Dear Editor:
Plasmacytoid dendritic cells (pDCs) are a specialized DC population1. They display plasma cell morphology and express CD4, CD123, HLA-DR, BDCA-2, and toll-like receptors (TLR)7 and TLR9 within endosomal compartments. pDCs are usually not present in normal skin, but infiltrate the skin in several cutaneous pathologies including inflammatory/autoimmune, infectious, and neoplastic entities1. Upon TLR stimulation, pDCs have the ability to secret type I interferons (IFNs) up to 1,000 times more than other cell types as well as proinflammatory cytokines including interleukin (IL)-6 and tumor necrosis factor (TNF)-α1. These lead mainly to an antiviral state and contribute to the regulation of the function of other immune cells such as myeloid DC, T-, B- and natural killer (NK) cells. Thus, pDCs provide protective immunity at the skin level by regulated sensing of microbial or self-nucleic acids upon skin damage. However, when excessive sensing of self-antigens occurs, pDCs may participate in an exaggerated self-directed immune responses contributing to the pathogenesis of different inflammatory/autoimmune cutaneous pathologies. Several studies have identified a significant role of pDCs in several inflammatory/autoimmune mucocutaneous disorders including lupus erythematosus (LE), psoriasis, and lichen planus (LP)1. However, the role of pDCs in the autoimmune blistering disorders has not been well-explored. Hence, we intend in this study to investigate pDC role in the pemphigus group of the autoimmune blistering skin diseases. This may allow us to uncover part of the underlying pathogenesis of these disorders. Our institutional review board approved the study (American University of Beirut IRB protocol DER.OA.24). Forty-six pemphigus cases (including 36 pemphigus vulgaris (PV) and 10 pemphigus foliaceus types) and 32 pemphigoid cases (29 bullous pemphigoid and 3 pemphigoid gestationis) as comparison group were retrieved from our database. Only straightforward cases that fit the clinicopathological and immunoflorescence features of the respective autoimmune blistering diseases were included. Immunohistochemical analysis was performed on sections obtained from formalin-fixed, paraffin-embedded tissue using antibodies to BDCA-2 (mouse immuno globulin G1, clone 124B3.13; Dendritics, Lyon, France) and myxovirus resistance protein A (MxA, M143; University of Freiburg, Freiburg, Germany). Anti-BDCA2 antibody is a specific pDC marker1, while anti-MxA antibody assesses type I IFN production by pDCs, since MxA is well established surrogate marker for local type I IFN production1. A semiquantitative scoring system was used to assess pDC recruitment and MxA expression (Table 1).
Table 1. pDCs presence and MxA expression in pemphigus versus pemphigoid group (%).
| Entity (case no.) | Age (yr) | Gender (ratio) | Frequency of pDC infiltration | pDC score* | MxA score† | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | ||||
| Pemphigus (n=46) | 6~81 | 20 M:26 F | 46 | 0 | 16 | 29 | 1 | 0 | 44 | 2 |
| Pemphigoid (n=32) | 47~91 | 14 M:18 F | 23 | 9 | 17 | 6 | 0 | 9 | 23 | 0 |
| p-value‡ | - | - | <0.05 | <0.05 | <0.05 | |||||
pDCs: plasmacytoid dendritic cells, MxA: myxovirus resistance A, M: male, F: female. *BDCA2+ pDC content was scored as percentage of total mononuclear infiltrate: 0 (no positive cells), 1 (1%~10% positive cells), 2 (10%~50% positive cells), 3 (>50% positive cells). †MxA staining was scored as: 0=negative, 1=patchy/weak, and 2=diffuse. ‡Statistical analysis was performed by using the Mann-Whitney test to analyze statistical differences in pDC and MxA scores between the 2 groups. A two-tailed p-value of <0.05 was considered statistically significant. Normal skin tissue served as negative control and cutaneous lupus erythematosus served as positive control.
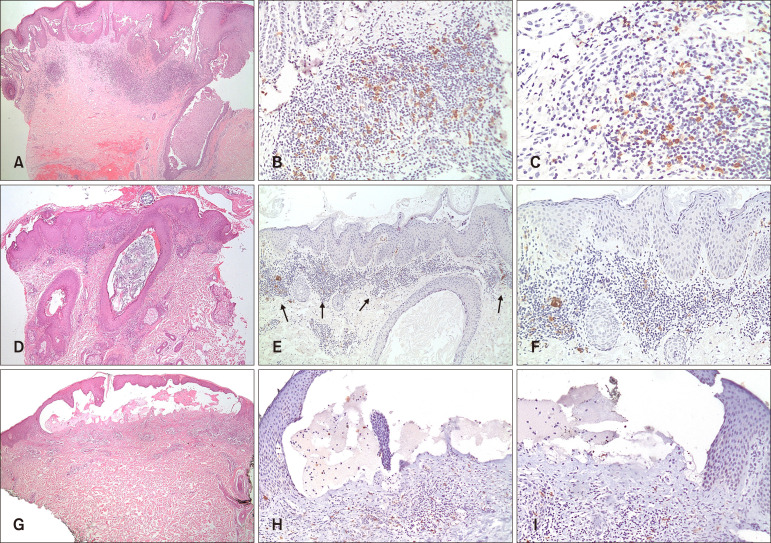
Results showed the pDCs to be present in all of the pemphigus cases (n=46) and 72% (n=23) of pemphigoid cases. However, pDCs were significantly more abundant in pemphigus cases (Fig. 1B, E) than in pemphigoid cases (Fig. 1H) with a significantly higher pDC score (p<0.05). MxA expression was mostly patchy in both pemphigus (n=44, 96%) (Fig. 1C, F) and pemphigoid (n=23, 72%) cases (Fig. 1I).
Fig. 1. (A~C) Pemphigus vulgaris. (A) Representative case showing suprabasal acantholysis with underlying dermal inflammatory infiltrate (H&E, ×40). (B, C) BDCA-2 immunostaining highlighted plasmacytoid dendritic cells (pDCs) in a superficial perivascular and interstitial distribution with pDCs making up more than 10% of the inflammatory infiltrate/pDC score of 2 (B: ×100, C: ×200). (D~F) Pemphigus foliaceus. (D) Representative case showing subcorneal acantholysis with underlying dermal inflammatory infiltrate (H&E, ×40). (E, F) BDCA-2 immunostaining highlighted pDCs in a superficial perivascular and interstitial distribution with pDCs making up more than 10% of the inflammatory infiltrate/pDC score of 2 (E: ×100, F: ×200). (G~I): Bullous pemphigoid. (G) Representative case with subepidermal blistering and underlying inflammatory infiltrate (H&E, ×100). (H, I) BDCA-2 immunostaining highlighted scattered pDCs in a perivascular and interstitial distribution with pDCs making up less than 10% of the inflammatory infiltrate/pDC score of 1 (H: ×100, I: ×100).
Our hypothesis in this study concerning pDCs role in the pemphigus group is based on several observations. First, several reports have described induction of autoimmune blistering disorders following administration of IFN-α, the endogenous local counterpart of which is mainly produced by pDCs2. Second, high-titer IFN-α antibodies have been detected in patients with autoimmune blistering disorders3. Third, imiquimod, an immunomodulator known to be a potent pDCs activator, and TNF inhibitors, known to be secondary inducers of IFN-α, have been reported to induce autoimmune blistering disorders4,5. Fourth, some autoimmune blistering disorders have been associated with viral infections. Since pDCs' mainly function in anti-viral resistance, their involvement in such autoimmune blistering disorders would not be surprising6. Finally, autoimmune blistering disorders have been associated with several inflammatory disorders such as LE, LP and psoriasis, in which evidence suggests significant pDC role in their underlying pathogenesis7.
Our study results support our hypothesis, especially in relation to a possible role for pDCs in pemphigus pathogenesis. While the contribution of the adaptive immune system has been well studied using animal models, the earlier mechanisms that contribute to the initial production of autoantibodies and loss of tolerance have not been well investigated7,8. One study demonstrated that, in the presence of Dsg3, NK cells interact with CD4+ T cells in the perilesional skin and peripheral blood of PV patients leading to the production of several cytokines, with especially high IL-6 levels. IL-6 is a pleiotropic cytokine important in the pathophysiology of inflammation and autoimmune disorders such as its role in the production of anti-DNA and chromatin autoantibodies in LE8. However, the contribution of pDCs, which can also secret IL-6, was not explored in this study8. In another study investigating the local inflammatory infiltrate in Darier's disease and using 14 PV cases as a comparison group, the authors reported the presence of CD123+ pDCs in low percentages (<5%) in PV cases9.
The results of our study and the known multifaceted immunological functions of the pDC indicate a possible role of this cell in the autoimmune blistering disorders, especially pemphigus group1. Especially with NK cells which have been implicated in the early pathogenic steps of PV pathogenesis, pDCs have been shown to have bidirectional interaction10. There is evidence that NK cells, upon cell-to-cell contact, promote pDC maturation and strongly enhance pDC production of IFN-α, TNF-α, and IL-6. On the other hand, pDCs can efficiently promote NK cell activation. In addition, several studies have shown that pDCs are critical for antibody responses through their role in promoting plasma cell differentiation from naive and memory B cells1. Actually, pDCs have been shown to induce plasma cell differentiation through the sequential action of type I IFNs and IL-610. This thus makes their possible role in the induction of autoantibodies in the autoimmune blistering disorders unsurprising.
The authors recognize that a relatively small number of cases have been studied and that the study is performed at only one point during the course of these autoimmune blistering disorders. Hence, these observations are considered preliminary.
In summary, we have shown that pDCs are recruited into the skin lesions of the autoimmune blistering disorders, with significantly higher content in the pemphigus group. Their consistent presence speaks in favor of an important role of these cells in their pathogenesis, possibly in the initial mechanisms leading to autoantibody production.
ACKNOWLEDGMENT
This research has been supported by a grant from the Medical Practice Plan (MPP) at the American University of Beirut Medical Center.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Saadeh D, Kurban M, Abbas O. Update on the role of plasmacytoid dendritic cells in inflammatory/autoimmune skin diseases. Exp Dermatol. 2016;25:415–421. doi: 10.1111/exd.12957. [DOI] [PubMed] [Google Scholar]
- 2.Niizeki H, Inamoto N, Nakamura K, Tsuchimoto K, Hashimoto T, Nishikawa T. A case of pemphigus foliaceus after interferon alpha-2a therapy. Dermatology. 1994;189(Suppl 1):129–130. doi: 10.1159/000246954. [DOI] [PubMed] [Google Scholar]
- 3.Prümmer O, Zillikens D, Porzsolt F. High-titer interferon-alpha antibodies in a patient with pemphigus foliaceus. Exp Dermatol. 1996;5:213–217. doi: 10.1111/j.1600-0625.1996.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 4.Lo Schiavo A, Sangiuliano S, Puca RV, Brunetti G, Ruocco E, Cozzi R. Contact pemphigus: a side-effect of imiquimod therapy. Int J Dermatol. 2008;47:765–767. doi: 10.1111/j.1365-4632.2008.03533.x. [DOI] [PubMed] [Google Scholar]
- 5.Boussemart L, Jacobelli S, Batteux F, Goulvestre C, Grange P, Carlotti A, et al. Autoimmune bullous skin diseases occurring under anti-tumor necrosis factor therapy: two case reports. Dermatology. 2010;221:201–205. doi: 10.1159/000318008. [DOI] [PubMed] [Google Scholar]
- 6.Ruocco E, Ruocco V, Lo Schiavo A, Brunetti G, Wolf R. Viruses and pemphigus: an intriguing never-ending story. Dermatology. 2014;229:310–315. doi: 10.1159/000365845. [DOI] [PubMed] [Google Scholar]
- 7.Vassileva S, Drenovska K, Manuelyan K. Autoimmune blistering dermatoses as systemic diseases. Clin Dermatol. 2014;32:364–375. doi: 10.1016/j.clindermatol.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Stern JN, Keskin DB, Barteneva N, Zuniga J, Yunis EJ, Ahmed AR. Possible role of natural killer cells in pemphigus vulgaris-preliminary observations. Clin Exp Immunol. 2008;152:472–481. doi: 10.1111/j.1365-2249.2008.03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miracco C, Pietronudo F, Mourmouras V, Pellegrino M, Onorati M, Mastrogiulio MG, et al. Possible implication of local immune response in Darier's disease: an immunohistochemical characterization of lesional inflammatory infiltrate. Mediators Inflamm. 2010;2010:350304. doi: 10.1155/2010/350304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wehner R, Dietze K, Bachmann M, Schmitz M. The bidirectional crosstalk between human dendritic cells and natural killer cells. J Innate Immun. 2011;3:258–263. doi: 10.1159/000323923. [DOI] [PubMed] [Google Scholar]