Abstract
Background
Several epidemiological studies have shown that the atopic tendency increases in the obese population.
Objective
The aim of this study was to confirm the effect of weight reduction on improvement of atopic dermatitis (AD) symptoms and to investigate the relationship between AD severity and the level of serum adipokines.
Methods
Forty subjects who were AD outpatients were recruited for this study. Obese patients were divided into a weight maintenance group and weight reduction group. During the study period, patient information was collected that included measured body mass index (BMI), Eczema Area and Severity Index (EASI), and visual analogue scale for pruritus. Adiponectin, leptin, eosinophil count, and total immunoglobulin E were also tested.
Results
In the weight reduction group, there was a significant improvement in the EASI score, however, no significant improvement was determined in the weight maintenance group. BMI and EASI showed positive correlation. The adiponectin level was lower in AD patients compared to healthy controls, and it was significantly lower in obese patients compared with normal weight patients. Serum levels of leptin were significantly different among control, obese patient group, and normal weight patient group. There was no statistically significant relationship between serum adipokine level and EASI.
Conclusion
In our study, weight reduction was associated with significant improvement of AD symptoms. Related adipokine levels were significantly different among the control, normal weight AD patient group, and obese AD patient group.
Keywords: Adiponectin, Atopic dermatitis, Body mass index, Leptin, Weight loss
INTRODUCTION
Atopic dermatitis (AD) is a recurrent, chronic, and pruritic inflammatory skin disorder featuring skin dryness1. The prevalence of AD has increased over the last few decades especially in industrialized countries2,3. Obesity has also increased over recent decades. Obesity is known to cause low-grade systemic inflammation and the underlying conditions for many related morbidities including type 2 diabetes, cancer, and cardiovascular diseases4. Previous researchers have proposed an association between obesity and AD. However, the results of these studies were limited and not consistent5,6. Moreover, most studies regarding the relationship between obesity and AD have been observational and epidemiologic research.
Although the underlying mechanism of association between obesity and AD remains obscure, possible roles for adipokines have been suggested. Leptin and adiponectin are the best defined adipokines. Leptin is a proinflammatory adipokine that arises though stimulation of a T-helper (Th) 1 related response7. Generally, adiponectin is regarded as an anti-inflammatory mediator. Previous investigators have shown that adiponectin reduces the production of inflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α8. Among the chronic inflammatory skin diseases, psoriasis has a well-known relation with obesity and the correlation between adipokines and clinical severity or response to therapy has been demonstrated in patients with psoriasis9,10. However, the number of studies showing roles for adipokines in AD is limited.
Therefore, we aimed to confirm the therapeutic effect of weight loss for AD by conducting a prospective randomized controlled study. To determine the detailed mechanism for the association between obesity and AD, the serum levels of adipokines was also examined.
MATERIALS AND METHODS
Subjects and ethics
Forty AD outpatients (≥18 years) who visited the Department of Dermatology at Hallym University Kangnam Sacred Heart Hospital in Seoul, Korea from April 2016 to May 2018 were enrolled in this study. AD was diagnosed based on the criteria of Hanifin et al.11 by dermatologists. Patients with other uncontrolled accompanying systemic diseases and other dermatologic disorders, as well as pregnant or nursing women were excluded. Healthy volunteers were also enrolled as controls. The study was approved by the Ethics Committee of Hallym University Kangnam Sacred Heart Hospital (IRB no.2015-12-150). Written consent forms were signed by the patients volunteering to participate in the study.
Study design
Normal weight AD patients (n=20) and obese (body mass index [BMI] of 25 kg/m2 or higher) AD patients (n=20) were enrolled in this study. Obese patients were randomly assigned to 12 weight maintenance groups and 8 weight reduction groups by table of random numbers. The weight reduction group received collaborative care for weight loss with the department of family medicine for 3 months, and the weight maintenance group did not receive collaborative care for weight loss. The subjects in both groups came in for an initial visit (week 0) and follow-up visits (weeks 1, 2, 4, 8, and 12). Under the collaborative care of the Department of Family Medicine, the weight reduction group was advised to lose weight and to this end, physical examination and history taking were repeated, and professional consultation was provided regarding weight loss. For the duration of the clinical study, the subjects had to maintain their exercise regimen. The regimen involved aerobic exercise, such as walking, three times per week for one hour. Patients were also recommended to follow a low-calorie diet. Furthermore, the dosage and types of medication for treating AD could be reduced but not increased. If a patient had to inevitably increase the dosage or the number of medications to control AD symptoms, that subject was excluded from the study. Subjects were allowed to apply moderate topical steroids and topical calcineurin inhibitors, and the emollients were maintained only for previously used.
Specific assessments
A. BMI measurement
At the baseline, and at week 1, 2, 4, 8, and 12, the BMI was calculated for each subject according to body weight (kg) / upright height (m).
B. Severity of AD (Eczema Area and Severity Index, EASI)
At the baseline, and at week 1, 2, 4, 8, and 12, the severity of AD was assessed according to the EASI score by dermatologists11.
C. Pruritus for visual analog scale (VAS)
At the baseline, and at week 1, 2, 4, 8, and 12, pruritus was evaluated by the subjects using the VAS. The patients were asked to indicate the level of pruritus they experienced for a week on a 10 cm line, with the left end being “none” and the right end being “most extreme”. The indication was then converted to a score on a scale from 0 (none) to 10 (very severe).
Serum total immunoglobulin E, eosinophil count, leptin, and adiponectin measurements
Blood sampling was performed at baseline visit. The fasting blood samples were collected in the morning between 7 AM and 9 AM following an overnight fast. Serum total immunoglobulin E (IgE) levels were measured using the paper radioimmunosorbent test (PRIST) kit (Behring, Marbug, Germany) according to the manufacturer instructions. Blood eosinophils were measured as part of the complete blood count plus differential, and these samples were analyzed at the local laboratory of Hallym University Kangnam Sacred Heart Hospital (Seoul, Korea). The serum concentrations of the target proteins were measured using enzyme-linked immunosorbent assays (ELISA) kits. Serum samples were diluted to 1:200 in assay diluent. ELISA were used to determine the levels of human leptin (Abcam, Cambridge, MA, USA) and human adiponectin (Abcam). All protocols provided by the manufacturer were followed for each ELISA kit.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics ver. 24.0 (IBM Corp., Armonk, NY, USA). Data were presented as a mean±standard deviation. Student's t-test was performed for comparisons between groups. The changes in the EASI score was tested by performing Student's t-test and repeated-measures ANOVA. For leptin and adiponectin, differences of statistical significance were identified in the average serum levels using ANOVA. Specification of the patient groups that showed statistically significant variances for the sample measured was achieved using the Turkey post-hoc test. Correlations were calculated using the Pearson's correlation test or Spearman rank-order correlation. For all tests, p-values <0.05 were considered statistically significant.
RESULTS
Demographic and clinical features of subjects
Demographic and clinical characteristics at the baseline are shown in Table 1. BMI and EASI were significantly lower in normal weight patients than in obese patients (p<0.001, p=0.003). There was no significant difference in VAS for pruritus, eosinophil count, and total IgE between normal weight patients and obese patients.
Table 1. Demographic data and clinical characteristics of patients at baseline visit.
| Characteristic | Normal weight group (n=20) | p-value* | Weight maintenance group (n=12) | Weight reduction group (n=8) | p-value† |
|---|---|---|---|---|---|
| Age (yr) | 28.05±5.70 | 0.044 | 42.42±15.70 | 26.25±11.11 | 0.022 |
| Sex | 0.744 | 0.085 | |||
| Male | 8 (40.0) | 6 (50.0) | 1 (12.5) | ||
| Female | 12 (60.0) | 6 (50.0) | 7 (87.5) | ||
| BMI at baseline (kg/m2) | 22.04±2.18 | <0.001 | 27.23±2.03 | 30.73±5.40 | 0.054 |
| EASI | 8.9±5.72 | 0.003 | 16.64±9.25 | 15.59±7.71 | 0.793 |
| VAS for pruritus | 5.05±2.33 | 0.076 | 6.83±1.40 | 5.38±2.07 | 0.075 |
| Eosinophil count (103/µl) | 0.61±0.70 | 0.414 | 0.34±0.31 | 0.59±0.57‡ | 0.262 |
| Total IgE (IU/ml) | 932.11±1,147.72§ | 0.374 | 1,580.23±960.58∥ | 1,185.4±1,180.68¶ | 0.585 |
| TARC | 303.19±83.52 | 0.007 | 259.36±65.14 | 193.66±68.75 | 0.044 |
Values are presented as mean±standard deviation or number (%). BMI: body mass index, EASI: Eczema Area and Severity Index, VAS: visual analogue scale, IgE: immunoglobulin E, TARC: thymus and activaton-regulated chemokine. *p-value between normal weight group and obese group, †p-value between weight maintenance group and weight loss group, ‡n=10, §n=16, ∥n=4, ¶n=7.
The mean age was significantly older (p=0.022) in the weight maintenance group (42.42±15.70) than in the weight reduction group (26.25±11.11). There was no significant difference between the two groups in BMI, VAS for pruritus, eosinophil count, and total IgE. Thymus and activaton-regulated chemokine (TARC) in atopic dermatitis of the normal weight group was higher than the obese group, and the TARC of the weight maintenance group was slightly higher than the weight reduction group.
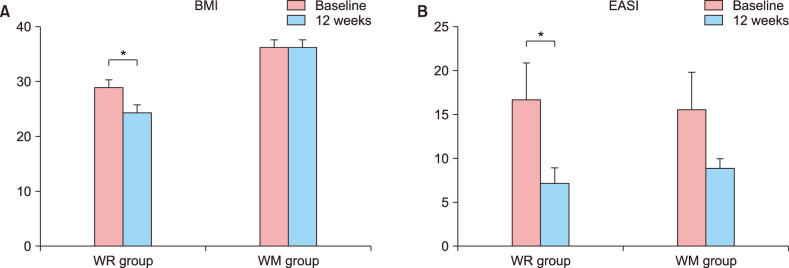
Weight reduction decreased EASI score in obese AD patients
Of the enrolled obese patients, two and five patients were followed-up loss in the weight maintenance group and the weight reduction group, respectively. For each group (obese and normal weight) 8 and 5 patients completed all 12 weeks of the program. When comparing conditions at the baseline and last visit (week 12), the mean BMI decreased significantly from 29.37 to 24.42 in the weight reduction group (p=0.043), but there was no significant change in the weight maintenance group (p=0.889) (Fig. 1). EASI scores showed a statistically significant decrease in the weight reduction group (p=0.043), but there was no statistically significant decrease in the weight maintenance group (p=0.183). Most of subjects remained on antihistamines from the baseline visit to the end of the study. All of the patients in the weight reduction group were taking cyclosporine at the baseline, but at the end of the study there was a decrease in the dose (two of them: 200 mg/d to 50 mg/d, one of them 100 mg/d to 50 mg/d, one of them 100 mg/d to 25 mg/d) in four patients and one patient stopped taking cyclosporine (50 mg/d at baseline). Three of the weight maintenance patients were taking cyclosporine at the baseline. Two of them sustained their dosages (100 mg/d and 25 mg/d) and one decreased the dosage (100 mg/d to 25 mg/d) by the end of the study.
Fig. 1. (A) Body mass index (BMI). (B) Eczema Area and Severity Index (EASI) at baseline and week 12 for weight reduction (WR) and weight maintenance (WM) groups. Data was compared using the paired t-test (*p<0.05). Only the weight reduction group showed significant BMI and EASI reduction.
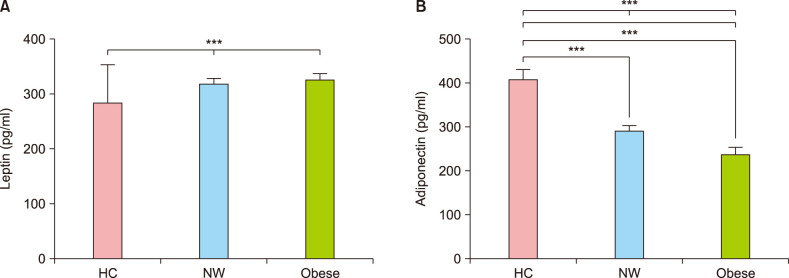
Serum adipokine levels were significantly different in subjects with obese AD
Normal weight (n=20) and obese (n=20) AD patients were selected for the ELISA test for serum adipokines. Six normal weight healthy people (without AD) were examined by blood sampling. Serum leptin levels were significantly different among the groups (Fig. 2A). Serum adiponectin levels were significantly lower in AD patients than in healthy normal weight subjects. It was also significantly lower in obese AD patients than in normal weight AD patients (Fig. 2B).
Fig. 2. (A) Serum leptin. (B) Adiponectin level of normal, normal weight, and obese patients. Serum leptin levels were significantly different between groups. Serum adiponectin levels were significantly lower in atopic dermatitis (AD) patients than in normal controls, and significantly lower in obese AD patients than in normal weight controls. HC: healthy control, NW: normal weight patients with AD. ***p<0.001.
Association of serum adipokines with AD severity
Table 2 shows the correlation between the clinical values of BMI, EASI, and VAS, with the laboratory values of eosinophil count and total IgE. There was a significant positive correlation between clinical severity (EASI score) and VAS score (r=0.486, p=0.001). BMI and EASI were also positively correlated (r=0.316, p=0.047). However, the other results were not correlated.
Table 2. Pearson's correlation coefficient of each measured value.
| Calculated statistical value | BMI | EASI | VAS | EOS | Total IgE |
|---|---|---|---|---|---|
| BMI | |||||
| Pearson's coefficient of correlation | 1 | 0.316 | 0.266 | −0.018 | 0.188 |
| p-value | 0.047* | 0.097 | 0.913 | 0.348 | |
| Number | 40 | 40 | 40 | 38 | 27 |
| EASI | |||||
| Pearson's coefficient of correlation | 0.316 | 1 | 0.486 | 0.072 | 0.342 |
| p-value | 0.047* | 0.001* | 0.667 | 0.081 | |
| Number | 40 | 40 | 40 | 38 | 27 |
| VAS | |||||
| Pearson's coefficient of correlation | 0.266 | 0.486 | 1 | −0.166 | −0.033 |
| p-value | 0.097 | 0.001* | 0.319 | 0.869 | |
| Number | 40 | 40 | 40 | 38 | 27 |
| EOS | |||||
| Pearson's coefficient of correlation | −0.018 | 0.072 | −0.166 | 1 | 0.096 |
| p-value | 0.913 | 0.667 | 0.319 | 0.635 | |
| Number | 38 | 38 | 38 | 38 | 27 |
| Total IgE | |||||
| Pearson's coefficient of correlation | 0.188 | 0.342 | −0.033 | 0.096 | 1 |
| p-value | 0.348 | 0.081 | 0.869 | 0.635 | |
| Number | 27 | 27 | 27 | 27 | 27 |
BMI: body mass index, EASI: Eczema Area and Severity Index, VAS: visual analogue scale, EOS: eosinophil count, IgE: immunoglobulin E. *p<0.05.
Table 3 shows the correlation between BMI, EASI, IgE, and eosinophil count with leptin and adiponectin. A statistically significant negative correlation was found between BMI and adiponectin (r=−0.373, p=0.018). IgE and leptin were positively correlated (r=0.393, p=0.043), but there was no statistical significance after control of BMI. There was no significant correlation between the other values. Overall, no significant correlations were observed after controlling for BMI.
Table 3. Relationship between leptin or adiponectin and severity scoring of atopic dermatitis (EASI), total IgE, EOS, and BMI.
| Calculated statistical value | BMI | EASI | IgE | EOS | EASI† | IgE† | EOS† |
|---|---|---|---|---|---|---|---|
| Leptin | |||||||
| Pearson's coefficient of correlation | 0.003 | 0.294 | 0.393 | 0.198 | 0.338 | 0.370 | 0.268 |
| p-value | 0.987 | 0.066 | 0.043* | 0.234 | 0.091 | 0.063 | 0.185 |
| Number | 40 | 40 | 27 | 38 | 40 | 27 | 38 |
| Adiponectin | |||||||
| Pearson's coefficient of correlation | −0.373* | −0.041 | −0.226 | −0.038 | −0.069 | −0.157 | 0.054 |
| p-value | 0.018 | 0.801 | 0.257 | 0.822 | 0.737 | 0.444 | 0.793 |
| Number | 40 | 40 | 27 | 38 | 40 | 27 | 38 |
EASI: Eczema Area and Severity Index, IgE: immunoglobulin E, EOS: eosinophil count, BMI: body mass index. *p<0.05. †Adjusted by BMI.
Serum adipokine level according to AD onset and extrinsic or intrinsic AD
Table 4 shows the serum leptin or adiponectin level according to the onset of disease and whether the AD was extrinsic or intrinsic. We divided the onset of disease into pre-adult onset (≤18 years old) and adult-onset (>18 years old). Extrinsic AD was defined as elevated total IgE among the patients who had a value of IgE. Patients with adult-onset AD had a significantly higher serum adiponectin level than those with pre-adult onset AD. There were no significant differences of the serum leptin or adiponectin level between extrinsic and intrinsic AD groups.
Table 4. Differences in serum level of leptin and adiponectin according to AD onset and extrinsic or intrinsic AD.
| Calculated statistical value | Pre-adult onset (n=28) | Adult-onset (n=12) | Extrinsic AD (n=18) | Intrinsic AD (n=4) |
|---|---|---|---|---|
| Leptin | ||||
| Mean±SD | 314.0±59.6 | 347.7±45.9 | 332.5±56.5 | 303.8±50.3 |
| p-value | 0.089 | 0.362 | ||
| Adiponectin | ||||
| Mean±SD | 253.2±67.0 | 306.2±64.4 | 284.6±60.6 | 297.2±84.1 |
| p-value | 0.026* | 0.729 | ||
The pre-adult-onset AD subgroup had onset of AD between birth and 18th year of life, and the adult-onset AD subgroup, including the 18th year of life and after. It was adjusted by BMI. AD: atopic dermatitis, SD: standard deviation, BMI: body mass index. *p<0.05.
DISCUSSION
In this study we reported that the weight reduction in obese AD patients reversed the AD symptoms. BMI and AD severity showed positive correlation. Moreover, in our study it was found that the serum levels of adiponectin and leptin were significantly different among healthy control, normal weight AD group, and obese weight AD group. A statistically significant negative correlation was found between BMI and adiponectin level. However, no associations were observed between serum adipokine levels and AD severity.
The majority of recent studies found that obesity is positively associated with the prevalence of AD. A systematic review and meta-analysis found that AD was related to higher risk of obesity in children and adults6,12. In Korean adulthood, Lee et al.13, found a positive correlation between obesity and AD in women, but not in men. Obesity increased the risk of AD in Korean children and adolescents14,15,16. However, several older studies did not find this relationship17,18,19. It is speculated that these results are inconsistent because of differences in the diagnostic criteria of AD, varying definitions of obesity, and regional differences in risk factors. Although most observational studies showed positive relationships between obesity and AD, there have been few studies regarding the impact of weight reduction intervention on the AD outcome. To confirm the effect of weight loss on the treatment of AD, we conducted a randomized controlled study. This study showed that weight reduction in obese AD patients decreased the severity of AD. All patients in the weight reduction group reduced their dosages of oral cyclosporine by the end of the study. This could be explained by the following hypothesis. The reductions might be a result of increased drug distribution in the body as a consequence of decreased body mass, or it could be affected by decreased proinflammatory cytokine release due to decrease in the number of adipocytes.
The underlying mechanisms relating obesity and AD remain poorly understood. Obesity is regarded as a state of low-grade systemic inflammation20. Adiponectin is a hormone produced by visceral and subcutaneous adipocytes and is secreted into the bloodstream. Associated with fat metabolism through adiponectin receptor types 1 and 2, adiponectin is a major factor in regulating insulin sensitivity. It has been reported that decrease in adiponectin is closely related with obesity, insulin sensitivity, and metabolic syndrome21. Adiponectin is also involved in anti-inflammatory actions by reducing the manifestation of inflammatory cytokines such as IL-6 and TNF-α, and by inducing the manifestation of anti-inflammatory cytokines such as IL-10 and IL-1 receptor antagonist22. It has been reported that decrease in adiponectin causes airway inflammation, resulting in the association of induced asthma, which is a type of allergic march23. Jung et al.24 demonstrated adiponectin-induced stimulation of lipid production in sebocytes. In AD, sebocyte proliferation and sebaceous gland activity are decreased, implying an association between decreased sebum production and skin barrier dysfunction25. A recent experimental study reported that adiponectin suppressed the expression of inflammatory mediators from keratinocytes and enhanced lipogenesis ability and epidermal differentiation in an AD-like reconstructed human epidermis model26.
Leptin is an adipokine widely used in research as a gauge of obesity. Contrary to the response of adiponectin, leptin has been known to escalate in obesity27. Leptin induces the activation of Th1 immunity and the secretion of inflammatory cytokines, such as TNF-α, IL-6, and IL-128,29. The positive correlation between levels of serum leptin and asthma was reported in recent meta-analysis30.
Data about the action of adiponectin and leptin in AD are rare. Nagel et al.31 reported that low adiponectin levels have been associated with increased prevalence of AD in children. They found no clear associations for circulating leptin concentration concerning AD prevalence, nor any correlation between serum leptin levels and AD symptoms. The result of Bostanci et al.32 was consistent with the study of Nagel et al31. Contrary to these studies, Kimata33 found that serum leptin level was elevated in AD children.
Another recent study found no significant differences in leptin and adiponectin levels according to AD severity34. Our study demonstrated results similar to those in previous studies. In our study, the serum adiponectin level was lower in AD patients compared to healthy controls, and the serum leptin level was higher in the AD group than in the controls. There were no associations between serum adiponectin and leptin levels and AD severity.
Seo et al.35 reported that serum leptin levels were higher in extrinsic AD than in intrinsic AD. Han et al.34 demonstrated that serum adiponectin levels in extrinsic AD were lower than in intrinsic AD. However, there were no significant differences of the serum leptin or adiponectin level between extrinsic and intrinsic AD groups in our study. In our previous study, obese patients with AD showed significantly higher EASI scores in the pre-adult-onset adult AD group, but not in the adult-onset adult AD group36. Based on previous study, we evaluated the differences of adipokine levels according to onset age. Interestingly, obese patients with pre-adult-onset AD had a significantly lower serum adiponectin level than those with adult-onset AD.
The blood eosinophil count and serum IgE level have been regarded as conventional biomarkers in AD. The leptin level was positively correlated with serum IgE, but there was no statistical significance after control of BMI. There was no significant correlation between serum adiponectin and the IgE or eosinophil count in this study. Based on the results of previous studies and our present study, we can assess the probability that increase in leptin and decrease in adiponectin are associated with the inflammatory reaction of AD. However, there might be other factors besides adiponectin and leptin involved in the mechanism of the beneficial effect of weight reduction for AD outcome.
There are some limitations in this study. First, the study was implemented using a small sample at a single health center. Second, we did not compare adipokine level with non-AD obesity individuals. Third, we could not check abdominal obesity, such as waist circumference, waist-to-height ratio, and waist-to-hip ratio. BMI cannot discriminate between muscle mass and body fat, nor can it reflect body fat distribution. Recently, abdominal obesity has come to be regarded as a key factor in at least some aspects of obesity-related health risks37,38. Despite the limitations mentioned above, to our knowledge, this is the first study to demonstrate that weight loss can improve the symptoms of AD.
In summary, we have found that weight reduction intervention had a positive AD outcome and that related adipokine levels were significantly different among the control, normal weight AD patient group, and obese AD patient group. These results suggest that weight loss could provide a safe and economical supplementary treatment for AD. Additional larger-scale studies are needed to confirm this.
ACKNOWLEDGMENT
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2018R1C1B6007998) and Hallym University Research Fund (HURF-2019-72).
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Nahm DH. Personalized immunomodulatory therapy for atopic dermatitis: an allergist's view. Ann Dermatol. 2015;27:355–363. doi: 10.5021/ad.2015.27.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251–1258.e23. doi: 10.1016/j.jaci.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109–1122. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- 4.Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sybilski AJ, Raciborski F, Lipiec A, Tomaszewska A, Lusawa A, Furmańczyk K, et al. Obesity--a risk factor for asthma, but not for atopic dermatitis, allergic rhinitis and sensitization. Public Health Nutr. 2015;18:530–536. doi: 10.1017/S1368980014000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali Z, Suppli Ulrik C, Agner T, Thomsen SF. Is atopic dermatitis associated with obesity? A systematic review of observational studies. J Eur Acad Dermatol Venereol. 2018;32:1246–1255. doi: 10.1111/jdv.14879. [DOI] [PubMed] [Google Scholar]
- 7.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Verbout NG, Benedito L, Williams AS, Kasahara DI, Wurmbrand AP, Si H, et al. Impact of adiponectin overexpression on allergic airways responses in mice. J Allergy (Cairo) 2013;2013:349520. doi: 10.1155/2013/349520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerdes S, Osadtschy S, Rostami-Yazdi M, Buhles N, Weichenthal M, Mrowietz U. Leptin, adiponectin, visfatin and retinol-binding protein-4-mediators of comorbidities in patients with psoriasis? Exp Dermatol. 2012;21:43–47. doi: 10.1111/j.1600-0625.2011.01402.x. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi H, Tsuji H, Takahashi I, Hashimoto Y, Ishida-Yamamoto A, Iizuka H. Plasma adiponectin and leptin levels in Japanese patients with psoriasis. Br J Dermatol. 2008;159:1207–1208. doi: 10.1111/j.1365-2133.2008.08823.x. [DOI] [PubMed] [Google Scholar]
- 11.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang A, Silverberg JI. Association of atopic dermatitis with being overweight and obese: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:606–616.e4. doi: 10.1016/j.jaad.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Han KD, Jung HM, Youn YH, Lee JY, Park YG, et al. Association between obesity, abdominal obesity, and adiposity and the prevalence of atopic dermatitis in young Korean adults: the Korea National Health and Nutrition Examination Survey 2008-2010. Allergy Asthma Immunol Res. 2016;8:107–114. doi: 10.4168/aair.2016.8.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim MS, Lee CH, Sim S, Hong SK, Choi HG. Physical activity, sedentary habits, sleep, and obesity are associated with asthma, allergic rhinitis, and atopic dermatitis in Korean adolescents. Yonsei Med J. 2017;58:1040–1046. doi: 10.3349/ymj.2017.58.5.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KS, Rha YH, Oh IH, Choi YS, Choi SH. Socioeconomic and sociodemographic factors related to allergic diseases in Korean adolescents based on the Seventh Korea Youth Risk Behavior Web-based Survey: a cross-sectional study. BMC Pediatr. 2016;16:19. doi: 10.1186/s12887-016-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SI, Shin MH, Lee HB, Lee JS, Son BK, Koh YY, et al. Prevalences of symptoms of asthma and other allergic diseases in Korean children: a nationwide questionnaire survey. J Korean Med Sci. 2001;16:155–164. doi: 10.3346/jkms.2001.16.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HY, Pizzichini MM, Becker AB, Duncan JM, Ferguson AC, Greene JM, et al. Disparate geographic prevalences of asthma, allergic rhinoconjunctivitis and atopic eczema among adolescents in five Canadian cities. Pediatr Allergy Immunol. 2010;21:867–877. doi: 10.1111/j.1399-3038.2010.01064.x. [DOI] [PubMed] [Google Scholar]
- 18.Van Gysel D, Govaere E, Verhamme K, Doli E, De Baets F. Body mass index in Belgian schoolchildren and its relationship with sensitization and allergic symptoms. Pediatr Allergy Immunol. 2009;20:246–253. doi: 10.1111/j.1399-3038.2008.00774.x. [DOI] [PubMed] [Google Scholar]
- 19.Silva MJ, Ribeiro MC, Carvalho F, Gonçalves Oliveira JM. Atopic disease and body mass index. Allergol Immunopathol (Madr) 2007;35:130–135. doi: 10.1157/13108223. [DOI] [PubMed] [Google Scholar]
- 20.Ali Z, Ulrik CS. Obesity and asthma: a coincidence or a causal relationship? A systematic review. Respir Med. 2013;107:1287–1300. doi: 10.1016/j.rmed.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Kadowaki T, Yamauchi T, Okada-Iwabu M, Iwabu M. Adiponectin and its receptors: implications for obesity-associated diseases and longevity. Lancet Diabetes Endocrinol. 2014;2:8–9. doi: 10.1016/S2213-8587(13)70120-7. [DOI] [PubMed] [Google Scholar]
- 22.Lago F, Dieguez C, Gómez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007;18:313–325. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118:389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Jung YR, Lee JH, Sohn KC, Lee Y, Seo YJ, Kim CD, et al. Adiponectin signaling regulates lipid production in human sebocytes. PLoS One. 2017;12:e0169824. doi: 10.1371/journal.pone.0169824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Firooz A, Gorouhi F, Davari P, Atarod M, Hekmat S, Rashighi-Firoozabadi M, et al. Comparison of hydration, sebum and pH values in clinically normal skin of patients with atopic dermatitis and healthy controls. Clin Exp Dermatol. 2007;32:321–322. doi: 10.1111/j.1365-2230.2007.02364.x. [DOI] [PubMed] [Google Scholar]
- 26.Seo HS, Seong KH, Kim CD, Seo SJ, Park BC, Kim MH, at al. Adiponectin attenuates the inflammation in atopic dermatitis-like reconstructed human epidermis. Ann Dermatol. 2019;31:186–195. doi: 10.5021/ad.2019.31.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 28.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 29.Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Yin Y, Zhang H, Zhong W, Zhang J. Association of asthma diagnosis with leptin and adiponectin: a systematic review and meta-analysis. J Investig Med. 2017;65:57–64. doi: 10.1136/jim-2016-000127. [DOI] [PubMed] [Google Scholar]
- 31.Nagel G, Koenig W, Rapp K, Wabitsch M, Zoellner I, Weiland SK. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol. 2009;20:81–88. doi: 10.1111/j.1399-3038.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 32.Bostanci I, Atli O, Celebi N, Taşar A, Alpkarakoç E, Dallar Y. Serum leptin level in children with atopic dermatitis-treated topical steroids. Pediatr Allergy Immunol. 2004;15:267–269. doi: 10.1111/j.1399-3038.2004.00145.x. [DOI] [PubMed] [Google Scholar]
- 33.Kimata H. Elevated serum leptin in AEDS. Allergy. 2002;57:179. doi: 10.1034/j.1398-9995.2002.1n3549.x. [DOI] [PubMed] [Google Scholar]
- 34.Han B, Wu WH, Bae JM, Son SJ, Lee JH, Han TY. Serum leptin and adiponectin levels in atopic dermatitis (AD) and their relation to disease severity. J Am Acad Dermatol. 2016;75:629–631. doi: 10.1016/j.jaad.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 35.Seo S, Yoon WS, Cho Y, Park SH, Choung JT, Yoo Y. Leptin and atopic dermatitis in Korean elementary school children. Iran J Allergy Asthma Immunol. 2016;15:138–144. [PubMed] [Google Scholar]
- 36.Son JH, Chung BY, Kim HO, Park CW. Clinical features of atopic dermatitis in adults are different according to onset. J Korean Med Sci. 2017;32:1360–1366. doi: 10.3346/jkms.2017.32.8.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics. 1997;99:804–807. doi: 10.1542/peds.99.6.804. [DOI] [PubMed] [Google Scholar]
- 38.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13:275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]