Abstract
Background
Skin diseases characterized by epithelial barrier dysfunction show altered sphingolipid metabolism, which results in changes in the stratum corneum intercellular lipid components and structure. Under pathological conditions, 1-deoxysphingolipids form as atypical sphingolipids from de novo sphingolipid biosynthesis.
Objective
This study investigated the potential role of 1-deoxysphingolipids in skin barrier dysfunction secondary to X-ray and ultraviolet B (UVB) irradiation in vitro and in vivo. It was also evaluated changes in the expression of 1-deoxysphingolipids in lesional human skin of atopic dermatitis.
Methods
In this study, the changes in these 1-deoxysphingolipids levels of skin and serum samples were investigated in skin barrier dysfunction associated with X-ray and UVB irradiation in vitro and in vivo.
Results
Increased 1-deoxysphingolipids were observed in cultured normal human epidermal keratinocytes after X-ray irradiation. X-ray or UVB irradiation increased the production of 1-deoxysphingosine in a reconstituted 3-dimensional (3D) skin model. Interestingly, treatment with a physiological lipid mixture (multi-lamellar emulsion contained pseudoceramide), which can strengthen the epidermal permeability barrier function, resulted in decreased 1-deoxysphingosine formation in a reconstituted 3D skin model. Further investigation using a hairless mouse model showed similar preventive effects of physiological lipid mixture against 1-deoxysphingosine formation after X-ray irradiation. An increased level of 1-dexoysphingosine in the stratum corneum was also observed in lesional skin of atopic dermatitis.
Conclusion
1-deoxysphingosine might be a novel biomarker of skin barrier dysfunction and a physiological lipid mixture treatment could prevent 1-deoxysphingosine production and consequent skin barrier dysfunction.
Keywords: Atopic dermatitis, Permeability, Radiation, Sphingolipids, X-ray
INTRODUCTION
Sphingolipids play an important role in maintaining the mechanical and biological integrity of the human skin1. In particular, the stratum corneum (SC) layer serves as an effective barrier between the environment and the human body. The SC is the outermost layer of the epidermis and contains corneocytes and extracellular lipids, including ceramides, cholesterol, and free fatty acids. These lipids in the SC provide an effective skin barrier and prevent excessive water loss2. Sphingolipids are lipids that include the sphingoid base, which is generated by the condensation of an amino acid and a fatty acid. The sphingoid bases are enzymatically converted to produce biologically active sphingolipids, such as ceramides, sphingomyelin, sphingosine-1-phosphate (S1P), ceramide-1-phosphate, and glycosphingolipids3,4. In keratinocytes, de novo sphingolipid synthesis begins with the action of serine palmitoyltransferase (SPT), which catalyzes the condensation of L-serine and palmitoyl-CoA (Pal-CoA) to 3-ketosphinganine. Next, C18-sphinganine is generated by the enzyme 3-Ketodihydrosphingosine reductase (KDHR). Subsequently, N-acylation to dihydroceramides completes the formation of the starting molecules, further forming complex sphingolipids, such as sphingomyelins or glycosphingolipids. These are secreted into the SC, where they are transformed into ceramides as a major component of the lipid barrier5,6.
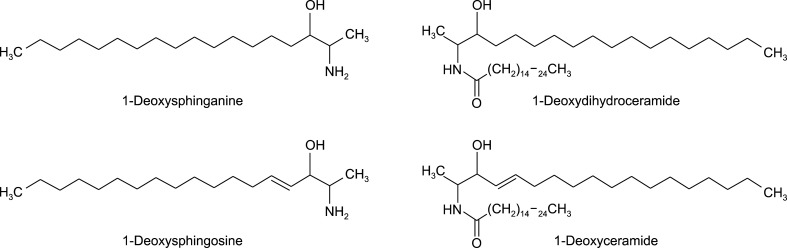
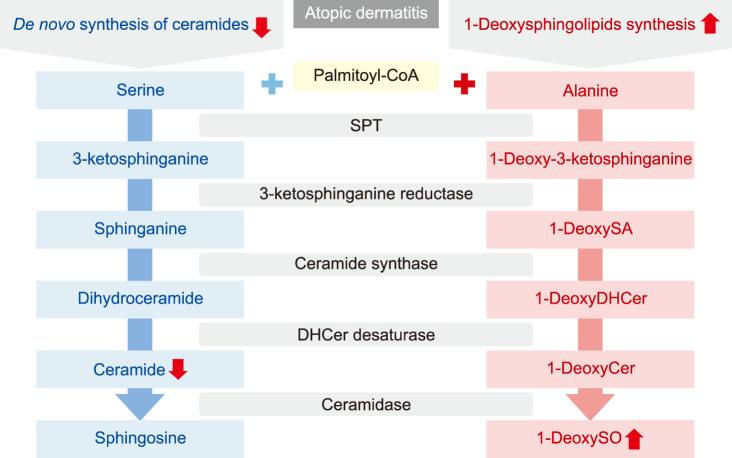
Various atypical sphingolipids, including 1-deoxysphingolipids (1-deoxySLs), are formed by the action of SPT on acyl-CoAs other than Pal-CoA or amino acids other than L-serine. When L-alanine, instead of L-serine, is incorporated into the SPT reaction, 1-deoxySLs, which lack the C1-hydroxyl (C1-OH) group of canonical sphingolipids are formed (Fig. 1). Notably, 1-deoxySLs are neurotoxic and pathologically high levels of 1-deoxySLs have been linked to hereditary sensory neuropathy type I (HSAN1), which is caused by missense mutations in the SPT genes. HSAN1 is a peripheral neuropathy characterized by sensory loss with painless injuries, chronic non-healing skin ulcers, gradual destruction of underlying bones, bone infections, muscle wasting, amputation of fingers or toes, and consequent motor deterioration7. The present study focused on skin involvement in HSAN1 and inferred that chronic skin ulcers associated with this condition may be related to the accumulation of 1-deoxySLs8.
Fig. 1. The structures of 1-deoxysphinganine, 1-deoxysphingosine, 1-deoxydihydroceramide, and 1-deoxyceramide.
Exposure to X-ray irradiation damages the skin barrier9, and ultraviolet irradiation of the skin is known to disrupt the epidermal permeability barrier10. Atopic dermatitis (AD) is a chronic inflammatory skin disease associated with genetic and environmental factors, particularly those that are linked to skin barrier dysfunction and immunological abnormalities11. Recent studies have demonstrated alterations in sphingolipid metabolism in the SC in patients with AD12,13. Therefore, it was hypothesized that atypical sphingolipid levels might be altered in dermatological conditions associated with barrier dysfunction for example in patients suffering from X-ray or ultraviolet B (UVB) irradiation-induced cutaneous injuries and AD. This study investigated the potential role of 1-deoxySLs in skin barrier dysfunction secondary to X-ray and UVB irradiation in vitro and in vivo. It was also evaluated changes in the expression of 1-deoxySLs in lesional human skin of AD.
MATERIALS AND METHODS
Cell culture
Normal human epidermal keratinocytes (NHEKs) obtained from Clonetics-BioWhittaker (San Diego, CA, USA) were grown in culture dishes at 37℃ and 5% CO2. NHEK cells were maintained in EpiLife medium supplemented with human keratinocyte growth supplement kit (Thermo Fisher Scientific, Waltham, MA, USA). The culture medium was replaced every 2-day. Near-confluent (70%~90%) cells were disaggregated with 0.25 mg/ml trypsin/0.01% ethylenediamine tetra-acetic acid and subcultured. Second-to-fourth-passage NHEKs were used in all the experiments.
Analysis of 1-deoxysphingolipids
To assess the levels of cellular 1-deoxySLs, cells were harvested and lysed in radioimmunoprecipitation assay (RIPA) buffer followed by extraction of sphingolipids, as reported previously11,12. The extracted lipids dried using a vacuum system (Vision, Seoul, Korea) were re-dissolved in methanol and analyzed using an LC-ESI-MS/MS (API 3200 QTRAP mass; SCIEX, Concord, ON, Canada) in selective ion monitoring mode. The deoxyceramide MS/MS transitions (m/z) were 522→266 for C16-deoxyceramide, 550→266 for C18-deoxyceramide, 578→266 for C20-deoxyceramide, 606→266 for C22-deoxyceramide, 642→266 for C24:1-deoxyceramide, and 644→266 for C24-deoxyceramide, respectively. The deoxy sphingoid bases MS/MS transitions (m/z) were 286→238 for C17 sphingosine as an internal standard, 284→266 for C18 deoxy sphingosine, and 286→268 for deoxy sphinganine. Data were acquired using Analyst 1.4.2 software (Applied Biosystems, Foster City, CA, USA). 1-deoxySL levels were expressed as pmol per mg protein.
Three-dimensional skin models and physiologic lipid mixture treatment
EpiDermFT™ (EFT-400) skin models were obtained from MatTek Corporation (Ashland, MA, USA). Upon arrival at the laboratories, skin models were acclimatized overnight in 6-well plates containing 1 ml maintenance medium (EFT-400-ASY; MatTek Corporation) in a humidified incubator at 37℃ and 5% CO2.
The physiologic lipid mixture used in our study was prepared according to a previously reported method14. The physiologic lipid components (pseudoceramide, stearic acid, and cholesterol), oils, common base (cetanol, 2-octyldodecanol, squalane, and fatty acid triglyceride), and emulsifiers (glyceryl monostearate and POE(15) glyceryl monostearate) were mixed and melted at 85℃~90℃. The water and water-soluble components (glycerine and 1,3-butylene glycol) were added slowly with vigorous agitation using a homomixer (T.K. Homomixer Mark II f-model; Tokyo Co. Ltd, Tokyo, Japan) at 70℃. After emulsifying, the mixtures were cooled to room temperature. The formulations and the concentrations of main components of physiologic lipid mixture are shown as Table 1.
Table 1. The formulations and the concentrations of main components of physiologic lipid mixture.
| Component | % |
|---|---|
| Pseudo-stratum corneum lipid | 2.3 |
| Pseudoceramide | 0.6 |
| Stearic acid | 1.5 |
| Cholesterol | 0.2 |
| Oils and common base | 8.0 |
| Cetanol | 3.5 |
| Squalene | 2.0 |
| Fatty acid triglyceride | 3.5 |
| Polyols | 10.0 |
| Glycerin | 5.0 |
| 1,3-butylene glycol | 5.0 |
| Emulsifiers | 4.5 |
| Glyceryl monostearate | 1.5 |
| Polyoxyethylene glyceryl monostearate/POE(15) | 3.0 |
| Thickener | 0.2 |
| Carbomer | 0.2 |
| Wate | 74.0 |
Pseudoceramide (INCI name: myristyl/palmityl-oxo-stearamidel arachimide MEA; Neopharma, Daejeon, Korea) is a mixture of myristyl-oxostearamide MEA (C34H67NO3, 1,172%), palmityl-oxostearamide MEA (C36H71NO3, 2,173%), myristyl-oxoarachamide MEA (C36H71NO3, 2,173%), and palmityl-oxoarachamide MEA (C36H75NO3, 4,773%). The cross-polarized microscopic result of the pseudoceramide confirmed its intrinsic characteristic of lamellar structure formation. Based on the results of skin irritation tests performed on animals and humans, the pseudoceramide was verified to be non-toxic, non-irritable, and non-sensitizing15.
On the day of treatment, skin models were placed in 1 ml fresh maintenance medium. Just before treatment, any moisture on the skin surface was removed with a cotton tip, followed by topical application of formulations (50 µl) directly on the surface of the EpiDerm™ skin model. During the treatment period, skin models were placed in a humidified incubator at 37℃ and 5% CO2.
Radiation and ultraviolet irradiation for the normal human epidermal keratinocytes and 3-dimensional skin model
NHEKs were radiated with a single dose of 3 Gy or 6 Gy at KAERI (the Korea Atomic Energy Research Institute), while skin models were radiated with a single dose of 2 Gy. Skin models were irradiated with a single dose of UVB irradiation (100 mJ/cm2) using a calibrated self-produced UVB irradiator to deliver 2 mJ/cm2/s of radiation.
Animals and radiation
Male hairless mice were purchased from Samtako Biokorea (Osan, Korea) at 8 and 12 weeks of age. All animals were housed 3~4 per cage, under standard controlled room conditions with food and water available ad libitum. Irradiation was performed at KAERI. In the 4-week irradiation treatment protocol, mice were exposed to radiation at a dose of 2 Gy under anesthesia once a week for 4 weeks, and fresh formulations were applied daily during the experiment. This study was approved by the Institutional Animal Care and Use Committees of Hallym University (H20150480).
Skin hydration and transepidermal water loss (TEWL) were evaluated in standardized conditions (temperature- and humidity-controlled room 23℃ and 30% of relative humidity and an acclimatization time of 40 minutes) and according to guidelines16 using a corneometer (Corneometer® CM 825; Courage+Khazaka Electronic GmbH, Cologne, Germany) and a vapometer (Tewameter TM 300; Courage+Khazaka Electronic GmbH) device before and after irradiation. Skin hydration was expressed in arbitrary units, and TEWL, expressed in g/m2/h.
After the end of the experiment, the mice were anesthetized, after which the dorsal skins were collected and fixed using 4% formalin for histological studies. The fixed dorsal skins were dehydrated in a graded ethanol series and paraffin embedded. Sections 5 mm thick were cut and mounted on glass slides. The paraffin was removed from the tissue sections using xylene, and the sections rehydrated in a decreasing graded ethanol series.
Skin samples
Two punch skin biopsies (5 mm diameter each) were taken from human patients with AD. The first was from lesional skin and the other from an apparently healthy site. Each specimen was fixed in formalin and embedded in paraffin to form paraffin blocks. This study was approved by the Institutional Review Board of Hallym University Kangnam Sacred Heart Hospital (IRB No. 2015-11-131).
Generation of anti-1-deoxysphingosine antibody
In this study, anti-1-deoxysphingosine antibody was produced using hybridoma technology, which is a common and efficient tool for generating high-quality monoclonal antibodies. It is usually preferred over other monoclonal antibody production techniques as it ensures the easy production of identical monoclonal antibodies in large numbers17. The brief methodology for the generation of anti-1-deoxysphingosine antibody is as follows18.
Six female BALB/c mice (5-week-old) were injected subcutaneously at multiple sites with 50 µg of the 1-deoxysphinosine conjugated with Ovalbumin (Imject™ maleimide activated ovalbumin; Thermo Fisher Scientific). A total of 3~5 injections were performed over a 2-week interval. One week after every injection, the serum was collected and was titrated using the enzyme-linked immunosorbent assay (ELISA) using 1-deoxysphingosine conjugated with Imject™ maleimide activated BSA (bovine serum albumin; Thermo Fisher Scientific). Considering the small molecular size of 1-deoxysphingosine, we used OVA conjugated 1-deoxysphingosine for immunization in mice and BSA conjugated 1-deoxysphinosine form was used for the titration. After the prime dose and checking for the adequate titer against 1-deoxysphingosine, booster doses were adjusted.
In order to product hybridomas, the splenocytes of the 10-week immunized BALB/c mice were fused with SP2/0 myeloma cells using polyethylene glycol 1500 (PEG 1500) (Roche, Basel, Switzerland). The hybridomas were then selected in RPMI 1640 medium supplemented with hypoxanthine, aminopterin and thymidine (HAT) (Sigma-Aldrich, Saint Lousis, MO, USA). Positive clones were identified using an indirect ELISA. After four subcloning's, hybridomas producing monoclonal antibodies were established and characterized. Further, chromosome analysis was performed for each of these hybridomas. Total four clones exhibiting optical density (OD) higher than 3.0 OD were selected and the supernatants from each cultured cells (1C11 [0.77 mg/ml], 1C17 [0.47 mg/ml], 4C24 [0.70 mg/ml], 5E2 [1.00 mg/ml]) were subjected to purification by Protein G beads for the separation of the monoclonal antibody. As a result, monoclonal antibody (5E2) was selected for immunohistochemistry (IHC). For checking the specificity of anti-1-deoxysphingosine antibody, ELISA was conducted.
Histology and immunohistochemical analysis
After the end of the experiment, skin models were fixed in 4% neutral buffered formalin overnight at 4℃ before being dehydrated in a graded ethanol series and paraffin embedded. Sections 5 mm thick were cut and mounted on glass slides. The tissue sections were deparaffinized in xylene and rehydrated in a decreasing graded ethanol series before routine staining with H&E.
For visualizing specific proteins within the 3-dimensional (3D) Skin model, hairless mouse skin, and skin tissues of AD patients; 5 mm-thick tissue sections were deparaffinized and rehydrated before heat-induced epitope revival treatment in antigen retrieval solution (ab937; Abcam, Cambridge, MA, USA) at pH 6.0 for 10 minutes at 97℃, followed by cooling for 10 minutes in a cooling chamber. Endogenous peroxidases were blocked with hydrogen peroxide solution (S2023; Dako, Glostrup, Denmark), and nonspecific primary antibody binding was blocked using a blocking reagent (X0909; Dako). The primary antibody was anti-1-deoxysphingosine antibody (anti-005 antibody, self-produced in-house). Primary antibody was diluted in antibody diluent solution (S3022; Dako) and incubated in a humidified chamber overnight at 4℃. A horseradish peroxidase-linked secondary antibody was then applied to the tissue samples and detected with a 3,3′-diaminobenzidine chromogen system (K4001; Dako). The tissue sections were counterstained using hematoxylin nuclear stain.
Statistical analysis
The statistical analysis was performed using IBM SPSS Statistics ver. 24.0 (IBM Corp., Armonk, NY, USA) and data presented as mean values±standard deviation. The statistical significance of any differences between groups was determined by the student's t-test and the analysis of variance (ANOVA), followed by the Tukey's test. Values of p<0.05 were considered statistically significant.
RESULTS
Elevated 1-deoxysphingolipid levels in cultured normal human keratinocytes following X-ray irradiation
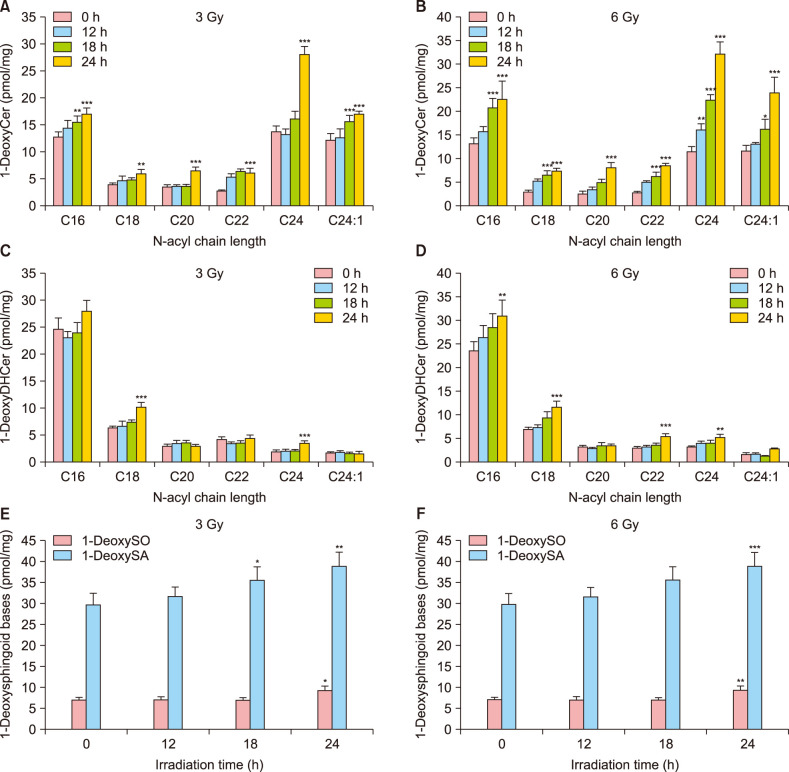
Initially, the level of 1-deoxySLs in cultured NHEKs exposed to X-ray irradiation was measured using liquid chromatography tandem-mass. The synthesis of 1-deoxyceramide (Table 2), 1-deoxydihydroceramide (Table 3), 1-deoxysphinganine, and 1-deoxysphingosine (Table 4) were increased after both, 3 Gy and 6 Gy radiation in cultured NHEK (Fig. 2).
Table 2. The production of 1-deoxyceramide after X-ray irradiation.
| X-ray dose (Gy) | Irradiation time (h) | N-acyl chain length | |||||
|---|---|---|---|---|---|---|---|
| C16 | C18 | C20 | C22 | C24 | C24:1 | ||
| 3 | 0 | 12.80±1.03 | 3.95±0.24 | 3.62±0.34 | 2.80±0.22 | 13.76±1.05 | 12.28±1.13 |
| 12 | 14.41±1.41 | 4.85±0.75 | 3.71±0.25 | 5.46±0.58 | 13.33±0.94 | 12.69±1.56 | |
| 18 | 15.50±1.21** | 4.96±0.34 | 3.71±0.34 | 6.49±0.45 | 16.30±1.21 | 15.68±1.09*** | |
| 24 | 17.11±1.07*** | 6.05±0.75** | 6.57±0.69*** | 6.28±0.69*** | 27.98±1.53*** | 17.07±0.46*** | |
| 6 | 0 | 13.26±1.24 | 3.08±0.33 | 2.65±0.53 | 2.86±0.25 | 11.58±1.03 | 11.75±1.04 |
| 12 | 15.80±1.08 | 5.34±0.42 | 3.57±0.43 | 5.20±0.13 | 16.12±1.29** | 13.20±0.25 | |
| 18 | 20.80±1.98*** | 6.63±0.78*** | 5.06±0.65 | 6.41±0.87*** | 22.42±1.22*** | 16.34±1.98* | |
| 24 | 22.52±3.87*** | 7.51±0.54*** | 8.17±1.09*** | 8.58±0.48*** | 32.14±2.52*** | 23.9±3.25*** | |
Values are presented as mean±standard deviation (pmol/mg). *p<0.05, **p<0.01, and ***p<0.001 compared to untreated normal human epidermal keratinocytes.
Table 3. The production of 1-deoxydihydroceramide after X-ray irradiation.
| X-ray dose (Gy) | Irradiation time (h) | N-acyl chain length | |||||
|---|---|---|---|---|---|---|---|
| C16 | C18 | C20 | C22 | C24 | C24:1 | ||
| 3 | 0 | 24.66±2.04 | 6.43±0.25 | 3.06±0.29 | 4.29±0.49 | 2.05±0.31 | 1.78±0.18 |
| 12 | 23.15±1.09 | 6.75±0.84 | 3.63±0.48 | 3.52±0.26 | 2.15±0.25 | 1.93±0.18 | |
| 18 | 24.01±1.87 | 7.54±0.36 | 3.72±0.36 | 3.69±0.36 | 2.24±0.21 | 1.69±0.24 | |
| 24 | 27.99±1.94 | 10.35±0.75*** | 3.04±0.33 | 4.59±0.47 | 3.63±0.37*** | 1.72±0.33 | |
| 6 | 0 | 23.58±1.83 | 6.88±0.42 | 3.15±0.34 | 3.00±0.16 | 3.09±0.24 | 1.59±0.36 |
| 12 | 26.35±2.52 | 7.42±0.45 | 2.86±0.21 | 3.20±0.21 | 3.96±0.45 | 1.69±0.25 | |
| 18 | 28.50±2.84 | 9.42±1.21 | 3.49±0.51 | 3.61±0.33 | 4.08±0.41 | 1.27±0.11 | |
| 24 | 30.93±3.21** | 11.61±1.25*** | 3.44±0.41 | 5.47±0.46*** | 5.20±0.58** | 2.80±0.14 | |
Values are presented as mean±standard deviation (pmol/mg). **p<0.01, and ***p<0.001 compared to untreated normal human epidermal keratinocytes.
Table 4. The production of 1-deoxysphingosine and 1-deoxysphinganine after X-ray irradiation.
| X-ray dose (Gy) | Irradiation time (hr) | 1-deoxysphingosine (pmol/mg) | 1-deoxysphinganine (pmol/mg) |
|---|---|---|---|
| 3 | 0 | 7.23±0.45 | 29.77±2.62 |
| 12 | 7.25±0.56 | 31.70±2.14 | |
| 18 | 7.13±0.47 | 35.66±3.01* | |
| 24 | 9.42±0.95* | 38.86±3.21** | |
| 6 | 0 | 7.31±0.62 | 29.50±3.09 |
| 12 | 7.94±0.46 | 29.99±2.41 | |
| 18 | 8.94±0.81 | 34.26±4.12 | |
| 24 | 11.27±0.91** | 44.26±3.24*** |
Values are presented as mean±standard deviation (pmol/mg). *p<0.05, **p<0.01, and ***p<0.001 compared to untreated normal human epidermal keratinocytes.
Fig. 2. Increase of the production of 1-deoxysphinganine (1-DeoxySA), 1-deoxysphingosine (1-DeoxySO), 1-deoxydihydroceramide (1-DeoxyDHCer), and 1-deoxyceramide (1-DeoxyCer) by 3 Gy or 6 Gy radiation in cultured normal human epidermal keratinocytes (NHEK). (A, B) 1-DeoxyCer, (C, D) 1-DeoxyDHCer, (E, F) 1-DeoxySA, and 1-DeoxySO. Each determination was made in triplicate, and data represent the mean±standard deviation. *p<0.05, **p<0.01, and ***p<0.001 compared to untreated NHEK.
Treatment with a physiological lipid mixture inhibited 1-deoxysphingosine production in a three-dimensional skin model exposed to X-ray and ultraviolet B-irradiation
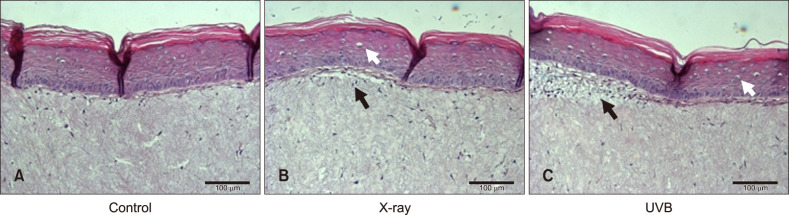
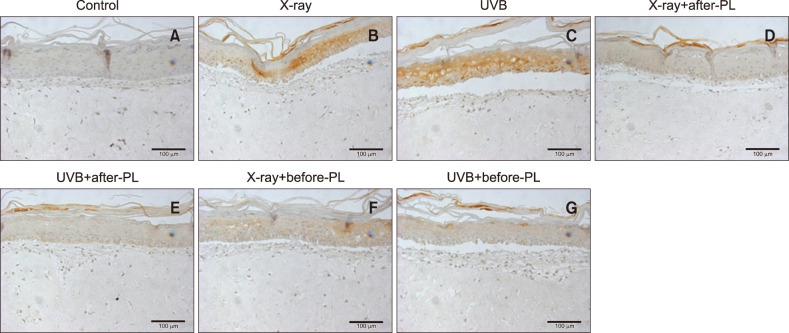
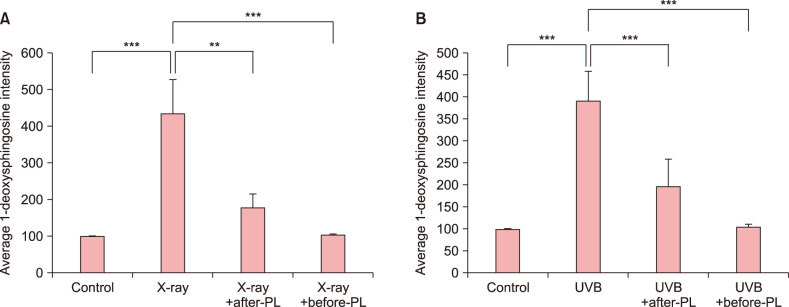
A 3D skin model exposed to both X-ray and UVB irradiation showed vacuolated and necrotic keratinocytes and superficial dermal edema (Fig. 3). IHC analysis showed increased 1-deoxysphingosine levels in the epidermis of a 3D skin model following exposure to X-ray (2 Gy) and UVB irradiation compared to controls (Fig. 4A~C, 5). Treatment with a physiological lipid mixture showed a decrease in 1-deoxysphingosine levels in both the X-ray and UVB-irradiated 3D skin models (Fig. 4D~G, 5).
Fig. 3. Histological findings of a three-dimensional skin model following exposure to X-ray and ultraviolet B (UVB) irradiation: X-ray radiation was done by 2 Gy (50 s). UVB irradiation was executed by 100 mJ/cm2 (2 mJ/cm2/s, 50 s). A three-dimensional skin model exposed to X-ray and UVB irradiation showed vacuolated and necrotic keratinocytes and superficial dermal edema. (A) Control, (B) X-ray irradiation, (C) UVB irradiation (H&E staining; original magnification: ×200). Black arrows indicated superficial dermal edema. White arrows indicated vacuolated and necrotic keratinocytes.
Fig. 4. Change of 1-deoxysphingosine levels following X-ray, ultraviolet B (UVB) irradiation, and the physiological lipid mixture (PL) treatment: X-ray radiation was done by 2 Gy (50 s). UVB irradiation was executed by 100 mJ/cm2 (2 mJ/cm2/s, 50 s). The 1-deoxysphingosine expression in the epidermis of a 3-dimensional (3D) skin model following exposure to X-ray (B) and UVB irradiation (C) was increased compared to controls (A). Treatment with PL showed a decrease in 1-deoxysphingosine levels in both the X-ray (D, F) and UVB-irradiated 3D skin models (E, G) (1-deoxysphinogosine staining; original magnification: ×200). X-ray before-PL, the group of that PL was treated before X-ray radiation, UVB before-PL, the group of that PL was treated before UVB irradiation. X-ray after-PL, the group of that PL was treated after X-ray radiation, UVB after-PL, the group of that PL was treated after UVB irradiation.
Fig. 5. Quantification of immunohistochemical analysis of 1-deoxysphinogosine in 3-dimensional (3D) skin model following X-ray, ultraviolet B (UVB) irradiation, and the physiological lipid mixture (PL) treatment. The 1-deoxysphingosine expression in the epidermis of a 3D skin model following exposure to (A) X-ray and (B) UVB irradiation was significantly increased compared to controls (p<0.001, p<0.001, respectively). Treatment with PL showed a significant decrease in 1-deoxysphingosine levels in both the (A) X-ray and (B) UVB-irradiated 3D skin models (X-ray vs. X-ray+after-PL, p<0.01, and X-ray vs. X-ray+before-PL, p<0.001). Expression levels of 1-deoxysphingosine staining were semiquantitatively analyzed using LASX software (Leica, Wetzlar, Germany). Results are expressed as mean±standard deviation of optical density of six different digital images. **p<0.01 and ***p<0.001. X-ray before-PL, the group of that PL was treated before X-ray radiation, UVB before-PL, the group of that PL was treated before UVB irradiation. X-ray after-PL, the group of that PL was treated after X-ray radiation, UVB after-PL, the group of that PL was treated after UVB irradiation.
Treatment with physiological lipid mixture decreased 1-deoxysphingosine production in the X-ray irradiated skin of a hairless mouse
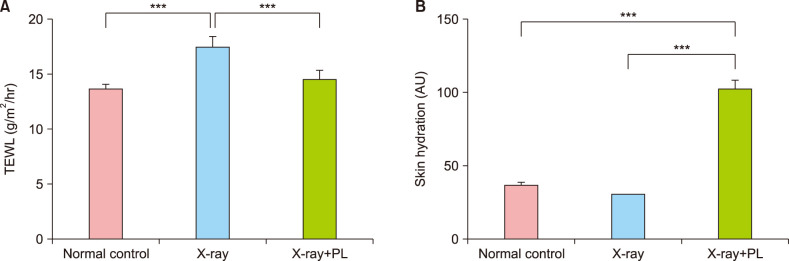
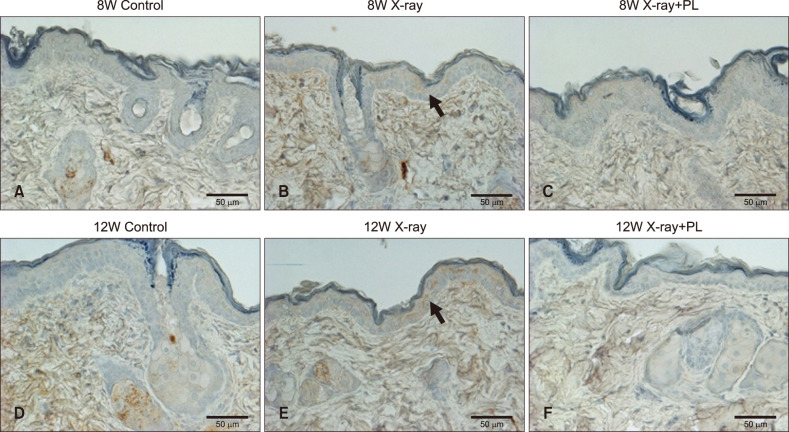
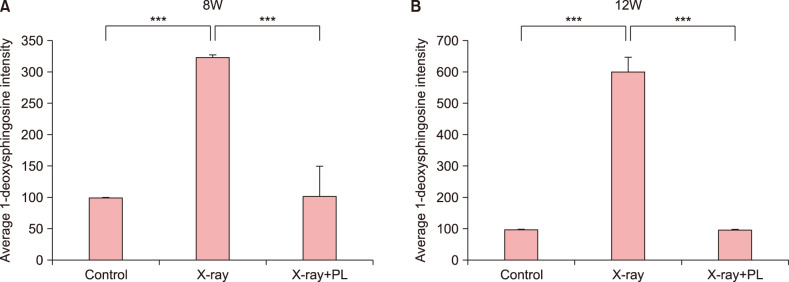
To confirm the results obtained from in vitro study, it was evaluated the changes in skin barrier function, skin hydration, 1-deoxysphingosine levels, and the effect of physiological lipid mixture treatment on 1-deoxysphingosine production in the skin of an X-ray irradiated hairless mouse. TEWL in the hairless mouse increased after X-ray irradiation, but not in the group treated with physiological lipid mixture (Fig. 6A). Skin hydration of the hairless mouse improved in the physiological lipid mixture treatment group (Fig. 6B). Skin in the X-ray irradiated group showed increased the accumulation of 1-deoxysphingosine (Fig. 7, 8). However, the group treated with the physiological lipid mixture did not show increased the production of 1-deoxysphingosine in skin following X-ray irradiation (Fig. 7, 8).
Fig. 6. The changes in skin barrier function, skin hydration, 1-deoxysphingosine levels, and the effect of physiological lipid mixture (PL) treatment on 1-deoxysphingosine production in the skin of an X-ray irradiated hairless mouse: (A) transepidermal water loss (TEWL) in the hairless mouse increased after X-ray irradiation (normal control vs. X-ray, p<0.001), but not in the group treated with physiological lipid mixture (X-ray vs. X-ray+PL, p<0.001). Each determination was made in triplicate, and data represent the mean±standard deviation (SD). (B) Skin hydration (arbitrary units, AU) of the hairless mouse improved in the physiological lipid mixture treatment group (normal control vs. X-ray+PL, p<0.001, X-ray vs. X-ray+PL, p<0.001). Each determination was made in triplicate, and data represent the mean±SD. ***p<0.001.
Fig. 7. Immunohistochemistry stain for 1-deoxysphingosine in the skin biopsy tissue of (A~C) 8-week-old (8W) and (D~F) 12-week-old (12W) hairless mice: Skin in the X-ray irradiated group showed increased the accumulation of 1-deoxysphingosine. The group treated with the physiological lipid mixture (PL) did not show increased the production of 1-deoxysphingosine in skin following X-ray irradiation (original magnification, ×400). Arrows indicate probed 1-deoxysphingosine. 8W X-ray: X-ray irradiated 8W hairless mouse, 8W X-ray+PL: X-ray irradiation with PL treatment in 8W hairless mouse, 12W X-ray: X-ray irradiated 12W hairless mouse 12W X-ray+PL: X-ray irradiation with PL treatment in 12W hairless mouse.
Fig. 8. Quantification of immunohistochemical analysis of 1-deoxysphinogosine in (A) 8-week-old (8W) and (B) 12-week-old (12W) hairless mice following X-ray and the physiological lipid mixture (PL) treatment. X-ray irradiated group showed significantly increased the accumulation of 1-deoxysphingosine in the skin compared to control in 8W and 12W hairless mice (p<0.001, p<0.001, respectively). The group treated with PL showed significantly lowered production of 1-deoxysphingosine in skin following X-ray irradiation compared to X-ray irradiation group without treatment of PL in 8W and 12W hairless mice (p<0.001, p<0.001, respectively). Expression levels of 1-deoxysphingosine staining were semiquantitatively analyzed using LASX software (Leica, Wetzlar, Germany). Results are expressed as mean±standard deviation of optical density of six different digital images. ***p<0.001.
Increased production of 1-deoxysphingosine in lesional skin of atopic dermatitis
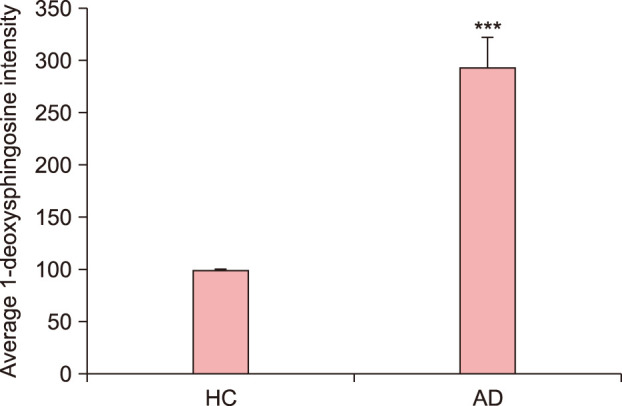
IHC analysis was performed to determine the degree of 1-deoxysphingosine production in the lesional skin of AD. The degree of 1-deoxysphingosine synthesis was higher in the lesional skin of AD than that observed in normal skin (Fig. 9, 10). 1-deoxysphingosine primarily stained the keratinocytes throughout the epidermis in the lesional skin of AD (Fig. 9).
Fig. 9. Immunohistochemistry analysis of the degree of 1-deoxysphingosine expression in the lesional skin of atopic dermatitis (AD): The degree of 1-deoxysphingosine expression was higher in the lesional skin of AD than that observed in normal skin. (A, B) normal skin (C, D) lesional skin of AD (A, C: 1-deoxysphinogosine staining, original magnification: ×100; B, D: 1-deoxysphinogosine staining, original magnification: ×200). The red circles in low magnification figures (A, C) indicated the areas of high magnification figures (B, D).
Fig. 10. Quantification of immunohistochemical analysis of 1-deoxysphinogosine in the lesional skin of atopic dermatitis (AD): The degree of 1-deoxysphingosine expression was significantly higher in the lesional skin of AD than that observed in normal skin. Expression levels of 1-deoxysphingosine staining were semiquantitatively analyzed using LASX software (Leica, Wetzlar, Germany). Results are expressed as mean±standard deviation of optical density of six different digital images. ***p<0.001 compared to healthy control (HC).
DISCUSSION
This study demonstrated that the production of 1-deoxysphingosine, an atypical sphingolipid, increased under conditions associated with skin barrier dysfunction, such as X-ray and UVB-irradiated skin and AD. In addition, it was observed that treatment with a physiological lipid mixture prevented the synthesis of 1-deoxysphingosine in an X-ray irradiated 3D skin model and in mice skin. In the pathway of 1-deoxySLs synthesis, 1-deoxysphingosine is the end-product of this pathway. Therefore, the present study using 3-D skin culture model and animal model was focused to determine the change of production of 1-deoxysphingosine. Sphingolipids, which have biological and structural roles in epidermis, are crucially involved in the preservation of the skin barrier function and control processes, such as proliferation, differentiation, and apoptosis of keratinocytes. Ceramides, as an element of extracellular lipids in the SC, are important components of the skin barrier. A side reaction that occurs during de novo sphingolipid biosynthesis generates atypical sphingolipids called 1-deoxySLs. Elevated production of these abnormal compounds is thought to be involved in the neuropathy of HSAN1 and type 2 diabetes mellitus (DM) patients19,20. In addition to neuropathy, patients with HSAN1 and DM show several skin manifestations of defective wound repair or diabetic foot ulcers19,20. Based on these findings, this study was focused on understanding how the production of 1-deoxySLs was associated with skin barrier dysfunction. Sphingolipid metabolism changes in the SC have been studied in several dermatological diseases, such as psoriasis, AD, and a few genetic disorders, all of which show skin barrier dysfunction21. However, the role of 1-deoxySL in dermatological diseases associated with skin barrier dysfunction has not been studied. To the best our knowledge, this study is first study to demonstrate that 1-deoxySLs are related to epidermal barrier damage in vivo and in vitro.
Although the effects of ultraviolet radiation on epidermal barrier function are poorly understood, UVB irradiation has been known to lead to skin dehydration and increased TEWL10. The mechanisms are suggested abnormalities in the production of lipids in the SC and disturbance in tight junction function in the granular layer22,23. Previous studies analyzing the effects of UV light on SC lipids emphasized mainly the change of ceramides. One group of authors showed that UV light leads to an increased amount of SC lipids, including most ceramide subgroups24. Another study showed that no other ceramide subgroups except ceramide 6 or the ceramide/cholesterol ratio, demonstrated a change following UV light exposure25. In fact, ceramide 6 is not known to differ among skin barrier dysfunction groups, such as patients with AD. Patient groups of AD and psoriasis, which are representative skin disorders with skin barrier dysfunction, have shown lower levels of ceramides 1 and 3 compared to healthy control subjects26. Present study demonstrated inceptively that pathologic sphingolipid production was elevated in the UVB-irradiated 3D skin model.
X-ray irradiation disturbs the normal formation and maturation of epithelial cells27, and also provokes local inflammatory reaction with recruitment of eosinophils and neutrophils that induce the damage to the protective skin barrier28. Radiation dermatitis has been known to cause skin barrier impairment with increased TEWL29. To our knowledge, there is no study regarding the direct effects of X-ray irradiation on the SC lipids. Notably, this study demonstrated that the production of 1-deoxysphingosine, an atypical sphingolipid, increased in irradiated skin.
Previous in vitro studies have reported that moisturizers effectively restore skin barrier function30,31. In a previous study, this physiologic lipid mixture was characterized as the lateral hexagonal phase, as well as both long and short periodicity lamellar phases, which showed a structural similarity with the native human SC intercellular lipid32.
The clinical study demonstrated that the physiologic lipid mixture used in our study was effective for AD. After four weeks of applying the physiologic lipid mixture to 37 AD patients, the efficacy on improvement of the skin symptoms was 60% based on SCORAD evaluation. The decreases in TEWL and skin pH after four weeks show statistical significance, and the levels of decrease were higher in severe AD cases14. In the present study, a physiological lipid mixture prevented skin barrier dysfunction in mouse skin exposed to X-ray irradiation, either through inhibition of TEWL or by preventing skin dehydration. Treatment with a physiological lipid mixture suppressed the production of 1-deoxysphingosine in both the X-ray and UVB-irradiated 3D skin models. In addition, 1-deoxysphingosine levels in irradiated mouse skin were lowered more in the physiological lipid mixture treatment group. This study data suggests that physiological lipid mixtures could be useful for the prevention and treatment of radiation dermatitis, and that 1-deoxysphingosine could be a biomarker of skin barrier dysfunction.
AD is a chronic, relapsing inflammatory skin disease characterized by itching, xerosis, and a broad spectrum of clinical manifestations. An increasing body of evidence shows that skin barrier dysfunction is an etiological contributor to AD33. Recent biochemical findings indicate that disturbances in the epidermal lipid compartment structures, particularly the ceramides, account for skin barrier dysfunction in atopic dry skin34,35. An alternative reaction of de novo biosynthesis of ceramide produces 1-deoxySLs. In this study, deoxysphingosine was elevated in the lesional skin of AD compared to normal skin. Based on the results of our study, we presented an altered skin sphingolipid metabolism pathway of AD that inhibited the de novo synthesis pathway of ceramide but upregulated the 1-deoxy-sphingolipids metabolism pathway. Therefore, the changed sphingomyelin metabolism in patients with AD might result in decrease in ceramide levels (Fig. 11). This could be an important etiological contributor to the continuous development of atopic dry skin with impaired barrier function observed in these patients.
Fig. 11. A possible mechanisms for ceramide alterations in atopic dermatitis (AD). Possible mechanisms may explain for the decreased ceramide content in the stratum corneum of AD patients, such as: (1) a decrease in de novo ceramide synthesis and (2) an increase 1-deoxysphingolipids synthesis. SPT: serine palmitoyltransferase, DHCer desaturase: dihydroceramide desaturase, 1-DeoxySA: 1-deoxysphinganine, 1-DeoxyDHCer: 1-deoxydihydroceramide, 1-DeoxyCer: 1-deoxyceramide, 1-DeoxySO: 1-deoxysphingosine.
The limitations of this study are as follows. First, the only experiment of quantification of the deoxySLs using liquid chromatography coupled to mass spectrometry was exerted on proliferative human keratinocytes in 2D culture. Therefore, further studies are needed to conduct the quantification of the deoxySLs using liquid chromatography coupled to mass spectrometry in an animal or human 3D skin model. Second, we haven't made a comparison with the effect of simple occlusion by non-physiological petrolatum. In order to confirm the hypothesis that ‘the skin barrier function is impaired by various causes and the condition of increased 1-deoxySLs is improved by application of physiological lipid mixture’, it is required to compare the effects of petrolatum cream and physiologic lipid mixture application on 1-deoxySL level and skin barrier function. Third, by evaluating that applying 1-deoxySLs to the skin might cause the skin barrier dysfunction, we can confirm more about the relationship between 1-deoxySL increase and skin barrier damage. Fourth, the effect of UVB irradiation on 1-deoxySL production was the strict focus on the 3D skin model.
In conclusion, 1-deoxysphingosine productions were observed to be elevated in conditions related to skin barrier dysfunction. A physiological lipid mixture inhibited 1-deoxysphingosine synthesis and consequent skin barrier dysfunction. These results suggest that this 1-deoxySL might be used as a novel biomarker in skin diseases with epidermal barrier dysfunction.
ACKNOWLEDGMENT
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2015M2A2A6A04044376, 2017R1A2B4006252, 2018R1C1B6007998), Korea Healthcare technology R&D project, funded by Ministry of Health & Welfare, Republic of Korea (HI17C0597) and Hallym University Research Fund 2019 (HURF-2019-72).
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006;580:5456–5466. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Meckfessel MH, Brandt S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J Am Acad Dermatol. 2014;71:177–184. doi: 10.1016/j.jaad.2014.01.891. [DOI] [PubMed] [Google Scholar]
- 3.Khavandgar Z, Murshed M. Sphingolipid metabolism and its role in the skeletal tissues. Cell Mol Life Sci. 2015;72:959–969. doi: 10.1007/s00018-014-1778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamanaka S, Hara M, Nishio H, Otsuka F, Suzuki A, Uchida Y. Human epidermal glucosylceramides are major precursors of stratum corneum ceramides. J Invest Dermatol. 2002;119:416–423. doi: 10.1046/j.1523-1747.2002.01836.x. [DOI] [PubMed] [Google Scholar]
- 6.Schurer NY, Elias PM. The biochemistry and function of stratum corneum lipids. Adv Lipid Res. 1991;24:27–56. doi: 10.1016/b978-0-12-024924-4.50006-7. [DOI] [PubMed] [Google Scholar]
- 7.Rotthier A, Auer-Grumbach M, Janssens K, Baets J, Penno A, Almeida-Souza L, et al. Mutations in the SPTLC2 subunit of serine palmitoyltransferase cause hereditary sensory and autonomic neuropathy type I. Am J Hum Genet. 2010;87:513–522. doi: 10.1016/j.ajhg.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bode H, Bourquin F, Suriyanarayanan S, Wei Y, Alecu I, Othman A, et al. HSAN1 mutations in serine palmitoyltransferase reveal a close structure-function-phenotype relationship. Hum Mol Genet. 2016;25:853–865. doi: 10.1093/hmg/ddv611. [DOI] [PubMed] [Google Scholar]
- 9.Denham JW, Hauer-Jensen M. The radiotherapeutic injury--a complex ‘wound’. Radiother Oncol. 2002;63:129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 10.Haratake A, Uchida Y, Schmuth M, Tanno O, Yasuda R, Epstein JH, et al. UVB-induced alterations in permeability barrier function: roles for epidermal hyperproliferation and thymocyte-mediated response. J Invest Dermatol. 1997;108:769–775. doi: 10.1111/1523-1747.ep12292163. [DOI] [PubMed] [Google Scholar]
- 11.Malajian D, Guttman-Yassky E. New pathogenic and therapeutic paradigms in atopic dermatitis. Cytokine. 2015;73:311–318. doi: 10.1016/j.cyto.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 12.van Smeden J, Janssens M, Gooris GS, Bouwstra JA. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim Biophys Acta. 2014;1841:295–313. doi: 10.1016/j.bbalip.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Bleck O, Abeck D, Ring J, Hoppe U, Vietzke JP, Wolber R, et al. Two ceramide subfractions detectable in Cer(AS) position by HPTLC in skin surface lipids of non-lesional skin of atopic eczema. J Invest Dermatol. 1999;113:894–900. doi: 10.1046/j.1523-1747.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- 14.Park BD, Youm JK, Lee SH. The clinical efficacy of multi-lamellar emulsion(MLE) contained pseudo-ceramide (PC-9S) on atopic dermatitis. J Skin Barrier Res. 2001;3:81–89. [Google Scholar]
- 15.Park BD, Lee M, Kim Y, Youm JK. Synthesis of N-ethanol-2-(myristyl/palmityl)-3-oxo(stearamide/arachidamide) and its physical properties for a cosmetic raw material. J Cosmet Sci. 2000;51:253–262. [Google Scholar]
- 16.Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164–178. doi: 10.1111/j.1600-0536.1990.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C. Hybridoma technology for the generation of monoclonal antibodies. Methods Mol Biol. 2012;901:117–135. doi: 10.1007/978-1-61779-931-0_7. [DOI] [PubMed] [Google Scholar]
- 18.Hnasko RM, Stanker LH. Hybridoma technology. Methods Mol Biol. 2015;1318:15–28. doi: 10.1007/978-1-4939-2742-5_2. [DOI] [PubMed] [Google Scholar]
- 19.Zuellig RA, Hornemann T, Othman A, Hehl AB, Bode H, Güntert T, et al. Deoxysphingolipids, novel biomarkers for type 2 diabetes, are cytotoxic for insulin-producing cells. Diabetes. 2014;63:1326–1339. doi: 10.2337/db13-1042. [DOI] [PubMed] [Google Scholar]
- 20.Auer-Grumbach M, Bode H, Pieber TR, Schabhüttl M, Fischer D, Seidl R, et al. Mutations at Ser331 in the HSN type I gene SPTLC1 are associated with a distinct syndromic phenotype. Eur J Med Genet. 2013;56:266–269. doi: 10.1016/j.ejmg.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilgram GS, Vissers DC, van der Meulen H, Pavel S, Lavrijsen SP, Bouwstra JA, et al. Aberrant lipid organization in stratum corneum of patients with atopic dermatitis and lamellar ichthyosis. J Invest Dermatol. 2001;117:710–717. doi: 10.1046/j.0022-202x.2001.01455.x. [DOI] [PubMed] [Google Scholar]
- 22.Jiang SJ, Chu AW, Lu ZF, Pan MH, Che DF, Zhou XJ. Ultraviolet B-induced alterations of the skin barrier and epidermal calcium gradient. Exp Dermatol. 2007;16:985–992. doi: 10.1111/j.1600-0625.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Kurasawa M, Hattori T, Maeda T, Nakano H, Sasaki H. Relationship between expression of tight junction-related molecules and perturbed epidermal barrier function in UVB-irradiated hairless mice. Arch Dermatol Res. 2008;300:61–68. doi: 10.1007/s00403-007-0817-y. [DOI] [PubMed] [Google Scholar]
- 24.Wefers H, Melnik BC, Flür M, Bluhm C, Lehmann P, Plewig G. Influence of UV irradiation on the composition of human stratum corneum lipids. J Invest Dermatol. 1991;96:959–962. doi: 10.1111/1523-1747.ep12476124. [DOI] [PubMed] [Google Scholar]
- 25.Jungersted JM, Høgh JK, Hellgren LI, Jemec GB, Agner T. The impact of ultraviolet therapy on stratum corneum ceramides and barrier function. Photodermatol Photoimmunol Photomed. 2011;27:331–333. doi: 10.1111/j.1600-0781.2011.00618.x. [DOI] [PubMed] [Google Scholar]
- 26.Jungersted JM, Hellgren LI, Jemec GB, Agner T. Lipids and skin barrier function--a clinical perspective. Contact Dermatitis. 2008;58:255–262. doi: 10.1111/j.1600-0536.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 27.Goldsmith LA. Physiology, biochemistry, and molecular biology of the skin 2. New York: Oxford University Press; 1991. pp. 1251–1278. [Google Scholar]
- 28.Panizzon RG. Radiation treatment and radiation reactions in dermatology. 2nd ed. Berlin: Springer; 2015. pp. 17–23. [Google Scholar]
- 29.Schmuth M, Sztankay A, Weinlich G, Linder DM, Wimmer MA, Fritsch PO, et al. Permeability barrier function of skin exposed to ionizing radiation. Arch Dermatol. 2001;137:1019–1023. [PubMed] [Google Scholar]
- 30.Held E, Lund H, Agner T. Effect of different moisturizers on SLS-irritated human skin. Contact Dermatitis. 2001;44:229–234. doi: 10.1034/j.1600-0536.2001.044004229.x. [DOI] [PubMed] [Google Scholar]
- 31.Mortz CG, Andersen KE, Halkier-Sørensen L. The efficacy of different moisturizers on barrier recovery in hairless mice evaluated by non-invasive bioengineering methods. A model to select the potentially most effective product. Contact Dermatitis. 1997;36:297–301. doi: 10.1111/j.1600-0536.1997.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 32.Park BD, Youm JK, Jeong SK, Choi EH, Ahn SK, Lee SH. The characterization of molecular organization of multilamellar emulsions containing pseudoceramide and type III synthetic ceramide. J Invest Dermatol. 2003;121:794–801. doi: 10.1046/j.1523-1747.2003.12470.x. [DOI] [PubMed] [Google Scholar]
- 33.McLean WH, Hull PR. Breach delivery: increased solute uptake points to a defective skin barrier in atopic dermatitis. J Invest Dermatol. 2007;127:8–10. doi: 10.1038/sj.jid.5700609. [DOI] [PubMed] [Google Scholar]
- 34.Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96:523–526. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto A, Serizawa S, Ito M, Sato Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch Dermatol Res. 1991;283:219–223. doi: 10.1007/BF01106105. [DOI] [PubMed] [Google Scholar]