
- 5.Rifaioglu EN, Sen BB, Ekiz Ö, Dogramaci AC. Mean platelet volume and eosinophilia relationship in patients with bullous pemphigoid. Platelets. 2014;25:264–267. doi: 10.3109/09537104.2013.784735. [DOI] [PubMed] [Google Scholar]
- 6.Herbst A, Bystryn JC. Patterns of remission in pemphigus vulgaris. J Am Acad Dermatol. 2000;42:422–427. doi: 10.1016/s0190-9622(00)90213-5. [DOI] [PubMed] [Google Scholar]
- 7.Lo Schiavo A, Ruocco E, Brancaccio G, Caccavale S, Ruocco V, Wolf R. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391–399. doi: 10.1016/j.clindermatol.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Kasperkiewicz M, Zillikens D. The pathophysiology of bullous pemphigoid. Clin Rev Allergy Immunol. 2007;33:67–77. doi: 10.1007/s12016-007-0030-y. [DOI] [PubMed] [Google Scholar]
- 9.Wick MR. Disorders characterized by predominant or exclusive dermal inflammation. Semin Diagn Pathol. 2017;34:273–284. doi: 10.1053/j.semdp.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Annu Rev Pathol. 2016;11:175–197. doi: 10.1146/annurev-pathol-012615-044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee EH, Kim YH, Kim S, Kim SE, Kim SC. Usefulness of enzyme-linked immunosorbent assay using recombinant BP180 and BP230 for serodiagnosis and monitoring disease activity of bullous pemphigoid. Ann Dermatol. 2012;24:45–55. doi: 10.5021/ad.2012.24.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salz M, Haeberle S, Hoffmann J, Enk AH, Hadaschik EN. Elevated IL-31 serum levels in bullous pemphigoid patients correlate with eosinophil numbers and are associated with BP180-IgE. J Dermatol Sci. 2017;87:309–311. doi: 10.1016/j.jdermsci.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Sitaru C, Schmidt E, Petermann S, Munteanu LS, Bröcker EB, Zillikens D. Autoantibodies to bullous pemphigoid antigen 180 induce dermal-epidermal separation in cryosections of human skin. J Invest Dermatol. 2002;118:664–671. doi: 10.1046/j.1523-1747.2002.01720.x. [DOI] [PubMed] [Google Scholar]
- 14.Giusti D, Le Jan S, Gatouillat G, Bernard P, Pham BN, Antonicelli F. Biomarkers related to bullous pemphigoid activity and outcome. Exp Dermatol. 2017;26:1240–1247. doi: 10.1111/exd.13459. [DOI] [PubMed] [Google Scholar]
- 15.de Graauw E, Sitaru C, Horn M, Borradori L, Yousefi S, Simon HU, et al. Evidence for a role of eosinophils in blister formation in bullous pemphigoid. Allergy. 2017;72:1105–1113. doi: 10.1111/all.13131. [DOI] [PubMed] [Google Scholar]
- 16.Giusti D, Gatouillat G, Le Jan S, Plée J, Bernard P, Antonicelli F, et al. Eosinophil cationic protein (ECP), a predictive marker of bullous pemphigoid severity and outcome. Sci Rep. 2017;7:4833. doi: 10.1038/s41598-017-04687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim TY, Park HJ, Kim CW. Eosinophil cationic protein (ECP) level and its correlation with eosinophil number or IgE level of peripheral blood in patients with various skin diseases. J Dermatol Sci. 1997;15:89–94. doi: 10.1016/s0923-1811(97)00614-2. [DOI] [PubMed] [Google Scholar]