Abstract
Background
Particulate matters (PM) comprise a heterogeneous mixture of particles suspended in air. A recent study found that urban PMs may penetrate into hair follicles via transfollicular and transdermal routes in dorsal skin.
Objective
To investigate the effects of PM on ex vivo cultured human scalp hair follicles and hair follicular keratinocytes in vitro.
Methods
TUNEL staining was employed to check cells undergoing apoptosis in cultured hair follicles after PM treatment. MTT assay was employed to check cell viability after PM treatment. Quantitative real-time PCR analysis was employed to quantitate the expression of inflammatory genes, matrix metalloproteinases (MMPs), and Duox1. Inflammatory cytokine levels were measured by ELISA after PM treatment. The level of reactive oxygen species (ROS) production was measured using a chemical fluorescent probe by a fluorescence plate reader.
Results
Abundant TUNEL-positive cells were observed in the keratinocyte region of hair including the epidermis, sebaceous gland, outer root sheath (ORS), inner root sheath (IRS), and bulb region. The viability of follicular cells, including the ORS, was found to be decreased upon PM exposure. mRNA expression and protein levels of inflammatory response genes and MMPs were upregulated in a dose-dependent manner by PM treatment. ROS levels were also increased by PM.
Conclusion
These data strongly suggest that penetrated PMs from air pollution may cause apoptotic cell death to follicular keratinocytes by increased production of ROS and inflammatory cytokines, which could impair hair growth.
Keywords: Apoptosis, Hair keratinocyte, Hair loss, Particulate matter, Reactive oxygen species
INTRODUCTION
Air born particulate matters (PMs) comprise a mixture of air particles composed of metals, organic compounds, biologically-originating ions, and a carbon particle core that varies in size. These chemical constituents are produced by various natural and industrial resources1. Concern regarding the health effects of urban air pollution has increased with the rapid industrialization of the modern era, and numerous epidemiologic studies have indicated that exposure to PM is associated with increased risk of diverse diseases such as cancers, pulmonary, respiratory, and cardiovascular diseases, as well as skin diseases2,3,4. Skin is the largest organ of human body and the outermost barrier between the environment and the body that provide powerful defensive capacity. Hair is a unique skin appendage that contains dermal cells and epidermal keratinocytes, which undergoes a sequential cycle of anagen (the active growth phase), catagen (the apoptotic regression phase), and telogen (the resting phase)5.
Various studies have been performed from multiple centers to investigate the impacts and consequences of urban pollution on skin in areas of Mexico and Shanghai6,7. These analyses demonstrated that urban pollution negatively impacted skin quality, and they identified biochemical parameters that caused changes in sebum secretion and the cutaneous structure. Moreover, Galliano et al.8 used the deposit and adherence test of PM to explore the impact of urban pollution on the human hair surface, and Naudin et al.9 suggested that polycyclic aromatic hydrocarbons, which co-exist with PMs in air pollutants, accelerate the ultrastructural degradation of hair fibers. These studies showed that pollution affects hair surface properties and alters hair quality in vitro and real-life condition. The signaling pathways related to skin barrier dysfunction upon exposure to PMs (standard reference material [SRM] 1649b) were explored in vivo and in vitro by Lee et al10.
A recent study by Jin et al.11 observed inflammation and detrimental effects upon PM penetration into barrier-disrupted skin in vivo via stimulation of inflammatory mediator and reactive oxygen species (ROS) production. In addition, a recent review suggested that systemic and direct dermal exposure and uptake of PMs can occur in specific populations12. These findings suggest that penetrated PMs might alert the hair growth mechanism. However there is no clear evidence of the effects of PM exposure on scalp skin and hair follicles, although recent studies have provided evidence that environmental cigarette smoke from air pollution is closely related with androgenetic alopecia13. Therefore, we explored the effects of PM on human hair using ex vivo organ culture and primary cultured human hair follicular cells.
MATERIALS AND METHODS
Cell culture
Hair biopsy specimens were obtained from the non-balding occipital scalp region of patients with androgenic alopecia during hair transplantation at Kyungpook National University Hospital (Daegu, Korea) with the patients' written informed consent. The Medical Ethical Committee of the Kyungpook National University Hospital approved all of the study protocol (IRB No. KNUH 2013-02-001-007). Hair follicles were isolated and cultured by the method described previously14. Dermal papilla (DP), dermal fibroblast (DF), dermal sheath (DS), and outer root sheath (ORS) cells were isolated from dissected anagen hair follicles and cultured as described previously15. Briefly, isolated primary human hair cells were cultured in keratinocyte growth medium for ORS and in DMEM low-glucose medium for DP, DS, and DF (Clonetics Inc., San Diego, CA, USA). The cells were maintained in a humidified incubator at 37℃ under 5% CO2. We used primary cultured cells at passage 2 to 3 in this study.
All data were analyzed using Prism 6 for Windows Version 6.01 (GraphPad Software Inc., San Diego, CA, USA). p-values of less than 0.05 were considered significant.
PM10 preparation and treatment
For the in vitro study, PM10 (PM10-like, European reference material ERM-CZ120) (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in phosphate buffered saline (PBS) at a concentration of 5 mg/ml and diluted to a range of concentrations with the basal medium of each cell type. The PM10 suspension was then sonicated for 30 minutes to prevent aggregation. Experiments were conducted within 1 hour of PM preparation to avoid variability in PM components at different concentrations between replicates.
Cell viability test
Cells were incubated with PM at the indicated concentration for 24 hours. Cell viability was assayed using MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) according to the manufacturer's protocol. Viability was compared with the value of the non-PM-treated control.
Quantitative real-time PCR
Total RNA was extracted from cultured primary human DP and ORS cells using RNAiso Plus (Takara Bio Inc., Shiga, Japan). Three micrograms of total RNA was used to synthesize cDNA using a First Strand cDNA Synthesis Kit (MBI Fermentas, Vilnius, Lithuania). Gene-specific forward and reverse primers were used in each well of a 96-well plate for quantitative real time-PCR (qPCR) using a Step OnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with SYBR Green PCR Master Mix (Takara Bio Inc.) according to the manufacturer's instructions. The PCR conditions were 95℃ for 2 minutes, followed by 40 cycles at 95℃ for 15 seconds and 60℃ for 1 minute. qPCR primer information is shown in Supplementary Table 1. The data were calculated using the comparative Ct method (ΔΔCt method) for to normalization.
ELISA
The protein levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1α, IL-1β, IL-8, and IL-6 were determined by ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol. Briefly, DP and ORS cells were seeded in 6-well plates (1.2×106 cells/well). After 24 hours starvation in serum-free media, cells were exposed to different concentrations of PM10 suspension for the indicated time. Then, the culture supernatant was collected at 4℃, centrifuged for 20 minutes at 1,000×g at 4℃, and stored at −20℃ for subsequent assays. Serial dilutions of recombinant human proteins were used to establish a standard curve for optical density measurements by an ELISA reader at 450 nm.
Reactive oxygen species measurement
Cells were seeded in a 96-well pate (4.5×104 cells/well) and serum-starved for 24 hours. After discarding the supernatant, the indicated concentration of PM10 suspension was applied in serum-free media. Plates were washed with PBS after 1 hour of exposure and then filled with 25 µM DCF-DA solution diluted in pre-warmed phenol-free HBSS (Gibco®; ThermoFisher, Waltham, MA, USA) and incubated at 37℃ for 1 hour protected from light. ROS was detected using a fluorescence plate reader excitation at 490 nm and emission at 525 nm. As a positive control of this experiment, cells were incubated with freshly prepared 0.5 mM tert-Butyl hydroperoxide (TBHP) for 20 minutes at 37℃.
TUNEL assay
A TUNEL kit (EMD Millipore, Billerica, MA, USA) was used according to the manufacture's protocol.
RESULTS
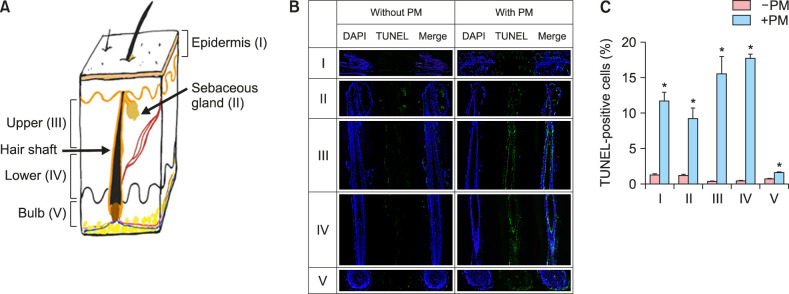
First, to investigate overall effects of PM10 on human hair, TUNEL assay was carried out using ex vivo organ culture in the presence of PM10 solution. Surprisingly, as compared to the control group, cells undergoing apoptosis were widely observed from nearly all keratinocyte regions in the hair follicle including the epidermis, sebaceous gland, ORS and inner root sheath (IRS) layer in both the upper and lower follicle (Fig. 1). In addition, some apoptotic cells were occasionally detected in the DP and DS in all three cases.
Fig. 1. Apoptotic cell death in ex vivo cultured human hair follicles after PM10 treatment. (A) Diagram of longitudinal dissection of skin with hair follicles. (B) Organ cultured hair follicles were treated with PM10 solution (100 µg/ml) for 5 days and subjected to TUNEL assay. Representative images from each hair follicle region are shown. DAPI (blue), TUNEL (green), and merged (cyan). (C) TUNEL-positive cells were quantified, and the data are presented as the mean±standard deviation from three independent experiments with hair follicles from three different individuals. *p<0.05 compared with the control by Student's t-test. PM: particulate matter.
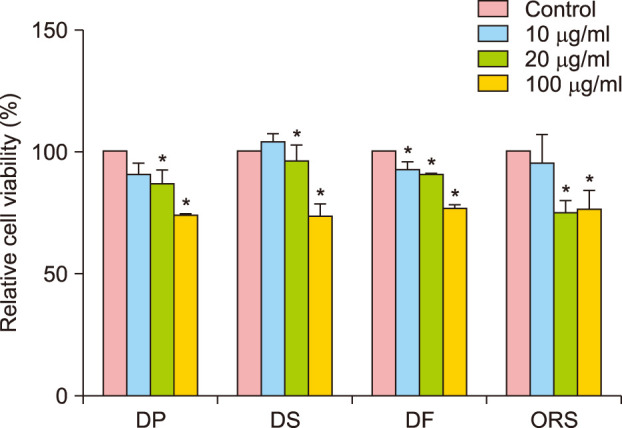
Subsequently, to confirm cellular cytotoxicity in the major component cells of hair follicles, we tested cell viability in the presence of PM10 in cultured human hair cells including DS, DF, DP, and ORS cells. All cells showed dose-dependent increased cytotoxicity upon PM10 treatment (Fig. 2); 100 µg/ml PM10 showed the maximum effect and was used for further study.
Fig. 2. Effect of PM10 on the cell viability of follicular cells. MTT assay was performed in cultured primary human dermal papilla (DP), dermal sheath (DS), dermal fibroblast (DF), and outer root sheath (ORS) cells. After 24 hours of serum starvation, cells were treated with suspended PM10 solution at the indicated concentrations for 24 hours. Data are presented as the mean±standard deviation from three independent experiments using cells from three individuals. *p<0.05 compared with the control by Student's t-test. PM: particulate matter.
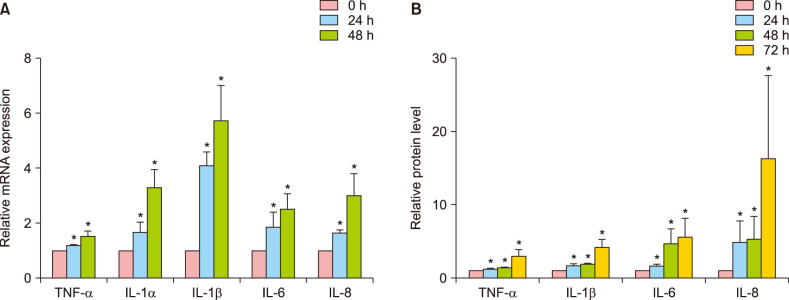
Several reports have highlighted the importance of cytokines such as TNF-α, IL-1α, IL-1β, IL-6, and IL-8, which have been implicated in the inflammatory response of HaCaT cells exposed to PM16. To determine the mRNA expression levels of these cytokines, qPCR was performed after PM10 exposure to cultured ORS cells. The expression levels of all inflammatory factors were increased (Fig. 3A). Subsequently, the protein levels of TNF-α, IL-1β, IL-6, and IL-8 were measured by ELISA. Interestingly, IL-6 and IL-8 protein were significantly increased by PM10 treatment as compared to the control group in ORS cells (Fig. 3B).
Fig. 3. Upregulation of inflammatory cytokines by PM10 treatment in cultured human outer root sheath (ORS) cells. Cells were treated with suspended PM10 (100 µg/ml) solution for the indicated times after 24 hours serum starvation. Inflammatory cytokine genes including tumor necrosis factor (TNF)-α, interleukin (IL)-1α, IL-1β, IL-6, and IL-8 mRNA expression levels were examined by quantitative real-time PCR (A), and their protein levels were measured from culture supernatants by ELISA (B). Data are shown as relative levels compared to the control group. Data are presented as means±standard deviation from three independent experiments using cells from three individuals. PM: particulate matter. *p<0.05 compared with the control by Student's t-test.
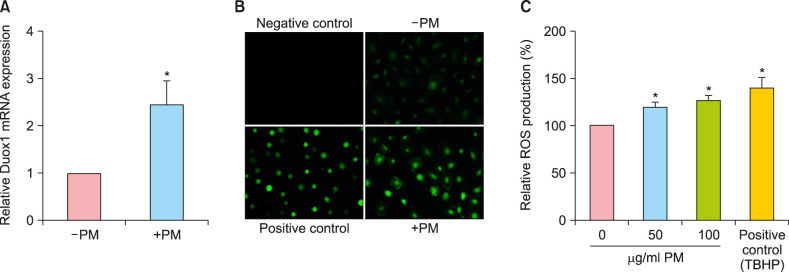
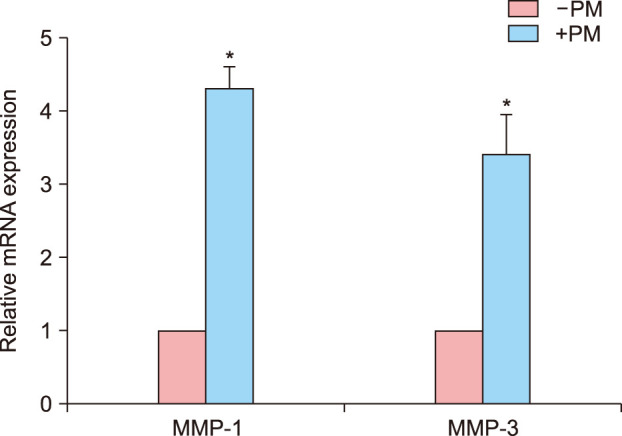
Numerous studies have reported that PM causes inflammatory mediator productions through the generation of ROS and free radicals. NADPH oxidase (NOX) family members produce ROS, and Duox1 is predominant NOX family member in ORS cells17. qPCR showed that Duox1 expression increased more than two-fold after 48-hour PM treatment compared to control cells (Fig. 4A). The level of ROS production was visualized by fluorescent microscopy (Fig. 4B) using a chemical fluorescent probe, CM-H2DCFDA18. Consistent with the above findings, intracellular ROS production was increased in ORS cells after PM10 treatment in a dose-dependent manner (Fig. 4C). Collectively, these data show that PM10 induced Duox1 and increased ROS production in human hair keratinocytes. In addition to proinflammatory cytokines, the expression levels of matrix metalloproteinases (MMPs) are increased by ROS19; therefore, qPCR was conducted after PM exposure in ORS cells. MMP-1 and MMP-3 were upregulated more than three-fold upon PM treatment (Fig. 5).
Fig. 4. Effect of PM10 on reactive oxygen species (ROS) production in cultured human outer root sheath (ORS) cells. (A) Cells were treated with PM10 (100 µg/ml) for 48 hours, and Duox1 mRNA expression levels were examined by quantitative real-time PCR. (B) Cells were treated with the fluorescent probe CM-H2DCFDA (20 µM) for 1 hour in the absence or presence of PM10, and ROS production was visualized by fluorescent microscopy. The negative control is without CM-H2DCFDA. For the positive control, cells were treated with TBHP (0.5 mM) for 20 minutes. (C) Intracellular ROS levels were measured, and data are shown as the relative levels compared to the control group. Data are presented as means±standard deviation from three independent experiments using cells from three individuals. PM: particulate matter. *p<0.05 compared with the control by Student's t-test.
Fig. 5. Matrix metalloprotease mRNA expression after PM10 treatment in cultured human outer root sheath (ORS) cells. Cells were serum-starved for 24 hours prior to PM10 (100 µg/ml) treatment for 48 hours. Data are the relative mRNA expression of metalloprotease-1 (MMP-1) and metalloprotease-3 (MMP-3) and are presented as means±standard deviation from three independent experiments using cells from three individuals. *p<0.05 compared with the control by Student's t-test. PM: particulate matter.
DISCUSSION
In this study, we observed that PMs induce apoptotic cell death, especially in follicular keratinocytes in ex vivo cultured human scalp hair follicles (Fig. 1). In agreement with this, PM treatment decreased the viability of follicular cells, including ORS keratinocytes (Fig. 2). In addition, inflammatory cytokine expression was increased after PM10 treatment in cultured keratinocytes (Fig. 3). Considering that high-dose proinflammatory cytokines induce apoptosis of hair bulb keratinocytes in vivo19 and our previous research showed that IL-6 inhibited hair shaft elongation in cultured human hair follicles and caused premature onset of catagen in mice20, these data suggest that PM may impair hair growth by inducing inflammatory cytokines.
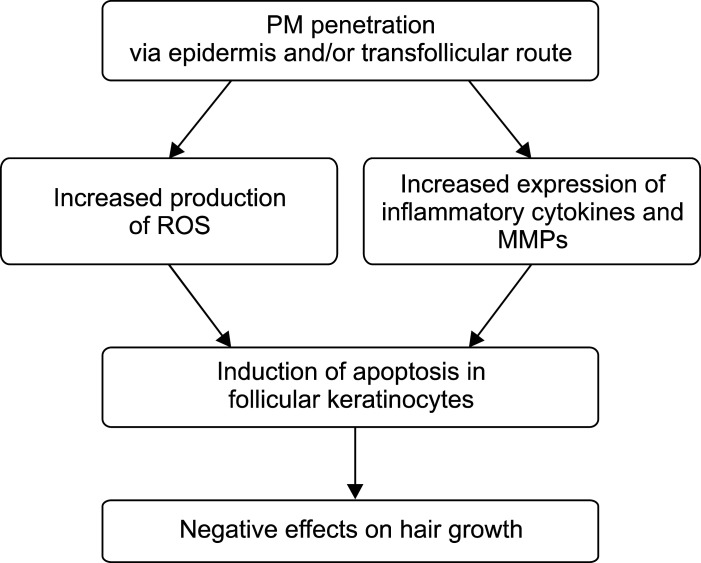
We also observed that PM treatment increased Duox1, an important NOX family member in ORS cells, as well as ROS production (Fig. 4). In addition, PM treatment increased MMP-1/3 expression in ORS cells (Fig. 5). ROS are chemically powerful molecules, and major biomolecules are susceptible to modification by ROS stress16. It is known that ROS can regulate redox-modulated matrix degradation via MMP gene expression and activation through keratinocyte signaling pathways21. Therefore, PM-generated ROS not only cause biomolecule modifications but may also activate MMPs, which together can cause inflammation and apoptosis in the skin, especially in epidermal keratinocytes. Whether PMs are able to penetrate the skin via the hair follicle remains under debate4,11,22. Considering that the diameter of a scalp hair is generally larger than the size of all PMs and that a recent finding of in vivo skin distribution of rhodamine B after SRM 1649b treatment showed that dye delivery occurred via the follicle rather than via intracellular or transcellular means, PMs may accumulate in the aperture of the hair follicles and penetrate into follicular cells via the transfollicular route23,24. Despite the complexity of PM10 components and the moderate variability in donor hair samples, our ex vivo organ culture and in vitro cell culture studies suggest that penetrated PMs from air pollution may cause apoptotic cell death to follicular keratinocytes through increased production of ROS and inflammatory cytokines, resulting in hair loss (Fig. 6).
Fig. 6. Proposed model based on this study. Penetrated PM enters hair component cells via epidermal and transfollicular routes to increase ROS production and upregulate inflammatory cytokines and MMPs in follicular keratinocytes, which in turn results in apoptotic cell death and inflammation. Thereby, penetrated PMs from air pollution may have negative effects on hair growth. PM: particulate matter, ROS: reactive oxygen species, MMPs: matrix metalloproteinases.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: This research was funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2018R1A2A2A05018574). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIALS
Supplementary data can be found via http://anndermatol.org/src/sm/ad-32-388-s001.pdf.
PCR primers used in this study
References
- 1.World Health Organization (WHO) Burden of disease from the joint effects of household and ambient air pollution for 2012. Geneva: World Health Organization (WHO); 2014. [Google Scholar]
- 2.Kim KH, Kabir E, Kabir S. A review on the human health impact of airborne particulate matter. Environ Int. 2015;74:136–143. doi: 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Thompson JE. Airborne particulate matter: human exposure and health effects. J Occup Environ Med. 2018;60:392–423. doi: 10.1097/JOM.0000000000001277. [DOI] [PubMed] [Google Scholar]
- 4.Kim KE, Cho D, Park HJ. Air pollution and skin diseases: adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016;152:126–134. doi: 10.1016/j.lfs.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Botchkarev VA, Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc. 2003;8:46–55. doi: 10.1046/j.1523-1747.2003.12171.x. [DOI] [PubMed] [Google Scholar]
- 6.Lefebvre MA, Pham DM, Boussouira B, Bernard D, Camus C, Nguyen QL. Evaluation of the impact of urban pollution on the quality of skin: a multicentre study in Mexico. Int J Cosmet Sci. 2015;37:329–338. doi: 10.1111/ics.12203. [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre MA, Pham DM, Boussouira B, Qiu H, Ye C, Long X, et al. Consequences of urban pollution upon skin status. A controlled study in Shanghai area. Int J Cosmet Sci. 2016;38:217–223. doi: 10.1111/ics.12270. [DOI] [PubMed] [Google Scholar]
- 8.Galliano A, Ye C, Su F, Wang C, Wang Y, Liu C, et al. Particulate matter adheres to human hair exposed to severe aerial pollution: consequences for certain hair surface properties. Int J Cosmet Sci. 2017;39:610–616. doi: 10.1111/ics.12416. [DOI] [PubMed] [Google Scholar]
- 9.Naudin G, Bastien P, Mezzache S, Trehu E, Bourokba N, Appenzeller BMR, et al. Human pollution exposure correlates with accelerated ultrastructural degradation of hair fibers. Proc Natl Acad Sci U S A. 2019;116:18410–18415. doi: 10.1073/pnas.1904082116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CW, Lin ZC, Hu SC, Chiang YC, Hsu LF, Lin YC, et al. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci Rep. 2016;6:27995. doi: 10.1038/srep27995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin SP, Li Z, Choi EK, Lee S, Kim YK, Seo EY, et al. Urban particulate matter in air pollution penetrates into the barrierd-isrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J Dermatol Sci. 2018;91:175–183. doi: 10.1016/j.jdermsci.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Araviiskaia E, Berardesca E, Bieber T, Gontijo G, Sanchez Viera M, Marrot L, et al. The impact of airborne pollution on skin. J Eur Acad Dermatol Venereol. 2019;33:1496–1505. doi: 10.1111/jdv.15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatherwright J, Liu MT, Gliniak C, Totonchi A, Guyuron B. The contribution of endogenous and exogenous factors to female alopecia: a study of identical twins. Plast Reconstr Surg. 2012;130:1219–1226. doi: 10.1097/PRS.0b013e31826d104f. [DOI] [PubMed] [Google Scholar]
- 14.Philpott MP, Sanders DA, Kealey T. Effects of insulin and insulin-like growth factors on cultured human hair follicles: IGF-I at physiologic concentrations is an important regulator of hair follicle growth in vitro. J Invest Dermatol. 1994;102:857–861. doi: 10.1111/1523-1747.ep12382494. [DOI] [PubMed] [Google Scholar]
- 15.Magerl M, Kauser S, Paus R, Tobin DJ. Simple and rapid method to isolate and culture follicular papillae from human scalp hair follicles. Exp Dermatol. 2002;11:381–385. doi: 10.1034/j.1600-0625.2002.110414.x. [DOI] [PubMed] [Google Scholar]
- 16.Seok JK, Lee JW, Kim YM, Boo YC. Punicalagin and (−)-epigallocatechin-3-gallate rescue cell viability and attenuate inflammatory responses of human epidermal keratinocytes exposed to airborne particulate matter PM10. Skin Pharmacol Physiol. 2018;31:134–143. doi: 10.1159/000487400. [DOI] [PubMed] [Google Scholar]
- 17.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 18.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, et al. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rückert R, Lindner G, Bulfone-Paus S, Paus R. High-dose proinflammatory cytokines induce apoptosis of hair bulb keratinocytes in vivo. Br J Dermatol. 2000;143:1036–1039. doi: 10.1046/j.1365-2133.2000.03784.x. [DOI] [PubMed] [Google Scholar]
- 20.Kwack MH, Ahn JS, Kim MK, Kim JC, Sung YK. Dihydrotestosterone-inducible IL-6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice. J Invest Dermatol. 2012;132:43–49. doi: 10.1038/jid.2011.274. [DOI] [PubMed] [Google Scholar]
- 21.Freitas-Rodríguez S, Folgueras AR, López-Otín C. The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochim Biophys Acta Mol Cell Res. 2017;1864(11 Pt A):2015–2025. doi: 10.1016/j.bbamcr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Yokota J, Kyotani S. Influence of nanoparticle size on the skin penetration, skin retention and anti-inflammatory activity of non-steroidal anti-inflammatory drugs. J Chin Med Assoc. 2018;81:511–519. doi: 10.1016/j.jcma.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Bronaugh RL, Maibach HI. Percutaneous absorption: drugs, cosmetics, mechanisms, methodology. 3rd ed. New York: Marcel Dekker; 1999. [Google Scholar]
- 24.Pan TL, Wang PW, Aljuffali IA, Huang CT, Lee CW, Fang JY. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J Dermatol Sci. 2015;78:51–60. doi: 10.1016/j.jdermsci.2015.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR primers used in this study
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.