Abstract
Background
Despite the autologous serum skin test (ASST) and autologous plasma skin test (APST) is widely used test accessing whether a patient with chronic spontaneous urticaria (CSU) has autoreactivity or not, the clinician often encounter difficulty making correlation between the test result and clinical implications.
Objective
This study was aimed to find any clinical and laboratory findings related to the ASST and APST response. Agreement and correlation between the two tests was also analyzed.
Methods
A retrospective study was conducted on 300 CSU patients who underwent ASST, APST. The subjects were divided into four groups according to the skin test result. Also, the degree of serum and plasma response was recorded.
Results
Both ASST and APST positive group had shorter duration of the disease, higher incidence of at least one episode of angioedema than negative group. There were no significant differences in the positivity for autoantibodies including antinuclear, ds-DNA, and thyroid-related between the two groups. The predicted positive rate of ASST and APST according to age showed bimodal peak and decreasing pattern according to disease duration. Predicted positivity of both tests declined with increase in total immunoglobuline E (IgE) level. In the correlation study, the two tests showed high correlation coefficients.
Conclusion
ASST and APST positivity may be related to disease duration and severity of CSU. The two tests showed a generally consistent result. Autoreactivity may be gradually lost as disease continues. We suggest the autoreactivity in CSU could arise independently from IgE mediated immune process.
Keywords: Autologous plasma skin test, Autologous serum skin test, Chronic spontaneous urticaria, Chronic urticaria
INTRODUCTION
Chronic urticaria (CU) is a skin disease characterized by the spontaneous recurrence of transient wheals with or without angioedema lasting for more than 6 weeks1. Although the disease is usually not life-threatening, it may impair the quality of life as patients with CU might have unexpected attack of wheals with extreme itching that can last for many years.
Several investigators have suggested that CU could be divided into two subgroups, chronic spontaneous urticaria (CSU) and inducible urticaria2. There have been many advances in identifying the causes of CSU. Among them, autoimmunity is considered to be an important etiology of CSU. It is characterized by the presence of functional autoantibodies that activate mast cells and basophils. Well known functional antibodies include an immunoglobuline G (IgG) autoantibody specific for the α-subunit of the IgE receptor (FcεRI) and an anti IgE antibody3. These functional antibodies against FcεRI or IgE are believed to be present in about 30% to 50% and 5% to 10% of patients in CSU, respectively1. The “gold standard” study for detecting functional antibodies in the serum of a CSU patient is basophil histamine releasing assay (BHRA). However, in the clinical setting, autologous serum skin test (ASST) or autologous plasma skin test (APST) is more commonly used to demonstrate circulating endogenous pro-inflammatory or wheal inducing factors in urticarial patients.
Although ASST and APST are simple and easy in vivo test for accessing the ‘autoreactivity’ of urticaria, a positive result in the tests may not always indicate that the patient has autoimmunity. The clinician may be confused in interpreting and adopting the test result in the clinical field. Therefore, it would be important if there is any difference in ASST or APST result in CSU patients. Thus, the objective of this study was to compare clinical and laboratory characteristics between ASST/APST positive (ASST+/APST+) and ASST/APST negative (ASST−/APST−) CSU subjects. Relationships between variables and degree of serum response in CSU patients were also accessed. At last, we analyzed the correlation between the two test results.
MATERIALS AND METHODS
Enrollment of patients
In this retrospective study, we examined clinical and laboratory findings of CSU patients who underwent both ASST and APST at the Department of Dermatology and the Allergy Clinic in the Department of Pulmonology in Hallym University Dongtan Sacred Heart Hospital, Korea. CU was diagnosed as daily appearance of wheals and associated itching sensation lasting for at least 6 weeks. We excluded patients with clinical evidence of urticarial vasculitis and inducible urticaria such as dermographism, cholinergic urticaria and cold/heat urticaria. This study was approved by the Institutional Review Board of Hallym University Dongtan Sacred Heart Hospital (IRB File No. 2017-11-001).
Clinical and laboratory data collection
We reviewed subjects' clinical characteristics including age, sex, duration of the CU, accompanying diseases, and treatment level, which was modified from guidelines for the treatment of CU2: Level 1, well controlled to one or two second-generation antihistamine(s); level 2, well controlled to increased dose (up to 4 times) of antihistamines or three or more second-generation antihistamines; level 3, well controlled to addition of omalizumab; level 4, patients whose urticaria was not adequately controlled with level 3 treatment and added cyclosporine. Laboratory data including CBC, routine chemistry, serum total IgE (normal <100 IU/ml), antinuclear antibody, anti-ds DNA antibody and antithyoid antibodies including anti thyroid peroxidase (anti-TPO) antibodies (normal <60 IU/ml), antithyroglobulin (anti-TG) antibodies (normal <60 IU/ml), and anti-thyroid stimulating hormone receptor (anti-TSH-R) antibodies (normal <1.75 IU/L) were reviewed.
Application of autologous serum and plasma skin test
ASST and APST were applied at least 5 cm apart onto the flexor surface of the forearm of patient. Approximately 0.05 ml of autologous serum and heparin anticoagulated plasma of a patient was injected into the dermis using a 1 ml insulin syringe. Additionally, 0.05 ml of histamine diphosphate (10 µg/ml) and 0.9% saline were injected as a positive and negative control, respectively. The result was accessed at 30 minutes after injection. When the mean maximum perpendicular diameter of the induration formed by autologous serum and plasma was at least 2 mm larger than the saline injection area, the response was accepted as a positive one4. In addition to judgement, we recorded differences in mean maximum perpendicular diameters of the induration of serum and saline injection sites. Degrees of serum response were recorded at 1 mm intervals. Reactions greater than 5 mm were included in the more than 5 mm group.
Statistical analysis
The study population was classified into four groups according to the results of ASST and APST. Clinical and laboratory characteristics of the each group were summarized as means, standard deviations, and ranges for continuous variables, and proportions and percentages for categorical variables. Comparisons between the four groups were performed using Wilcoxon rank sum test for continuous variables, and Fisher's exact test for categorical variables. The positive rates of ASST and APST were compared with McNemar test, and the overall agreement between the two tests was assessed using Cohen kappa statistic. Univariable analyses for the associations between the skin test results and each clinical or laboratory characteristic were conducted using simple logistic regression or penalized logistic regression. For continuous variables, generalized additive models were also fit to detect the nonlinear relationships between the positive rates of the skin tests and patient characteristics. Because of skewed distributions, Yeo-Johnson transformations were applied to the continuous variables in the generalized additive models. Multivariable analysis was performed using multiple logistic regression which includes independent variables that were statistically significant in the univariable analysis for ASST or APST. The correlation in diameters of the induration between ASST and APST was evaluated using scatter plot and Pearson correlation coefficient, and the agreement between them was assessed using concordance correlation coefficient. A p-value less than 0.05 was considered statistically significant. All statistical analyses were performed using R version 3.6.2 (the R foundation for statistical computing, Vienna, Austria).
RESULTS
Characteristics of study subjects and the test results
Among a total of 300 subjects, the average age was 35.5±12.66. A total of 187 subjects were ASST negative while 113 were ASST positive. A total of 232 subjects were APST negative while 68 were APST positive. Positive rate of ASST (37.7%) was significantly higher than that of APST (22.7%) (p<0.001). However, the test agreement between the two tests was moderate, with 0.47 on the Cohen kappa coefficient (95% confidence interval, 0.37 to 0.57). Patients' characteristics and test results are summarized in Table 1.
Table 1. Comparison of study variables with positive and negative autologous serum and positive test groups.
| Study variable | ASST−/APST− | ASST−/APST+ | ASST+/APST− | ASST+/ASST+ | p-value |
|---|---|---|---|---|---|
| Patients counts (n=300) | 175 | 12 | 57 | 56 | |
| Age | 35.97±12.12 | 38.08±12.18 | 34.11±12.87 | 35.29±14.28 | 0.697 |
| Sex | 0.230 | ||||
| Male | 81 (46.3) | 2 (16.7) | 26 (45.6) | 28 (50.0) | |
| Female | 94 (53.7) | 10 (83.3) | 31 (54.4) | 28 (50.0) | |
| Duration (wk) | 95.95±175.74 | 70.08±147.37 | 56.30±101.89 | 45.86±117.01 | 0.004* |
| Episode(s) of angioedema | 11 (6.3) | 2 (16.7) | 11 (19.3) | 11 (19.6) | 0.005* |
| History of atopy | 20 (11.4) | 3 (25.0) | 9 (15.8) | 5 (8.9) | 0.336 |
| Treatment level | 0.432 | ||||
| 1 | 142 (84.0) | 11 (91.7) | 50 (89.3) | 51 (92.7) | |
| 2 | 21 (12.4) | 1 (8.3) | 4 (7.1) | 2 (3.6) | |
| 3 | 3 (1.8) | 0 (0.0) | 2 (3.6) | 0 (0.0) | |
| 4 | 3 (1.8) | 0 (0.0) | 0 (0.0) | 2 (3.6) | |
| C3 (mg/dl) | 111.37±18.88 | 103.37±13.58 | 104.64±20.42 | 108.41±22.22 | 0.497 |
| C4 (mg/dl) | 26.99±7.38 | 29.66±9.60 | 26.13±5.89 | 24.93±9.18 | 0.439 |
| Total IgE (IU/ml) | 262.60±368.57 | 163.50±140.86 | 225.72±201.72 | 173.23±116.62 | 0.598 |
| Eosinophil count (%) | 2.73±1.83 | 2.85±1.51 | 2.67±1.75 | 2.29±1.46 | 0.375 |
| Platelet count (109/L) | 253.96±54.03 | 234.00±50.58 | 259.43±51.44 | 257.63±65.84 | 0.313 |
| Thyroid function test | |||||
| TSH (mU/L) | 2.04±1.74 | 1.63±1.14 | 2.24±1.94 | 4.91±16.36 | 0.460 |
| T3 (ng/dl) | 111.65±29.99 | 107.00±14.70 | 113.28±19.46 | 108.60±21.71 | 0.445 |
| Free T4 (ng/dl) | 1.28±0.35 | 1.28±0.19 | 1.27±0.18 | 1.27±0.27 | 0.812 |
| Thyroid antibodies | |||||
| Anti-TSH-R (U/L) | 0.62±1.17 | 0.70±0.38 | 0.43±0.25 | 0.48±0.27 | 0.079 |
| Anti-TPO (IU/ml) | 120.98±509.32 | 71.69±173.70 | 145.30±371.63 | 157.60±498.87 | 0.059 |
| Anti-TG (IU/ml) | 71.07±279.95 | 29.68±39.15 | 32.88±45.84 | 71.67±186.24 | 0.593 |
Values are presented as number only, number (%), or mean±standard deviation. APST: autologous plasma skin test, ASST: autologous serum skin test, −: negative, +: positive, TG: thyroglobulin, TPO: thyroid peroxidase, TSH: thyroid stimulating hormone, TSH-R: thyroid stimulating hormone receptor, IgE: immunoglobuline E. *p-value of <0.05.
Association of ASST/APST response with clinical and laboratory parameters in chronic spontaneous urticaria
There were no significant differences in age or sex between test positive and negative groups. However, as shown in the Table 1, the duration of the disease was longer in the both ASST− and APST− group (94.29±173.83 and 86.21±161.47 weeks) than in the ASST+ and APST+ group (51.12±109.27 and 50.13±122.03 weeks) (p<0.001). There were no significant differences between the two groups in treatment levels in both tests. Regarding the severity of the disease, 19.5% of subjects in the ASST+ group had at least one episode of angioedema during CU while only 7.0% of the subjects in the ASST− group experienced at least one episode, showing significant difference between the two groups (p<0.001). In the APST, the same analysis yielded 19.1% and 9.5%, respectively (p=0.05). However, there were no significant differences in the incidence of atopic dermatitis, allergic asthma, or allergic rhinitis between the two groups in both tests.
Laboratory results showed no significant differences between the two groups in both tests. Also, there were no significant differences in the positivity for anti-thyroid (TSH-R, TPO, and TG) antibodies, antinuclear, or anti-ds DNA antibodies.
Univariable and multivariable analysis of study variables with ASST/APST positivity
In our logistic regression analysis, disease duration and episode of angioedema showed significant correlation with ASST positivity and only disease duration with APST (Table 2). We also performed multiple logistic regression analysis which included age, disease duration, and angioedema that were statistically significant in the univariable analysis and generalized additive model analysis (Table 3). In the ASST, age group of less than 30 year old and 45 or more had significantly higher odd ratio compared to a group of 30 to less than 45. In the disease duration, a group of less than 8 weeks had significantly higher odd ratio than other duration groups.
Table 2. Univariable analysis for the association between autologous serum and plasma skin test results and clinical and laboratory characteristics in chronic spontaneous urticaria.
| Variable | Category | ASST | APST | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age | 0.99 (0.97~1.01) | 0.349 | 1.00 (0.98~1.02) | 0.877 | |
| Sex | 0.567 | 0.771 | |||
| Male | Reference | Reference | |||
| Female | 0.87 (0.55~1.39) | 0.567 | 1.08 (0.63~1.88) | 0.771 | |
| Eosinophil count | 0.91 (0.79~1.05) | 0.225 | 0.88 (0.73~1.05) | 0.178 | |
| TSH level | 1.07 (0.99~1.26) | 0.383 | 1.05 (0.99~1.22) | 0.313 | |
| T3 level | 1.00 (0.99~1.01) | 0.899 | 0.99 (0.98~1.01) | 0.442 | |
| fT4 level | 0.93 (0.31~2.37) | 0.875 | 0.93 (0.24~2.64) | 0.907 | |
| ANA positivity | 0.142 | 0.848 | |||
| 0 | Reference | Reference | |||
| 1 | 0.37 (0.14~0.87) | 0.022 | 0.65 (0.22~1.62) | 0.376 | |
| 2 | 0.78 (0.13~3.60) | 0.754 | 0.83 (0.08~4.27) | 0.837 | |
| 3 | 0.47 (0.00~8.88) | 0.625 | 1.01 (0.01~19.26) | 0.994 | |
| C3 level | 0.99 (0.97~1.01) | 0.273 | 0.99 (0.97~1.01) | 0.471 | |
| C4 level | 0.97 (0.92~1.01) | 0.173 | 0.99 (0.94~1.04) | 0.639 | |
| Treatment level | 0.330 | 0.213 | |||
| 1 | Reference | Reference | |||
| 2 | 0.41 (0.15~1.00) | 0.064 | 0.42 (0.11~1.19) | 0.109 | |
| 3 | 1.01 (0.13~6.20) | 0.991 | 0.28 (0.00~2.53) | 0.308 | |
| 4 | 1.01 (0.13~6.20) | 0.991 | 2.20 (0.36~11.57) | 0.363 | |
| Angioedema | 0.002 | 0.033 | |||
| Absent | Reference | Reference | |||
| Present | 3.24 (1.58~6.89) | 0.002 | 2.26 (1.05~4.71) | 0.033 | |
| History of atopy | 0.911 | 0.799 | |||
| Absent | Reference | Reference | |||
| Present | 0.96 (0.46~1.92) | 0.911 | 0.90 (0.37~1.98) | 0.799 | |
| High TPO | 0.470 | 0.754 | |||
| Absent | Reference | Reference | |||
| Present | 1.36 (0.58~3.08) | 0.470 | 1.17 (0.41~2.92) | 0.754 | |
| High TG | 0.269 | 0.212 | |||
| Absent | Reference | Reference | |||
| Present | 0.63 (0.27~1.38) | 0.269 | 0.50 (0.14~1.34) | 0.212 | |
| High TSH-R | 0.129 | 0.413 | |||
| Absent | Reference | Reference | |||
| Present | 0.17 (0.00~1.51) | 0.129 | 0.35 (0.00~3.15) | 0.413 | |
| Platelet count | 1.21 (0.79~1.85) | 0.389 | 0.95 (0.57~1.55) | 0.829 | |
| Total IgE level | 0.93 (0.82~1.02) | 0.147 | 0.84 (0.70~0.98) | 0.057 | |
| Disease duration | 0.79 (0.63~0.95) | 0.025 | 0.81 (0.61~1.01) | 0.099 | |
APST: autologous plasma skin test, ASST: autologous serum skin test, ANA: antinuclear antibody, OR: odd ratio, CI: confidence interval, TG: thyroglobulin, TPO: thyroid peroxidase, TSH: thyroid stimulating hormone, TSH-R: thyroid stimulating hormone receptor, IgE: immunoglobuline E.
Table 3. Multivariable analysis for the association between autologous serum and plasma skin test result and age, disease duration, angioedema in chronic spontaneous urticaria.
| Variable | Category | ASST | APST | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age (yr) | <0.001 | 0.421 | |||
| 30 to <45 | Reference | Reference | |||
| <30 | 3.00 (1.69~5.39) | <0.001 | 1.45 (0.76~2.78) | 0.259 | |
| ≥45 | 2.49 (1.32~4.72) | 0.005 | 1.48 (0.71~3.01) | 0.286 | |
| Duration (wk) | 0.038 | 0.031 | |||
| <8 | Reference | Reference | |||
| 8 to <14 | 0.65 (0.33~1.29) | 0.220 | 1.00 (0.49~2.06) | 0.998 | |
| 14 to <52 | 0.59 (0.28~1.23) | 0.159 | 0.68 (0.30~1.51) | 0.346 | |
| ≥52 | 0.35 (0.17~0.71) | 0.004 | 0.32 (0.13~0.74) | 0.009 | |
| Angioedema | 0.013 | 0.107 | |||
| Absent | Reference | Reference | |||
| Present | 2.63 (1.23~5.78) | 0.013 | 1.88 (0.85~4.02) | 0.107 | |
APST: autologous plasma skin test, ASST: autologous serum skin test, OR: odd ratio, CI: confidence interval. Age groups were divided into three groups based on 30 and 45 years old. Duration group were divided into four groups based on 8, 14, 52 weeks.
Predicted positivity of ASST/APST and study variables
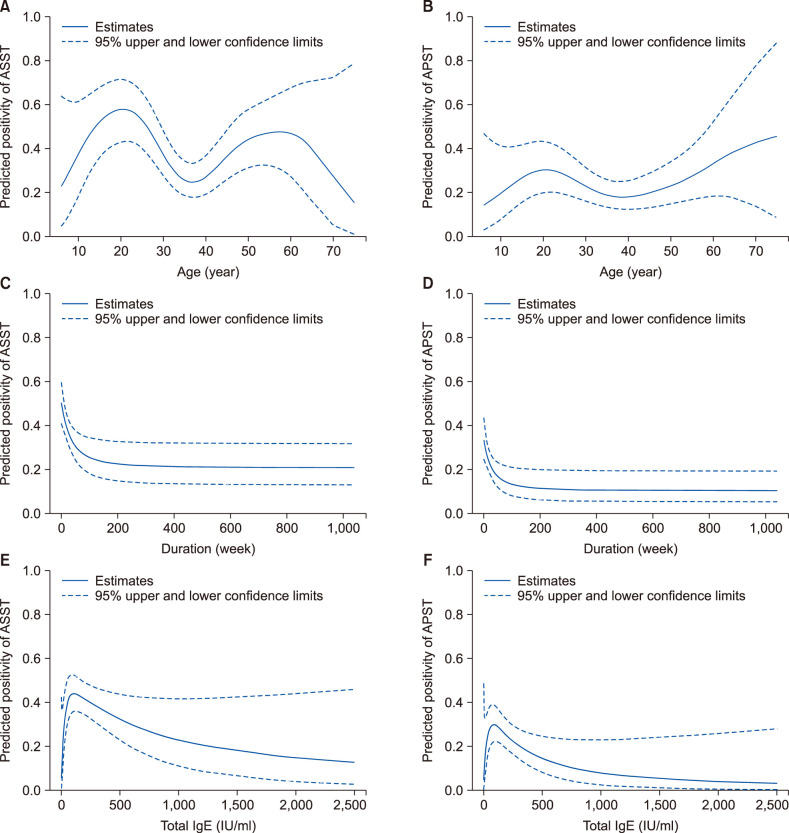
The predicted positive rate of ASST and APST according to age showed a bimodal distribution (Fig. 1A, B). The predicted positive rate of ASST and APST showed a decreasing pattern according to duration of the disease, in particular, during the first year of the disease (Fig. 1C, D). The predicted positive rate of ASST and APST declined with an increase in the total IgE level (Fig. 1E, F).
Fig. 1. Predicted positivity of ASST and APST by study variables by using generalized additive model. (A, B) By age (C, D) by disease duration (E, F) by total IgE level. APST: autologous plasma skin test, ASST: autologous serum skin test, IgE: immunoglobuline E.
Correlation and agreement analysis of ASST and APST
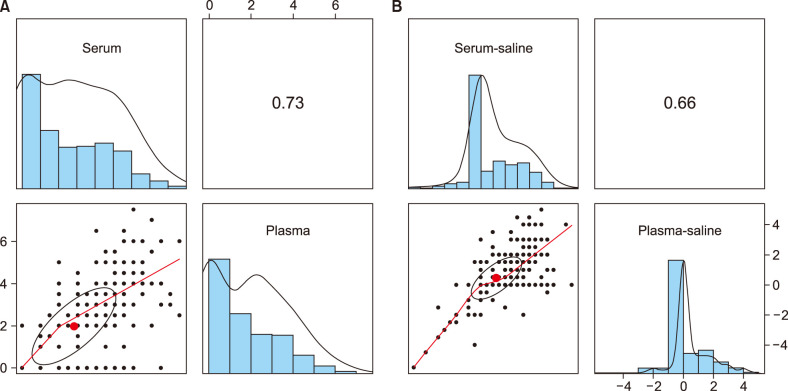
In the correlation analysis, coefficient value of recorded diameter (mean maximum perpendicular) of serum and plasma in the skin test was 0.73, which is accepted to have strong correlation (Fig. 2A). Also coefficient value of diameter difference between serum and saline (serum–saline) and between plasma and saline (plasma–saline) was 0.66 (Fig. 2B).
Fig. 2. Scatter plot of (A) diameters of the induration of serum (horizontal axis) and plasma (vertical axis) injection sites (B) differences in diameters of the induration between serum and saline injection sites (horizontal axis) and those between plasma and saline injection sites (vertical axis).
Concordance correlation coefficients of diameter of serum and plasma in the study subjects are summarized in Table 4.
Table 4. Concordance correlation coefficients of recorded diameter of serum, plasma, and saline in the study subjects.
| Variable | Serum | Plasma | Saline | Serum–saline |
|---|---|---|---|---|
| Plasma | 0.66 (0.58~0.73) | |||
| Saline | 0.32 (0.24~0.40) | 0.59 (0.50~0.67) | ||
| Serum–Saline | 0.64 (0.59~0.69) | 0.36 (0.26~0.45) | −0.16 (−0.29~−0.02) | |
| Plasma–Saline | 0.25 (0.20~0.31) | 0.45 (0.38~0.51) | −0.13 (−0.25~0.00) | 0.55 (0.44~0.65) |
Values are presented as odd ratio (95% confidence interval).
DISCUSSION
From a pathophysiological point of view, there have been many efforts to find the cause of CSU. Significant progress has made, although the exact mechanism of CSU is still unclear. Activation and degranulation of mast cells may occur by immunological, non-immunological, or mixed mechanisms. Autoimmune reaction and synthesis of autoantibodies against IgE and/or the α-subunit of FcεRI on mast cells or basophils are known to account for about 30% to 50% in the causes of CSU development5,6.
The ASST and APST is a simple screening method to see whether a patient's CU has an autoreactive nature. A positive test result suggests that there are functional autoantibodies or histamine releasing factors in the serum7. Functional autoantibodies need to be confirmed by BHRA. Although these autologous tests has limited positive predicted value for a positive BHRA, the low cost and simplicity make ASST and APST a practical screening tool in actual clinical practice4. Autoimmunity and autoreactivity are not synonymous. However, one can estimate that there exists autoimmunity stimulates mast cells based on the autoreactivity shown by these skin tests. In contrast, some authors have questioned the usefulness of the tests because various results can be obtained depending on the researcher.
Studies of clinical characteristics of test positive group and test negative group have been ongoing as an interesting topic. However, to the best of our knowledge, this is the first study to not only compare ASST/APST responders and non-responders, but also to perform subgroup analysis of predicted positivity for the subjects based on the measured degree of skin response. In most studies, there was no significant relationship between test response and gender, age, personal history or family history of atopy8,9,10. Some studies have reported that the ASST or APST positive group shows more severe disease activity such as more wheals, higher itch scores, more systemic symptoms, greater frequency and requiring significantly more antihistamines3,11,12.
In our study, we found no association between skin test results and gender, age, history or presence of atopic diseases such as atopic dermatitis, allergic asthma, or allergic rhinitis. In this study, we not only compared clinical characteristics between skin test+ group and skin test− group but elucidated the relationship between the degree of serum response and the variables, revealed no significant findings.
Regarding disease activity or severity and ASST/APST response, similar to one previous study13, we found that the test positive group had significantly more episodes of angioedema than the test negative group. In univariable and multivariable regression analysis, both assays clearly showed a high risk of angioedema with ASST and APST positivity. Therefore, patients with positive ASST or APST result should be warned more about the possibility of angioedema. We also compared disease activity by treatment level, revealing no significant difference between the two groups in both tests. Additional studies that include the urticaria activity score (UAS) as disease severity may provide a clearer explanation of this result.
In the study, the ASST+ group had significantly shorter disease duration compared to the ASST− group. Such significant results were similarly derived from regression analysis. However, the same analysis did not produce significant results with APST. There are several studies comparing disease duration and ASST positivity, and the results are heterogeneous9,14. One meta-analysis indicated that the two groups displayed no statistically significant differences in duration15.
Interestingly, the predicted positivity of both tests showed a decreasing pattern according to the duration of disease, especially, during the first year of the disease. Few studies have longitudinally observed ASST or APST positivity in CSU patients12. This result may need further consideration. Nevertheless, based on our finding, it can be assumed that ‘autoreactivity’ may be gradually lost which might be due to decreased ability to stimulate mast cells or reduced autoantibodies as disease continues for longer period.
Regarding the relationship between the presence of autoantibodies against thyroid antigens, (TSH, TPO, and thyroglobulin) and response to ASST or APST, results have been controversial8,9. One recent meta-analysis study has shown that a positive ASST response is more likely to be accompanied by the presence of thyroid autoantibodies as well as angioedema15. In contrast, we did not reveal any significant difference in the frequency of thyroid autoantibodies or antinuclear antibody between the skin test+ group and the skin test− group. Therefore, in addition to thyroid autoantigens, there might be unknown different types of autoantibodies that can stimulate mast cells via receptors on the surface.
In the study, we calculated the predicted positivity of ASST and APST according to variables using a generalized additive model. Interestingly, bimodal peak was shown for those in the twenties and sixties of age. This pattern is usually seen in a combination of two heterogeneous groups. Therefore there might be different types of affecting autoantibodies or histamine releasing factors by age. Since it has not been tightly characterized, finding autoantibodies that vary with age may also be an important factor for consideration for future biological treatment of CSU. Another variable affecting the predicted positivity of the test was total IgE level. As total IgE increased, the predicted positive rate of ASST and APST significantly decreased. Based on these findings, it can be assumed that at least the autoreactivity seen in the CSU can act independently of IgE. Similar to several previous studies, recent multinational, multicenter study of CSU patients revealed that autoimmune chronic spontaneous urticaria patients had markedly lower togal IgE levels16. To support such hypothesis, one can estimate autoantibodies specific for the receptor on the mast cell or IgE itself may block further production of IgE by unknown pathway. Also, there have been several studies in which highlighted the link of low IgE levels and lower rates of response to anti-IgE treatment with omalizumab in CSU patients17,18. Taken together, the results of these studies and our study suggest that ASST/APST positivity may be a factor in reducing the responsiveness of anti-IgE treatment. There will be an opportunity to confirm our hypothesis in the anti-IgE treatment and control group studies. In the study, we analyzed how the two tests, ASST and APST, matched or showed different results in this study. The hypothesis that coagulation pathways will play a part in the pathogenesis of chronic urticaria has long been suggested19. Asero et al.20 hypothesized that coagulation cascade was involved in the wheal and flare reaction, induced by autologous plasma. Considering this, APST should be more sensitive than ASST, but the results differ depending on the researchers21. Although the positive rates of the two tests showed statistically significant differences, comparison of the diameters demonstrated that the two tests were sufficiently correlated. Meanwhile concordance correlation analysis of diameters showed borderline result of 0.66, interpretation of this result may require further controversy.
This study has some limitations. First, UAS was not evaluated as a disease activity. Second, a comparison of ASST results before and after treatment for CSU was not performed. Such study is needed in the future. Third, since gold standard test such as BHRA were not performed in this retrospective study, the superiority of ASST and APST could not be determined.
In conclusion, results of the present study indicated that CSU with positive ASST or APST response had shorter duration of disease and more severe clinical feature such as angioedema. Regression analysis especially showed a high risk of angioedema with ASST and APST positivity. We could not find any association of laboratory parameters with both skin tests positivity. The predicted positivity of ASST and APST decreased with increasing of serum total IgE level and disease duration, while showed a bimodal distribution in which peaks was shown for twenties and sixties of age. Although positive rate of ASST was significantly higher than that of APST, ASST and APST showed strong correlation regardless of sensitivity.
ACKNOWLEDGMENTS
This research was supported by Hallym University Research Fund.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270–1277. doi: 10.1016/j.jaci.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 2.Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA²LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 3.Sabroe RA, Seed PT, Francis DM, Barr RM, Black AK, Greaves MW. Chronic idiopathic urticaria: comparison of the clinical features of patients with and without anti-FcepsilonRI or anti-IgE autoantibodies. J Am Acad Dermatol. 1999;40:443–450. doi: 10.1016/s0190-9622(99)70495-0. [DOI] [PubMed] [Google Scholar]
- 4.Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P, Grattan CE. EAACI/GA(2)LEN task force consensus report: the autologous serum skin test in urticaria. Allergy. 2009;64:1256–1268. doi: 10.1111/j.1398-9995.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 5.Grattan CE, Sabroe RA, Greaves MW. Chronic urticaria. J Am Acad Dermatol. 2002;46:645–657. doi: 10.1067/mjd.2002.122759. quiz 657-660. [DOI] [PubMed] [Google Scholar]
- 6.Gruber BL, Baeza ML, Marchese MJ, Agnello V, Kaplan AP. Prevalence and functional role of anti-IgE autoantibodies in urticarial syndromes. J Invest Dermatol. 1988;90:213–217. doi: 10.1111/1523-1747.ep12462239. [DOI] [PubMed] [Google Scholar]
- 7.Sabroe RA, Greaves MW. Chronic idiopathic urticaria with functional autoantibodies: 12 years on. Br J Dermatol. 2006;154:813–819. doi: 10.1111/j.1365-2133.2006.07183.x. [DOI] [PubMed] [Google Scholar]
- 8.Kumar YH, Bhaskar S, Shankar K. Comparative study of positive versus negative autologous serum skin test in chronic spontaneous urticaria and its treatment outcome. N Am J Med Sci. 2016;8:25–30. doi: 10.4103/1947-2714.175195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boonpiyathad T, Sangasapaviliya A. Autologous serum and plasma skin test to predict 2-year outcome in chronic spontaneous urticaria. Asia Pac Allergy. 2016;6:226–235. doi: 10.5415/apallergy.2016.6.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Hamamy HR, Hameed AF, Abdulhadi AS. Autologous serum skin test as a diagnostic aid in chronic idiopathic urticaria. ISRN Dermatol. 2013;2013:291524. doi: 10.1155/2013/291524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staubach P, Onnen K, Vonend A, Metz M, Siebenhaar F, Tschentscher I, et al. Autologous whole blood injections to patients with chronic urticaria and a positive autologous serum skin test: a placebo-controlled trial. Dermatology. 2006;212:150–159. doi: 10.1159/000090656. [DOI] [PubMed] [Google Scholar]
- 12.Caproni M, Volpi W, Giomi B, Cardinali C, Antiga E, Melani L, et al. Chronic idiopathic and chronic autoimmune urticaria: clinical and immunopathological features of 68 subjects. Acta Derm Venereol. 2004;84:288–290. doi: 10.1080/00015550410026939. [DOI] [PubMed] [Google Scholar]
- 13.Nettis E, Dambra P, D'Oronzio L, Cavallo E, Loria MP, Fanelli M, et al. Reactivity to autologous serum skin test and clinical features in chronic idiopathic urticaria. Clin Exp Dermatol. 2002;27:29–31. doi: 10.1046/j.0307-6938.2001.00962.x. [DOI] [PubMed] [Google Scholar]
- 14.Ye YM, Park JW, Kim SH, Ban GY, Kim JH, Shin YS, et al. Prognostic factors for chronic spontaneous urticaria: a 6-month prospective observational study. Allergy Asthma Immunol Res. 2016;8:115–123. doi: 10.4168/aair.2016.8.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niu XL, Zhu LL, Shi MH, Zhang YJ, Gao XH, Qi RQ. Association of positive and negative autologous serum skin test responses with clinical features of chronic spontaneous urticaria in Asian patients: a systematic review and meta-analysis. Exp Ther Med. 2019;17:2603–2613. doi: 10.3892/etm.2019.7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoepke N, Asero R, Ellrich A, Ferrer M, Gimenez-Arnau A, Grattan CEH, et al. Biomarkers and clinical characteristics of autoimmune chronic spontaneous urticaria: results of the PURIST Study. Allergy. 2019;74:2427–2436. doi: 10.1111/all.13949. [DOI] [PubMed] [Google Scholar]
- 17.Weller K, Ohanyan T, Hawro T, Ellrich A, Sussman G, Koplowitz J, et al. Total IgE levels are linked to the response of chronic spontaneous urticaria patients to omalizumab. Allergy. 2018;73:2406–2408. doi: 10.1111/all.13586. [DOI] [PubMed] [Google Scholar]
- 18.Straesser MD, Oliver E, Palacios T, Kyin T, Patrie J, Borish L, et al. Serum IgE as an immunological marker to predict response to omalizumab treatment in symptomatic chronic urticaria. J Allergy Clin Immunol Pract. 2018;6:1386–1388.e1. doi: 10.1016/j.jaip.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tedeschi A, Kolkhir P, Asero R, Pogorelov D, Olisova O, Kochergin N, et al. Chronic urticaria and coagulation: pathophysiological and clinical aspects. Allergy. 2014;69:683–691. doi: 10.1111/all.12389. [DOI] [PubMed] [Google Scholar]
- 20.Asero R, Tedeschi A, Riboldi P, Cugno M. Plasma of patients with chronic urticaria shows signs of thrombin generation, and its intradermal injection causes wheal-and-flare reactions much more frequently than autologous serum. J Allergy Clin Immunol. 2006;117:1113–1117. doi: 10.1016/j.jaci.2005.12.1343. [DOI] [PubMed] [Google Scholar]
- 21.Kocatürk E, Kavala M, Kural E, Sarıgul S, Zındancı I. Autologous serum skin test vs autologous plasma skin test in patients with chronic urticaria: evaluation of reproducibility, sensitivity and specificity and relationship with disease activity, quality of life and anti-thyroid antibodies. Eur J Dermatol. 2011;21:339–343. doi: 10.1684/ejd.2011.1294. [DOI] [PubMed] [Google Scholar]