Abstract
Purpose:
The microvascular capillary network is ensheathed by cells called pericytes - a heterogeneous population of mural cells derived from multiple lineages. Pericytes play a multifaceted role in the body, including in vascular structure and permeability, regulation of local blood flow, immune and wound healing functions, induction of angiogenesis, and generation of various progenitor cells. Here, we consider the role of pericytes in capillary de-recruitment, a pathophysiologic phenomenon that is observed following hyperemic stimuli in the presence of a stenosis and attenuates the hyperemic response.
Recent Findings:
We discuss recent observations that conclusively demonstrate pericytes to be the cellular structures that contract in response to hyperemic stimuli when an upstream arterial stenosis is present. This response constricts capillaries, which is likely aimed at maintaining capillary hydrostatic pressure, an important factor in tissue homeostasis. Nonetheless, the ensuing attenuation of the hyperemic response can lead to a decrease in energy supply and negatively impact tissue health.
Summary:
Therapeutics aimed at preventing pericyte-mediated capillary de-recruitment may prove beneficial in conditions such as coronary stenosis and peripheral arterial disease by reducing restriction in hyperemic flow. Identification of the pericyte subtypes involved in this de-recruitment and the underlying molecular mechanisms regulating this process will greatly assist this purpose.
Keywords: pericyte, arterial stenosis, microvascular blood flow, capillary, capillary de-recruitment, myocardial ischemia, coronary stenosis, peripheral arterial disease
Introduction
The principles of tissue homeostatis were defined by Starling in 1896 and were further refined over the years [1]. The four starling forces that govern tissue fluid homeostasis are the hydrostatic and oncotic pressures in the capillary and interstitium, respectively, and their balance defines net fluid filtration as well as cell size. Of these forces, the one most likely to fluctuate acutely is the capillary hydrostatic pressure (CHP) because it can be influenced by systemic pressure. In order to maintain a constant CHP at a mean of about 30 mmHg, the upstream resistance arterioles, 100–300 μm in diameter, change their tone in response to aortic pressure. Thus, they dilate when aortic pressure falls and constrict when the aortic pressure rises, in order to maintain constant CHP and blood flow, a phenomenon termed autoregulation [2, 3]. This phenomenon is noted in all vital organs, including the heart, and serves to maintain constant pressure within capillaries. In conditions of hyperemia under stenosis, this drive to maintain CHP results in attenuation of hyperemic blood flow. This review focuses on what is known about the cellular structures that control capillary contractility and hence CHP, namely pericytes, and points towards future research directions necessary to better understand the molecular mechanisms regulating this process with a view towards developing therapeutics for conditions featuring stenosis.
Relationship between aortic pressure and capillary hydrostatic pressure
In the heart, autoregulation maintains a constant CHP when the mean aortic pressure (and thus, the mean epicardial coronary pressure) ranges from approximately 45 mmHg to 120 mmHg, the physiologic autoregulatory range. At 45 mmHg aortic pressure, the low end of this range, the arterioles are fully dilated and cannot dilate anymore, while at the high end - 120 mmHg - they are fully constricted and cannot constrict anymore [4]. At these extremes, the flow through the coronary vasculature is driven directly by aortic pressure in comparison to the autoregulatory range where flow is maintained constant despite variations in aortic pressure.
Stenosis and capillary de-recruitment
In the presence of a non-critical stenosis, the hyperemic response is attenuated without alterations in resting perfusion pressure (Figure 1) [5]. This occurs because autoregulation decreases downstream vascular resistance in a manner inversely proportional to the increase in resistance caused by the stenosis. In this setting, the total resistance does not change but the decrease in arteriolar resistance allows maintenance of CHP. When hyperemia is induced by exercise or exogenously administered vasodilators, the increased flow through the stenosis causes a pressure drop beyond it, thus imperiling CHP. In response, a phenomenon we termed capillary ‘de-recruitment’ is observed [6*]. Capillary de-recruitment under physiological or pharmacological stress results in a reversible perfusion defect on myocardial contrast echocardiography (Figure 2) [7–9] and other imaging modalities [10**], which allows their use in detecting physiologically significant coronary artery disease. Presumably, this de-recruitment increases capillary resistance, which helps maintain CHP, but the ensuing attenuation of hyperemic blood flow through the tissue is likely to have a negative impact on tissue metabolism and health. Although ‘pre-capillary sphincters’ had been suggested as mediating capillary de-recruitment [6*], the exact nature of these sphincters and, hence, the mechanism responsible for capillary de-recruitment under stenosis remained elusive until recently, largely because of a lack of knowledge regarding the cellular structures that impart contractility to capillaries.
Figure 1.
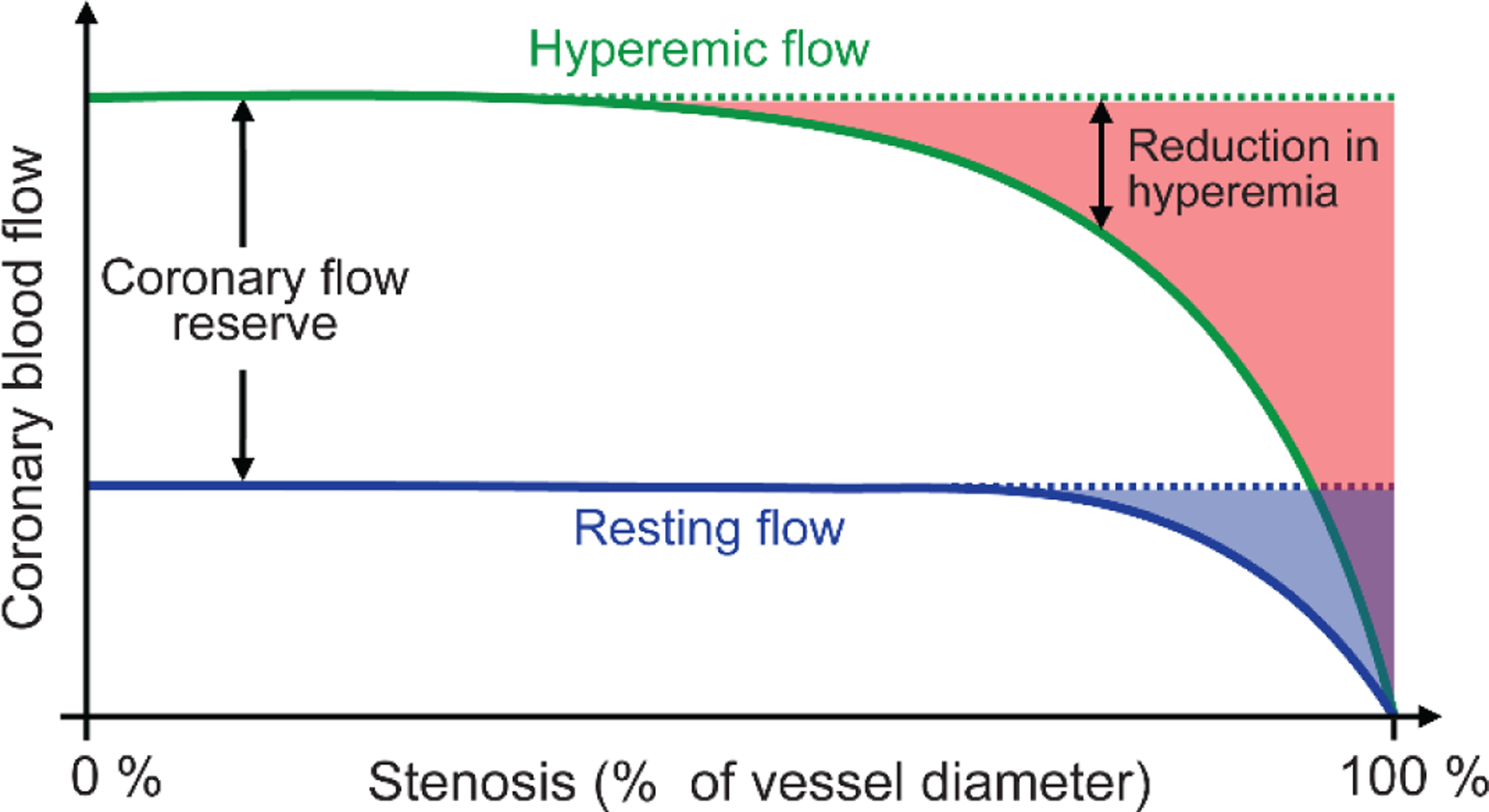
Relationship between percent narrowing of the coronary artery diameter (stenosis, x-axis) and coronary blood flow (y-axis) during rest (dashed blue line) and hyperemia (solid green line). The resting flow is maintained at normal levels (dotted black line) up to 85–90% stenosis because of autoregulation. During hyperemia, however, increases in flow are attenuated at 50% or greater stenosis, with more severe stenosis resulting in greater attenuation of hyperemia flow. The shaded blue region denotes the decrease in resting flow d the shaded red region denotes reduction in hyperemic flow. Adapted with permission from Gould et al. [5]†.
† Reprinted from The American Journal of Cardiology, Vol. 34, Gould, K. L. and Lipscomb, K., Effect of coronary stenosis on coronary flow reserve and resistance, Pages P48–55, DOI: 10.1016/0002-9149(74)90092-7. Copyright (1974), with permission from Elsevier; License # 4850441356925 (June, 2020).
Figure 2.
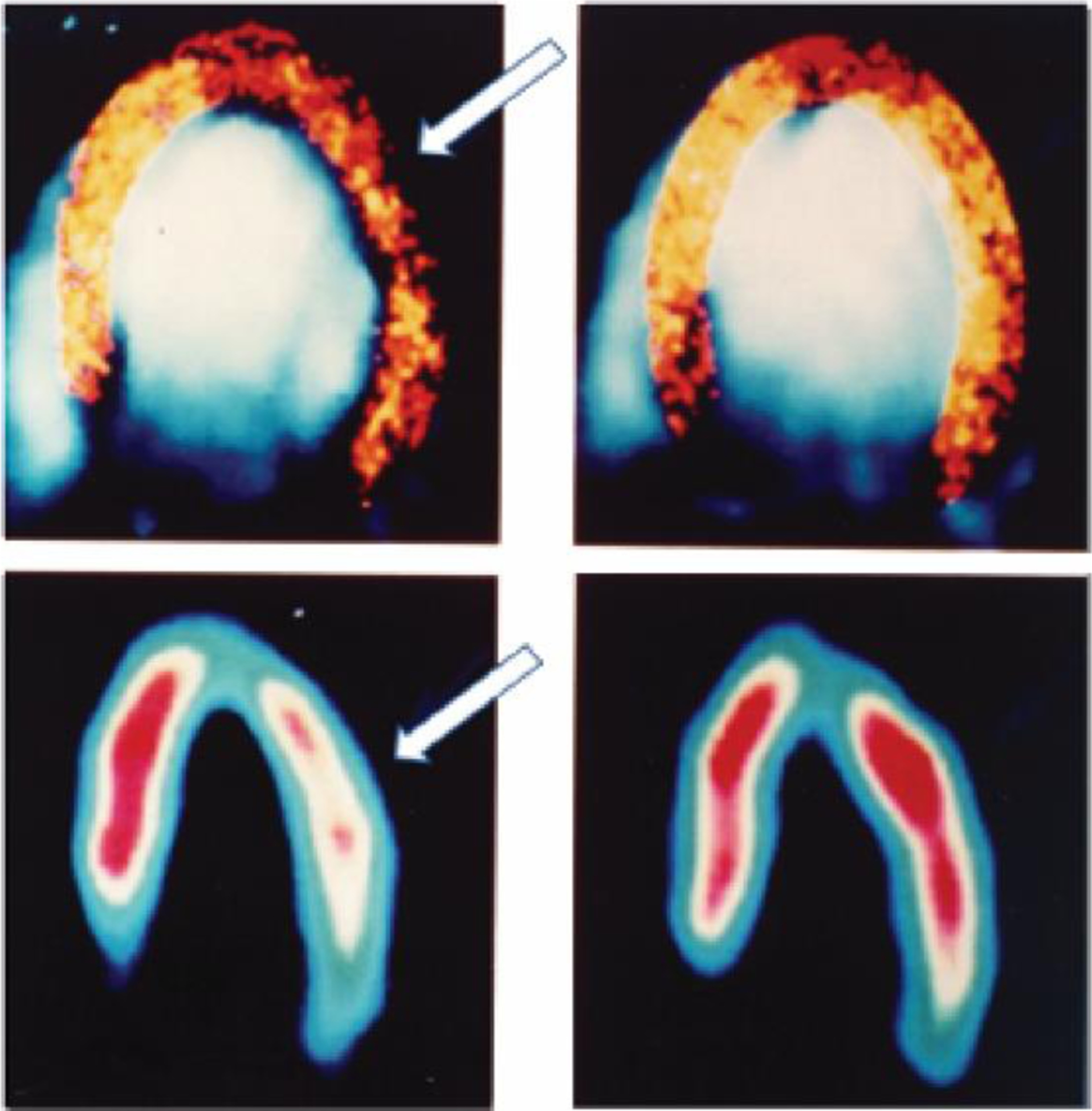
Myocardial contrast echocardiography (top) and 99mTc-sestamibi SPECT images (bottom) at rest (right panel) and stress (left panel). White arrows depict reversible perfusion defects that are caused by capillary de-recruitment in a patient with left circumflex artery stenosis. At rest, perfusion (capillary density) was normal and equal to the contralateral left anterior descending artery bed while during hyperemia the capillary density decreased in the left circumflex compared to the left anterior descending artery bed. Reprinted with permission from Kaul et al. [9]§.
§Reprinted from Kaul S., Senior, R., Dittrich, H., Raval, U., Khattar, R. and Lahiri, A. Detection of coronary artery disease with myocardial contrast echocardiography: comparison with 99mTc-sestamibi single-photon emission computed tomography. Circulation, 1997. 96(3): p. 785–92. DOI: 10.1161/01.CIR.96.3.785. Copyright (1997), with permission from Wolters Kluwer Health, Inc.; License # 4850970686370 (June, 2020).
Pericytes
Pericytes were first described by Rouget in 1874, two decades before Starling published his paper on tissue homeostasis, as cells that form the contractile tunic of the vessels [11]. Half a century later, in 1923, Zimmerman assigned them the name ‘pericytes’ based on their location and described them as cells that clung to the outside of capillaries [12]. During this same period, Krogh’s Nobel prize winning work had already established that capillary diameter could change independent of their upstream arteries to regulate local blood flow [13, 14] and pericytes were proposed as the cellular substrate mediating these changes [15]. Despite these seminal observations, pericytes were overlooked in vascular physiology research for a large part of the twentieth century. Fortunately, they have enjoyed a renewed interest in the last few decades, producing a flourishing body of work on pericyte phenotype and biology, including their contribution to vascular structure and permeability, blood flow regulation, immune and wound healing functions, progenitor capacity, and, in the central nervous system (CNS), maintenance of the blood-brain barrier [16–26].
Pericytes can be identified by their relatively high expression of the platelet-derived growth factor receptor β (PDGFRβ) and neuron-glia antigen 2 (NG2), a chondroitin sulfate proteoglycan [23]. Pericytes also express desmin (also found in myocytes), CD13 and CD146 [24, 27–31]. Although these markers are not entirely unique to pericytes, they can nonetheless be used to reliably identify pericytes in conjunction with their morphology and location along the vascular tree. Unlike vascular smooth muscle cells, which cover the arterioles in a contiguous fashion, pericytes are located on capillaries as individual cells with a bump-on-a-log morphology, where their soma appear as bumps along the vasculature at approximately 30 μm intervals in the brain and retina [21, 24], and 60 μm intervals in the heart [32*] (Figure 3). Indeed, the characterization of these markers has driven the development of many tools that allow the study of pericytes more widely, e.g. mice expressing fluorescent reporters under the PDGFRβ or NG2 promoters are now commonly used in pericyte research [33–35].
Figure 3.
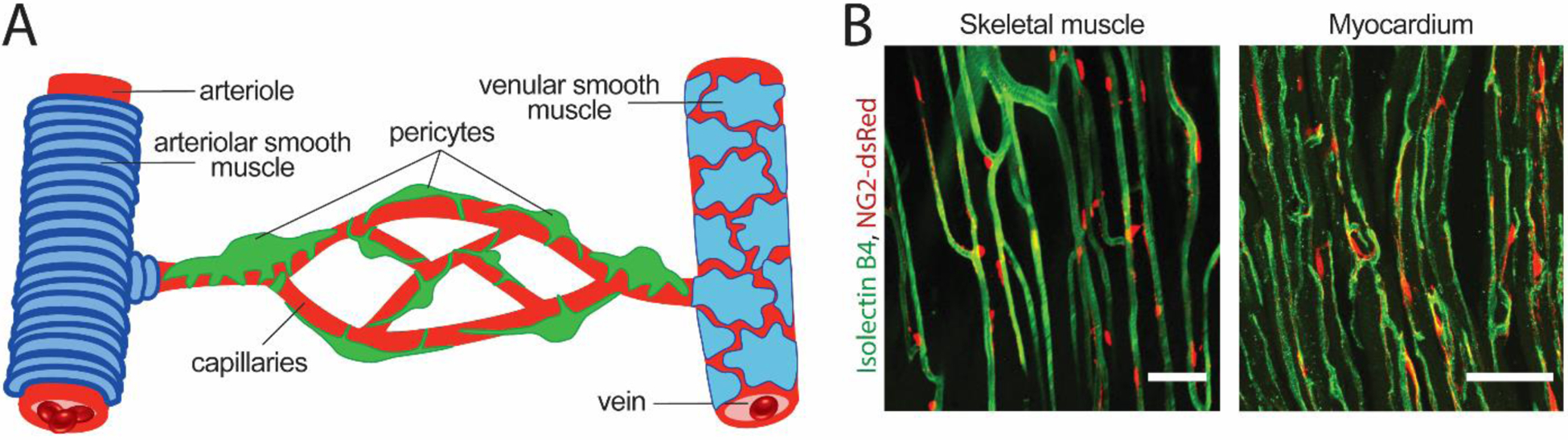
Pericyte coverage of the capillary network. (A) A schematic cartoon of the vasculature showing arteriole on the left ensheathed by arteriolar smooth muscle cells (blue), the capillary network in the center enwrapped by pericytes (green) of different morphologies as described by Hartmann et al. [37], and the draining venule covered by a thin, sparse layer of smooth muscle cells on the right. (B) Images of the microvascular network in a skeletal muscle (left) and cardiac tissue (right). These images were obtained from NG2-dsRed transgenic mice in which pericytes express the red fluosrescent protein dsRed under the NG2 promoter [33] and vessels were labeled by intravascular administration of Isolectin B4 (in green) to label the vascular basement membrane. Scale bar = 50 μm.
Organizational heterogeneity of pericytes
Zimmermann classified pericytes into three distinct classes based on their organization along the vascular tree and their corresponding morphology, namely precapillary pericytes, capillary pericytes and postcapillary pericytes (schematized in Figure 3A) [12]. These pericytes have been described in more detail in recent studies [36, 37]. Precapillary pericytes, also known as ensheathing or mesh pericytes, exist at the transitional zone between arteriole and capillaries, extend circumferential processes that wrap the vessel, and express alpha smooth muscle actin (α-SMA) [23, 36, 38]. A further subset of these are located at the transitional branch-points from arterioles to capillaries and are proposed to be the cellular substrates of pre-capillary sphincters [39]. Precapillary pericytes play an active role in microvascular regulation. At least in the CNS, they are shown to regulate activity-dependent changes in regional blood flow [17, 21, 40] and to constrict in response to ischemia and other injuries [21, 22, 41, 42]. These precapillary pericytes also harbor other specializations that contribute to their hemodynamic function. For example, although all CNS pericytes express Kir6.1 channels [43], which is involved in regulating resting perfusion [44], Kir6.1 channels in pericytes proximal to retinal arterioles were much more weakly rectifying compared to those closer to venules due to intracellular regulation [45]. The loss of this rectification gradient across the capillary network important in diabetic retinopathy [45], a condition characterized by capillary dysfunction, suggests that it is for microvascular dynamics.
In contrast, mid-capillary pericytes located in the capillary bed possess long spindly processes that twist around the endothelial tube, aptly renamed thin strand/helical pericytes [36]. In the CNS, mid-capillary pericytes are most studied in the context of blood-brain barrier development and maintenance [19, 46]. They are believed not to regulate blood flow largely due to their lack of smooth muscle actin [38], but this view has been challenged by a recent study that reported that α-SMA is indeed expressed in mid-capillary pericytes, albeit at low levels, which renders it difficult to detect using traditional fixation methods [47]. Another study reports that capillaries in the mid-capillary bed are contractile but with slower dynamics than ensheathing pericytes, regardless of branching order or α-SMA expression [48]. Indeed, mid-capillary pericytes may express actin isoforms other than α-SMA [49], such as the β- or γ-SMA as shown in the retina [50] and the heart [32*], and clearly contain other proteins such as myosins [38, 51] and tropomyosin [52] that bestow contractility. Indeed, their role in regulating blood flow has also been demonstrated in the skeletal and renal microcirculation [53, 54**].
Much less is known about the function of postcapillary pericytes on the venules, but regulation of immune extravasation has been proposed. Postcapillary pericytes often reside on top of the endothelial cell junctions on venules (and sometimes on capillaries as well) [26]. Upon stimulation by inflammatory mediators, pericytes migrate along the venule to cap the endothelial junction openings even further to limit [25, 55, 56] or regulate [57] extravasation of immune cells.
Functional heterogeneity of pericytes
Pericyte heterogeneity and distribution also depends on which part of the body is under study. Studies in human and equine samples show that distal capillaries further away from the heart have more pericytes than those closer to the heart [58, 59], with pericytes increasing in both number and coverage in a head-to-foot direction. This observation holds true within at least two different organ systems, the skin and skeletal muscle, although variation exists between organs in pericyte numbers and coverage. This prompts the hypothesis that perhaps the higher pericyte investment of capillaries further away from the heart is necessary to maintain physiologic capillary blood flow under increased hydrostatic pressure [58], although this idea has yet to be tested empirically. It is also noteworthy that pericyte coverage depends on the organ system: capillaries in the skin possess more pericytes and are more completely ensheathed than are capillaries in the skeletal muscle [58]. The skin microvascular bears a higher internal pressure compared to skeletal muscle capillaries [58], which may necessitate this higher pericyte coverage. The skin also plays a crucial role in the regulation of body temperature via dilation and constriction of the dermal capillary bed; hence, it is tempting to speculate that perhaps the higher pericyte coverage of skin capillaries is necessary for thermoregulation.
Even within the same tissue system, pericytes may differ in their functional specialization. Recently, pericytes from several organ systems–skeletal muscle, lung, heart, kidney, spinal cord and heart–were reported to classify into two main sub-types: type 1 pericytes that are NG2+/Nestin− and type 2 pericytes that are NG2+/Nestin+ [60, 61]. Type 1 pericytes tend to contribute to fibrosis in most organs, but their collagen producing ability is organ-dependent [61, 62]. Similarly, in the spinal cord, a subset of pericytes that are negative for contractile proteins have been shown to contribute to scar tissue formation [63], cementing their role in wound healing [64]. This is perhaps one reason why wound healing is slowed in conditions like diabetes, where pericyte loss is observed. Type 2 pericyte, on the other hand, tended to induce angiogenesis in vivo [65]. Further, both pericyte subtypes could give rise to α-SMA+ contractile pericytes, but only the type 2 cells could be guided to generate Tuj1+ cells lacking α-SMA that are reminiscent of neural progenitors under some in vitro conditions [60]. The attempts at pericyte classification thus far are commendable, yet the fact that the scar-forming pericytes in the spinal cord are α-SMA negative while type 1 pericytes that are fibrotic in other organ systems are more closely related to α-SMA positive cells suggests that the work is far from over.
Pericytes have also long been proposed to have immune functions [66], and this idea is now supported by several studies demonstrating that subsets of pericytes can give rise to microglia-like cells following injury [67, 68]. Further, brain pericytes can sense systemic inflammation and secrete chemokines to alter brain function [69] and activate local microglia [70]. Even more interestingly, a sub-population of pericytes in the CNS and the skin vasculature are generated from myeloid cells [71, 72], further stressing their heterogeneity in both lineage and function. The remainder of this review will focus on the role of pericytes in capillary flow regulation during arterial stenosis; however, the diversity of pericytes must be kept in mind when studying and interpreting their functions.
Pericytes in the coronary vasculature
Pericytes are also an integral component of the capillary network in the heart and other organs (Figure 3B). Most studies relating to heart pericytes have focused on cardiac regeneration, immune surveillance, and cardiotoxicity induced by cancer therapies [73–75]. The role of pericytes in controlling coronary blood flow has not been described as extensively. Similar to brain pericytes, approximately 40% of heart pericytes also express alpha smooth muscle actin [32]. A much larger proportion have been found to also express beta and gamma smooth muscle actins (~60% and ~80% of pericytes, respectively) [32, 50], suggesting that pericyte contractility in the heart may be mediated by multiple actin isoforms, potentially in a location dependent manner.
Pericytes in capillary de-recruitment
Two recent studies have suggested that pericyte-dependent capillary constriction may be a mechanism underlying capillary de-recruitment. O’Farrell et al. reported capillary constrictions in regions apposed to pericytes after acute myocardial infarction and suggested this constriction to be the underlying cause of no reflow [32*]. However, this study examined ischemic myocardial tissue and did not differentiate whether capillary constriction was due to direct effect of ischemia on pericytes or due to the pericytes sensing the change in perfusion pressure induced by coronary occlusion and actively responding by de-recruiting the capillaries.
Ideally, visualization of the pericyte contractions during coronary stenosis would be invaluable to prove the active role of these cells in capillary de-recruitment; however, this is near impossible to image in the heart due to the tissue movement caused by the heartbeat. Hence, in a recent study, we used a skeletal muscle preparation as a substitute system to conclusively show that capillary de-recruitment is caused by contraction of pericytes [54**]. When a stenosis was produced in the femoral artery and hyperemic challenge administered, a significant reduction in red blood cell flux through the downstream capillary bed resulted, reflecting capillary de-recruitment. In-vivo 2-photon microscopy demonstrated a significant increase in the number of constricting capillaries under these conditions. Importantly, the capillary regions that constricted were invested with pericytes and capillary de-recruitment was prevented in a transgenic mouse model featuring partial pericyte depletion. This was the first direct in vivo visualization of hyperemia-induced capillary de-recruitment distal to an arterial stenosis and the results establish pericytes as the contractile cell responsible for capillary de-recruitment.
In cases of coronary stenosis, similarly, a pressure drop would occur distal to the stenosis at baseline. Hyperemic stimulation under this situation, such as exercise, is expected to cause capillary constriction due to pericyte contraction, which would attenuate hyperemic blood flow in the myocardial at the time most necessary to sustain function. Such a response would cause undue metabolic stress on cardiac tissue over time and increase the likelihood of developing heart failure due to repetitive episodes of myocardial stunning [76]. Therefore, targeting pericytes to increase myocardial perfusion and restore energy supply to active cardiac tissue should be considered a viable therapeutic target for future study. Further, although the first indications that capillary de-recruitment contributes to vascular defects following stenosis came from observations made in the coronary system [6, 77], the findings discussed above [54**] suggest that this phenomenon likely also contributes to reduced peripheral perfusion observed in peripheral arterial disease, a common condition in older patients with diabetes, atherosclerosis and other cardiovascular morbidities [78, 79]. Hence, therapeutics aimed at preventing pericyte-mediated capillary de-recruitment may also prove beneficial in peripheral arterial disease [80]. Whether such capillary de-recruitment is mediated by all pericytes equally or whether only certain subpopulations of pericytes are involved remains unknown. Further studies to identify these pericyte populations and the mechanisms that trigger them to de-recruit capillaries will prove imperative in this endeavor.
Conclusions
Pericytes are a heterogeneous population of mural contractile cells that play a multifaceted role in tissue homeostasis. Unlike the brain and the retina, where the role of pericytes in blood flow control has been investigated over the past decade or two, the role of pericytes in the control of myocardial hemostasis is still largely ignored. The recent development of new tools and techniques allow better specificity in terms of pericyte identification in tissue as well as cell culture, thus opening the doors to future research in elucidating the role of pericytes in capillary blood flow and hydrostatic pressure maintenance not only in coronary stenosis but also in ischemic injury and peripheral arterial disease. Sparse but tantalizing recent discoveries imply pericyte contraction in capillary de-recruitment following coronary stenosis and myocardial ischemia. We believe that pericytes could play a very important role in local regulation of myocardial and skeletal muscle blood flow during both rest and under stress. Pharmacological manipulation of pericytes could prove to be of enormous benefit for human health and, as such, efforts towards identifying the molecular mechanisms by which pericytes regulate microvascular blood flow should be the focus of much future research.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest statement. The authors declare no conflicts of interest.
References
- 1.Starling EH, On the Absorption of Fluids from the Connective Tissue Spaces. J Physiol, 1896. 19(4): p. 312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarhult J and Mellander S, Autoregulation of capillary hydrostatic pressure in skeletal muscle during regional arterial hypo- and hypertension. Acta Physiol Scand, 1974. 91(1): p. 32–41. [DOI] [PubMed] [Google Scholar]
- 3.Johnson PC, Autoregulation of blood flow. Circ Res, 1986. 59(5): p. 483–95. [DOI] [PubMed] [Google Scholar]
- 4.Rouleau J, et al. , The role of autoregulation and tissue diastolic pressures in the transmural distribution of left ventricular blood flow in anesthetized dogs. Circ Res, 1979. 45(6): p. 804–15. [DOI] [PubMed] [Google Scholar]
- 5.Gould KL and Lipscomb K, Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol, 1974. 34(1): p. 48–55. [DOI] [PubMed] [Google Scholar]
- 6.*.Jayaweera AR, et al. , Role of capillaries in determining CBF reserve: new insights using myocardial contrast echocardiography. Am J Physiol, 1999. 277(6): p. H2363–72. [DOI] [PubMed] [Google Scholar]; First paper to show capillary derecruitment and its biophysics when hyperermia is induced in the presence of stenonsis.
- 7.Kaul S, Myocardial contrast echocardiography: 15 years of research and development. Circulation, 1997. 96(10): p. 3745–60. [DOI] [PubMed] [Google Scholar]
- 8.Kaul S, Myocardial contrast echocardiography: a 25-year retrospective. Circulation, 2008. 118(3): p. 291–308. [DOI] [PubMed] [Google Scholar]
- 9.Kaul S, et al. , Detection of coronary artery disease with myocardial contrast echocardiography: comparison with 99mTc-sestamibi single-photon emission computed tomography. Circulation, 1997. 96(3): p. 785–92. [PubMed] [Google Scholar]
- 10.**.Wei K, et al. , Mechanism of reversible (99m)Tc-sestamibi perfusion defects during pharmacologically induced vasodilatation. Am J Physiol Heart Circ Physiol, 2001. 280(4): p. H1896–904. [DOI] [PubMed] [Google Scholar]; First paper to show that capillary derecruitment during hyperemia in the presence of stenosis decreases capillary surface area leading to reversible perfusion defects that form the basis of noninvasive diagnosis of coronary artery disease.
- 11.Rouget C, Note sur le développement de la tunique contractile des vaisseaux, in Compt.rend.acad.sci 1874, Paris. p. 559–562. [Google Scholar]
- 12.Zimmermann KW, Der feinere Bau der Blutkapillaren. Z Anat Entwicklungsgesch, 1923. 68: p. 29–109. [Google Scholar]
- 13.Krogh A, Studies on the physiology of capillaries: II. The reactions to local stimuli of the blood-vessels in the skin and web of the frog. J Physiol, 1921. 55(5–6): p. 412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krogh A, The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol, 1919. 52(6): p. 457–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boas EA, The clinical significance of recent studies of the capillaries. The Boston Medical and Surgical Journal, 1925. 192(23): p. 1085–90. [Google Scholar]
- 16.Lindahl P, et al. , Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science, 1997. 277(5323): p. 242–5. [DOI] [PubMed] [Google Scholar]
- 17.Mishra A, et al. , Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dore-Duffy P and Cleary K, Morphology and properties of pericytes. Methods Mol.Biol, 2011. 686: p. 49–68. [DOI] [PubMed] [Google Scholar]
- 19.Daneman R, et al. , Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature, 2010. 468(7323): p. 562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armulik A, Genove G, and Betsholtz C, Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev.Cell, 2011. 21(2): p. 193–215. [DOI] [PubMed] [Google Scholar]
- 21.Hall CN, et al. , Capillary pericytes regulate cerebral blood flow in health and disease. Nature, 2014. 508(7494): p. 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yemisci M, et al. , Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat.Med, 2009. 15(9): p. 1031–1037. [DOI] [PubMed] [Google Scholar]
- 23.Attwell D, et al. , What is a pericyte? J Cereb Blood Flow Metab, 2016. 36(2): p. 451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra A, et al. , Imaging pericytes and capillary diameter in brain slices and isolated retinae. Nat.Protoc, 2014. 9(2): p. 323–336. [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Flores L, et al. , Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol, 2009. 24(7): p. 909–69. [DOI] [PubMed] [Google Scholar]
- 26.Sims DE, Diversity within Pericytes. Clinical and Experimental Pharmacology and Physiology, 2000. 27: p. 842–6. [DOI] [PubMed] [Google Scholar]
- 27.Dvoretskiy S, et al. , The impact of skeletal muscle contraction on CD146(+)Lin(−) pericytes. Am J Physiol Cell Physiol, 2019. 317(5): p. C1011–C1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehra A, et al. , A High Output Method to Isolate Cerebral Pericytes from Mouse. J Vis Exp, 2020(155). [DOI] [PubMed] [Google Scholar]
- 29.Gerlach JC, et al. , Perivascular mesenchymal progenitors in human fetal and adult liver. Stem Cells Dev, 2012. 21(18): p. 3258–69. [DOI] [PubMed] [Google Scholar]
- 30.Smyth LCD, et al. , Markers for human brain pericytes and smooth muscle cells. J Chem Neuroanat, 2018. 92: p. 48–60. [DOI] [PubMed] [Google Scholar]
- 31.Ma Q, et al. , Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-beta42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol Neurodegener, 2018. 13(1): p. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.*.O’Farrell FM, et al. , Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]; First paper to show that capillary constrcition due to pericyte contraction contributes to no reflow phenomenon following myocardial ischemia.
- 33.Zhu X, Bergles DE, and Nishiyama A, NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development, 2008. 135(1): p. 145–157. [DOI] [PubMed] [Google Scholar]
- 34.Sono T, et al. , Platelet Derived Growth Factor Receptor-beta (PDGFRbeta) lineage tracing highlights perivascular cell to myofibroblast transdifferentiation during post-traumatic osteoarthritis. J Orthop Res, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kisler K, et al. , Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci, 2017. 20(3): p. 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant RI, et al. , Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J Cereb Blood Flow Metab, 2019. 39(3): p. 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartmann DA, et al. , Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics, 2015. 2(4): p. 041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nehls V and Drenckhahn D, Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol, 1991. 113(1): p. 147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grubb S, et al. , Precapillary sphincters maintain perfusion in the cerebral cortex. Nat Commun, 2020. 11(1): p. 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peppiatt CM, et al. , Bidirectional control of CNS capillary diameter by pericytes. Nature, 2006. 443(7112): p. 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dore-Duffy P, et al. , Pericyte-mediated vasoconstriction underlies TBI-induced hypoperfusion. Neurol.Res, 2011. 33(2): p. 176–186. [DOI] [PubMed] [Google Scholar]
- 42.Alarcon-Martinez L, et al. , Retinal ischemia induces alpha-SMA-mediated capillary pericyte contraction coincident with perivascular glycogen depletion. Acta Neuropathol Commun, 2019. 7(1): p. 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bondjers C, et al. , Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J, 2006. 20(10): p. 1703–5. [DOI] [PubMed] [Google Scholar]
- 44.Hosford PS, et al. , A critical role for the ATP-sensitive potassium channel subunit KIR6.1 in the control of cerebral blood flow. J Cereb Blood Flow Metab, 2019. 39(10): p. 2089–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsushita K and Puro DG, Topographical heterogeneity of K(IR) currents in pericyte-containing microvessels of the rat retina: effect of diabetes. J Physiol, 2006. 573(Pt 2): p. 483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armulik A, Mae M, and Betsholtz C, Pericytes and the blood-brain barrier: recent advances and implications for the delivery of CNS therapy. Ther.Deliv, 2011. 2(4): p. 419–422. [DOI] [PubMed] [Google Scholar]
- 47.Alarcon-Martinez L, et al. , Capillary pericytes express alpha-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. Elife, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartmann DA, et al. , Brain capillary pericytes exert a substantial but slow influence on blood flow. bioRxiv, 2020: p. 2020.03.26.008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herman IM and D’Amore PA, Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol, 1985. 101(1): p. 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeNofrio D, Hoock TC, and Herman IM, Functional sorting of actin isoforms in microvascular pericytes. J Cell Biol, 1989. 109(1): p. 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joyce NC, Haire MF, and Palade GE, Contractile proteins in pericytes. II. Immunocytochemical evidence for the presence of two isomyosins in graded concentrations. J.Cell Biol, 1985. 100(5): p. 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joyce NC, Haire MF, and Palade GE, Contractile proteins in pericytes. I. Immunoperoxidase localization of tropomyosin. J.Cell Biol, 1985. 100(5): p. 1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spiranec K, et al. , Endothelial C-Type Natriuretic Peptide Acts on Pericytes to Regulate Microcirculatory Flow and Blood Pressure. Circulation, 2018. 138(5): p. 494–508. [DOI] [PubMed] [Google Scholar]
- 54.**.Methner C, et al. , Pericyte constriction underlies capillary derecruitment during hyperemia in the setting of arterial stenosis. Am J Physiol Heart Circ Physiol, 2019. 317(2): p. H255–H263. [DOI] [PMC free article] [PubMed] [Google Scholar]; First in vivo optical documentation that pericytes are responsible for capillary derecruitment during hyperemia in the presence of stenosis.
- 55.Sims DE, et al. , Interleukin-2 alters the positions of capillary and venule pericytes in rat cremaster muscle. J Submicrosc Cytol Pathol, 1994. 26(4): p. 507–13. [PubMed] [Google Scholar]
- 56.Sims DE, et al. , Ultrastructure of pericytes in early stages of histamine-induced inflammation. J Morphol, 1990. 206(3): p. 333–42. [DOI] [PubMed] [Google Scholar]
- 57.Proebstl D, et al. , Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med, 2012. 209(6): p. 1219–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sims D, et al. , Heterogeneity of pericyte populations in equine skeletal muscle and dermal microvessels: a quantitative study. Anat Histol Embryol, 1994. 23(3): p. 232–8. [DOI] [PubMed] [Google Scholar]
- 59.Tilton RGF, A. M; Hoffmann PL; Kilo C; Williamson IR, Acellular Capillaries and Increased Pericyte Degeneration in the Diabetic Extremity. Frontiers in Diabetes, 1987. 8: p. 186–9. [Google Scholar]
- 60.Birbrair A, et al. , Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res, 2013. 10(1): p. 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Birbrair A, et al. , Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Ther, 2014. 5(6): p. 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Birbrair A, et al. , Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol, 2013. 305(11): p. C1098–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goritz C, et al. , A pericyte origin of spinal cord scar tissue. Science, 2011. 333(6039): p. 238–42. [DOI] [PubMed] [Google Scholar]
- 64.Bodnar RJ, et al. , Pericytes: A newly recognized player in wound healing. Wound Repair Regen, 2016. 24(2): p. 204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Birbrair A, et al. , Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol, 2014. 307(1): p. C25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas WE, Brain macrophages: on the role of pericytes and perivascular cells. Brain Res Brain Res Rev, 1999. 31(1): p. 42–57. [DOI] [PubMed] [Google Scholar]
- 67.Sakuma R, et al. , Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J Neuroinflammation, 2016. 13(1): p. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ozen I, et al. , Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol, 2014. 128(3): p. 381–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duan L, et al. , PDGFRbeta Cells Rapidly Relay Inflammatory Signal from the Circulatory System to Neurons via Chemokine CCL2. Neuron, 2018. 100(1): p. 183–200 e8. [DOI] [PubMed] [Google Scholar]
- 70.Matsumoto J, et al. , TNF-alpha-sensitive brain pericytes activate microglia by releasing IL-6 through cooperation between IkappaB-NFkappaB and JAK-STAT3 pathways. Brain Res, 2018. 1692: p. 34–44. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto S, et al. , A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci Rep, 2017. 7(1): p. 3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamazaki T, et al. , Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-beta Signaling in Developing Skin Vasculature. Cell Rep, 2017. 18(12): p. 2991–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chintalgattu V, et al. , Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci Transl Med, 2013. 5(187): p. 187ra69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee LL and Chintalgattu V, Pericytes in the Heart. Adv Exp Med Biol, 2019. 1122: p. 187–210. [DOI] [PubMed] [Google Scholar]
- 75.Stark K, Pekayvaz K, and Massberg S, Role of pericytes in vascular immunosurveillance. Front Biosci (Landmark Ed), 2018. 23: p. 767–781. [DOI] [PubMed] [Google Scholar]
- 76.Firoozan S, et al. , A canine model of chronic ischemic cardiomyopathy: characterization of regional flow-function relations. Am J Physiol, 1999. 276(2): p. H446–55. [DOI] [PubMed] [Google Scholar]
- 77.Bin JP, et al. , Direct effects of dobutamine on the coronary microcirculation: comparison with adenosine using myocardial contrast echocardiography. J Am Soc Echocardiogr, 2003. 16(8): p. 871–9. [DOI] [PubMed] [Google Scholar]
- 78.Criqui MH and Aboyans V, Epidemiology of peripheral artery disease. Circ Res, 2015. 116(9): p. 1509–26. [DOI] [PubMed] [Google Scholar]
- 79.Criqui MH, et al. , Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med, 1992. 326(6): p. 381–6. [DOI] [PubMed] [Google Scholar]
- 80.Murray IR, et al. , Skeletal and cardiac muscle pericytes: Functions and therapeutic potential. Pharmacol Ther, 2017. 171: p. 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


