Abstract
Background
Aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor, is important for xenobiotic metabolism and binds to various endogenous and exogenous ligands in the skin. However, the functional role of AhR in patients with psoriasis (PS) and atopic dermatitis (AD) remains unclear.
Objective
We aimed to determine whether AhR-regulated factors (AhR, CYP1A1, interleukin [IL]-17, IL-22) were affected by AhR ligands (2,3,7,8-tetrachlorodibenzo-p-dioxin, TCDD) in chronic inflammatory skin diseases such as PS and AD.
Methods
The expression levels of AhR-related factors were determined by quantitative PCR, western blotting, and immunocytochemistry. Specific siRNA targeting AhR was used to inhibit gene expression in human peripheral blood mononuclear cells (PBMC). Cytokine assays were performed to determine the protein production of CD4+ T cells.
Results
In comparison with healthy controls, TCDD-treated PBMCs and CD4+ T cells from patients with PS and AD showed an increase in AhR gene levels as well as significantly increased expression of AhR-related factors (such as AhR, CYP1A1, IL-17, and IL-22). In contrast, 6-formyl indolo [3,2-b] carbazole (FICZ) inversely affected the differentiation of CD4+ T cells and their cytokine expression levels as compared with TCDD. CD4+ T cells from patients with AD and PS showed higher expression levels of AhR, CYP1A1, IL-17, and IL-22.
Conclusion
Our results suggest that TCDD-induced AhR-related factor upregulation in AD and PS patients may increase the expression of AhR-regulatory genes, thereby contributing to the development of AD and PS.
Keywords: 6-formyl indolo [3,2-b] carbazole (FICZ) psoriasis; Dermatitis; atopic; Polychlorinated dibenzodioxins; Psoriasis; Receptor; aryl hydrocarbon
INTRODUCTION
Environmental pollution is known to contribute to the increased prevalence of inflammatory skin diseases such as atopic dermatitis (AD) and psoriasis (PS)1. The incidence of AD has greatly increased in the last few decades in western countries2 and AD prevalence is higher in developed countries and urban areas than in developing countries and rural areas3. While the causes underlying PS are not completely understood, environmental risk factors4, including tobacco smoking, are known to be associated with this pathology5,6.
The biological response to many environmental pollutants involves their direct interactions with a receptor of xenobiotics, aryl hydrocarbon receptor (AhR), which binds to several exogenous ligands such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) as well as endogenous ligands such as 6-formyl indolo [3,2-b] carbazole (FICZ)1. TCDD is a major environmental pollutant in automobile exhaust, cigarette smoke, various foods, and industrial wastes1,7. FICZ is formed from tryptophan in the presence of riboflavin and ultraviolet (UV) light, which explains the reported UVdependent activation of CYP1 enzymes in human skin8. Although evidence suggests a relationship between AhR ligands and cutaneous inflammatory disorders, the precise underlying mechanisms remain unclarified9.
AhR has been recently recognized as a major transcription factor involved in the development of Th17 and Th22 cells7,10,11 and is thought to be essential for the production of interleukin (IL)-227,12.
In the present study, we hypothesized that the stimulation of AhR with exogenous and endogenous AhR ligands could be associated with the pathogenesis of chronic inflammatory skin diseases. Therefore, we aimed to determine whether AhR-regulated genes (AhR, CYP1A1, IL-17, IL-22) are affected by AhR ligands (TCDD) in chronic inflammatory skin diseases such as PS and AD.
MATERIALS AND METHODS
Subjects
We recruited 18 patients of each category, AD, PS, and healthy volunteers, who visited the clinic between January 2012 and January 2016. The clinician's diagnosis of AD was carried out according to the criteria proposed by Hanifin and Rajka13. The diagnosis of PS was performed based on observations of the skin, and microscopic examinations of biopsied samples10. The clinical severity of AD was assessed by the Eczema Area and Severity Index (EASI) (Table 1)10. The severity of PS was assessed according to the Psoriasis Area and Severity Index (PASI) (Table 1)11. The study protocol was approved by the Institutional Review Board (2012-05-45) and all subjects were required to sign informed consent forms.
Table 1. Severity of disease in patients participating in the study.
| Sample | Psoriasis (PASI)* | Atopic dermatitis (EASI)† |
|---|---|---|
| PBMC | ||
| 1 | 10.3 | 18.8 |
| 2 | 9.9 | 13 |
| 3 | 8.1 | 15.2 |
| 4 | 19.2 | 21.6 |
| CD4+ T cell | ||
| 1 | 4.4 | 10.6 |
| 2 | 10.3 | 11.5 |
| 3 | 5.7 | 15.6 |
| 4 | 3.2 | 10.7 |
| 5 | 3.2 | 37 |
| 6 | 9.8 | 28.9 |
| 7 | 14.4 | 25.5 |
| 8 | 13.8 | 18.8 |
| 9 | 13.2 | 13 |
| 10 | 13.2 | 37 |
| 11 | 12.1 | 29.1 |
| 12 | 12 | 28.9 |
| 13 | 11.5 | 23.7 |
| 14 | 10.3 | 22.4 |
PBMC: peripheral blood mononuclear cells, PASI: Psoriasis Area and Severity Index, EASI: Eczema Area and Severity Index. *The severity of psoriasis was assessed according to the PASI. †The clinical severity of atopic dermatitis was assessed by the EASI.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
A total of 1×104 cells in 0.1 ml of culture medium were seeded into 96 well and cultured for 24 hours. These cells were incubated with medium alone, medium plus TCDD at various concentrations (0, 0.1, 1, 10, and 100 nmol/L), or medium plus FICZ at various concentrations (0, 0.1, 1, 10, and 100 nmol/L) for another 12, 24, or 48 hours. Subsequently, 20 µl of MTT (2 mg/ml; Duchefa, Haarlem, The Netherlands) was added to each well and incubation was continued for 4 hours at 37℃. Absorbance was determined using a THERMOmax microplate reader (Molecular Devices, Oxnard, CA, USA) at 540 nm. Cell viability percentage was calculated as follows: Viability percentage (%)=(absorption value of TCDD- or PCB-treated group)/(absorption value of the control group)×100.
PBMC culture
As there is still controversy regarding the effect of AhR ligands on particular cell subsets (naïve CD4+ T cells, T cells during cytokine induced differentiation, etc.), Peripheral blood mononuclear cells (PBMC) were evaluated with respect to the effects of TCDD in an environment where all these cell types were represented and could interact. Blood from each subject, four AD, four PS patients, and four healthy controls, was drawn and prepared in an ethylenediaminetetraacetic acid tube. PBMC were isolated using Ficoll-Paque™ PREMIUM (GE Healthcare, Uppsala, Sweden) by the density gradient centrifugation method. Cells were dispensed into a 96-well plate at 1×106/ml and cultivated for one day in RPMI 1640 medium (Biowest, Kansas, MO, USA) containing 10% fetal bovine serum (FBS; Biowest) and 1% penicillin/streptomycin (Biowest). To compare the expressions of AhR-related genes, cells were treated with the appropriate dilutions of TCDD (Sigma, Bellefonte, PA, USA). Cells were then cultivated for two days.
CD4+ T cell culture
Blood from each subject, 14 AD and 14 PS patients and 14 healthy controls, was drawn and CD4+ T cells were isolated and divided into a control group, TCDD-treated group (10 nmol/L of TCDD, seven patients from each group), and a FICZ-treated group (0.3 µmol/L of FICZ, seven patients from each group). After isolation of PBMC by density gradient centrifugation using Ficoll-Paque™ PREMIUM (GE Healthcare), isolated PBMC were mixed with anti-human CD4+ T cell microbeads (Miltenyi Biotec, Auburn, CA, USA) and incubated at 4℃ for 15 minutes. The samples washed with magnetic activated cell sorting (MACS) buffer, and then CD4+ T cells were isolated using a MACS separator. Isolated CD4+ T cells were washed with HBSS (Gibco, Grand Island, NY, USA) and cultured in RPMI 1640 medium (Gibco) containing inactivated 10% FBS (Gibco) and 1% antibiotic-antimycotic (Gibco). Anti-CD3 at 0.5 µg/ml (R&D Systems, Minneapolis, MN, USA) and anti-CD28 (R&D Systems) at 1 µg/ml were added to activate CD4+ T cells (24-well plate, 1×105/well and 6-well plate, 1×106/well, respectively). TCDD and FICZ were diluted to achieve a final concentration of 10 nM. Two days after incubation, both recombinant human IL-23 (PeproTech, Inc., Rocky Hills, NJ, USA) and recombinant human IL-2 (PeproTech) were diluted to achieve a final concentration of 10 nM. At day seven, cell differentiation was analyzed using fluorescence-activated cell sorting, and cytokine expression was assessed by performing enzyme-linked immunosorbent assay (ELISA) on cell supernatants.
CD4+ T cell cytokine assay
To measure the concentration of cytokines such as tumor necrosis factor-alpha (TNF-α), interferon gamma (IFN-γ), IL-4, IL-13, IL-17, and IL-22, a cytokine ELISA Immunoassay kit (R&D Systems) was used. The absorbance of the reacted solution was quantified with DTX880 Multimode Detector (Molecular Devices, Sunnyvale, CA, USA) at a wavelength of 450 nm.
Immunocytochemistry
Using Cytopro® Cytocentrifuge (ELITech, Logan, UT, USA), CD4+ T cells were attached to a slide glass. Methanol-fixed cells were washed with phosphate buffered saline (PBS), and treated with Triton X-100 for 10 minutes to detect intracellular materials. To prevent nonspecific antibody binding, pretreatment with bovin serum albumin (BSA) was performed for 30 minutes. Then samples were treated with 1:50 anti-IL-17 (Abcam, Cambridge, MA, USA) and anti-IL-22 (Abcam) for one hour at room temperature. After washing three times with PBS, samples were stained with 1:100 anti-mouse Alexa Fluor 594 and 1:100 anti-rabbit Alexa Flour 488 (Invitrogen, Eugene, OR, USA) for one hour at room temperature. After washing five times using PBS, VECTASHIELD Mounting Medium (Vector, Burlingame, CA, USA) including 4′,6-diamidino-2-phenylindole, dihydrochloride was added. Cells were observed by a Cell® illuminator system (Olympus, Tokyo, Japan).
Western blot
We used radioimmunoprecipitation assay buffer lysis buffer (Biosesang, Seoul, Korea) to extract proteins. Cells were treated with the following primary antibodies: beta-tubulin (Millipore, Billerica, MA, USA), IL-17 (Santa Cruz, Santa Cruz, CA, USA), and IL-22 (Abcam). For horseradish peroxidase (HRP) detection, a chemiluminescent substrate (Thermo, Waltham, MA, USA) was used along with an X-ray film. The protein bands were determined by ImageJ program.
AhR sequence-specific interfering RNA (siRNA) transfection
Specific siRNA targeting AhR (Santa Cruz) was used to inhibit gene expression in human PBMC. After one day of cell culture in serum free media, the following processes for transfection were performed using a siRNA reagent system (Santa Cruz). Mixed siRNA for AhR and transfection reagent were reacted for 45 minutes at room temperature, diluted in transfection media, and applied to cells without culture media. After five hours in a CO2 incubator at 37℃, samples were cultured for one day in media containing FBS and a twofold greater quantity of antibiotics than previously added. Thereafter, culture media was removed, replenished with new media, and cultured for an additional day.
Quantitative PCR
Quantitative PCR (qPCR) was performed as previously described. Briefly, mRNA was isolated by using an RNeasy Mini Kit (QiAGEN, Hilden, Germany) according to the manufacturer's instructions and the RNA density and purity were measured using a DU 730 UV/Vis spectrophotometer (Beckman Coulter, St. Charles, IL, USA). Reverse transcription was performed using 400 ng RNA and a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster, CA, USA). cDNA was synthesized in a VeritiTM 96-well Thermal Cycler (Applied Biosystems). After mixing 2.5 µl synthesized cDNA with a probe and reagent supplied by QuantiFast Probe Assay (QiAGEN), qPCR was performed using a Light Cycler 480 II (Roche, Rotkreuz, Switzerland) programmed to 95℃, 30 seconds for denaturation, 62℃, 30 seconds for annealing, and 72℃, ten second for extension for 30 cycles.
Statistical analysis
Results were obtained from at least three independent experiments. For statistical analysis, results are shown as mean±standard error of the mean. Using Prism software for Windows (GraphPad Software Inc., La Jolla, CA, USA), statistical significance of differences between groups was assessed by one-way analysis of variance (ANOVA) for factorial comparisons and by the Tukey's multiple comparison tests for multiple comparisons. Results of immunohistochemical staining were analyzed using the Mann–Whitney test. The differences in the positivity rates were evaluated with Fisher's exact test. Statistical analyses were carried out using the SPSS statistical software (ver. 12.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Effect of TCDD on AhR gene expression in PBMC of patients with PS and AD
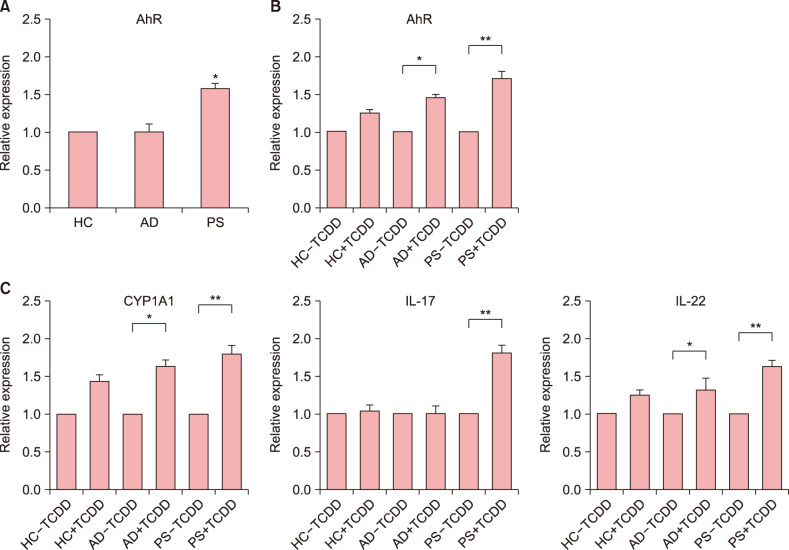
Results showed that compared to the levels of AhR in healthy controls, the levels of AhR were higher in patients with PS and lower in those with AD (Fig. 1A). To investigate the effect of TCDD on AhR expression, we were performed qPCR, accordingly. These results showed that expression of AhR was higher in TCDD-treated PBMC in PS than in healthy controls (Fig. 1B). These results indicated that TCDD-treated PBMC significantly induced AhR activation in PS compare to healthy controls. To investigate the effect of TCDD on AhR-related factors (CYP1A1, IL-17, IL-22), we were performed qPCR, respectively (Fig. 1C). The TCDD treatment of PBMC from patients with PS and AD resulted in a significantly increase in the expression of AhR-related factors (AhR, CYP1A1, IL-22). But the expression of IL-17 was significantly different between the two groups. The differences were higher in patients with PS and AD than in healthy control. Patients with AD and PS may be more sensitive to AhR ligands.
Fig. 1. (A) mRNA expression levels of aryl hydrocarbon receptor (AhR) in peripheral blood mononuclear cells (PBMC) from patients with atopic dermatitis (AD), psoriasis (PS), and healthy controls (HC) were examined by quantitative PCR (qPCR). (B) mRNA expression levels of AhR in PBMC from patients with AD, PS, and HC treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) were examined by qPCR. (C) mRNA expression levels of cytochrome P450 family 1 member A1 (CYP1A1), interleukin (IL)-17, and IL-22 in PBMC from patients with AD, PS, and HC treated with TCDD were examined by qPCR. Values are presented as mean±standard error of mean. *p<0.05 vs. control, **p<0.01 vs. control.
Effect of silencing AhR on TCDD-treated PBMC of patients with PS and AD
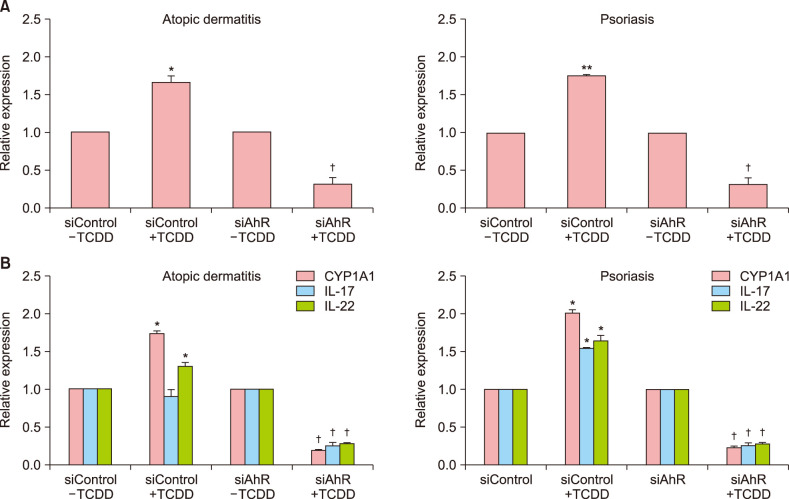
To further confirm the effect of AhR, PBMC were transfected with control siRNA or AhR-siRNA. The results showed that AhR-siRNA significantly inhibited TCDD-induced AhR expression compared with control siRNA (Fig. 2). These results suggest that AhR plays a regulating role in patients with AD and PS. AhR-siRNA significantly inhibited TCDD-induced AhR-related factors (AhR, CYP1A1, IL-17, and IL-22) in patients with AD and PS (Fig. 2B).
Fig. 2. Effect of silencing aryl hydrocarbon receptor (AhR) with AhR-specific small interference RNA as determined by quantitative PCR. Cells were transfected with specific interfering control (siControl) or specific interfering AhR (siAhR) for 24 hours and stimulated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) for 24 hours. IL: interlukin. Values are presented as mean±standard error of mean. *p<0.05 vs. siControl+TCDD; **p<0.01 vs. siControl+TCDD; †p<0.05 vs. siAhR+TCDD.
Effect of TCDD on AhR gene expression in CD4+ T cells of patients with PS and AD
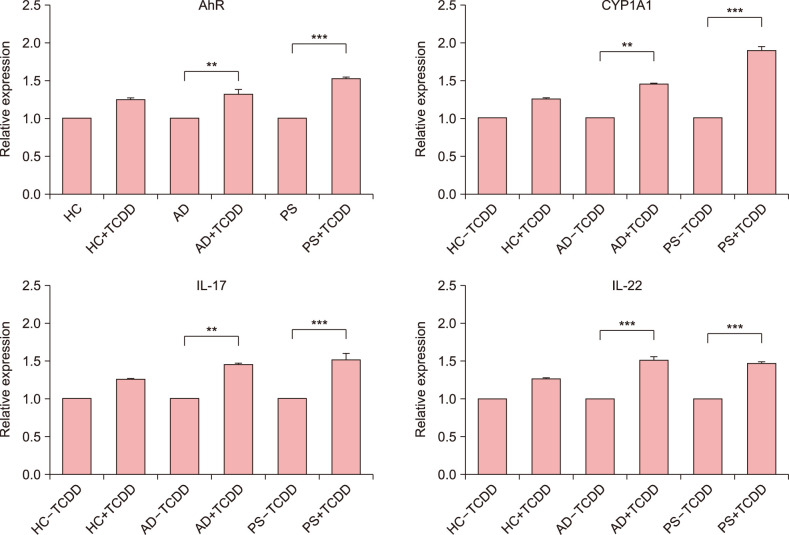
To investigate the effect of TCDD on AhR expression in CD4+ T cells, we performed qPCR accordingly. Our results showed that the expression of genes coding for AhR-related factors (AhR, CYP1A1, IL-17, and IL-22) was significantly upregulated in TCDD-treated CD4+ T cells of patients with AD and PS than in healthy controls (Fig. 3).
Fig. 3. mRNA expression levels of aryl hydrocarbon receptor (AhR), CYP1A1, interleukin (IL)-17, and IL-22 in CD4+ T cells from patients with atopic dermatitis (AD), psoriasis (PS), and healthy controls (HC) were examined by quantitative PCR. **p<0.01 vs. control, ***p<0.001 vs. control.
Effect of TCDD on cytokine expression levels in CD4+ T cells
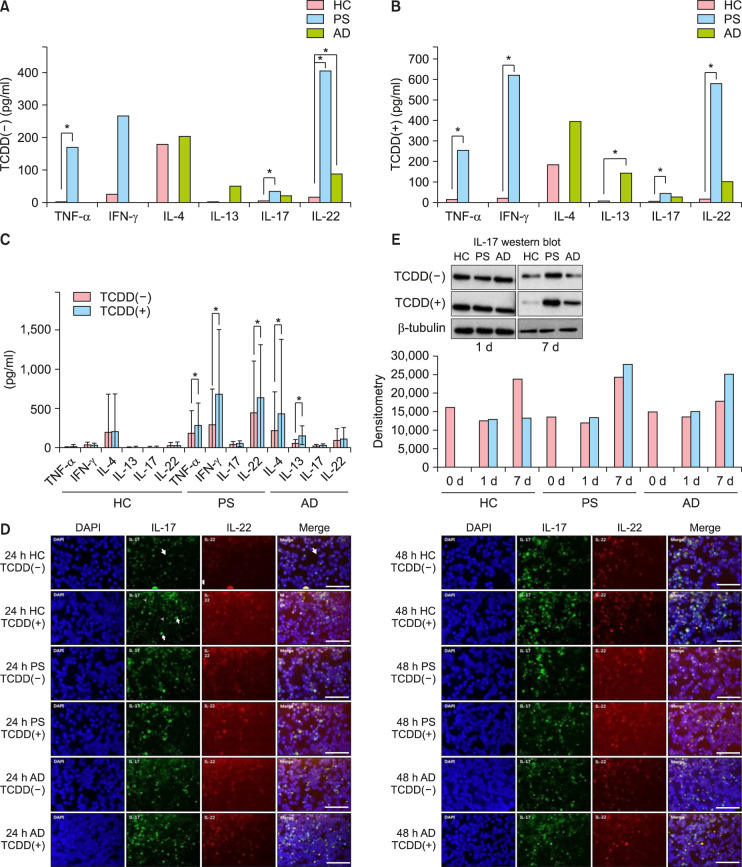
To confirm the effects of AhR activation on the polarization of different T cells, we analyzed the production of IFN-γ, IL-4 and IL-13, IL-17, and TNF-α and IL-22. These results showed that compared to healthy controls, patients with PS had significantly higher levels of TNF-α, IFN-γ, IL-17, and IL-22, and patients with AD had significantly higher levels of IL-4, IL-13, IL-17, and IL-22 (Fig. 4A). TCDD treatment of CD4+ T cells resulted in increased levels of TNF-α, IL-4, IL-13, IL-17, and IL-22, but decreased levels of IFN-γ in healthy controls. The production of TNF-α, IFN-γ, IL-17, and IL-22, but not IL-17, significantly increased in patients with PS. Among TCDD-treated groups, patients with PS showed higher levels of TNF-α, IFN-γ, IL-17, and IL-22, and those with AD showed significantly higher levels of IL-17 than healthy controls (Fig. 4B). Furthermore, the levels of IL-4, IL-13, IL-17, and IL-22 increased among patients with AD, and a significant increase in the production of IL-4 and IL-13 was reported (Fig. 4C). The levels of IL-17 and IL-22 were higher in patients with PS and AD than in healthy controls (Fig. 4D). The production of IL-17 and IL-22 increased in all three groups when CD4+ T cells were treated with TCDD. IL-17 expression was usually detected inside the cells, while IL-22 expression was observed on the cell surface. In CD4+ T cells treated with TCDD, the levels of IL-17 noticeably increased in patients with PS and IL-22 levels prominently increased in patients with AD. However, no differences were observed after day seven (Fig. 4D). Western blot analysis revealed an increase only in IL-17 levels in patients with PS and AD after treatment of CD4+ T cells with TCDD. On the other hand, no bands were observed for IL-22 (Fig. 4E). These results suggest that TCDD significantly regulates the expression of AhR-related factors in CD4+ T cells of PS and AD.
Fig. 4. (A~C) Cytokine expression levels of CD4+ T cells after treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Cytokine levels were assessed in the supernatants at day seven of culture. (A) Significantly higher levels of tumor necrosis factor-alpha (TNF-α), interferon gamma (IFN-γ), interleukin (IL)-17, and IL-22 were observed among patients with psoriasis (PS) compared to healthy controls (HC). (B) In TCDD(+) groups, the levels of TNF-α, IFN-γ, IL-17, and IL-22 were significantly higher in patients with PS and the level of IL-13 was significantly higher in patients with atopic dermatitis (AD) than in HC. (C) Among TCDD-treated groups, the levels of TNF-α, IFN-γ, and IL-22 were significantly higher in patients with PS and those of IL-4 and IL-13 were significantly increased in patients with AD compared to HC. (D) Intracellular levels of IL-17 and IL-22 in CD4+ T cells from patients with AD, PS, and HC after 24 and 48 hours of treatment with TCDD. After 24 hours treatment of CD4+ T cells with TCDD, the production of IL-17 and IL-22 increased in the three groups. After 48 hours treatment of CD4+ T cells with TCDD, the production of IL-17 noticeably increased in patients with PS, and IL-22 levels prominently increased in patients with AD. Scale bar=25 µm. (E) Western blot results of IL-17 in CD4+ T cells from patients with AD, PS, and HC after one, two, and seven days of treatment with TCDD. After treatment of CD4+ T cells with TCDD, the production of IL-17 increased in patients with PS and AD. *Statistically significant (p<0.05).
Effect of FICZ on cytokine expression levels in CD4+ T cells
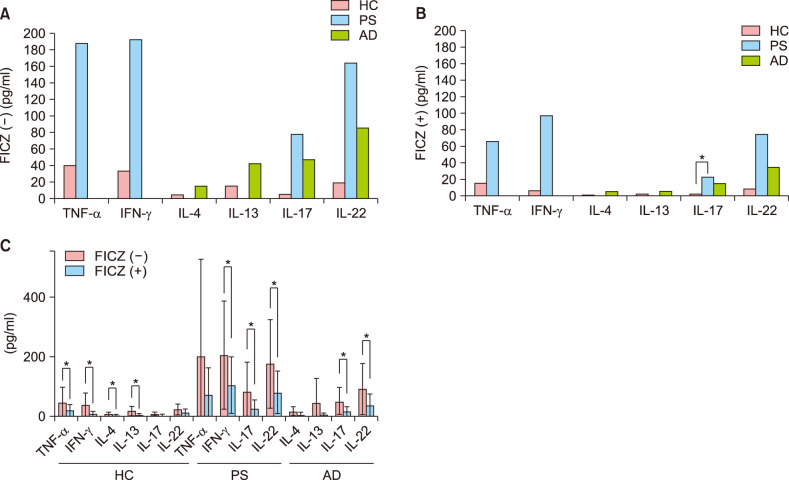
In the control group, the levels of TNF-α, IFN-γ, IL-17, and IL-22 were higher in patients with PS, and IL-4, IL-13, IL-17, and IL-22 were higher in patients with AD than in healthy controls (Fig. 5A). After treatment with FICZ, the levels of TNF-α, IFN-γ, IL-4, IL-13, IL-17, and IL-22 decreased in healthy controls; these differences were statistically significant except for IL-17 and IL-22. In patients with PS, the decreases in IFN-γ, IL-17, and IL-22 levels with FICZ treatment were statistically significant, except for TNF-α. Lastly, in patients with AD, the decreases in IL-17 and IL-22 levels with FICZ treatment were statistically significant, except for IL-4 and IL-13 (Fig. 5B). Furthermore, the level of IL-17 was significantly higher in patients with PS than in healthy controls and patients with AD after FICZ treatment (Fig. 5C). These results show that TCDD and FICZ inversely regulated the production of cytokines in CD4+ T cells.
Fig. 5. Cytokine expression of CD4+ T cells after treatment with 6-formyl indolo [3,2-b] carbazole (FICZ). Cytokine levels were assessed in the supernatants at day seven of culture. (A) In FICZ(–) group, the levels of tumor necrosis factor-alpha (TNF-α), interferon gamma (IFN-γ), interleukin (IL)-17, and IL-22 were higher in patients with psoriasis (PS) than in healthy controls (HC), while the levels of IL-4, IL-13, IL-17, and IL-22 were higher in patients with atopic dermatitis (AD) than in HC. (B) In FICZ(+) group, the level of IL-17 was significantly higher in patients with PS than in those with AD and HC. (C) After FICZ treatment, the levels of TNF-α, IFN-γ, IL-4, IL-13, IL-17, and IL-22 decreased in HC, and those of TNF-α, IFN-γ, IL-4, and IL-13 significantly decreased as compared to those in the control group. In patients with PS, the levels of TNF-α, IFN-γ, IL-17, and IL-22 decreased and a statistically significant difference was observed except for TNF-α level. In patients with AD, the levels of IL-4, IL-13, IL-17, and IL-22 decreased and a statistically significant difference was reported for all except IL-4 and IL-13 levels. *Statistically significant (p<0.05).
DISCUSSION
Our previous study demonstrated higher expression levels of AhR-related factors in lesional skin from patients with PS and AD than in samples from the control group9. We investigated the role of AhR and its ligands in PBMC from AD and PS, and analyzed the polarization of CD4+ T cells. The TCDD treatment of PBMC from patients with PS resulted in an increase in the expression levels of AhR-related factors, IL-22 and IL-17, and the same findings were observed for IL-22 in PBMC from patients with AD. Activated PBMC from patients with PS and AD showed higher expression levels of AhR-related factors and cytokines such as IL-22 and IL-17 than those from healthy controls. After TCDD treatment, the levels of AhR-related factors, IL-22 and IL-17, showed greater differences in patients with PS and AD than in healthy subjects. To confirm the effects of AhR activation on the polarization of different T cells, we analyzed the production of IFN-γ, IL-4 and IL-13, IL-17, and TNF-α and IL-2212,14. ELISA revealed that treatment with TCDD induced an increase in the production of TNF-α, IFN-γ, and IL-22 by CD4+ T cells in patients with PS, and IL-4 and IL-13 by CD4+ T cells in patients with AD. Notably, the production of IL-22 and IL-4 increased in both patient types. On the other hand, FICZ treatment decreased the levels of IFN-α, IL-17, and IL-22 in patients with PS, and IL-17 and IL-22 in patients with AD. These results show that TCDD and FICZ reciprocally regulated the production of cytokines in CD4+ T cells. PS is sustained by pro-inflammatory CD4+ T helper cells mainly belonging to the Th1, Th17, and Th22 lineages14,15. In AD, Th22 cells play a role in skin barrier impairment through IL-22, and AD is often considered as a Th2/Th22-dominant allergic disease16. While PS is emerging as an IL-23/Th17-skewed disease and AD is considered as a Th2-centered disease, both share a common Th22 component17,18. In AD, the importance of Th17 appears to differ depending on race17.
Recent data suggest that AhR is mainly involved in the development of Th17 and Th22 subsets1,19. In a study by Di Meglio et al.19, lack of AhR was shown to cause hyperinflammation, whereas AhR activation with FICZ improved the inflammatory profile in both human PS skin samples and a mouse model of PS skin inflammation20. These results are consistent with our study results with FICZ, which reduced the production of circulating inflammatory cytokines, and TCDD induced the differentiation of T cells into Th22 cells and increased the production of IL-22. The role of AhR ligands present in cigarette smoke on immune activation and pathogenesis of PS and AD has been revealed21. Tobacco smoke extract induced Th17 generation from central memory T cells in vitro and increased IL-17 and IL-22 expression, resulting in PS exacerbation22.
Although AhR has been consistently involved in IL-22 production by T cells, its role in IL-17 production is still controversial. For instance, TCDD and FICZ have been reported to induce IL-17 production in mice but inhibit IL-17 level in humans7,11,23. According to a previous study that generated transgenic mice constitutively expressing AhR in keratinocyte, severe skin lesions with itching resemble typical AD developed postnatally in mice24,25. More AhR studies should be done in AD patients in the future. Our conclusion is that TCDD-induced AhR gene induction in AD and PS patients may increase the expression of AhR-regulatory genes, thereby contributing to the development of AD and PS.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: The study was funded by National Research Foundation of Korea (NRF) [grant numbers NRF-2017R1A2B4006252], the Korea Healthcare Technology R&D Project funded by the Ministry of Health & Welfare, Republic of Korea [grant number HI17C0597], Korea Disease Control and Prevention Agency (KDCA) [grant number 2020ER671400] and the Hallym University Research Fund [grant number HURF-2017-83].
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Plé C, Fan Y, Ait Yahia S, Vorng H, Everaere L, Chenivesse C, et al. Polycyclic aromatic hydrocarbons reciprocally regulate IL-22 and IL-17 cytokines in peripheral blood mononuclear cells from both healthy and asthmatic subjects. PLoS One. 2015;10:e0122372. doi: 10.1371/journal.pone.0122372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams HC. Epidemiology of atopic dermatitis. Clin Exp Dermatol. 2000;25:522–529. doi: 10.1046/j.1365-2230.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 3.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(6 Suppl):S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 5.Wang IJ, Hsieh WS, Wu KY, Guo YL, Hwang YH, Jee SH, et al. Effect of gestational smoke exposure on atopic dermatitis in the offspring. Pediatr Allergy Immunol. 2008;19:580–586. doi: 10.1111/j.1399-3038.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong AW, Armstrong EJ, Fuller EN, Sockolov ME, Voyles SV. Smoking and pathogenesis of psoriasis: a review of oxidative, inflammatory and genetic mechanisms. Br J Dermatol. 2011;165:1162–1168. doi: 10.1111/j.1365-2133.2011.10526.x. [DOI] [PubMed] [Google Scholar]
- 7.Brembilla NC, Ramirez JM, Chicheportiche R, Sorg O, Saurat JH, Chizzolini C. In vivo dioxin favors interleukin-22 production by human CD4+ T cells in an aryl hydrocarbon receptor (AhR)-dependent manner. PLoS One. 2011;6:e18741. doi: 10.1371/journal.pone.0018741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furue M, Takahara M, Nakahara T, Uchi H. Role of AhR/ARNT system in skin homeostasis. Arch Dermatol Res. 2014;306:769–779. doi: 10.1007/s00403-014-1481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HO, Kim JH, Chung BY, Choi MG, Park CW. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23:278–281. doi: 10.1111/exd.12350. [DOI] [PubMed] [Google Scholar]
- 10.Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez JM, Brembilla NC, Sorg O, Chicheportiche R, Matthes T, Dayer JM, et al. Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur J Immunol. 2010;40:2450–2459. doi: 10.1002/eji.201040461. [DOI] [PubMed] [Google Scholar]
- 12.Rohlman D, Pham D, Yu Z, Steppan LB, Kerkvliet NI. Aryl hydrocarbon receptor-mediated perturbations in gene expression during early stages of CD4+ T-cell differentiation. Front Immunol. 2012;3:223. doi: 10.3389/fimmu.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudzki E, Samochocki Z, Rebandel P, Saciuk E, Gałecki W, Raczka A, et al. Frequency and significance of the major and minor features of Hanifin and Rajka among patients with atopic dermatitis. Dermatology. 1994;189:41–46. doi: 10.1159/000246781. [DOI] [PubMed] [Google Scholar]
- 14.Mu Z, Zhao Y, Liu X, Chang C, Zhang J. Molecular biology of atopic dermatitis. Clin Rev Allergy Immunol. 2014;47:193–218. doi: 10.1007/s12016-014-8415-1. [DOI] [PubMed] [Google Scholar]
- 15.Quaglino P, Bergallo M, Ponti R, Barberio E, Cicchelli S, Buffa E, et al. Th1, Th2, Th17 and regulatory T cell pattern in psoriatic patients: modulation of cytokines and gene targets induced by etanercept treatment and correlation with clinical response. Dermatology. 2011;223:57–67. doi: 10.1159/000330330. [DOI] [PubMed] [Google Scholar]
- 16.Noda S, Suárez-Fariñas M, Ungar B, Kim SJ, de Guzman Strong C, Xu H, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136:1254–1264. doi: 10.1016/j.jaci.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 17.van Beelen AJ, Teunissen MB, Kapsenberg ML, de Jong EC. Interleukin-17 in inflammatory skin disorders. Curr Opin Allergy Clin Immunol. 2007;7:374–381. doi: 10.1097/ACI.0b013e3282ef869e. [DOI] [PubMed] [Google Scholar]
- 18.Baba N, Rubio M, Kenins L, Regairaz C, Woisetschlager M, Carballido JM, et al. The aryl hydrocarbon receptor (AhR) ligand VAF347 selectively acts on monocytes and naïve CD4+ Th cells to promote the development of IL-22-secreting Th cells. Hum Immunol. 2012;73:795–800. doi: 10.1016/j.humimm.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Di Meglio P, Duarte JH, Ahlfors H, Owens ND, Li Y, Villanova F, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989–1001. doi: 10.1016/j.immuni.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue J, Zhao Q, Sharma V, Nguyen LP, Lee YN, Pham KL, et al. Aryl hydrocarbon receptor ligands in cigarette smoke induce production of interleukin-22 to promote pancreatic fibrosis in models of chronic pancreatitis. Gastroenterology. 2016;151:1206–1217. doi: 10.1053/j.gastro.2016.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 22.Hidaka T, Ogawa E, Kobayashi EH, Suzuki T, Funayama R, Nagashima T, et al. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat Immunol. 2017;18:64–73. doi: 10.1038/ni.3614. [DOI] [PubMed] [Google Scholar]
- 23.Ito T, Inouye K, Nohara K, Tohyama C, Fujimaki H. TCDD exposure exacerbates atopic dermatitis-related inflammation in NC/Nga mice. Toxicol Lett. 2008;177:31–37. doi: 10.1016/j.toxlet.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Torii K, Saito C, Furuhashi T, Nishioka A, Shintani Y, Kawashima K, et al. Tobacco smoke is related to Th17 generation with clinical implications for psoriasis patients. Exp Dermatol. 2011;20:371–373. doi: 10.1111/j.1600-0625.2010.01224.x. [DOI] [PubMed] [Google Scholar]
- 25.Haarmann-Stemmann T, Esser C, Krutmann J. The Janusfaced role of aryl hydrocarbon receptor signaling in the skin: consequences for prevention and treatment of skin disorders. J Invest Dermatol. 2015;135:2572–2576. doi: 10.1038/jid.2015.285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.