Abstract
Objective:
The objective of the study was to compare maintenance versus single course of intravesical Bacillus Calmette–Guerin (BCG) in the management of high-risk nonmuscle invasive bladder cancer (NMIBC) regarding recurrence, progression, survival, and complications.
Patients and Methods:
After transurethral resection of bladder tumor (TURBT), Group I patients (33) received weekly doses of 90 mg of live attenuated Pasteur strain of BCG. The course was started 14 days after the second TURBT for 6 consecutive weeks. In Group II: 35 patients, the induction schedule was followed by 3 weekly instillations at months 3, 6, and 12 as a maintenance course. Recurrence, progression rates, survival, and toxicity were assessed in both the groups.
Results:
Patients with induction therapy alone had significantly higher recurrence rate than those received maintenance therapy (55.6% vs. 19.2%, P = 0.01). The 5-year recurrence-free survival rate was 41% and 78% in both the groups, respectively. There was no significant difference regarding the progression rate for both the groups. The mean 5-year progression-free time was comparable between the two groups. The 5-year progression-free survival was 69.8% for patients who underwent induction therapy alone compared to 70.7% for maintenance therapy. Overall local adverse events were significantly higher in patients who underwent maintenance treatment protocol.
Statistical Analysis Used:
SPSS package version 20 and Kaplan–Meier curves were used to evaluate the survival rate.
Conclusions:
Maintenance doses of BCG significantly decrease and delay the recurrence of high-risk NMIBC. However, there is no significant favor as regards tumor progression. Maintenance doses of BCG are significantly associated with a higher incidence of local adverse effects than induction doses alone.
Keywords: Bacillus Calmette–Guerin toxicity, Bacillus Calmette-Guerin, high-risk nonmuscle invasive bladder cancer
INTRODUCTION
Intravesical Bacillus Calmette–Guerin (BCG) proved to be more effective than other intravesical chemotherapeutic agents in terms of prevention/slowing down of recurrence and progression of nonmuscle invasive bladder cancer (NMIBC).[1,2,3,4] Several studies confirmed the superiority of 1–3 years of maintenance doses over the standard six induction doses.[5,6] Other reports found that maintenance doses may be associated with more adverse events and drug toxicity, without adding benefits than the induction doses alone.[7,8,9,10]
The standard regimen of BCG after transurethral resection of bladder tumor (TURBT) is still controversial.[5,6,7,11]
In the current study, maintenance and single course of intravesical BCG in management of high-risk NMIBC were compared to evaluate the recurrence, progression, survival, and complication rates of both the regimens.
PATIENTS AND METHODS
This was a prospective randomized comparative study including 68 patients with high-risk NMIBC. Patients were divided into two groups: Group I: 33 patients (48.5%) belonged to the BCG-induction therapy alone and Group II: 35 patients (51.5%) underwent BCG-maintenance protocol. The inclusion criteria were T1 TCC, high-grade Ta TCC, and patients with carcinoma in situ (CIS) either primary or concurrent with Ta or T1. The exclusion criteria included patients with muscle invasive TCC, low-grade Ta tumors, previous intravesical treatment with BCG or chemotherapy, previous radiotherapy for bladder tumor, and immunocompromised or immunosuppressed patients. Of the 68 patients who were enrolled in the current study, 53 patients were included in the final analysis, including 27 patients (50.9%) who underwent BCG-induction therapy alone and 26 (49.1%) who underwent maintenance therapy [Figure 1]. Patients were assigned to each treatment approach on a randomized basis according to a 1:1 ratio. Randomization was performed using a closed envelope method within 24 h after transurethral resection of the tumor and prior to receipt of the histology report.
Figure 1.
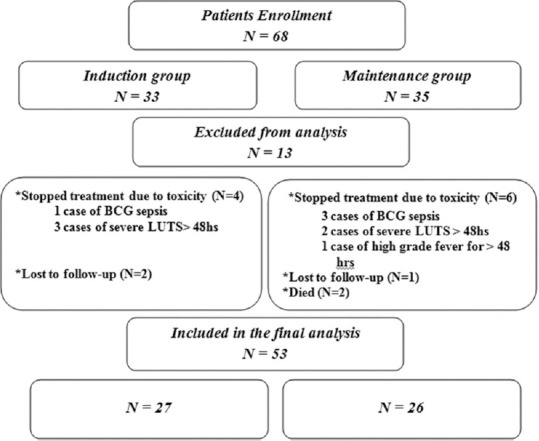
Patients enrollment and flow chart
The sample size was calculated before conducting the study to avoid statistical errors using the equation of the difference between the recurrence rate of tumor in high-grade TCC in each group.[12]
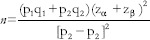
N = sample size for each group
p1 = prevalence of the outcome in the group 1 = 0.12 (12% recurrence rate of tumor in high grade TCC postinduction intravesical BCG followed by maintenance course) (Chung et al., 2008)
q1 = (1 − P1)
p2 = prevalence of the outcome in the group 2 = 0.45 (45% recurrence rate of tumor in high-grade TCC postinduction BCG without maintenance course) (Chung et al., 2008)
q2 = (1 − P2)
Zα = 1.96 and Zβ = 0.84
Sample size calculated per group = 22 (at least 22 patients should be included in each group).
The study was approved by the ethical committee for research at the Faculty of Medicine, Suez Canal University, under registration number 775 dated November 07, 2012. Informed consent was obtained from all patients prior to their enrolment. The enrolled patients were subjected to clinical evaluation with complete history taking and physical examination. Laboratory investigations included urine analysis, urine culture, and sensitivity. Serum creatinine, fasting blood glucose, bleeding profile, complete blood count, and liver enzymes were done. Radiological studies with pelvic abdominal ultrasonography and spiral computed tomography of the abdomen and pelvis with and without contrast were done before TURBT. TURBT was performed followed by a single dose of an intravesical instillation of 50 mg of doxorubicin diluted in 50 ml of saline given within 6 h after TURBT. The second-look TURBT was arranged for all patients after 4 weeks.
After the second-look TURBT, patients assigned to the BCG-induction therapy alone (Group I) received weekly doses of 90 mg of live attenuated Pasteur strain of BCG. The course was started 14 days after the second TURBT for 6 consecutive weeks. In Group II, the induction schedule was followed by 3 weekly instillations at 3, 6, and 12 months as maintenance course. Follow-up cystoscopy after 3 months was performed for all patients. Cystoscopies were repeated every 3 months for 1 year, every 4 months in the 2nd years, every 6 months in the 3rd year, and yearly afterward. The mean follow-up period was 40 months for the induction group and 35 months for the maintenance protocol group. BCG protocol was stopped when there was twice recurrence of tumor within <1 year or progression to muscle-invasive disease. Evaluation and outcome measurement were done through a comparison of both the groups regarding the percentage of recurrence, progression, recurrence-free duration, upstaging, and upgrading of free interval. Complications and toxicity of each treatment regimen were reported. IBM SPSS Statistics for Windows, Version 20.0.(Armonk, NY: IBM Corp) was used for statistical analysis and Kaplan–Meier curves were used to evaluate disease-free survival. The study database registered under the thesis sector at the Egyptian Universities Libraries Consortium-Registration Bib ID number: 12455591.
RESULTS
In the current study, 68 patients with high-risk NMIBC were prospectively enrolled and randomized to have 1 year of maintenance intravesical BCG, after the postoperative induction course, or a single induction course alone. Fifteen cases were excluded due to missed follow-up, drug toxicity, and death. Therefore, 53 patients were included in the final analysis, including 27 patients (50.9%) who underwent BCG-induction therapy alone and 26 (49.1%) who underwent maintenance therapy [Figure 1]. Patients in both the treatment protocols were comparable in terms of demographic parameters and tumor characteristics [Table 1].
Table 1.
Demographic parameters and tumor characteristics
| Variable | Induction therapy alone (n=27), n (%) | Maintenance therapy (n=26), n (%) | P |
|---|---|---|---|
| Age (years), mean±SD | 57.26±9.48 | 62.23±11.12 | 0.08 |
| Sex | |||
| Male | 24 (88.9) | 26 (100) | 0.08 |
| Female | 3 (11.1) | 0 (0) | |
| Presentation | |||
| Hematuria | 13 (48.1) | 14 (53.8) | 0.55 |
| LUTS | 2 (7.4) | 3 (11.5) | |
| Combined | 12 (44.4) | 9 (34.6) | |
| Risk factors | |||
| None | 12 (44.4) | 14 (53.8) | 0.71 |
| Smoking | 6 (22.2) | 7 (26.9) | |
| Bilharziasis | 2 (7.4) | 1 (3.8) | |
| Combined | 7 (25.9) | 4 (15.4) | |
| Tumor number | |||
| Single | 13 (48.2) | 9 (34.6) | 0.41 |
| Multifocal | 14 (51.8) | 17 (65.4) | |
| Tumor size (cm) | |||
| ≤3 | 12 (44.4) | 11 (42.3) | 0.88 |
| >3 | 15 (55.6) | 15 (57.7) | |
| Stage and grade | |||
| Ta high grade | 7 (25.9) | 4 (15.4) | 0.34 |
| T1 high grade | 14 (51.9) | 14 (53.8) | |
| T1 low grade | 6 (22.2) | 8 (30.8) | |
| Associated CIS | 7 (25.9) | 4 (15.4) | 0.35 |
LUTS: Lower urinary tract symptoms, BN: Bladder neck, CIS: Carcinoma in situ, SD: Standard deviation
The overall recurrence rate was 37.7% (20 patients). The 5-year overall recurrence-free survival was 59% with a recurrence-free time of approximately 40 months. Patients who received induction therapy alone had a significantly higher recurrence rate than those of maintenance therapy (55.6% vs. 19.2%, P = 0.01). The mean recurrence-free time was significantly shorter in the induction only group (30.23 vs. 41.69 months, P = 0.004). The 5-year recurrence-free survival was 41% and 78% in both the groups, respectively [Table 2]. Progression rates were comparable between both the groups (29.6% vs. 23.1%, P = 0.59), respectively. The 5-year progression-free survival was 69.8% for patients who underwent induction therapy alone compared to 70.7% for those who underwent maintenance therapy.
Table 2.
Recurrence and progression parameters between the two treatment protocols
| Variable | n (%)/mean±SD | P | |
|---|---|---|---|
| Induction therapy alone (n=27) | Maintenance therapy (n=26) | ||
| Follow-up (months) | 40.11±13.52 | 34.73±9.00 | 0.09 |
| Recurrence rate | 15 (55.6) | 5 (19.2) | 0.01* |
| Progression rate to T2 | 8 (29.6) | 6 (23.1) | 0.59 |
| Recurrence-free duration (months) | 21.11±17.95 | 27.88±12.82 | 0.12 |
| Progression-free duration (months) | 33.07±18.29 | 31.54±11.73 | 0.72 |
*P ≤ 0.05 is significant. SD: Standard deviation
Using the Kaplan–Meier curves, the 5-year overall recurrence-free survival was 59.07 ± 0.07% and the 5-year overall progression-free survival was 71.03 ± 0.07%. The mean overall recurrence-free time (95% confidence interval [CI]) was 39.75 (33.11–46.39) months. The mean 5-year recurrence-free time (95% CI) was significantly shorter in patients undergoing induction therapy alone than those who underwent maintenance therapy (30.23 [20.60–39.85] vs. 41.69 [35.91–47.48] months, P = 0.004), respectively [Figure 2]. The 5-year recurrence-free survival rate was 41.4% ± 0.10% for patients undergoing induction therapy alone and 78.02% ± 0.09% for those who underwent maintenance therapy.
Figure 2.
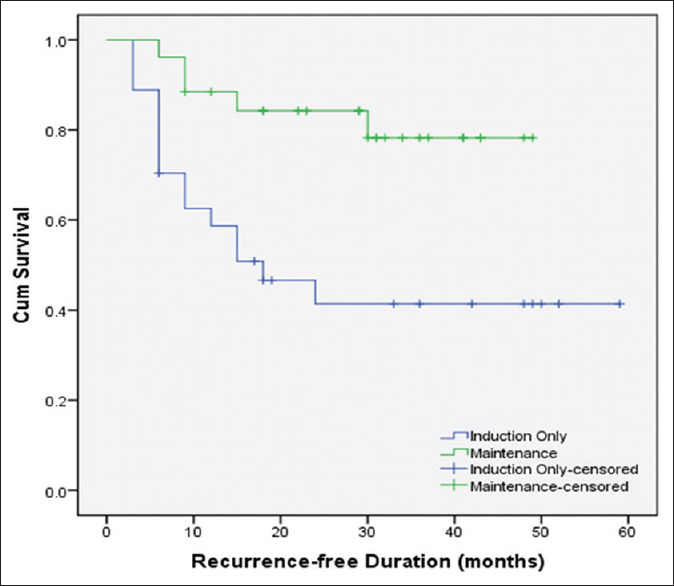
Recurrence-free duration between both treatment regimens
The progression rates were comparable between both the groups (29.6% vs. 23.1%, P = 0.59), with respective progression-free interval of 33.07 ± 18.29 and 31.54 ± 11.73 months (P = 0.72). The mean overall progression-free interval (95% CI) was 50.35 (44.20–56.51) months. The mean 5-year progression-free interval (95% CI) was comparable between patients undergoing induction therapy alone and those who underwent maintenance therapy (48.35 [39.20–57.49] vs. 43.44 [37.40–49.48] months, P = 0.55), respectively [Figure 3]. The 5-year progression-free survival was 69.8% ± 0.09% for patients who underwent induction therapy alone and 70.7% ± 0.11% for maintenance therapy.
Figure 3.
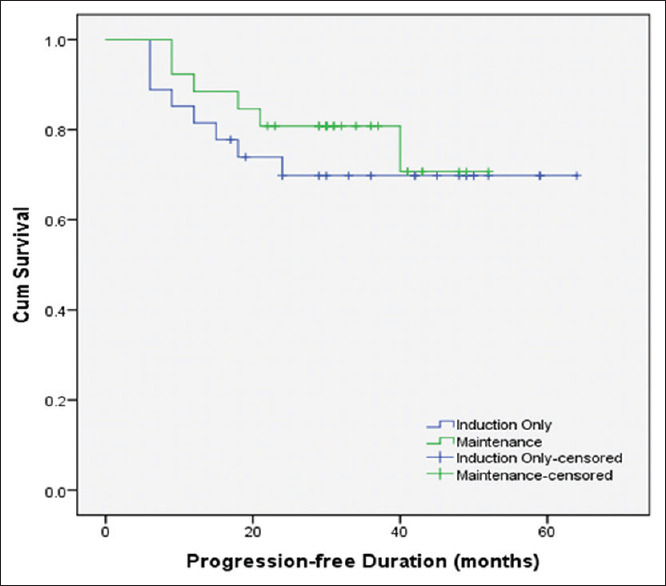
Kaplan–Meier curve showing progression free survival in the study cohort
Overall local adverse events due to intravesical BCG instillation were detected in 47 (69.1%) patients, including hematuria (8.8%), chemical/bacterial cystitis (32.4%), and combined hematuria/cystitis in 17.6% of the patients. Twenty-seven patients (39.7%) developed systemic adverse events mostly fever of <48 h and general malaise in 32.3% of the patients. Ten cases (14.7%) (6 in maintenance arm and 4 in induction only group) stopped BCG due to toxicity. Persistent severe LUTS longer than 48 h was found in 5 (7.3%) patients, four patients (5.9%) of BCG sepsis, and one case (1.5%) of high-grade fever for >48 h. The incidence of local adverse events (P = 0.03) and severity of the total side effects (P = 0.026) were significantly higher in patients who underwent maintenance treatment protocol.
DISCUSSION
Intravesical BCG has been proven as the most effective and the gold standard treatment for patients with intermediate- and high-risk NMIBC.[13] In the current study, patients who underwent induction therapy alone had a significantly higher recurrence rate than those who received maintenance therapy (55.6% vs. 19.2%, respectively, P = 0.01). The mean recurrence-free time was significantly shorter in the induction only group. These results are comparable to reported literature.[13]
Herr et al.[11] assessed 1021 patients with high-risk NMIBC, who received a 6-week induction course of BCG therapy. Only patients who responded initially to induction BCG therapy continued the study. The 2- and 5-year recurrence-free survival rates were 73% and 46%, respectively, where the median recurrence time was 41 months. This 5-year recurrence-free rate is comparable to the findings of the current study despite the exclusion of patients who did not respond to the induction course in the latter study.
Phase III EORTC trials in intermediate- and high-risk NMIBC showed respective 1- and 5-year recurrence rates of 25.9% and 41.3% in patients who received 1–3 year maintenance BCG.[14] A Spanish multicenter prospective trial included 397 patients randomized to have either an induction protocol only or maintenance BCG. The overall recurrence in this study was 37.3%.[7] These results are comparable to our findings.
Okamura et al.[15] studied seventy-five patients with NMIBC. The 5-year recurrence-free rates were significantly higher in those who underwent maintenance therapy (83% vs. 51.9%, P = 0.006). These results were higher compared to our study, and this can be explained as patients with primary and concomitant CIS were excluded from their study.
Martínez-Piñeiro et al.[7] reported a progression rate of 11.4% and 18.5% in the maintenance and induction only arm, respectively. There was an insignificant difference between both the groups as regards the progression rate and time to progression at 5 years (P = 0.3).
A meta-analysis of six trials did not recommend the regular use of maintenance BCG, as it had no superiority to induction BCG treatments in preventing or delaying tumor progression; however, it prolongs treatment duration and adds more toxicity.[16] This is in accordance with our findings.
In a critical review of seven randomized studies, Ehdaie et al.[3] reported that maintenance BCG doses were beneficial in reducing disease recurrence and delaying progression compared to induction only group. However, the optimal duration of BCG treatment remains unknown and should be the subject of further trials.
The controversy in the progression outcomes can be explained by dissimilar characteristics of the studied populations, periods of follow-up, methodology, and types of statistical analysis of different trials.
Chemical cystitis, storage lower urinary tract symptoms, and hematuria were the most prevalent local adverse effects of intravesical BCG in our study. Twenty-seven patients (39.7%) developed systemic adverse events mostly fever of <48 h and general malaise in 32.3% of the patients. These results were comparable to the reported literature, denoting that the most frequent local side effects were bacterial and/or chemical cystitis (56.2%), hematuria (46.0%), and frequency (45.1%), whereas general malaise (15.5%) and fever (8.1%) are the most frequent systemic side effects.[17]
The discontinuation rate in the current study (14.7%) is similar to the reported literature. Sylvester[8] reported cessation of further intravesical instillation in 20% of patients due to local and systemic side effects.
Similarly, Brausi et al.[18] reported an overall 69.5% of local or systemic side effects, and 7.8% of the patients stopped treatment for BCG toxicity.
The incidence of local adverse events and severity of the total side effects were significantly higher in patients who underwent maintenance treatment protocol. This could be explained by the cumulative nature of toxic effects due to BCG instillation. Herr et al.[11] did not demonstrate a strong evidence for routine use of maintenance BCG doses. They considered maintenance BCG as a salvage therapy only for relapsing patients to avoid exposure of all patients to the toxicity associated with it.
Ali-El-Dein et al. in their trial to get the benefits of BCG and reducing the toxicity encountered with it, they reduced the frequency of BCG instillations by half and replaced the second half with epirubicin. They reported a lower toxicity with that regimen while maintaining the same efficacy of BCG.[19,20]
Lower doses with more frequent instillations of BCG, new BCG regimens, maintenance BCG on demand, and combined immunochemotherapy are all possible lines of therapy that need to be thoroughly investigated, especially in the era of shortage of BCG supply.
Limitations
Despite being a prospective controlled study, the current study might have some limitations. The small sample size may preclude some significant statistical differences between both the groups. The study has short-term follow-up although it is consistent with that previously reported in most relevant studies. Further studies are recommended to specify a given category of high-risk tumors with less variable recurrence and progression scores.
CONCLUSIONS
Maintenance doses of BCG significantly decrease and delay the recurrence of high-risk NMIBC. Although maintenance protocols are recommended in high-risk NMIBC, patients need to be counseled about its limited benefits on tumor progression and bothering side effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Böhle A, Jocham D, Bock PR. Intravesical Bacillus calmette-guerin versus mitomycin C for superficial bladder cancer: A formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90–5. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 2.Böhle A, Bock PR. Intravesical bacille calmette-guérin versus mitomycin C in superficial bladder cancer: Formal meta-analysis of comparative studies on tumor progression. Urology. 2004;63:682–6. doi: 10.1016/j.urology.2003.11.049. [DOI] [PubMed] [Google Scholar]
- 3.Ehdaie B, Sylvester R, Herr HW. Maintenance Bacillus calmette-guérin treatment of non-muscle-invasive bladder cancer: A critical evaluation of the evidence. Eur Urol. 2013;64:579–85. doi: 10.1016/j.eururo.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Fuge O, Vasdev N, Allchorne P, Green JS. Immunotherapy for bladder cancer. Res Rep Urol. 2015;7:65–79. doi: 10.2147/RRU.S63447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinotsu S, Akaza H, Naito S, Ozono S, Sumiyoshi Y, Noguchi S, et al. Maintenance therapy with Bacillus calmette-guérin Connaught strain clearly prolongs recurrence-free Survival following transurethral resection of bladder tumour for non-muscle-invasive bladder cancer. BJU Int. 2011;108:187–95. doi: 10.1111/j.1464-410X.2010.09891.x. [DOI] [PubMed] [Google Scholar]
- 6.Pfister C, Kerkeni W, Rigaud J, Le Gal S, Saint F, Colombel M, et al. Efficacy and tolerance of one-third full dose Bacillus calmette-guérin maintenance therapy every 3 months or 6 months: Two-year results of URO-BCG-4 multicenter study. Int J Urol. 2015;22:53–60. doi: 10.1111/iju.12609. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Piñeiro L, Portillo JA, Fernández JM, Zabala JA, Cadierno I, Moyano JL, et al. Maintenance therapy with 3-monthly Bacillus calmette-guérin for 3 years is not superior to standard induction therapy in high-risk non-muscle-invasive urothelial bladder carcinoma: Final results of randomised CUETO study 98013. Eur Urol. 2015;68:256–62. doi: 10.1016/j.eururo.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 8.Sylvester RJ. Bacillus calmette-guérin treatment of non-muscle invasive bladder cancer. Int J Urol. 2011;18:113–20. doi: 10.1111/j.1442-2042.2010.02678.x. [DOI] [PubMed] [Google Scholar]
- 9.Weizer AZ, Tallman C, Montgomery JS. Long-term outcomes of intravesical therapy for non-muscle invasive bladder cancer. World J Urol. 2011;29:59–71. doi: 10.1007/s00345-010-0617-4. [DOI] [PubMed] [Google Scholar]
- 10.Koga H, Ozono S, Tsushima T, Tomita K, Horiguchi Y, Usami M, et al. Maintenance intravesical Bacillus calmette-guérin instillation for Ta, T1 cancer and carcinoma in situ of the bladder: Randomized controlled trial by the BCG Tokyo strain study group. Int J Urol. 2010;17:759–66. doi: 10.1111/j.1442-2042.2010.02584.x. [DOI] [PubMed] [Google Scholar]
- 11.Herr HW, Dalbagni G, Donat SM. Bacillus calmette-guérin without maintenance therapy for high-risk non-muscle-invasive bladder cancer. Eur Urol. 2011;60:32–6. doi: 10.1016/j.eururo.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 12.Chung J, Jung S. BCG intravesical therapy in bladder tumor: 6 week course versus modified 6+3 maintenance therapy. Urology. 72 101016/jurology200808189. [Google Scholar]
- 13.Alhunaidi O, Zlotta AR. The use of intravesical BCG in urothelial carcinoma of the bladder. Ecancermedicalscience. 2019;13:905. doi: 10.3332/ecancer.2019.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance Bacillus calmette-guérin. Eur Urol. 2016;69:60–9. doi: 10.1016/j.eururo.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 15.Okamura T, Akita H, Ando R, Ikegami Y, Naiki T, Kawai N, et al. Single monthly Bacillus calmette-guérin intravesical instillation is effective maintenance therapy to prevent recurrence in Japanese patients with non-muscle-invasive bladder cancer. Int J Clin Oncol. 2012;17:477–81. doi: 10.1007/s10147-011-0314-3. [DOI] [PubMed] [Google Scholar]
- 16.Herr HW. Is maintenance Bacillus calmette-guérin really necessary? Eur Urol. 2008;54:971–3. doi: 10.1016/j.eururo.2008.06.062. [DOI] [PubMed] [Google Scholar]
- 17.Oddens J, Brausi M, Sylvester R, Bono A, van de Beek C, van Andel G, et al. Final results of an EORTC-GU cancers group randomized study of maintenance Bacillus calmette-guérin in intermediate- and high-risk ta, T1 papillary carcinoma of the urinary bladder: One-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63:462–72. doi: 10.1016/j.eururo.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Brausi M, Oddens J, Sylvester R, Bono A, van de Beek C, van Andel G, et al. Side effects of Bacillus calmette-guérin (BCG) in the treatment of intermediate- and high-risk ta, T1 papillary carcinoma of the bladder: Results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol. 2014;65:69–76. doi: 10.1016/j.eururo.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Ali-El-Dein B, Nabeeh A, Ismail EH, Ghoneim MA. Sequential Bacillus calmette-guerin and epirubicin versus Bacillus calmette-guerin alone for superficial bladder tumors: A randomized prospective study. J Urol. 1999;162:339–42. [PubMed] [Google Scholar]
- 20.Ali-El-Dein B, Barakat TS, Nabeeh A, Ibrahiem el-HI. Weekly intravesical Bacillus calmette-guerin (BCG) alternating with epirubicin in ta and T1 urothelial bladder cancer: An approach to decrease BCG toxicity. Urol Ann. 2013;5:103–8. doi: 10.4103/0974-7796.110008. [DOI] [PMC free article] [PubMed] [Google Scholar]


