Tissue-resident memory T cells (TRM) patrol peripheral tissues and mediate a long-term protective immunity. Okła et al. review recent advances in TRM in the context of tumor immunity and immunotherapy.
Abstract
Tissue-resident memory T cells (TRM) represent a heterogeneous T cell population with the functionality of both effector and memory T cells. TRM express residence gene signatures. This feature allows them to traffic to, reside in, and potentially patrol peripheral tissues, thereby enforcing an efficient long-term immune-protective role. Recent studies have revealed TRM involvement in tumor immune responses. TRM tumor infiltration correlates with enhanced response to current immunotherapy and is often associated with favorable clinical outcome in patients with cancer. Thus, targeting TRM may lead to enhanced cancer immunotherapy efficacy. Here, we review and discuss recent advances on the nature of TRM in the context of tumor immunity and immunotherapy.
Introduction
Tissue-resident memory T cells (TRM) reside in peripheral tissues, patrol their surroundings, and rapidly respond to alarming signals (Jiang et al., 2012; Schenkel et al., 2014; Park and Kupper, 2015; Clark, 2015; Dijkgraaf et al., 2019). These features enable them to potentially serve as critical players in antitumor surveillance and immunity. Early studies in viral infections have revealed that T cells were retained in tissues, including human and murine skin as well as murine small intestine and lung tissues (Zhu et al., 2007; Gebhardt et al., 2009; Masopust et al., 2010; Teijaro et al., 2011). In support of TRM tissue retention, TRM down-regulate the expression of markers for tissue egress and express surface molecules that enable their retention in tissues (Kumar et al., 2017; Park et al., 2019; Raphael et al., 2020; Byrne et al., 2020). TRM differentiation is guided by transcription programs common to both effector and memory T cells (Milner and Goldrath 2018), consistent with their persistence in tissues as memory T cells yet retaining rapid effector function for immune protection. TRM are defined in multiple infection models to be retained in tissues, as shown in parabiosis experiments in mice, antibody in vivo labeling, and in other approaches that mark and monitor tissue T cells (Szabo et al., 2019; Masopust and Soerens, 2019). Of note, the transcriptome signature of TRM suggests a “tissue-tailored” retention model rather than a “one size fits all” model, highlighting tissue- and organ-specific immune regulation, also named tissue- and organ-specific “immunostats” (Amsen et al., 2018; Pao et al., 2018). Tissue-specific adaptations may enable TRM to maintain homeostasis in a site-specific fashion. Intriguingly, transcriptionally and functionally distinct TRM subsets and TRM precursors have been observed in the murine small intestine during infection (Kurd et al., 2020). This suggests a high heterogeneity and complexity of TRM populations.
Compared with their protective role in infectious diseases (Wu et al., 2018; Wilk and Mills, 2018; Muruganandah et al., 2018; O’Hara et al., 2020), the importance and significance of TRM in tumor immunity are not adequately characterized. Nevertheless, recent studies in cancer-bearing mouse models have indicated a pivotal role of TRM in antitumor immunity in several tumor types (Nizard et al., 2017; Milner et al., 2017; Malik et al., 2017; Enamorado et al., 2017). Moreover, human studies show an association between tumor TRM and improved responses to immunotherapy and favorable clinical outcome in patients with cancer (Table 1). These findings fuel interest in TRM research in cancer immunology and immunotherapy. Here, we review the current understanding of TRM from mouse infection models and human studies and how these findings have been used to guide studies of TRM in tumor immunity, as well as implications for the role of TRM in immune surveillance and immunotherapies.
Table 1. Clinical relevance of TRM in patients with cancer.
| Cancer types | TRM phenotype | Correlation with clinical features | References |
|---|---|---|---|
| Breast cancer | CD8+, CD103+ | Intratumoral CD103+CD8+ T cells are positively associated with RFS and OS in estrogen receptor–negative basal-like breast cancer. | Wang et al., 2016 |
| CD8+ TRM gene signature is associated with improved DFS and OS in patients with TNBC. | Savas et al., 2018 | ||
| Levels of CD103+CD8+ T cells are higher in TNBCs in patients without tumor relapse than with tumor relapse. | Egelston et al., 2019 | ||
| Bladder cancer | CD8+, CD103+, CD69+, CD49a+, PD-1+ | Levels of CD103+CD8+ T cells are negatively associated with tumor size and are positively associated with OS and RFS. | Wang et al., 2015 |
| High TRM tumor infiltration is associated with lower tumor stage. | Hartana et al., 2018 | ||
| Cervical cancer | CD8+, CD103+, PD-1+, GzmB+ | CD103 expression is associated with improved DSS. | Komdeur et al., 2017 |
| Prognostic benefit of increased CD103 expression is observed in patients treated with radiotherapy. | |||
| Abundance of intraepithelial CD103+ cells is associated with improved DSS and DFS in patients with or without radio(chemo)therapy. | |||
| Colorectal cancer | CD8+, CD103+ | High density of CD103+ cells is associated with DFS. | Huang et al., 2017 |
| High density of CD103+ cells is negatively associated with OS and DFS in patients with KRAS WT tumors. | |||
| Endometrial cancer | CD8+, CD103+, PD-1+ | CD103+ cells are associated with prolonged DSS. | Workel et al., 2016 |
| Esophageal cancer | CD8+, CD103+, PD-1+, TIM3+ | High density of CD103hiCD8hi cells is associated with improved OS. | Han et al., 2020 |
| Gastric cancer | CD103+, CD69+, PD-1+, TIGIT+, CD39+ | Presence of CD8+CD103+ cells is associated with improved OS. | Lin et al., 2020 |
| Hepatocellular carcinoma | CD8+, CD103+ | High density of CD8+CD103+ cells is associated with improved OS. | Lim et al., 2019 |
| Head and neck cancer | CD8+, CD103+, CD39+ | High frequencies of CD103+CD39+ cells are associated with improved OS. | Duhen et al., 2018 |
| High expression of CD103 is associated with improved OS. | |||
| Lung cancer | CD8+, CD103+ CD69+, CD49a+, PD-1+, 4-1BB+, CXCR6+ CD39+, TIM3+ | High levels of CD103+ cells are associated with improved OS in total tumor and stromal region. | Djenidi et al., 2015 |
| High levels of CD103+ cells are associated with improved DFS in total tumor, epithelial tumor islets, and stromal region. | Ganesan et al., 2017 | ||
| High density of CD103+ cells is associated with improved OS. | Duhen et al., 2018 | ||
| High expression of both CD103 and CD39, as well as CD103 alone, is associated with improved OS. | Koh et al., 2017 | ||
| High numbers of intratumoral, but not stromal, CD103+ cells are associated with prolonged DFS and OS. | Clarke et al., 2019 | ||
| PD-1+TIM3+ cells are enriched in responders to anti–PD-1 therapy. | Nizard et al., 2017 | ||
| High intratumoral CD103+ cells are associated with improved OS. | |||
| Melanoma | CD8+, CD103+, CD69+, CD49a+, PD-1+, LAG-3+, GzmB+ | High levels of CD49a expression are associated with improved DFS and OS. | Murray et al., 2016; Edwards et al., 2018; Menares et al., 2019 |
| Increased numbers of CD69+CD103+CD8+ cells are associated with improved OS in immunotherapy-naive patients. | |||
| Enrichment of TRM gene signature is associated with improved OS. | |||
| Ovarian cancer | CD8+, CD103+, PD-1+, CD3+ | Presence of CD103+ cells is associated with increased DSS in HGSOC and mucinous cancers. | Webb et al., 2014 |
| Patients with HGSOC, containing both CD103+ and PD-1+ cells, are associated with increased DSS. | Webb et al., 2015 | ||
| CD103+ cell numbers are associated with improved DSS in patients treated with PS. | Bösmüller et al., 2016 | ||
| High infiltration of CD103+ cells in tumor parenchyma of primary tumors is associated with improved 10-yr OS. | Komdeur et al., 2016 | ||
| Santoiemma et al., 2016 | |||
| Pancreatic cancer | CD8+, CD103+ | Increased ratio of CD8+CD103+ cells to CD8+CD103− cells is associated with improved DFS. | Lohneis et al., 2017 |
DFS, disease-free survival; DSS, disease-specific survival; HGSOC, high-grade serous ovarian cancer; OS, overall survival; PS, primary surgery and adjuvant chemotherapy; RFS, relapse-free survival; TIGIT, T cell immunoreceptor with immunoglobulin and ITIM domains; TNBC, triple-negative breast cancer.
Identifying TRM
TRM are defined based on the expression of specific markers, a distinct transcriptional profile, and their functional retention in tissues, all properties that distinguish TRM from circulating memory T cells, including effector memory T cells (TEM) and central memory T cells (TCM). Based on this definition, CD8+ TRM are found to be a component of tumor-infiltrating lymphocytes (TILs) in patients with solid tumors (Byrne et al., 2020). However, it is important to note that TRM are loosely defined in different studies in the context of tumor immunity.
TRM express tissue retention markers, including CD69 (C-type lectin), αE(CD103)β7 (E-cadherin receptor), and α1(CD49a)β1 (VLA-1), and exhibit reduced expression of migration and tissue egress markers, such as CCR7, CD62L, and sphingosine-1-phosphate receptor 1 (S1PR1; Schenkel and Masopust, 2014; Kumar et al., 2017; Szabo et al., 2019). Other core markers identified in both human and mouse TRM include the chemokine receptor CXCR6, the inhibitory receptor programmed death receptor 1 (PD-1), and CD101, which have inhibitory function (Mackay et al., 2016; Kumar et al., 2017; Wein et al., 2019). The expression of TRM-associated markers differs between organs and tissue sites. CD103 is most highly expressed by CD8+ TRM in mucosal and barrier sites, is variably expressed by TRM in lung and skin, and is not expressed by TRM in lymphoid sites (Kumar et al., 2017; Steinert et al., 2015). PD-1 is highly expressed by human TRM in exocrine sites, such as the pancreas, and to a lesser extent by lymphoid TRM, but is not highly expressed by intestinal TRM (Weisberg et al., 2019). In human tumors, TRM phenotype can also vary according to the tumor type (Table 1). Interestingly, human TRM associated with multiple tumor types in different sites express CD103, possibly due to the epithelial origin of many solid tumors, while lung tumor–associated TRM also express CXCR6 mRNA (Ganesan et al., 2017). PD-1 is expressed by TRM in tumor tissues and healthy tissues. Tumor-associated myeloid APCs, including macrophages and myeloid dendritic cells (DCs), highly express programmed death-ligand 1 (PD-L1; B7-H1), engage PD-1+ T cells, and mediate immune suppression in spontaneous and immunotherapy-induced tumor immunity (Curiel et al., 2004; Zou et al., 2016; Lin et al., 2018). It is unknown if PD-L1+ APCs interact with PD-1+ TRM, resulting in TRM functional alteration in the tumor-draining LNs and tumor microenvironment. Additionally, whether tumor-associated TRM in a particular tissue site differ phenotypically from TRM in the identical healthy tissue site requires further investigation.
Transcriptome profiling of the population and single-cell RNA sequencing (scRNAseq) have elucidated core features of TRM in mouse models and humans and further revealed TRM heterogeneity and tissue-specific signatures. Population-level RNAseq of mouse and human TRM has revealed conserved signatures that include genes encoding the surface and intracellular molecules described above, as well as transcriptional regulators (Mackay et al., 2016; Kumar et al., 2016; Hombrink et al., 2016). In mice, the transcription factors (such as Hobit, Blimp-1, Runx3, and Id2 family members) have been reported to play a role in TRM biology (Mackay et al., 2016; Milner et al., 2017; Milner et al., 2020). Human TRM do not express elevated levels of Hobit, although Notch expression is up-regulated and is related to TRM establishment (Hombrink et al., 2016; Kumar et al., 2017). A “master regulator” defining human TRM development has not yet been identified. However, single-cell transcriptome profiling of TRM has begun to reveal new insights into distinguished features of TRM, including their heterogeneity and tissue-specific variations. In humans, scRNAseq analysis of T cells from lung and lymphoid sites has revealed a TRM-associated tissue gene signature, including cell–cell communication, cell structure, and cell–cell matrix interactions (Szabo et al., 2019). This suggests that cell structure and cell interaction may regulate TRM formation and maintenance. Transcriptome analysis has also indicated considerable heterogeneity among mouse TRM populations (Milner et al., 2020).
Transcriptome analysis has likewise identified tumor-associated TRM subsets in the tumor microenvironment. For example, single-cell sequencing suggests different TRM subsets in human breast cancer (Savas et al., 2018). Interestingly, PD-1–expressing TRM may possess superior functionality when compared with PD-1–expressing non-TRM, as suggested in transcriptome profiling of human lung cancer (Clarke et al., 2019). Based on our current understanding of PD-L1 and PD-1 in tumor immunity (Curiel et al., 2004; Zou et al., 2016; Lin et al., 2018), this finding is unexpected. Transcriptionally distinct subsets of TRM, including Blimp1hi and Id3hi subpopulations, were also identified in a mouse melanoma model (Fig. 1; Milner et al., 2020). Although transcriptome studies have generated some insight into TRM in tumors, it is important and critical to phenotypically and functionally define the different subsets of tumor-associated TRM, including PD-1+ TRM. Notably, although tumor-infiltrating lymphocytes show a TRM-like signature, there are no specific phenotypic and functional markers to define TRM among different memory T cell subsets in the tumor microenvironment (Sasson et al., 2020). Thus, functional studies following single-cell phenotyping are critical to resolve discrepancies regarding different tumor-infiltrating T cell subsets, including TRM.
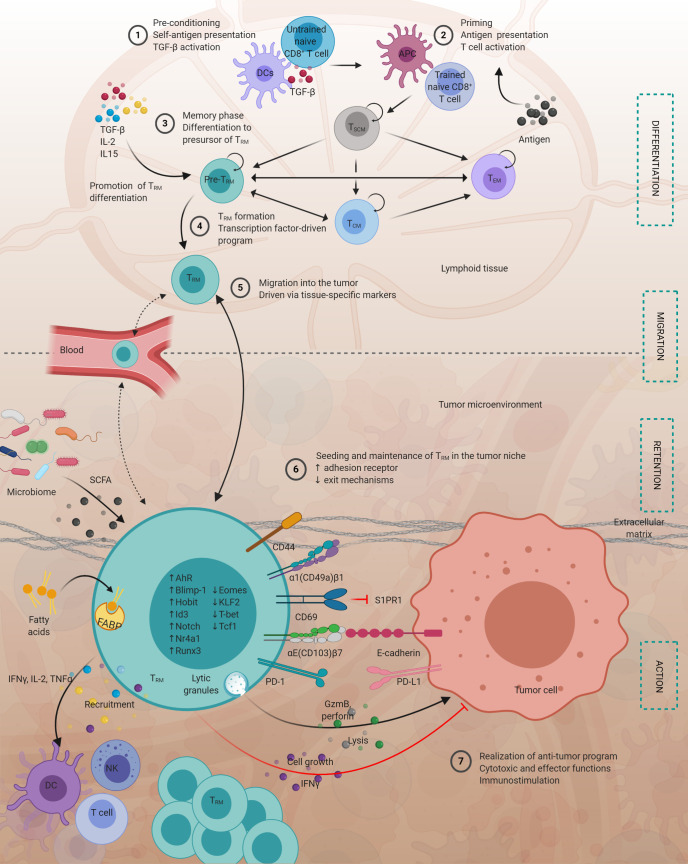
Figure 1.
TRM in tumor immunity. (1 and 2) Before specific antigen priming, CD8+ naive T cells may interact with DCs in the presence of TGF-β. This process may prepare TRM formation. (3) During memory phase, TSCM may give rise to different memory subsets, including TRM. IL-2, IL-15, and TGF-β, which may provide optimal signaling for TRM formation. (4) TRM precursors may undergo a transcription factor–driven generation program. (5) TRM can traffic into the tumor microenvironment, and they are maintained in situ without (or with minimal) recirculation. (6) TRM retention in tumors may be related to certain integrins, including CD44, α1(CD49a)β1, αE(CD103)β7, and CD69. CD44 and α1(CD49a)β1 tether TRM to the extracellular matrix. αE(CD103)β7 anchors TRM via interacting with E-cadherin on the epithelial cell surface. CD69 blocks S1PR1-mediated “exit” signaling. Blockade of TRM egress from the tissue may be promoted by up-regulation of some transcription factors (including Blimp, Hobit, Notch, and Runx) and by down-regulation of Krüppel-like factor 2 (KLF2). Microbiome-derived SCFA may promote long-term maintenance of TRM. FABP and FFA uptake may favor TRM survival and antitumor functionality. TRM may express immune checkpoint proteins, suggesting that TRM likely responds to checkpoint blockade. (7) TRM may release perforin and GzmB and directly kill target tumor cells. TRM-derived cytokines (IFN-γ, IL-2, and TNF-α) promote infiltration of DCs, T and B cells, and natural killer cells, indirectly boosting antitumor immunity.
TRM retention
TRM were originally identified in mice using parabiosis models and in vivo antibody labeling (Anderson et al., 2014; Szabo et al., 2019). Multiple molecules (including CD44, CD69, integrins [CD49a, CD103]), some transcription factors (i.e., Notch, Runx3, Blimp1, Hobit), fatty acid–binding proteins (FABPs), and microbiome-derived metabolites (such as short-chain fatty acid [SCFA]) are reported to be involved in TRM retention in the tissue. CD44 is a receptor for extracellular matrix and assists TRM interaction with epithelial cells and collagen (Amsen et al., 2018). CD69 restrains the function of S1PR1 signaling and blocks TRM egress from tissue (Skon et al., 2013; Mackay et al., 2015). Similarly, CD49a and CD103 function as “anchors” to arrest TRM within the tissues (Chen and Shen, 2020; Byrne et al., 2020).
The pathways controlling TRM retention in tumors are poorly defined. Different levels of CD8+CD103+ TRM are found in tumor epithelium and tumor stroma in different tumor types in patients (Cresswell et al., 2001; Ling et al., 2007; Djenidi et al., 2015; Wang et al., 2015; Workel et al., 2016; Nizard et al., 2017), suggesting that CD103 may be particularly important for TRM retention in tumors. Antibody blockade of CD103 or genetic deficiency of CD103 results in reduced tumor-infiltrating T cells and accelerated tumor progression in mice (Sandoval et al., 2013; Murray et al., 2016; Sun et al., 2016; Malik et al., 2017), supporting a role for CD103 in T cell tumor retention. Notch plays a role in controlling maximal CD103 expression in tumor-associated TRM (Hombrink et al., 2016). Transcription factors, including Runx3, Blimp, Hobit, and KLF2, have been shown to down-regulate homing receptor expression for egress in TRM and promote TRM tissue retention in mouse infection models (Milner et al., 2017; Mackay et al., 2016; Skon et al., 2013). The potential role of these transcription factors in TRM tumor retention remains to be established.
It appears that TRM retention is subject to their adaptation to and regulation by their tissue of residency. Consistent with this concept, tissue-tailored, variable, and malleable profiles of FABP isoforms are found in murine TRM after viral infection (Frizzell et al., 2020). Interestingly, microbiota-derived SCFA favors CD8+ T cell long-term survival and memory (Bachem et al., 2019). Altogether, these early studies suggest that it is crucial to decode molecular mechanisms by which TRM gain tissue-tailored “labels” and to characterize the “area code” to control TRM memory, survival, and function in the tumor microenvironment in different tumor types.
Generation and maintenance of TRM
Several models (including linear, asymmetrical, self-renewal, simultaneous, “one cell, one fate,” and “one cell, multiple fates” programs) are proposed to explain TRM differentiation (Enamorado et al., 2018; Raphael et al., 2020). Moreover, TRM differentiation is influenced by different factors at the earliest priming stage in the LN, the cytokine environment during differentiation and activation, and finally through tissue-specific influences. At the level of priming, murine Batf3-dependent DCs and human CD1c+/CD163+ TGF-β–producing DCs can prime T cells for TRM generation in lymphoid tissues (Mami-Chouaib et al., 2018; Amsen et al., 2018; Bourdely et al., 2020). DC-specific DNGR-1 (CLEC9A) provides optimal signal for murine TRM generation (Iborra et al., 2016). Even before antigen encounter, naive T cells can undergo “training” in the LNs via interacting with migratory αVβ8+ DCs. These DCs activate and present TGF-β to naive CD8+ T cells, resulting in TRM-like features, including up-regulation of CD103 expression and epigenetic modifications of TRM-related genes (Mani et al., 2019). It is speculated that DCs in the tumor-draining LNs may similarly affect TRM development in the tumor microenvironment.
TRM undergo a unique, hybrid effector cell–memory cell differentiation program driven by transcription factors associated with both memory and effector cell characteristics. For example, Blimp1 and Notch are required for TRM and favor TEM, whereas Runx3 and Nr4a1 promote TRM and support TCM (Milner and Goldrath, 2018). Conversely, T-bet and Eomes inhibit TRM formation but promote TEM and TCM differentiation, respectively. Furthermore, TRM seem to be reminiscent effector cells via expression of PD-1, IFNγ, perforin, and granzyme B (GzmB) on both mRNA and protein levels (Szabo et al., 2019; Milner and Goldrath, 2018; Ganesan et al., 2017), and they share properties of stem cells, as they may be long-lived and not terminally differentiated (Milner and Goldrath, 2018). Overall, coexistence of these memory-, effector-, and stem-like properties may reinforce the antitumor functionality of TRM.
Cytokines at the tissue site or during priming can influence TRM formation
Notably, TGF-β promotes CD103 expression and is critical for formation of TRM in the gut, skin, and lungs (Zhang and Bevan, 2013; Raphael et al., 2020). TGF-β can be highly expressed in the tumor microenvironment and may promote TRM establishment. Tissue-specific factors are also important for TRM establishment at specific sites. The cutaneous lymphocyte antigen and chemokine receptors, including CCR4, CCR8, CCR10, and CCR19, are expressed on TRM resident in the skin, whereas CCR9, CXCR3, and integrin α4β7 are revealed in intestinal-resident TRM (Farber et al., 2014; Amsen et al., 2018; Sun et al., 2019). Murine TRM in the kidney have enhanced expression of E-selectin and P-selectin (Ma et al., 2017). CXCR6 controls TRM trafficking to murine lungs (Wein et al., 2019) and is expressed in TRM in human lung cancer (Ganesan et al., 2017), while murine TRM migrate to the brain via CCR5 and CXCL10 (Glass et al., 2005; Klein et al., 2005). However, α4β7+ TRM are also present in the murine skin (Ohmatsu et al., 2010). CXCR3 can “navigate” TRM to the lungs, skin, and vagina, whereas P-selectin plays a role in migration of CD4+ TRM to the intestine (Haddad et al., 2003; Jeyanathan et al., 2017; Chen and Shen, 2020). Future studies are required to precisely characterize TRM-related functional states in different tumors.
Once established in the tissues, there is evidence that TRM can be maintained for a long period of time. Mouse studies in a parabiosis model show that CD4+ and CD8+ TRM generated from site-specific infection do not emerge into the circulation and enter peripheral organs during homeostasis for several weeks (Teijaro et al., 2011; Jiang et al., 2012; Steinert et al., 2015). Additionally, TRM are identified from intravenous antibody labeling due to their presence in tissues, not in the vasculature (Anderson et al., 2012; Turner et al., 2014). In humans, long-term persistence of CD4+ and CD8+ TRM has been demonstrated in transplanted organs in which donor TRM in lungs and intestines were maintained for over a year after transplant but were not detected in blood (Snyder et al., 2019; Bartolomé-Casado et al., 2019; Bartolomé-Casado et al., 2020; Pallett et al., 2020). Moreover, TRM frequencies in multiple mucosal barriers and lymphoid sites are stably maintained throughout decades of adult life (Kumar et al., 2018). Together, these findings suggest that TRM maintenance is tissue specific and integral for tissue homeostasis.
Studies in mice suggest that TRM persistence and stability in tissues can be variable
Lung CD8+ TRM generated after infection tend to wane over time, unless persistent antigen stimulation is provided (Uddbäck et al., 2021). Over time, lung TRM were also found to migrate out of the lung to the associated lymphoid tissue (Stolley et al., 2020). Recent studies have found low levels of potential TRM in healthy human peripheral blood (Klicznik et al., 2019; Guggino et al., 2019) and in the synovial fluid of individuals with spondyloarthritis (Guggino et al., 2019; Qaiyum et al., 2019). In line with this, scRNAseq analysis identifies a tissue gene signature in a minor fraction of peripheral blood T cells (Szabo et al., 2019). Thus, it seems that TRM emergence into the circulation may occur, but it may be a rare event. In mice, reactivation of TRM in secondary infection can result in migration of effector progeny to the local draining LNs (Fonseca et al., 2020; Stolley et al., 2020). Adoptive transfer of purified mouse TRM can enable TRM to migrate to and populate different tissue sites in response to systemic virus challenge (Fonseca et al., 2020). While these results suggest TRM plasticity for reactivation, further studies are needed to investigate the trafficking and retention properties of TRM in different sites; this is an important research area in TRM biology.
Tumor-infiltrating T cells have been studied before the identification of TRM
Tumor-associated TRM may consist of TRM already in the original tissue site before tumorigenesis and T cells that migrate from the periphery, which become TRM due to certain tumor environmental factors such as TGF-β (Fig. 1). Based on results in murine infection models, we propose that tumor-specific TRM reside primarily in the tumor milieu, where they may locally proliferate in response to antigen encounter in situ without or with minimal exiting from the tumor site. This concept is in agreement with the data that tumor-associated antigen-specific T cells are largely found in tumor tissue rather than in the circulation (Webb et al., 2014; Smazynski and Webb, 2018). Recall immune responses could be initiated at nonlymphoid tissues, such as tumor tissues (Zou, 2005). If so, tumor-infiltrating TRM may be locally activated to proliferate and combat tumor cells in the tumor microenvironment. Subsequently, they may exit the primary tumor niche and inhabit new sites within the tissue of origin—for instance, metastatic tumor tissues. Given that the majority of cancer patients die from tumor metastases, boosting TRM-mediated long-term local and systemic memory would be meaningful. Nonetheless, the potential mechanisms controlling tumor-associated TRM maintenance, replenishment, and function remain to be dissected.
Role of TRM in tumor immune surveillance and immunity
Due to their long-term retention in multiple tissues, TRM can play an important role in both tumor immune surveillance and immunity at diverse sites, analogous to their role in immune protection to pathogens (Amsen et al., 2018; Gebhardt et al., 2018; Byrne et al., 2020). Recent studies in mice have pointed toward the functional importance of TRM in immune responses (Park et al., 2018; Beura et al., 2018a; Klicznik et al., 2019; Fonseca et al., 2020). One intriguing possibility is that TRM may eliminate transformed cells in situ, thereby preventing tumor initiation. However, once a tumor is established, tumor cells outcompete tumor-infiltrating T cells for nutrients, resulting in impaired T cell functionality (Zhang et al., 2017; Bian et al., 2020). Correspondingly, human gastric cancer cells outcompete TRM for lipid uptake, resulting in TRM death (Lin et al., 2020). The data suggest that fatty acids may be required for TRM survival in the tumor niche.
TRM mediate antitumor immunity directly through production of effector and cytolytic mediators and through the release of cytokines and chemokines for immune cell recruitment and activation. CD8+ TRM can be reactivated by both hematopoietic and nonhematopoietic APCs within the sites, which can shape their functionality (Low et al., 2020). Once stimulated, TRM release lytic granules containing perforin and GzmB and kill tumor cells, similarly to effector CD8+ T cells (Amsen et al., 2018). Furthermore, TRM can support immune equilibrium in a melanoma mouse model and contribute to tumor control (Park et al., 2019a).
Interestingly, tumor-infiltrating CD8+CD39+CD103+ TRM elicit more potent cytotoxic and effector functions compared with CD103− counterparts (Franciszkiewicz et al., 2013; Djenidi et al., 2015; Enamorado et al., 2017; Malik et al., 2017; Nizard et al., 2017; Duhen et al., 2018; Park et al., 2019; Sasson et al., 2020). In line with this, CD8+CD103+ TRM in tumor, but not in tumor stroma, are a better prognostic factor in patients with cancer (Koh et al., 2017; Dhodapkar, 2018). Human tumor-infiltrating T helper type 17 (Th17) cells are affected by TGFβ in the tumor microenvironment, express CD49, are long-lived memory cells, and mediate potent antitumor immunity (Kryczek et al., 2009; Kryczek et al., 2011). These features suggest that human tumor-infiltrating Th17 cells exhibit and/or gain a TRM phenotype. Correspondingly, CD4+ TRM with a Th17 signature have been observed in patients with autoimmune disease (Krebs et al., 2020). Interestingly, CD49a+ TRM are the most effective tumor killers among T cells in a melanoma mouse model (Le Floc’h et al., 2007; Djenidi et al., 2015; Murray et al., 2016). It is tempting to speculate that TRM may possess superior antitumor activity compared with other tumor-associated lymphocytes.
In addition to tumor killing via their direct cytotoxic activity, TRM function as an immune stimulator. TRM-derived effector cytokines stimulate local DCs, natural killer cells, and T cells to boost antitumor immune responses (Schenkel et al., 2013; McMaster et al., 2015; Hombrink et al., 2016; Glasner et al., 2018). Furthermore, TRM more rapidly respond to antigen reexposure compared with circulating memory T cells (Mackay et al., 2012; Schenkel et al., 2014; Ariotti et al., 2014). Therefore, TRM may play a crucial role against tumor recurrence. Collectively, activated TRM initiate a system of a rapid, tissue-wide state of alarm for optimal immune protection. Unexpectedly, although TRM express inhibitory checkpoint receptors, their cytotoxic and effector functionalities are maintained (Ganesan et al., 2017; Savas et al., 2018; Boddupalli et al., 2016). Treatment with PD-1 and PD-L1 blockade results in TRM proliferation in patients with melanoma (Edwards et al., 2018), and production of high levels of GzmB, TNF-α, and IFN-γ (Djenidi et al., 2015; Ganesan et al., 2017; Behr et al., 2019). However, it has also been reported that TRM isolated from normal human lung tissue may be more effective in effector cytokine production than their counterparts isolated from tumor lung tissue (Bengsch et al., 2018). A tolerogenic signature has been observed in CD8+CD103+ T cells with high expression of IL-10 and CTLA-4 and low expression of TNF-α, IFN-γ, and GzmB in a melanoma mouse model (Gabriely et al., 2017). Nevertheless, TRM are associated with favorable prognosis in many types of human cancer (Table 1). Intriguingly, mouse infection models have recently shown that lung TRM can migrate to the draining LNs (Beura et al., 2018b) and that stimulation of lung TRM led to enhanced responses in the lung-draining LNs (Paik and Farber, 2021). These studies suggest that TRM may coordinate local immunity through fortification of the immune response in the neighboring LNs. If TRM existed in the tumor-draining LNs, TRM would be an ideal cell population to inhibit tumor lymphatic spread and metastases. Collectively, all of the above multifunctional TRM activities make them ideal effector T cells in antitumor immune responses.
TRM in tumor immunotherapy
There is evidence that TRM are involved in tumor immunotherapy. Checkpoint therapy boosts TRM formation in melanoma-bearing mice (Enamorado et al., 2017). PD-1 blockade in combination with TCM transfer results in 10-fold increase in TRM and inhibits B16 and MC38 tumor growth (Enamorado et al., 2017). Consistent with mouse studies, PD-1 blockade of TRM from human non–small cell lung cancer promotes ex vivo cytolysis of TRM to autologous tumors (Djenidi et al., 2015). Anti–PD-1 therapy leads to potent proliferation of CD8+CD103+ TRM in patients with melanoma, and the levels of CD8+CD103+ TRM are associated with improved patient survival (Edwards et al., 2018). Therefore, checkpoint blockade supports the notion that targeting TRM may be therapeutically meaningful. Multiple strategies have been suggested to modulate TRM to enhance cancer therapy efficacy (Fig. 2 and Table 2).
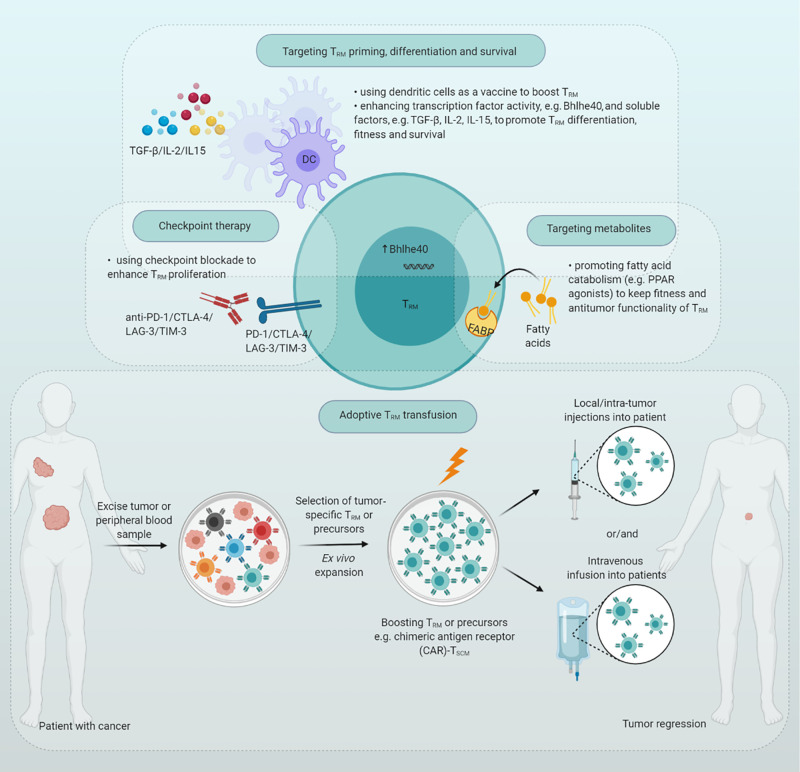
Figure 2.
TRM in cancer immunotherapy. Several strategies have been proposed to enhance TRM activity. Checkpoint blockade may enhance intratumoral proliferation of TRM. DCs may be used as a vaccine to induce functional TRM. Targeting specific transcription factors and cytokines may drive TRM formation and may boost induction of active TRM. Targeting particular metabolites (e.g., FFA and FABP) may promote TRM pool and antitumor activity. Adoptive transfer of preprogramed TRM or TSCM may improve TRM seeding or differentiation, thereby enhancing antitumor immunity. PPAR, peroxisome proliferator–activated receptor.
Table 2. Role of TRM in cancer immunotherapy.
| Tumor types | Treatment strategy | Effects | References |
|---|---|---|---|
| Cervical cancer | Vaccination + radiotherapy | HPV E6/E7-targeted therapeutic vaccination in combination with radiotherapy results in increased intratumoral number of CD8+CD103+ cells. | Komdeur et al., 2017 |
| Colorectal cancer | Anti–TGF-β mAb + radiotherapy | TGF-β contributes to TRM radioresistance. | Arina et al., 2019 |
| Esophageal cancer | Anti–PD-L1 mAb | PD-L1 blockade increases the number of CD8+CD103+ cells in the tumor. | Han et al., 2020 |
| Gastric cancer | Anti–PD-L1 mAb | PD-L1 blockade increases FABP4 and 5 expression in TRM, favoring lipid uptake by TRM and resulting in improved cell survival. | Lin et al., 2020 |
| PD-L1 blockade unleashes TRM in the PDX mice. | |||
| Non-responder PDX mice to PD-L1 blockade have less TRM than responders. | |||
| Head and neck cancer | Vaccination, anti–TGF-β mAb | STxB-E7 vaccination induces TRM and inhibits tumor growth. | Nizard et al., 2017 |
| TFG-β blockade inhibits TRM formation after vaccine immunization, resulting in lower vaccine efficacy. | |||
| Melanoma | “Prime-boost” immunization (CpG ODN 1826, OVA) | Subcutaneous antigen injection and epicutaneous CpG ODN adjuvant administration correlate with enhanced numbers of CD103+CD8+ cells in the skin, enhance TCIRC in the blood, and prevent tumor development. | Lai et al., 2019 |
| Vaccination (pVAX-OVA/DNA-OVA and pcDNA-GP100/DNA-GP100) | Vaccination-induced TRM strongly suppress the growth of melanoma cells independently of TCIRC. | Gálvez-Cancino et al., 2018 | |
| Anti–PD-1 mAb (nivolumab, pembrolizumab) | CD103+ cells significantly expand early during treatment. | Edwards et al., 2018 | |
| Adoptive T cell transfer | Runx3-deficient CD8+ cells fail to infiltrate the tumor, resulting in higher tumor growth and mortality. | Milner et al., 2017 | |
| Runx3 overexpression enhances CD8+ cell tumor infiltration, inhibits tumor growth, and prolongs OS. | |||
| T reg depletion and tumor removal | Skin-resident TRM are necessary for rejection of tumor rechallenge and long-lived melanoma immune protection. | Malik et al., 2017 | |
| Adoptive T cell transfer, anti–PD-1 mAb | Anti–PD-1 boosts TRM tumor infiltration and improves antitumor immunity after TCM transfer. | Enamorado et al., 2017 | |
| Anti-CD103, anti–VLA-1 mAb | Blockade of CD103 or VLA-1 on TRM impairs tumor control. | Murray et al., 2016 | |
| Adoptive T cell transfer, anti-CD103 mAb | Transfer of CD8+CD103+ cells enhances tumor growth, whereas CD103 blockade inhibits tumorigenesis. | Gabriely et al., 2017 |
HPV, human papillomavirus; ODN, oligodeoxynucleotide; OS, overall survival; PDX, patient-derived xenograft; STxB, B subunit of Shiga toxin; TCIRC, circulating memory T cells; VLA-1, very late antigen 1.
Targeting TRM priming, differentiation, and survival
DC priming is essential for TRM formation in different models, including tumors (Yu et al., 2013; Wu et al., 2014; Iborra et al., 2016; Shin et al., 2016; Enamorado et al., 2017). CD103+ DCs are required for optimal generation of TRM (Iborra et al., 2016). Both CD1c+ DCs (Yu et al., 2013) and CD301b+ DCs (Shin et al., 2016) promote TRM generation from effector CD8+ T cells. Given the importance of DCs in TRM formation, DCs may be used as a vaccine to induce TRM. Furthermore, IL-15 promotes CD8+ TRM in situ (Sowell et al., 2017). Cytokine nanogel allows transference of high doses of IL-15 to the tumor microenvironment (Tang et al., 2018; Xie et al., 2019) and enhancement of the TRM pool in the tumor. Interestingly, Bhlhe40 (a transcription factor) orchestrates TRM survival and functionality and is critical for immunotherapy efficacy (Li et al., 2019). Thus, targeting Bhlhe40 may be an option for cancer immunotherapy. In addition, TGF-β supports TRM formation (Zhang and Bevan, 2013) and promotes radiation resistance of TRM (Arina et al., 2019). Blockade of TGF-β results in reduced numbers of TRM after vaccination (Nizard et al., 2017). Given the general immune-suppressive role of TGF-β, it is challenging to specifically target TGF-β-signaling in TRM to improve cancer therapy. Future studies will determine whether critical and specific potential TGF-β downstream gene(s) for TRM formation can be identified.
Adoptive TRM transfusion
Adoptive transfusion of preprogrammed TRM may be a cancer immunotherapy strategy. Adoptive transfer of tumor-infiltrating T cells with overexpression of Runx3 promotes TRM development, inhibits tumor growth, and improves mouse survival in a melanoma murine model (Milner et al., 2017). T memory stem cells (TSCM) differentiate into TRM (Kondo et al., 2017). Adoptive transfusion of TSCM and chimeric antigen receptor TSCM (Kondo et al., 2020) may lead to increased TRM in cancers. Furthermore, in murine models of melanoma, the presence of both TRM and circulating T cells offers improved protection against tumor challenge compared with only one alone (Enamorado et al., 2017). Thus, adoptive TRM transfusion may be a reasonable approach in combination with other cancer therapies.
Targeting metabolites
T cells compete with tumor cells for glucose, amino acids, and free fatty acids (FFAs; Zhao et al., 2016; Molodtsov and Turk, 2018; Chen and Huang, 2019; Bian et al., 2020, Wang and Zou, 2020). TRM express high levels of FFA-binding proteins FABP4 and FABP5 (Pan et al., 2017; Lin et al., 2020). Peroxisome proliferator–activated receptor agonists promote FFA catabolism, accelerate T cell–mediated antitumor immunity, and sensitize anti–PD-1 treatment (Zhang et al., 2017; Chowdhury et al., 2018). The antitumor effect may be partially attributed to TRM responses. Checkpoint blockade promotes FABP4 and FABP5 expression in TRM, resulting in higher lipid uptake by TRM and enhancing TRM survival (Lin et al., 2020). As such, targeting FFA and FABP can affect TRM and improve antitumor immunity. Future studies will generate insight into TRM metabolic features and how to target TRM metabolism for cancer therapy.
Conclusions
Current evidence shows that TRM can evoke potent antitumor immune responses. However, our understanding of their phenotype, differentiation, trafficking, tissue retention, and effector function remains in its infancy. This is particularly the case in patients with cancer. Substantial human studies rely on expression of CD49, CD69, and CD103 to define TRM. A number of mouse tumor-bearing models and human samples have revealed the presence of TRM phenotype cells within tumors. These TRM may arise de novo from the tissue or infiltrate as part of the antitumor immunosurveillance. Evidence thus far shows that TRM presence in a tumor or in response to immunotherapy can be a useful prognostic indicator for improved outcomes. Using high-throughput technologies, including single-cell sequencing, microscopic tissue spatial analysis, and multi-omics studies, we may be able to fruitfully study limited clinical materials to gain critical and novel information on TRM, leading to developing new cancer treatments via targeting TRM.
Acknowledgments
This work was supported in part by research grants from the National Cancer Institute of the National Institutes of Health (CA248430, CA217648, CA123088, CA099985, CA193136, and CA152470 to W. Zou), the National Institutes of Health through a University of Michigan Rogel Cancer Center support grant (P30CA46592), and other National Institutes of Health grants (AI106697, AI128949, HL145547, and AI150680 to D.L. Farber). K. Okła was supported in part by the ETIUDA 7 Research Scholarship of the National Science Center in Poland.
Author contributions: Conceptualization, writing, review, and editing: K. Okła, D.L. Farber, and W. Zou.
References
- Amsen, D., van Gisbergen K.P.J.M., Hombrink P., and van Lier R.A.W.. 2018. Tissue-resident memory T cells at the center of immunity to solid tumors. Nat. Immunol. 19:538–546. 10.1038/s41590-018-0114-2 [DOI] [PubMed] [Google Scholar]
- Anderson, K.G., Sung H., Skon C.N., Lefrancois L., Deisinger A., Vezys V., and Masopust D.. 2012. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J. Immunol. 189:2702–2706. 10.4049/jimmunol.1201682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, K.G., Mayer-Barber K., Sung H., Beura L., James B.R., Taylor J.J., Qunaj L., Griffith T.S., Vezys V., Barber D.L., and Masopust D.. 2014. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc. 9:209–222. 10.1038/nprot.2014.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arina, A., Beckett M., Fernandez C., Zheng W., Pitroda S., Chmura S.J., Luke J.J., Forde M., Hou Y., Burnette B., et al. 2019. Tumor-reprogrammed resident T cells resist radiation to control tumors. Nat. Commun. 10:3959. 10.1038/s41467-019-11906-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti, S., Hogenbirk M.A., Dijkgraaf F.E., Visser L.L., Hoekstra M.E., Song J.-Y., Jacobs H., Haanen J.B., and Schumacher T.N.. 2014. T cell memory. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science. 346:101–105. 10.1126/science.1254803 [DOI] [PubMed] [Google Scholar]
- Bachem, A., Makhlouf C., Binger K.J., de Souza D.P., Tull D., Hochheiser K., Whitney P.G., Fernandez-Ruiz D., Dähling S., Kastenmüller W., et al. 2019. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity. 51:285–297.e5. 10.1016/j.immuni.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Bartolomé-Casado, R., Landsverk O.J.B., Chauhan S.K., Richter L., Phung D., Greiff V., Risnes L.F., Yao Y., Neumann R.S., Yaqub S., et al. 2019. Resident memory CD8 T cells persist for years in human small intestine. J. Exp. Med. 216:2412–2426. 10.1084/jem.20190414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomé-Casado, R., Landsverk O.J.B., Chauhan S.K., Sætre F., Hagen K.T., Yaqub S., Øyen O., Horneland R., Aandahl E.M., Aabakken L., et al. 2020. CD4+ T cells persist for years in the human small intestine and display a TH1 cytokine profile. Mucosal Immunol. 10.1038/s41385-020-0315-5 [DOI] [PubMed] [Google Scholar]
- Behr, F.M., Kragten N.A.M., Wesselink T.H., Nota B., van Lier R.A.W., Amsen D., Stark R., Hombrink P., and van Gisbergen K.P.J.M.. 2019. Blimp-1 rather than Hobit drives the formation of tissue-resident memory CD8+ T cells in the lungs. Front. Immunol. 10:400. 10.3389/fimmu.2019.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengsch, B., Ohtani T., Khan O., Setty M., Manne S., O’Brien S., Gherardini P.F., Herati R.S., Huang A.C., Chang K.-M., et al. 2018. Epigenomic-guided mass cytometry profiling reveals disease-specific features of exhausted CD8 T cells. Immunity. 48:1029–1045.e5. 10.1016/j.immuni.2018.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura, L.K., Mitchell J.S., Thompson E.A., Schenkel J.M., Mohammed J., Wijeyesinghe S., Fonseca R., Burbach B.J., Hickman H.D., Vezys V., et al. 2018a. Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat. Immunol. 19:173–182. 10.1038/s41590-017-0029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura, L.K., Wijeyesinghe S., Thompson E.A., Macchietto M.G., Rosato P.C., Pierson M.J., Schenkel J.M., Mitchell J.S., Vezys V., Fife B.T., et al. 2018b. T cells in non-lymphoid tissues give rise to lymph-node-resident memory T cells. Immunity. 48:327–338.e5. 10.1016/j.immuni.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian, Y., Li W., Kremer D.M., Sajjakulnukit P., Li S., Crespo J., Nwosu Z.C., Zhang L., Czerwonka A., Pawłowska A., et al. 2020. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature. 585:277–282. 10.1038/s41586-020-2682-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddupalli, C.S., Bar N., Kadaveru K., Krauthammer M., Pornputtapong N., Mai Z., Ariyan S., Narayan D., Kluger H., Deng Y., et al. 2016. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight. 1:e88955. 10.1172/jci.insight.88955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bösmüller, H.-C., Wagner P., Peper J.K., Schuster H., Pham D.L., Greif K., Beschorner C., Rammensee H.-G., Stevanović S., Fend F., and Staebler A.. 2016. Combined immunoscore of CD103 and CD3 identifies long-term survivors in high-grade serous ovarian cancer. Int. J. Gynecol. Cancer. 26:671–679. 10.1097/IGC.0000000000000672 [DOI] [PubMed] [Google Scholar]
- Bourdely, P., Anselmi G., Vaivode K., Ramos R.N., Missolo-Koussou Y., Hidalgo S., Tosselo J., Nuñez N., Richer W., Vincent-Salomon A., et al. 2020. Transcriptional and functional analysis of CD1c+ human dendritic cells identifies a CD163+ subset priming CD8+CD103+ T cells. Immunity. 53:335–352.e8. 10.1016/j.immuni.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, A., Savas P., Sant S., Li R., Virassamy B., Luen S.J., Beavis P.A., Mackay L.K., Neeson P.J., and Loi S.. 2020. Tissue-resident memory T cells in breast cancer control and immunotherapy responses. Nat. Rev. Clin. Oncol. 17:341–348. 10.1038/s41571-020-0333-y [DOI] [PubMed] [Google Scholar]
- Chen, M., and Huang J.. 2019. The expanded role of fatty acid metabolism in cancer: new aspects and targets. Precis. Clin. Med. 2:183–191. 10.1093/pcmedi/pbz017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L., and Shen Z.. 2020. Tissue-resident memory T cells and their biological characteristics in the recurrence of inflammatory skin disorders. Cell. Mol. Immunol. 17:64–75. 10.1038/s41423-019-0291-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, P.S., Chamoto K., Kumar A., and Honjo T.. 2018. PPAR-induced fatty acid oxidation in T cells increases the number of tumor-reactive CD8+ T cells and facilitates anti-PD-1 therapy. Cancer Immunol. Res. 6:1375–1387. 10.1158/2326-6066.CIR-18-0095 [DOI] [PubMed] [Google Scholar]
- Clark, R.A. 2015. Resident memory T cells in human health and disease. Sci. Transl. Med. 7:269rv1. 10.1126/scitranslmed.3010641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, J., Panwar B., Madrigal A., Singh D., Gujar R., Wood O., Chee S.J., Eschweiler S., King E.V., Awad A.S., et al. 2019. Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. J. Exp. Med. 216:2128–2149. 10.1084/jem.20190249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell, J., Robertson H., Neal D.E., Griffiths T.R.L., and Kirby J.A.. 2001. Distribution of lymphocytes of the α(E)β(7) phenotype and E-cadherin in normal human urothelium and bladder carcinomas. Clin. Exp. Immunol. 126:397–402. 10.1046/j.1365-2249.2001.01652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel, T.J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P., Evdemon-Hogan M., Conejo-Garcia J.R., Zhang L., Burow M., et al. 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10:942–949. 10.1038/nm1093 [DOI] [PubMed] [Google Scholar]
- Dhodapkar, K.M. 2018. Role of tissue-resident memory in intra-tumor heterogeneity and response to immune checkpoint blockade. Front. Immunol. 9:1655. 10.3389/fimmu.2018.01655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkgraaf, F.E., Matos T.R., Hoogenboezem M., Toebes M., Vredevoogd D.W., Mertz M., van den Broek B., Song J.-Y., Teunissen M.B.M., Luiten R.M., et al. 2019. Tissue patrol by resident memory CD8+ T cells in human skin. Nat. Immunol. 20:756–764. 10.1038/s41590-019-0404-3 [DOI] [PubMed] [Google Scholar]
- Djenidi, F., Adam J., Goubar A., Durgeau A., Meurice G., de Montpréville V., Validire P., Besse B., and Mami-Chouaib F.. 2015. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J. Immunol. 194:3475–3486. 10.4049/jimmunol.1402711 [DOI] [PubMed] [Google Scholar]
- Duhen, T., Duhen R., Montler R., Moses J., Moudgil T., de Miranda N.F., Goodall C.P., Blair T.C., Fox B.A., McDermott J.E., et al. 2018. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9:2724. 10.1038/s41467-018-05072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, J., Wilmott J.S., Madore J., Gide T.N., Quek C., Tasker A., Ferguson A., Chen J., Hewavisenti R., Hersey P., et al. 2018. CD103+ tumor-resident CD8+ T cells are associated with improved survival in immunotherapy-naïve melanoma patients and expand significantly during anti-PD-1 treatment. Clin. Cancer Res. 24:3036–3045. 10.1158/1078-0432.CCR-17-2257 [DOI] [PubMed] [Google Scholar]
- Egelston, C.A., Avalos C., Tu T.Y., Rosario A., Wang R., Solomon S., Srinivasan G., Nelson M.S., Huang Y., Lim M.H., et al. 2019. Resident memory CD8+ T cells within cancer islands mediate survival in breast cancer patients. JCI Insight. 4:e130000. 10.1172/jci.insight.130000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enamorado, M., Iborra S., Priego E., Cueto F.J., Quintana J.A., Martínez-Cano S., Mejías-Pérez E., Esteban M., Melero I., Hidalgo A., and Sancho D.. 2017. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat. Commun. 8:16073. 10.1038/ncomms16073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enamorado, M., Khouili S.C., Iborra S., and Sancho D.. 2018. Genealogy, dendritic cell priming, and differentiation of tissue-resident memory CD8+ T cells. Front. Immunol. 9:1751. 10.3389/fimmu.2018.01751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber, D.L., Yudanin N.A., and Restifo N.P.. 2014. Human memory T cells: generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 14:24–35. 10.1038/nri3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca, R., Beura L.K., Quarnstrom C.F., Ghoneim H.E., Fan Y., Zebley C.C., Scott M.C., Fares-Frederickson N.J., Wijeyesinghe S., Thompson E.A., et al. 2020. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat. Immunol. 21:412–421. 10.1038/s41590-020-0607-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciszkiewicz, K., Le Floc’h A., Boutet M., Vergnon I., Schmitt A., and Mami-Chouaib F.. 2013. CD103 or LFA-1 engagement at the immune synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell effector functions. Cancer Res. 73:617–628. 10.1158/0008-5472.CAN-12-2569 [DOI] [PubMed] [Google Scholar]
- Frizzell, H., Fonseca R., Christo S.N., Evrard M., Cruz-Gomez S., Zanluqui N.G., von Scheidt B., Freestone D., Park S.L., McWilliam H.E.G., et al. 2020. Organ-specific isoform selection of fatty acid-binding proteins in tissue-resident lymphocytes. Sci. Immunol. 5:eaay9283. 10.1126/sciimmunol.aay9283 [DOI] [PubMed] [Google Scholar]
- Gabriely, G., da Cunha A.P., Rezende R.M., Kenyon B., Madi A., Vandeventer T., Skillin N., Rubino S., Garo L., Mazzola M.A., et al. 2017. Targeting latency-associated peptide promotes antitumor immunity. Sci. Immunol. 2:eaaj1738. 10.1126/sciimmunol.aaj1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez-Cancino, F., López E., Menares E., Díaz X., Flores C., Cáceres P., Hidalgo S., Chovar O., Alcántara-Hernández M., Borgna V., et al. 2018. Vaccination-induced skin-resident memory CD8+ T cells mediate strong protection against cutaneous melanoma. OncoImmunology. 7:e1442163. 10.1080/2162402X.2018.1442163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan, A.-P., Clarke J., Wood O., Garrido-Martin E.M., Chee S.J., Mellows T., Samaniego-Castruita D., Singh D., Seumois G., Alzetani A., et al. 2017. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat. Immunol. 18:940–950. 10.1038/ni.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt, T., Wakim L.M., Eidsmo L., Reading P.C., Heath W.R., and Carbone F.R.. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10:524–530. 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- Gebhardt, T., Palendira U., Tscharke D.C., and Bedoui S.. 2018. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol. Rev. 283:54–76. 10.1111/imr.12650 [DOI] [PubMed] [Google Scholar]
- Glasner, A., Levi A., Enk J., Isaacson B., Viukov S., Orlanski S., Scope A., Neuman T., Enk C.D., Hanna J.H., et al. 2018. NKp46 receptor-mediated interferon-γ production by natural killer cells increases fibronectin 1 to alter tumor architecture and control metastasis. Immunity. 48:107–119.e4. 10.1016/j.immuni.2017.12.007 [DOI] [PubMed] [Google Scholar]
- Glass, W.G., Lim J.K., Cholera R., Pletnev A.G., Gao J.-L., and Murphy P.M.. 2005. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J. Exp. Med. 202:1087–1098. 10.1084/jem.20042530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggino, G., Rizzo A., Mauro D., Macaluso F., and Ciccia F.. 2019. Gut-derived CD8+ tissue-resident memory T cells are expanded in the peripheral blood and synovia of SpA patients. Ann. Rheum. Dis.:annrheumdis-2019-216456. 10.1136/annrheumdis-2019-216456 [DOI] [PubMed] [Google Scholar]
- Haddad, W., Cooper C.J., Zhang Z., Brown J.B., Zhu Y., Issekutz A., Fuss I., Lee H.O., Kansas G.S., and Barrett T.A.. 2003. P-selectin and P-selectin glycoprotein ligand 1 are major determinants for Th1 cell recruitment to nonlymphoid effector sites in the intestinal lamina propria. J. Exp. Med. 198:369–377. 10.1084/jem.20020691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, L., Gao Q.-L., Zhou X.-M., Shi C., Chen G.-Y., Song Y.-P., Yao Y.-J., Zhao Y.-M., Wen X.-Y., Liu S.-L., et al. 2020. Characterization of CD103+ CD8+ tissue-resident T cells in esophageal squamous cell carcinoma: may be tumor reactive and resurrected by anti-PD-1 blockade. Cancer Immunol. Immunother. 69:1493–1504. 10.1007/s00262-020-02562-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartana, C.A., Ahlén Bergman E., Broomé A., Berglund S., Johansson M., Alamdari F., Jakubczyk T., Huge Y., Aljabery F., Palmqvist K., et al. 2018. Tissue-resident memory T cells are epigenetically cytotoxic with signs of exhaustion in human urinary bladder cancer. Clin. Exp. Immunol. 194:39–53. 10.1111/cei.13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombrink, P., Helbig C., Backer R.A., Piet B., Oja A.E., Stark R., Brasser G., Jongejan A., Jonkers R.E., Nota B., et al. 2016. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat. Immunol. 17:1467–1478. 10.1038/ni.3589 [DOI] [PubMed] [Google Scholar]
- Huang, A., Huang P., Luo Y., Wang B., Luo X., Zheng Z., Yuan K., Huang Z., Peng S., Yu H., et al. 2017. CD 103 expression in normal epithelium is associated with poor prognosis of colorectal cancer patients within defined subgroups. Int. J. Clin. Exp. Pathol. 10:6624–6634. [Google Scholar]
- Iborra, S., Martínez-López M., Khouili S.C., Enamorado M., Cueto F.J., Conde-Garrosa R., Del Fresno C., and Sancho D.. 2016. Optimal generation of tissue-resident but not circulating memory T cells during viral infection requires crosspriming by DNGR-1+ dendritic cells. Immunity. 45:847–860. 10.1016/j.immuni.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyanathan, M., Afkhami S., Khera A., Mandur T., Damjanovic D., Yao Y., Lai R., Haddadi S., Dvorkin-Gheva A., Jordana M., et al. 2017. CXCR3 signaling is required for restricted homing of parenteral tuberculosis vaccine-induced T cells to both the lung parenchyma and airway. J. Immunol. 199:2555–2569. 10.4049/jimmunol.1700382 [DOI] [PubMed] [Google Scholar]
- Jiang, X., Clark R.A., Liu L., Wagers A.J., Fuhlbrigge R.C., and Kupper T.S.. 2012. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 483:227–231. 10.1038/nature10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, R.S., Lin E., Zhang B., Luster A.D., Tollett J., Samuel M.A., Engle M., and Diamond M.S.. 2005. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 79:11457–11466. 10.1128/JVI.79.17.11457-11466.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klicznik, M.M., Morawski P.A., Höllbacher B., Varkhande S.R., Motley S.J., Kuri-Cervantes L., Goodwin E., Rosenblum M.D., Long S.A., Brachtl G., et al. 2019. Human CD4+CD103+ cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci. Immunol. 4:eaav8995. 10.1126/sciimmunol.aav8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, J., Kim S., Kim M.-Y., Go H., Jeon Y.K., and Chung D.H.. 2017. Prognostic implications of intratumoral CD103+ tumor-infiltrating lymphocytes in pulmonary squamous cell carcinoma. Oncotarget. 8:13762–13769. 10.18632/oncotarget.14632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komdeur, F.L., Wouters M.C.A., Workel H.H., Tijans A.M., Terwindt A.L.J., Brunekreeft K.L., Plat A., Klip H.G., Eggink F.A., Leffers N., et al. 2016. CD103+ intraepithelial T cells in high-grade serous ovarian cancer are phenotypically diverse TCRαβ+ CD8αβ+ T cells that can be targeted for cancer immunotherapy. Oncotarget. 7:75130–75144. 10.18632/oncotarget.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komdeur, F.L., Prins T.M., van de Wall S., Plat A., Wisman G.B.A., Hollema H., Daemen T., Church D.N., de Bruyn M., and Nijman H.W.. 2017. CD103+ tumor-infiltrating lymphocytes are tumor-reactive intraepithelial CD8+ T cells associated with prognostic benefit and therapy response in cervical cancer. OncoImmunology. 6:e1338230. 10.1080/2162402X.2017.1338230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo, T., Morita R., Okuzono Y., Nakatsukasa H., Sekiya T., Chikuma S., Shichita T., Kanamori M., Kubo M., Koga K., et al. 2017. Notch-mediated conversion of activated T cells into stem cell memory-like T cells for adoptive immunotherapy. Nat. Commun. 8:15338. 10.1038/ncomms15338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo, T., Ando M., Nagai N., Tomisato W., Srirat T., Liu B., Mise-Omata S., Ikeda M., Chikuma S., Nishimasu H., et al. 2020. The NOTCH-FOXM1 axis plays a key role in mitochondrial biogenesis in the induction of human stem cell memory-like CAR-T cells. Cancer Res. 80:471–483. 10.1158/0008-5472.CAN-19-1196 [DOI] [PubMed] [Google Scholar]
- Krebs, C.F., Reimers D., Zhao Y., Paust H.-J., Bartsch P., Nuñez S., Rosemblatt M.V., Hellmig M., Kilian C., Borchers A., et al. 2020. Pathogen-induced tissue-resident memory TH17 (TRM17) cells amplify autoimmune kidney disease. Sci. Immunol. 5:eaba4163. 10.1126/sciimmunol.aba4163 [DOI] [PubMed] [Google Scholar]
- Kryczek, I., Banerjee M., Cheng P., Vatan L., Szeliga W., Wei S., Huang E., Finlayson E., Simeone D., Welling T.H., et al. 2009. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 114:1141–1149. 10.1182/blood-2009-03-208249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek, I., Zhao E., Liu Y., Wang Y., Vatan L., Szeliga W., Moyer J., Klimczak A., Lange A., and Zou W.. 2011. Human TH17 cells are long-lived effector memory cells. Sci. Transl. Med. 3:104ra100. 10.1126/scitranslmed.3002949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, V., Patel S., Tcyganov E., and Gabrilovich D.I.. 2016. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 37:208–220. 10.1016/j.it.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, B.V., Ma W., Miron M., Granot T., Guyer R.S., Carpenter D.J., Senda T., Sun X., Ho S.-H., Lerner H., et al. 2017. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 20:2921–2934. 10.1016/j.celrep.2017.08.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, B.V., Connors T.J., and Farber D.L.. 2018. Human T cell development, localization, and function throughout life. Immunity. 48:202–213. 10.1016/j.immuni.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurd, N.S., He Z., Louis T.L., Milner J.J., Omilusik K.D., Jin W., Tsai M.S., Widjaja C.E., Kanbar J.N., Olvera J.G., et al. 2020. Early precursors and molecular determinants of tissue-resident memory CD8+ T lymphocytes revealed by single-cell RNA sequencing. Sci. Immunol. 5:eaaz6894. 10.1126/sciimmunol.aaz6894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, J.C.Y., Cheng W.K., Hopkins P.D., Komba M., Carlow D.A., and Dutz J.P.. 2019. Topical adjuvant application during subcutaneous vaccination promotes resident memory T cell generation. J. Immunol. 203:2443–2450. 10.4049/jimmunol.1900199 [DOI] [PubMed] [Google Scholar]
- Le Floc’h, A., Jalil A., Vergnon I., Le Maux Chansac B., Lazar V., Bismuth G., Chouaib S., and Mami-Chouaib F.. 2007. α E β 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J. Exp. Med. 204:559–570. 10.1084/jem.20061524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., Zhu B., Son Y.M., Wang Z., Jiang L., Xiang M., Ye Z., Beckermann K.E., Wu Y., Jenkins J.W., et al. 2019. The transcription factor Bhlhe40 programs mitochondrial regulation of resident CD8+ T cell fitness and functionality. Immunity. 51:491–507.e7. 10.1016/j.immuni.2019.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, C.J., Lee Y.H., Pan L., Lai L., Chua C., Wasser M., Lim T.K.H., Yeong J., Toh H.C., Lee S.Y., et al. 2019. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut. 68:916–927. 10.1136/gutjnl-2018-316510 [DOI] [PubMed] [Google Scholar]
- Lin, H., Wei S., Hurt E.M., Green M.D., Zhao L., Vatan L., Szeliga W., Herbst R., Harms P.W., Fecher L.A., et al. 2018. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J. Clin. Invest. 128:805–815. 10.1172/JCI96113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, R., Zhang H., Yuan Y., He Q., Zhou J., Li S., Sun Y., Li D.Y., Qiu H.-B., Wang W., et al. 2020. Fatty acid oxidation controls CD8+ tissue-resident memory T-cell survival in gastric adenocarcinoma. Cancer Immunol. Res. 8:479–492. 10.1158/2326-6066.CIR-19-0702 [DOI] [PubMed] [Google Scholar]
- Ling, K.-L., Dulphy N., Bahl P., Salio M., Maskell K., Piris J., Warren B.F., George B.D., Mortensen N.J., and Cerundolo V.. 2007. Modulation of CD103 expression on human colon carcinoma-specific CTL. J. Immunol. 178:2908–2915. 10.4049/jimmunol.178.5.2908 [DOI] [PubMed] [Google Scholar]
- Lohneis, P., Sinn M., Bischoff S., Jühling A., Pelzer U., Wislocka L., Bahra M., Sinn B.V., Denkert C., Oettle H., et al. 2017. Cytotoxic tumour-infiltrating T lymphocytes influence outcome in resected pancreatic ductal adenocarcinoma. Eur. J. Cancer. 83:290–301. 10.1016/j.ejca.2017.06.016 [DOI] [PubMed] [Google Scholar]
- Low, J.S., Farsakoglu Y., Amezcua Vesely M.C., Sefik E., Kelly J.B., Harman C.C.D., Jackson R., Shyer J.A., Jiang X., Cauley L.S., et al. 2020. Tissue-resident memory T cell reactivation by diverse antigen-presenting cells imparts distinct functional responses. J. Exp. Med. 217:e20192291. 10.1084/jem.20192291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, C., Mishra S., Demel E.L., Liu Y., and Zhang N.. 2017. TGF-β controls the formation of kidney-resident T cells via promoting effector T cell extravasation. J. Immunol. 198:749–756. 10.4049/jimmunol.1601500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay, L.K., Stock A.T., Ma J.Z., Jones C.M., Kent S.J., Mueller S.N., Heath W.R., Carbone F.R., and Gebhardt T.. 2012. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. USA. 109:7037–7042. 10.1073/pnas.1202288109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay, L.K., Braun A., Macleod B.L., Collins N., Tebartz C., Bedoui S., Carbone F.R., and Gebhardt T.. 2015. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 194:2059–2063. 10.4049/jimmunol.1402256 [DOI] [PubMed] [Google Scholar]
- Mackay, L.K., Minnich M., Kragten N.A.M., Liao Y., Nota B., Seillet C., Zaid A., Man K., Preston S., Freestone D., et al. 2016. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 352:459–463. 10.1126/science.aad2035 [DOI] [PubMed] [Google Scholar]
- Malik, B.T., Byrne K.T., Vella J.L., Zhang P., Shabaneh T.B., Steinberg S.M., Molodtsov A.K., Bowers J.S., Angeles C.V., Paulos C.M., et al. 2017. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci. Immunol. 2:eaam6346. 10.1126/sciimmunol.aam6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mami-Chouaib, F., Blanc C., Corgnac S., Hans S., Malenica I., Granier C., Tihy I., and Tartour E.. 2018. Resident memory T cells, critical components in tumor immunology. J. Immunother. Cancer. 6:87. 10.1186/s40425-018-0399-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani, V., Bromley S.K., Äijö T., Mora-Buch R., Carrizosa E., Warner R.D., Hamze M., Sen D.R., Chasse A.Y., Lorant A., et al. 2019. Migratory DCs activate TGF-β to precondition naïve CD8+ T cells for tissue-resident memory fate. Science. 366:eaav5728. 10.1126/science.aav5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust, D., and Soerens A.G.. 2019. Tissue-resident T cells and other resident leukocytes. Annu. Rev. Immunol. 37:521–546. 10.1146/annurev-immunol-042617-053214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust, D., Choo D., Vezys V., Wherry E.J., Duraiswamy J., Akondy R., Wang J., Casey K.A., Barber D.L., Kawamura K.S., et al. 2010. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207:553–564. 10.1084/jem.20090858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster, S.R., Wilson J.J., Wang H., and Kohlmeier J.E.. 2015. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-γ production. J. Immunol. 195:203–209. 10.4049/jimmunol.1402975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menares, E., Gálvez-Cancino F., Cáceres-Morgado P., Ghorani E., López E., Díaz X., Saavedra-Almarza J., Figueroa D.A., Roa E., Quezada S.A., and Lladser A.. 2019. Tissue-resident memory CD8+ T cells amplify anti-tumor immunity by triggering antigen spreading through dendritic cells. Nat. Commun. 10:4401. 10.1038/s41467-019-12319-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner, J.J., and Goldrath A.W.. 2018. Transcriptional programming of tissue-resident memory CD8+ T cells. Curr. Opin. Immunol. 51:162–169. 10.1016/j.coi.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner, J.J., Toma C., Yu B., Zhang K., Omilusik K., Phan A.T., Wang D., Getzler A.J., Nguyen T., Crotty S., et al. 2017. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature. 552:253–257. 10.1038/nature24993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner, J.J., Toma C., He Z., Kurd N.S., Nguyen Q.P., McDonald B., Quezada L., Widjaja C.E., Witherden D.A., Crowl J.T., et al. 2020. Heterogenous populations of tissue-resident CD8+ T cells are generated in response to infection and malignancy. Immunity. 52:808–824.e7. 10.1016/j.immuni.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molodtsov, A., and Turk M.J.. 2018. Tissue resident CD8 memory T cell responses in cancer and autoimmunity. Front. Immunol. 9:2810. 10.3389/fimmu.2018.02810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, T., Fuertes Marraco S.A., Baumgaertner P., Bordry N., Cagnon L., Donda A., Romero P., Verdeil G., and Speiser D.E.. 2016. Very late antigen-1 marks functional tumor-resident CD8 T cells and correlates with survival of melanoma patients. Front. Immunol. 7:573. 10.3389/fimmu.2016.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruganandah, V., Sathkumara H.D., Navarro S., and Kupz A.. 2018. A systematic review: the role of resident memory T cells in infectious diseases and their relevance for vaccine development. Front. Immunol. 9:1574. 10.3389/fimmu.2018.01574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizard, M., Roussel H., Diniz M.O., Karaki S., Tran T., Voron T., Dransart E., Sandoval F., Riquet M., Rance B., et al. 2017. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat. Commun. 8:15221. 10.1038/ncomms15221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara, J.M., Redhu N.S., Cheung E., Robertson N.G., Patik I., Sayed S.E., Thompson C.M., Herd M., Lucas K.B., Conaway E., et al. 2020. Generation of protective pneumococcal-specific nasal resident memory CD4+ T cells via parenteral immunization. Mucosal Immunol. 13:172–182. 10.1038/s41385-019-0218-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmatsu, H., Kadono T., Sugaya M., Tomita M., Kai H., Miyagaki T., Saeki H., Tamaki K., Steeber D.A., Tedder T.F., and Sato S.. 2010. α4β7 Integrin is essential for contact hypersensitivity by regulating migration of T cells to skin. J. Allergy Clin. Immunol. 126:1267–1276. 10.1016/j.jaci.2010.08.048 [DOI] [PubMed] [Google Scholar]
- Paik, D.H., and Farber D.L.. 2021. Anti-viral protective capacity of tissue resident memory T cells. Curr. Opin. Virol. 46:20–26. 10.1016/j.coviro.2020.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallett, L.J., Burton A.R., Amin O.E., Rodriguez-Tajes S., Patel A.A., Zakeri N., Jeffery-Smith A., Swadling L., Schmidt N.M., Baiges A., et al. 2020. Longevity and replenishment of human liver-resident memory T cells and mononuclear phagocytes. J. Exp. Med. 217:e20200050. 10.1084/jem.20200050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Y., Tian T., Park C.O., Lofftus S.Y., Mei S., Liu X., Luo C., O’Malley J.T., Gehad A., Teague J.E., et al. 2017. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature. 543:252–256. 10.1038/nature21379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao, W., Ooi C.-H., Birzele F., Ruefli-Brasse A., Cannarile M.A., Reis B., Scharf S.H., Schubert D.A., Hatje K., Pelletier N., et al. 2018. Tissue-specific immunoregulation: a call for better understanding of the “Immunostat” in the context of cancer. Cancer Discov. 8:395–402. 10.1158/2159-8290.CD-17-1320 [DOI] [PubMed] [Google Scholar]
- Park, C.O., and Kupper T.S.. 2015. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 21:688–697. 10.1038/nm.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S.L., Zaid A., Hor J.L., Christo S.N., Prier J.E., Davies B., Alexandre Y.O., Gregory J.L., Russell T.A., Gebhardt T., et al. 2018. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 19:183–191. 10.1038/s41590-017-0027-5 [DOI] [PubMed] [Google Scholar]
- Park, S.L., Gebhardt T., and Mackay L.K.. 2019. Tissue-resident memory T cells in cancer immunosurveillance. Trends Immunol. 40:735–747. 10.1016/j.it.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Park, S.L., Buzzai A., Rautela J., Hor J.L., Hochheiser K., Effern M., McBain N., Wagner T., Edwards J., McConville R., et al. 2019a. Tissue-resident memory CD8+ T cells promote melanoma-immune equilibrium in skin. Nature. 565:366–371. 10.1038/s41586-018-0812-9 [DOI] [PubMed] [Google Scholar]
- Qaiyum, Z., Gracey E., Yao Y., and Inman R.D.. 2019. Integrin and transcriptomic profiles identify a distinctive synovial CD8+ T cell subpopulation in spondyloarthritis. Ann. Rheum. Dis. 78:1566–1575. 10.1136/annrheumdis-2019-215349 [DOI] [PubMed] [Google Scholar]
- Raphael, I., Joern R.R., and Forsthuber T.G.. 2020. Memory CD4+ T cells in immunity and autoimmune diseases. Cells. 9:531. 10.3390/cells9030531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval, F., Terme M., Nizard M., Badoual C.. Bureau M.-F., Freyburger L., Clement O., Marcheteau E., Gey A., Fraisse G., et al. 2013. Mucosal imprinting of vaccine-induced CD8+ T cells is crucial to inhibit the growth of mucosal tumors. Sci. Transl. Med. 5:172ra20. 10.1126/scitranslmed.3004888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoiemma, P.P., Reyes C., Wang L.-P., McLane M.W., Feldman M.D., Tanyi J.L., and Powell D.J. Jr. 2016. Systematic evaluation of multiple immune markers reveals prognostic factors in ovarian cancer. Gynecol. Oncol. 143:120–127. 10.1016/j.ygyno.2016.07.105 [DOI] [PubMed] [Google Scholar]
- Sasson, S.C., Gordon C.L., Christo S.N., Klenerman P., and Mackay L.K.. 2020. Local heroes or villains: tissue-resident memory T cells in human health and disease. Cell. Mol. Immunol. 17:113–122. 10.1038/s41423-019-0359-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas, P., Virassamy B., Ye C., Salim A., Mintoff C.P., Caramia F., Salgado R., Byrne D.J., Teo Z.L., Dushyanthen S., et al. Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab) . 2018. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 24:986–993. 10.1038/s41591-018-0078-7 [DOI] [PubMed] [Google Scholar]
- Schenkel, J.M., and Masopust D.. 2014. Tissue-resident memory T cells. Immunity. 41:886–897. 10.1016/j.immuni.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel, J.M., Fraser K.A., Vezys V., and Masopust D.. 2013. Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol. 14:509–513. 10.1038/ni.2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel, J.M., Fraser K.A., Beura L.K., Pauken K.E., Vezys V., and Masopust D.. 2014. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 346:98–101. 10.1126/science.1254536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, H., Kumamoto Y., Gopinath S., and Iwasaki A.. 2016. CD301b+ dendritic cells stimulate tissue-resident memory CD8+ T cells to protect against genital HSV-2. Nat. Commun. 7:13346. 10.1038/ncomms13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skon, C.N., Lee J.-Y., Anderson K.G., Masopust D., Hogquist K.A., and Jameson S.C.. 2013. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 14:1285–1293. 10.1038/ni.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smazynski, J., and Webb J.R.. 2018. Resident memory-like tumor-infiltrating lymphocytes (TILRM): latest players in the immuno-oncology repertoire. Front. Immunol. 9:1741. 10.3389/fimmu.2018.01741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, M.E., Finlayson M.O., Connors T.J., Dogra P., Senda T., Bush E., Carpenter D., Marboe C., Benvenuto L., Shah L., et al. 2019. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci. Immunol. 4:eaav5581. 10.1126/sciimmunol.aav5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell, R.T., Goldufsky J.W., Rogozinska M., Quiles Z., Cao Y., Castillo E.F., Finnegan A., and Marzo A.L.. 2017. IL-15 complexes induce migration of resting memory CD8 T cells into mucosal tissues. J. Immunol. 199:2536–2546. 10.4049/jimmunol.1501638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert, E.M., Schenkel J.M., Fraser K.A., Beura L.K., Manlove L.S., Igyártó B.Z., Southern P.J., and Masopust D.. 2015. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell. 161:737–749. 10.1016/j.cell.2015.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolley, J.M., Johnston T.S., Soerens A.G., Beura L.K., Rosato P.C., Joag V., Wijeyesinghe S.P., Langlois R.A., Osum K.C., Mitchell J.S., and Masopust D.. 2020. Retrograde migration supplies resident memory T cells to lung-draining LN after influenza infection. J. Exp. Med. 217:e20192197. 10.1084/jem.20192197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y.-Y., Peng S., Han L., Qiu J., Song L., Tsai Y., Yang B., Roden R.B.S., Trimble C.L., Hung C.-F., and Wu T.C.. 2016. Local HPV recombinant vaccinia boost following priming with an HPV DNA vaccine enhances local HPV-specific CD8+ T-cell-mediated tumor control in the genital tract. Clin. Cancer Res. 22:657–669. 10.1158/1078-0432.CCR-15-0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H., Sun C., Xiao W., and Sun R.. 2019. Tissue-resident lymphocytes: from adaptive to innate immunity. Cell. Mol. Immunol. 16:205–215. 10.1038/s41423-018-0192-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo, P.A., Miron M., and Farber D.L.. 2019. Location, location, location: Tissue resident memory T cells in mice and humans. Sci. Immunol. 4:eaas9673. 10.1126/sciimmunol.aas9673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, L., Zheng Y., Melo M.B., Mabardi L., Castaño A.P., Xie Y.-Q., Li N., Kudchodkar S.B., Wong H.C., Jeng E.K., et al. 2018. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 36:707–716. 10.1038/nbt.4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro, J.R., Turner D., Pham Q., Wherry E.J., Lefrançois L., and Farber D.L.. 2011. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 187:5510–5514. 10.4049/jimmunol.1102243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, D.L., Bickham K.L., Thome J.J.T., Kim C.Y., D’Ovidio F., Wherry E.J., and Farber D.L.. 2014. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 7:501–510. 10.1038/mi.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddbäck, I., Cartwright E.K., Schøller A.S., Wein A.N., Hayward S.L., Lobby J., Takamura S., Thomsen A.R., Kohlmeier J.E., and Christensen J.P.. 2021. Long-term maintenance of lung resident memory T cells is mediated by persistent antigen. Mucosal Immunol. 14:92–99. 10.1038/s41385-020-0309-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., and Zou W.. 2020. Amino acids and their transporters in T cell immunity and cancer therapy. Mol. Cell. 80:384–395. 10.1016/j.molcel.2020.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B., Wu S., Zeng H., Liu Z., Dong W., He W., Chen X., Dong X., Zheng L., Lin T., and Huang J.. 2015. CD103+ tumor infiltrating lymphocytes predict a favorable prognosis in urothelial cell carcinoma of the bladder. J. Urol. 194:556–562. 10.1016/j.juro.2015.02.2941 [DOI] [PubMed] [Google Scholar]
- Wang, Z.-Q., Milne K., Derocher H., Webb J.R., Nelson B.H., and Watson P.H.. 2016. CD103 and intratumoral immune response in breast cancer. Clin. Cancer Res. 22:6290–6297. 10.1158/1078-0432.CCR-16-0732 [DOI] [PubMed] [Google Scholar]
- Webb, J.R., Milne K., Watson P., Deleeuw R.J., and Nelson B.H.. 2014. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin. Cancer Res. 20:434–444. 10.1158/1078-0432.CCR-13-1877 [DOI] [PubMed] [Google Scholar]
- Webb, J.R., Milne K., and Nelson B.H.. 2015. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol. Res. 3:926–935. 10.1158/2326-6066.CIR-14-0239 [DOI] [PubMed] [Google Scholar]
- Wein, A.N., McMaster S.R., Takamura S., Dunbar P.R., Cartwright E.K., Hayward S.L., McManus D.T., Shimaoka T., Ueha S., Tsukui T., et al. 2019. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J. Exp. Med. 216:2748–2762. 10.1084/jem.20181308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg, S.P., Carpenter D.J., Chait M., Dogra P., Gartrell-Corrado R.D., Chen A.X., Campbell S., Liu W., Saraf P., Snyder M.E., et al. 2019. Tissue-resident memory T cells mediate immune homeostasis in the human pancreas through the PD-1/PD-L1 pathway. Cell Rep. 29:3916–3932.e5. 10.1016/j.celrep.2019.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk, M.M., and Mills K.H.G.. 2018. CD4 TRM cells following infection and immunization: implications for more effective vaccine design. Front. Immunol. 9:1860. 10.3389/fimmu.2018.01860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workel, H.H., Komdeur F.L., Wouters M.C.A., Plat A., Klip H.G., Eggink F.A., Wisman G.B.A., Arts H.J.G., Oonk M.H.M., Mourits M.J.E., et al. 2016. CD103 defines intraepithelial CD8+ PD1+ tumour-infiltrating lymphocytes of prognostic significance in endometrial adenocarcinoma. Eur. J. Cancer. 60:1–11. 10.1016/j.ejca.2016.02.026 [DOI] [PubMed] [Google Scholar]
- Wu, T.-C., Xu K., Banchereau R., Marches F., Yu C.I., Martinek J., Anguiano E., Pedroza-Gonzalez A., Snipes G.J., O’Shaughnessy J., et al. 2014. Reprogramming tumor-infiltrating dendritic cells for CD103+ CD8+ mucosal T-cell differentiation and breast cancer rejection. Cancer Immunol. Res. 2:487–500. 10.1158/2326-6066.CIR-13-0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X., Wu P., Shen Y., Jiang X., and Xu F.. 2018. CD8+ resident memory T cells and viral infection. Front. Immunol. 9:2093. 10.3389/fimmu.2018.02093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Y.-Q., Arik H., Wei L., Zheng Y., Suh H., Irvine D.J., and Tang L.. 2019. Redox-responsive interleukin-2 nanogel specifically and safely promotes the proliferation and memory precursor differentiation of tumor-reactive T-cells. Biomater. Sci. 7:1345–1357. 10.1039/C8BM01556B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, C.I., Becker C., Wang Y., Marches F., Helft J., Leboeuf M., Anguiano E., Pourpe S., Goller K., Pascual V., et al. 2013. Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-β. Immunity. 38:818–830. 10.1016/j.immuni.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N., and Bevan M.J.. 2013. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 39:687–696. 10.1016/j.immuni.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Kurupati R., Liu L., Zhou X.Y., Zhang G., Hudaihed A., Filisio F., Giles-Davis W., Xu X., Karakousis G.C., et al. 2017. Enhancing CD8+ T cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cancer Cell. 32:377–391.e9. 10.1016/j.ccell.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, E., Maj T., Kryczek I., Li W., Wu K., Zhao L., Wei S., Crespo J., Wan S., Vatan L., et al. 2016. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat. Immunol. 17:95–103. 10.1038/ni.3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J., Koelle D.M., Cao J., Vazquez J., Huang M.L., Hladik F., Wald A., and Corey L.. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 204:595–603. 10.1084/jem.20061792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, W. 2005. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 5:263–274. 10.1038/nrc1586 [DOI] [PubMed] [Google Scholar]
- Zou, W., Wolchok J.D., and Chen L.. 2016. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 8:328rv4. 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]