Abstract
Background:
Diabetes mellitus is an established risk factor for bacterial infections, but its role in cryptococcosis is unclear. The study aimed to determine whether uncontrolled diabetes (HbA1c >7%) was an independent risk factor for mortality in cryptococcosis.
Methods:
A retrospective case–control study partially matched by age and gender was performed in patients tested for Cryptococcus infection at the University of Colorado Hospital from 2000 to 2019. A multivariable logistic regression model was used to identify mortality predictors. Cox proportional hazard model was used for survival analysis.
Results:
We identified 96 cases of cryptococcosis and 125 controls. Among cases, cryptococcal meningitis (49.0%) and pneumonia (36.5%) constituted most infections. Cases with pulmonary cryptococcosis with uncontrolled diabetes had a higher mortality at 10 weeks (50% versus 7%, p = 0.006) and 1 year (66.7% versus 13.8%, p = 0.005) compared to pulmonary cases with controlled or no diabetes. Unadjusted Cox proportional hazard model found an increased rate of death for uncontrolled diabetes at 10 weeks [hazard ratio 8.4, confidence interval (CI): 1.4–50.8, p = 0.02] and 1 year (hazard ratio 7.0, CI: 1.7–28.4, p = 0.007) among pulmonary cryptococcosis cases. Multivariable analysis showed a significantly increased odds of 10 weeks [odds ratio (OR) = 4.3, CI: 1.1–16.5, p = 0.035] and 1 year (OR = 5.0, CI: 1.4–18.3, p = 0.014) mortality for uncontrolled diabetes among pulmonary cryptococcosis cases. After adjustment for gender, age, and case/control, for every 1% increase in HbA1c levels, the odds of pulmonary cryptococcosis mortality at 1 year increased by 11% (OR = 1.6, CI 95%: 1.1–2.3, p = 0.006).
Conclusion:
Uncontrolled diabetes is associated with worse outcomes in pulmonary cryptococcosis, including a 4-fold and 6-fold increased odds of death at 10 weeks and 1 year, respectively. Glucose control interventions should be explored to improve clinical outcomes in patients with pulmonary cryptococcosis.
Keywords: cryptococcosis, Cryptococcus, diabetes mellitus, glycemic levels, mortality, risk factors
Introduction
Diabetes mellitus (diabetes) is one of the most prevalent non-communicable diseases in the United States. The American Diabetes Association estimates that 34.2 million Americans (10.5% of the population) are affected,1 and the prevalence increases with age. Among those aged 65 or older, diabetes is present in 26.8%. A common complication among patients with diabetes is infections, which carries a higher risk of mortality compared with adults without diabetes.2 Pneumonia and sepsis are the most common infections associated with mortality in diabetes. The pathogenesis of hyperglycemia-induced immune dysfunction includes reduced neutrophil chemotaxis, adhesion, and migration, and decreased intracellular production of hydrogen peroxide.3,4 Diabetes is also a known risk factor for invasive fungal infections, such as mucormycosis.5 However, we lack reports of diabetes or increased glucose levels as independent risk factors for opportunistic infections commonly affecting advanced immunocompromised states, including Cryptococcus.
Cryptococcosis continues to be a source of morbidity and mortality—up to 20%—among immunocompromised hosts.6 Cryptococcal meningitis has a significant global impact with 181,100 deaths each year among HIV-infected individuals.7 Moreover, developing countries have seen an increasing incidence of the disease among non-HIV immunocompromised hosts as well.8,9 Solid-organ transplantation, systemic lupus erythematosus, malignancy, sarcoidosis, and cirrhosis are conditions of functional immunosuppression known to increase the risk for Cryptococcus spp. infection.10–15 Diabetes is often listed as an additional risk factor for pulmonary cryptococcosis, especially among HIV-negative patients.16,17
Diabetes is a common comorbidity among adult patients in the U.S. with cryptococcosis. However, the role of diabetes in the prevalence and associated mortality of Cryptococcus infections is not well defined. The study aimed to determine whether diabetes and hemoglobin A1c (HbA1c) levels were independent risk factors for mortality in Cryptococcus infection.
Methods
Patients and data collection
We collected data on all patients with culture from any site that grew Cryptococcus or with detection of antigens from serum or cerebrospinal fluid (CSF) diagnosed at the University of Colorado Hospital microbiology laboratory between January 2000 and October 2019.
Controls were randomly chosen among patients with negative serum and, when done, CSF cryptococcal antigen and culture. A manual chart review was performed to exclude clinical diagnoses of cryptococcosis in these patients. Controls were partially matched to cases by specimen collection date, age, and biological sex. Controls were mainly hospitalized but also ambulatory patients from the same community served by the University of Colorado Hospital. Most control patients had suspicions of having cryptococcosis based on their underlying immunosuppressive state and clinical concerns for meningitis or abnormal pulmonary findings.
The cryptococcal antigen tests used were an enzyme immunoassay test (Meridian Premier Cryptococcal assay) before 2013 and the lateral flow assay [CrAg® LFA, Immuno-Mycologics Inc. (IMMY), Norman, OK, USA] after 2013. Cryptococcal meningitis was diagnosed based on a positive cryptococcal CSF antigen or positive CSF culture. The assay did not differentiate the species of Cryptococcus. Medical reports were manually accessed to collect clinical and laboratory variables for all patients. The following data was retrospectively collected through RedCap (electronic data capture tools hosted at the University of Colorado Denver): demographics (gender, race, and age); symptoms (constitutional, headaches, altered mental status, respiratory abnormalities, fever, and others), medical history (smoking, lung disease, diabetes, hypertension, lupus, malignancy, sarcoidosis, cirrhosis, HIV infection, solid organ transplant, use of calcineurin inhibitors or corticosteroids, and prednisone dose); transplant (type and time since transplant); vital signs at collection times (systolic blood pressure, diastolic blood pressure, pulse, and temperature); weight and body mass index (BMI); diagnosis of cryptococcal meningitis; laboratory results (complete blood cell count, comprehensive metabolic panel, baseline renal function, and blood culture), and outcomes of cryptococcal infection: 10 weeks, 1 year, and overall mortality (per consensus criteria established by the Mycoses Study Group and European Organization for Research and Treatment of Cancer18), cognitive deficits, muscle weakness, speech difficulties, hearing impairment, stroke by magnetic resonance imaging results; and use of ventriculoperitoneal shunts. We included recorded deaths by all causes. Uncontrolled diabetes was defined as the most recent HbA1c level >7%. Electronic medical records (EPIC, Verona, WI, USA) were automatically interrogated for HbA1c levels and date of death from the cohort of patients through software supported by Health Data Compass Data Warehouse project (healthdatacompass.org). The most recent HbA1c available before admission was chosen for analysis. The vast majority of patients with a diagnosis of diabetes had an HbA1c level available.
Statistical analysis
Statistical analyses were performed using STATA software, version 12.1 (StataCorp, College Station, TX, USA). We had crude mortality as our primary outcome at 10 weeks and 1 year. The means and standard deviations for continuous variables were calculated. For categorical variables, frequencies and percentages were calculated. We initially performed a bivariate analysis for dichotomous outcomes variables using chi-squared, and the Fisher exact test for dichotomous and nominal independent variables, respectively. For interval independent variables, we used the t-test. The uncontrolled diabetes group was compared with patients with controlled diabetes (HbA1c ⩽7%) or no diabetes. For the multivariable analysis, we selected age, sex, commonly known cryptococcosis risk factors (HIV, solid organ transplant, cirrhosis, malignancy, and steroids), smoking, factors associated with increased mortality in cryptococcosis (altered mental status and positive blood cultures for Cryptococcus), and case versus control status. We did not include co-linear variables or variables with a significant number of missing values. We did not use any data imputation calculation for those variables with significant missing values. Selected variables were included in a multivariable, forward, stepwise logistic regression model. A parallel conditional logistic regression model was run for comparison. Kaplan–Meier survival curves were constructed to show cumulative mortality over the study period for cryptococcosis cases by uncontrolled diabetes mellitus status. Cox regression was used to estimate the mortality rate ratio among pulmonary cryptococcosis cases with and without uncontrolled diabetes. Due to heterogeneous controls, we also ran a propensity score matching analysis. Linearity between HbA1c and days from diagnosis to death was checked using a scatter plot and R-squared/adjusted R-squared from analysis of variance (ANOVA).
Data access
The corresponding author had full access to data in the study and had final responsibility for the decision to submit the manuscript for publication. The datasets generated and analyzed in the current study are available from the corresponding author on reasonable request.
Results
We included a total of 96 cases and 125 controls. At least 55 cases and 112 controls were matched by specimen collection date, age, and gender.
Clinical characteristics of patients with cryptococcosis
Most subjects were men (79%). The average age was 54.1 years, and most subjects were Caucasian with an upper limit of normal BMI. Diabetes was present in about one-quarter of the cases, with no statistical difference with controls. Among cases, cryptococcal meningitis (49.0%) and pneumonia (36.5%) constituted most infections, followed by skin (8.3%) and asymptomatic antigenemia (6.3%). Other common risk factors among cases were HIV (38.9%), steroid use (24.7%), malignancy (21.1%), solid organ transplantation (18.1%), and cirrhosis (5.2%). Overall mortality was similar among cases and controls (38.5% versus 39.2%, p = 0.921).
Clinical characteristics of patients with pulmonary cryptococcosis
Pulmonary cryptococcosis cases (n = 35) were older and were more likely to be HIV negative (Table 1). They commonly presented with respiratory symptoms as opposed to headaches and altered mental status. Controls shared similar demographics and risk factors. Mean hemoglobin and platelet levels were within or close to normal limits and similar among the two groups. Creatinine levels were similar among cases and controls. Although HbA1c levels were higher among pulmonary cryptococcosis cases, they were not statistically significant. Intensive care unit (ICU) stay, overall mortality, and mortality at 10 weeks and 1 year were similar to controls.
Table 1.
Patient clinical characteristics for cases and controls.
| Patient characteristics—n (%), mean ± SD | N | Controls n = 125* |
Pulmonary cryptococcosis cases n = 35* |
p-value |
|---|---|---|---|---|
| Demographics | ||||
| Gender, male | 221 | 106 (84.8%) | 27 (77.1%) | 0.285 |
| Age, years | 221 | 53.6 ± 14.9 | 59.5 ± 14.5 | 0.038 |
| Race, White | 126 | 73 (63.5%) | 22 (64.7%) | 0.242 |
| BMI, kg/m2 | 147 | 25.3 ± 5.9 | 24.9 ± 4.7 | 0.757 |
| Risk factors | ||||
| Smoking, current | 111 | 30 (24.4%) | 4 (11.4%) | 0.223 |
| Transplant | 219 | 28 (22.4%) | 9 (26.5%) | 0.618 |
| Diabetes mellitus | 221 | 25 (20%) | 9 (25.7%) | 0.465 |
| Uncontrolled diabetes mellitus | 211 | 9 (7.6%) | 6 (17.1%) | 0.093 |
| HIV | 220 | 46 (36.8%) | 6 (17.7%) | 0.035 |
| Malignancy | 220 | 31 (24.8%) | 12 (35.3%) | 0.222 |
| Cirrhosis | 221 | 6 (4.8%) | 1 (2.9%) | 0.619 |
| Steroids | 217 | 27 (21.8%) | 9 (27.3%) | 0.504 |
| Symptoms at presentation | ||||
| Headaches | 219 | 33 (26.6%) | 2 (5.9%) | 0.010 |
| Altered mental status | 221 | 48 (38.4%) | 4 (11.4%) | 0.003 |
| Respiratory | 220 | 32 (25.8%) | 28 (80%) | <0.001 |
| Labs | ||||
| Hemoglobin, g/dL | 215 | 12.3 ± 2.6 | 11.9 ± 2.9 | 0.513 |
| Platelets, 109/L | 214 | 207.9 ± 135.2 | 207.7 ± 84 | 0.992 |
| Creatinine, mg/dl | 208 | 1.3 ± 1.2 | 1.3 ± 0.8 | 0.843 |
| CD4 count, cells/µL | 86 | 206 ± 224.9 | 200.9 ± 319.1 | 0.952 |
| HbA1c, % | 96 | 6.24 ± 1.8 | 6.8 ± 1.7 | 0.252 |
| Outcome | ||||
| ICU stay | 53 | 27 (36%) | 7 (30.4%) | 0.624 |
| Overall death | 221 | 49 (39.2%) | 15 (42.9%) | 0.696 |
| Death within 10 weeks | 221 | 12 (9.6%) | 5 (14.3%) | 0.427 |
| Death within 1 year | 221 | 28 (22.4%) | 8 (22.9%) | 0.954 |
BMI, body mass index; HbA1c, hemoglobin A1c; ICU, intensive care unit.
some percentages were calculated on a different number due to missing values.
Clinical factors associated with an increase of 10-weeks and 1-year mortality from cryptococcosis
We found 10-weeks and 1-year mortality of 19% and 22% among cryptococcosis cases. The 10-weeks mortality was significantly higher in cases compared with controls (18.8% versus 9.6%, p = 0.049). At 10 weeks, diabetes (44.4% versus 21.8%, p = 0.048) and uncontrolled diabetes (31.3% versus 7.9%, p = 0.009) were more commonly present in those who died. Conversely, transplant recipients were less likely to die shortly after diagnosis (5.6% versus 21%, p = 0.001). Altered mental status, anemia, and lower mean platelet count associate with an increase in 10-weeks mortality (Table 2). At 1 year, transplant recipients maintained lower mortality compared with non-transplant recipients (5.9% versus 29.9%, p = 0.04). Patients with a history of malignancy were more like to die at 1 year (40.9% versus 20.6%, p = 0.05), as well as patients with a history of uncontrolled diabetes (54.5% versus 19.8%, p = 0.01) and patients with altered mental status (47.8% versus 17.8%, p = 0.004) or respiratory symptoms (34.8% versus 17%, p = 0.04). At 1 year, non-survivors had lower hemoglobin levels (10.8 ± 2.4 versus 12.1 ± 2.6 mg/dL, p = 0.03) and higher HbA1c levels (7.8 ± 1.9% versus 6.1 ± 1.1%, p = 0.003). Cryptococcosis patients who died at 10 weeks and 1 year had more commonly a history of speech difficulties and ICU stay. Among cryptococcosis cases, the 10-weeks and 1-year mortality was higher among those with uncontrolled diabetes: 45.5% versus 13.6%, p = 0.009, and 54.5% versus 19.8%, p = 0.01 respectively.
Table 2.
Patient clinical characteristics among survivors and non-survivors at 10 weeks.
| Patient characteristics, n (%), mean ± SD | N | Cases, survivors at 10 weeks n = 78** |
Cases, non-survivors at 10 weeks n = 18** |
p-value* |
|---|---|---|---|---|
| Type of infection | 96 | |||
| Cryptococcal meningitis | 36 (46.2%) | 11 (61.1%) | 0.818 | |
| Pulmonary disease | 30 (38.5%) | 5 (27.8%) | ||
| Skin and others | 7 (9%) | 1 (5.6%) | ||
| Asymptomatic antigenemia | 5 (6.4%) | 1 (5.6%) | ||
| Symptoms at presentation | ||||
| Headaches | 219 | 27 (34.6%) | 5 (29.4%) | 0.681 |
| Altered mental status | 221 | 14 (18%) | 9 (50%) | 0.004 |
| Respiratory | 220 | 32 (41%) | 11 (61.1%) | 0.122 |
| Labs | ||||
| Hemoglobin, g/dL | 215 | 12.1 ± 2.6 | 10.4 ± 2.0 | 0.007 |
| Platelets, 109/L | 214 | 216 ± 92.1 | 151 ± 161 | 0.025 |
| Creatinine, mg/dl | 208 | 1.3 ± 0.8 | 1.5 ±1.1 | 0.309 |
| CD4 count, cells/µL | 86 | 155 ± 250.8 | 202 ± 409 | 0.679 |
| HbA1c, % | 96 | 6.2 ± 1.1 | 7.8 ± 2.0 | 0.005 |
| CSF profile | ||||
| Opening pressure, cm H2O | 54 | 28.4 ± 11.6 | 14.8 ± 1.9 | 0.055 |
| CSF WBC, 106/L | 131 | 447 ± 2376 | 55.4 ± 83.1 | 0.589 |
| CSF glucose, mg/dL | 130 | 43.9 ± 21 | 73 ± 58.3 | 0.009 |
| CSF protein, mg/dL | 132 | 121 ± 193 | 120 ± 159 | 0.988 |
| Outcome | ||||
| ICU stay | 53 | 9 (20%) | 8 (100%) | <0.001 |
| Overall death | 221 | 19 (24.4%) | 18 (100%) | <0.001 |
| Death within 10 weeks | 221 | 0 (0%) | 18 (100%) | N/A |
| Death within 1 year | 221 | 6 (7.7%) | 18 (100%) | <0.001 |
| VP shunt | 83 | 3 (4.0%) | 1 (11%) | 0.351 |
| Cognitive deficits | 78 | 12 (16.4%) | 2 (40%) | 0.184 |
| Hearing impairment | 78 | 6 (8.1%) | 0 (0%) | 0.553 |
| Speech difficulties | 80 | 2 (2.7%) | 1 (20%) | 0.048 |
| Muscle weakness | 79 | 18 (24%) | 1 (25%) | 0.964 |
| Stroke | 34 | 5 (19.2%) | 3 (37.5%) | 0.287 |
CSF, cerebrospinal fluid; HbA1c, hemoglobin A1c; ICU, intensive care unit; VP, ventriculoperitoneal; WBC, white blood cell.
p-value sigificant <0.05.
some percentages were calculated on a different number due to missing values.
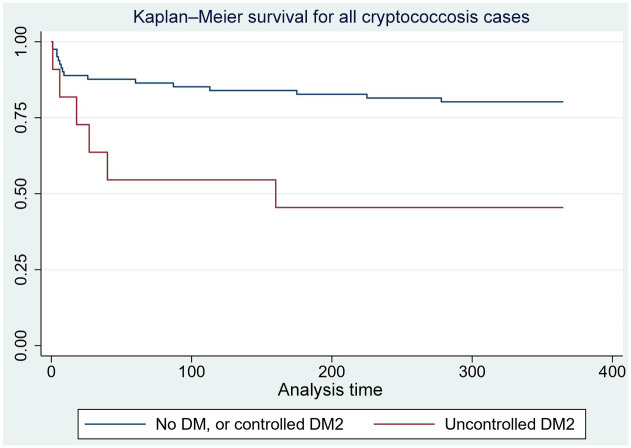
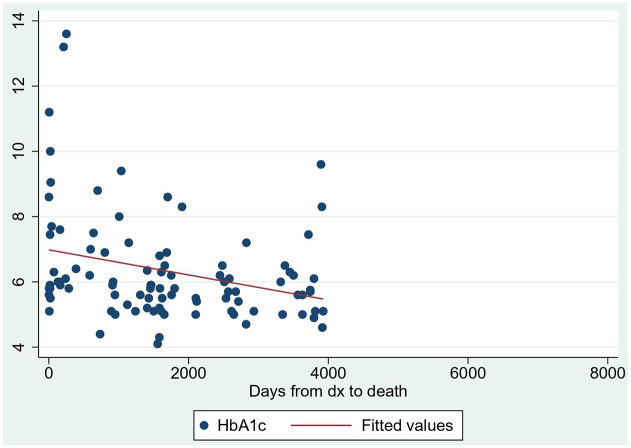
Unadjusted Cox proportional hazard model found an increased rate of death for uncontrolled diabetes at 10 weeks [hazard ratio 3.7, confidence interval (CI): 1.3–10.8, p = 0.01] and 1 year (hazard ratio 3.4, CI: 1.3–8.8, p = 0.01) (Figure 1). After adjustment for gender, age, and case/control, for every 1% increase in HbA1c levels, the odds of mortality increased by 40% [odds ratio (OR) = 1.4, 95% CI: 1.0–1.9, p = 0.045]. The scatter plot showed some degree of linearity, and the ANOVA R-squared/adjusted R-squared were 95% and 6%, respectively (Figure 2).
Figure 1.
Survival curves at 1 year of cryptococcosis cases by controlled or uncontrolled diabetes mellitus.
DM, diabetes mellitus; DM2: type 2 diabetes mellitus. X-axis: time in days; Y-axis: fraction survival.
Figure 2.
Scatter plot of HbA1c levels and days from diagnosis to death.
dx, diagnosis; HbA1c, hemoglobin A1c. Y-axis: HbA1c (%).
The multivariable analysis adjusted by gender, age, case/control, common risk factors for cryptococcosis (solid organ transplantation, steroid use, HIV infection, and smoking history), and factors associated with increased mortality (altered mental status and positive blood cultures for Cryptococcus) revealed an independent risk of death with uncontrolled diabetes (HbA1c >7%) at 10 weeks (OR = 3.6, CI: 1.1–12.3, p = 0.037) and 1 year (OR = 6.6, CI: 2.0–21.4, p = 0.002) (Table 3).
Table 3.
Multivariable analysis of mortality predictors.
| 10 weeks | |||
|---|---|---|---|
| Variable | Odds ratio | Confidence interval | p-value |
| Uncontrolled DM* | 3.6 | 1.1–12.3 | 0.03 |
| Malignancy | 3.4 | 13.–9.1 | 0.01 |
| Cryptococcosis case | 4.0 | 1.5–10.5 | 0.005 |
| Altered mental status | 1.4 | 0.8–5.2 | 0.157 |
| 1 year | |||
| Variable | Odds ratio | Confidence interval | p-value |
| Malignancy | 6.8 | 2.8–16.4 | 0.0001 |
| Uncontrolled DM* | 6.6 | 2.0–21.4 | 0.002 |
| Positive blood cultures** | 2.6 | 0.8–8.5 | 0.106 |
| HIV | 1.9 | 0.8–4.5 | 0.159 |
HbA1c >7%.
For Cryptococcus spp.
DM, diabetes mellitus; HbA1c, hemoglobin A1c.
The propensity score matching with the exposure confounders revealed an average treatment effect (ATE) of 0.3 (p = 0.03) and 0.4 (p = 0.0001) at 10 weeks and 1 year respectively for uncontrolled diabetics. Expressing these results on a percentage scale, the chance of dying at 10 weeks and 1 year is higher by 30% and 40% percentage points for uncontrolled diabetics respectively. A multivariable sensitivity analysis of the cohort—excluding controls with controlled diabetes (HbA1c <7%)—showed also an independent risk of death with uncontrolled diabetes (HbA1c >7%) among cases at 1 year (OR = 7.1, CI: 1.8–28.7, p = 0.006). The multivariable (MV) analysis also showed a significant increase in 10-weeks (OR: 6.0, CI: 1.5–24.1, p = 0.012) and 1-year mortality (OR: 4.4, CI: 1.5–13.0), p = 0.008) using a different HbA1c cutoff (HbA1c >8%) as uncontrolled diabetes. Pulmonary cases versus controls sensitivity analysis—among the subgroup of patients with well-controlled diabetes or without diabetes—showed mortality at 10 weeks of 7% versus 9%, p = 0.708 and at 1 year of 13.8% versus 20.9%, p = 0.389.
Clinical factors associated with an increase of 10-weeks and 1-year mortality from pulmonary and meningeal cryptococcosis
For cryptococcal meningitis cases only, the mortality was higher for those with uncontrolled diabetes at 10 weeks (50% versus 19.1%, p = 0.289) and 1 year (50% versus 26.2%, p = 0.46), although not statistically significant. Cases with pulmonary cryptococcosis with uncontrolled diabetes had a higher mortality at 10 weeks (50% versus 7%, p = 0.006) and 1 year (66.7% versus 13.8%, p = 0.005) compared to pulmonary cryptococcosis cases with controlled or no diabetes.
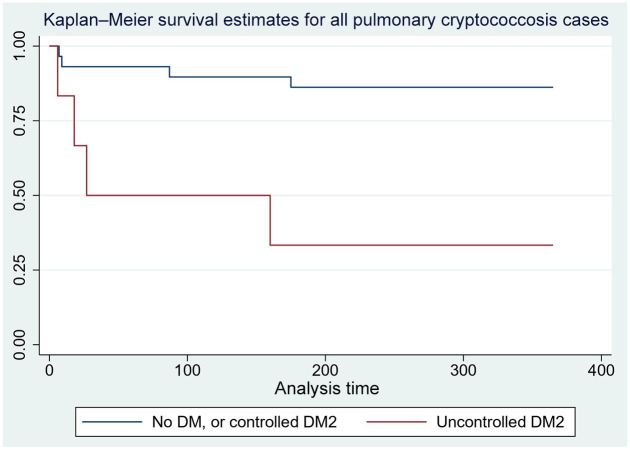
Unadjusted Cox proportional hazard model found an increased rate of death for uncontrolled diabetes at 10 weeks (hazard ratio 8.4, CI: 1.4–50.8, p = 0.02) and 1 year (hazard ratio 7.0, CI: 1.7–28.4, p = 0.007) among pulmonary cryptococcosis cases (Figure 3).
Figure 3.
Survival curves at 1 year of pulmonary cryptococcosis cases by controlled or uncontrolled diabetes mellitus.
DM, diabetes mellitus; DM2: type 2 diabetes mellitus. X-axis: time in days; Y-axis: fraction survival.
Multivariable analysis showed a significantly increased odds of 10-weeks (OR = 4.3, CI: 1.1–16.5, p = 0.035) and 1-year (OR = 5.0, CI: 1.4–18.3, p = 0.014) mortality for uncontrolled diabetes among pulmonary cryptococcosis cases only. Malignancy was also independently associated with increased mortality.
Clinical features and outcomes of cryptococcosis cases with diabetes and uncontrolled diabetes
Diabetes was the only known risk factor in six cases (6.3%), with four of these cases complicated by chronic kidney disease; diabetes coexisted with additional risk factors in 19 cases (19.8%). Mean HbA1c values were 5.4%, 6.9%, and 8.5% among cases without diabetes, cases with diabetes and additional risk factors, and cases with diabetes only as a risk factor, respectively. Cases with diabetes as a risk factor were older and had higher BMIs. They also were more likely to have a solid organ transplant as a risk factor, and less likely to be HIV-positive or current smokers. HbA1c levels were higher than 7% in 31.4% of the cryptococcosis cases. Labs showed a statistically significant lower mean of hemoglobin levels (p = 0.007) and an increase in the mean of creatinine levels (p = 0.002) among cryptococcal cases with diabetes. Cases with diabetes also had a non-statistically significant higher rate of paucicellular CSF values. Only one of the six cases with only diabetes as a risk factor had disseminated disease or cryptococcal meningitis. Patients with diabetes showed no statistically significant higher rates of ICU stay, death within 10 weeks, or death within 1 year, but they have an increased overall death rate (any death recorded during the study period) (60% versus 31%, p = 0.01, rate ratio 2.5, CI: 1.3–4.8, p = 0.007). Stroke as a complication was seen more often in patients with diabetes, but this association was not statistically significant.
Uncontrolled diabetes patients had a lower rate of HIV infection (9% versus 45%, p = 0.02), were older (64 ± 8.8 versus 52 ± 14.6, p = 0.008), and had most likely a pulmonary cryptococcal infection (54% versus 38.8%, p = 0.02). Their BMI was also higher than controlled or non-diabetics, although this was not statistically significant.
Discussion
We found in this single retrospective case–control study of 35 patients with pulmonary cryptococcosis an association of uncontrolled diabetes (HbA1c >7%) with an increase of 10-weeks and 1-year mortality. These findings remained after adjusting for commonly known cryptococcosis risk factors and other factors associated with disease mortality. Although the association remained for all cryptococcosis cases, the effect seems to be driven mainly by the pulmonary cases.
Survival curves demonstrated a decreased chance of survival among patients with cryptococcosis—and also pulmonary cryptococcosis—and uncontrolled diabetes compared with cryptococcosis patients without diabetes or with controlled diabetes. This difference was evident early in the diagnosis. Death from cryptococcosis among people with diabetes in our cohort approached 20% at 10 weeks and 22% at 1 year, which was comparable to other cohorts. Uncontrolled diabetes correlates with increased age, end-organ failure, the presence of comorbidities, and a suboptimal immune response. Poor glycemic control associates with increased rates of serious infections and infection-related hospitalizations.19 These factors could play a role in the observed increased mortality in cryptococcosis.
We also found diabetes to be a common comorbidity among patients with Cryptococcus infection, and it often accompanied other established risk factors, particularly transplant status and malignancy. Diabetes as the only identifiable risk factor was relatively uncommon, although it was frequently complicated by chronic kidney disease. A recent case series identified cryptococcal meningitis as a complication of patients with nephrotic syndrome.20 The presence of diabetes mellitus translated into an observed increased chance of overall death, but it was not associated with an increase in 10-weeks or 1-year mortality.
Other US cryptococcosis cohorts have reported a prevalence of diabetes of around 20%,8 similar to ours of 25%. These numbers correlate with the number of people living with diabetes based on age in the US.1 An Argentinian study found a marked increase in mortality in cryptococcal meningitis patients with AIDS and diabetes compared with those with AIDS without diabetes (85.7% versus 21.4%).21 Matched cohort studies have identified a 20% increase in the risk of any infection among patients with diabetes.22 This risk is modulated by increasing HbA1c levels.19 Since compromised cell-mediated immunity is present in most patients affected with cryptococcosis, one possible mechanistic explanation is the altered function of CD8+ T-cells and natural killer cells in patients with diabetes.23 Decreased macrophage and cytokine function (such as interleukin-12) may play a role as well.24,25 Patients with diabetic nephropathy may also confer additional immune response impairments. Reports have shown impairments in cell-mediated immunity in chronic kidney disease,26 including decreased CD4 and CD8 cell lines27 and T-cell proliferation.28 Solid organ transplant recipients often receive steroids as part of their immunosuppressive regimens, which in turn increases the risk to develop diabetes. The presence of diabetes in invasive infections often increases the risk of multiorgan injury and death.29
Some cohorts have not found diabetes as a predictor of mortality with cryptococcosis,6,30 but those cohorts did not list the HbA1c levels, which is more specific for diabetes-associated complications. In a case series of 30 cases of cryptococcosis in diabetics in mainland China, 57% of patients did not list an additional underlying risk factor, and 40% had non-disseminated pulmonary cryptococcosis.31 A case–control study in Taiwan found that HIV-negative patients with cryptococcosis were more likely to have diabetes (OR: 1.5), and the presence of diabetes was also associated with an increase in 1-year mortality from cryptococcosis.32 Finally, a case series in Japan found diabetes mellitus in 32% of patients with pulmonary cryptococcosis.17
We also found that non-survivors at 1 year had lower hemoglobin levels and higher HbA1c levels. The relationship between HbA1c levels and mortality may not be completely linear. There is evidence to suggest that HbA1C values are affected by changes in the erythrocyte lifespan. Iron-deficiency anemia, which increases the lifespan of erythrocytes resulting in increased glycation, is associated with falsely elevated HbA1C values. Other types of anemia, such as hemolytic anemia, decrease the lifespan of erythrocytes. This is associated with falsely decreased HbA1C levels.33,34 In this study, uncontrolled diabetes (HbA1C level >7%) was found to be a significant predictor of mortality in patients with Cryptococcus infection. Since non-survivors had a mean hemoglobin level of 10.4, anemia could be a possible confounder.
Uncontrolled diabetes in pulmonary cryptococcosis was independently associated with an increase in 10-weeks and 1-year mortality risk. Although cryptococcal meningitis had higher mortality rates at the same timepoints, it did not reach statistical difference. Possible explanations are the low number of cases studied or intrinsic factors of hosts with pulmonary cryptococcosis driving the mortality differences. Malignancy can be more common among patients with pulmonary cryptococcosis as opposed to cryptococcal meningitis. However, the association of mortality with uncontrolled diabetes remained after adjusting for this variable.
Another finding was that transplant recipients had lower mortality at 1 year compared with non-transplant patients. This is consistent with the literature, in which transplant status is associated with lower mortality compared with non-transplant status.35,36 Delay in diagnosis in HIV-negative, non-transplant patients compared with transplant recipients associates with worse outcomes.
There are few limitations to this study. The retrospective selection of data limits the reliability of the predictors and limits the number of variables analyzed. We could not control for all variables associated with cryptococcal mortality due to missing available data in our cohort. Some selection bias may exist as different observers worked on data collection. Also, the number of cases with uncontrolled diabetes was relatively low, contributing to a lack of power. We employed measures to reduce biases by adjusting for confounders. Controls were identified by a negative cryptococcal antigen, which may not be representative of the overall at-risk population and biases towards the null underestimating of the possible effects. Finally, since data was collected over two decades and some out-of-the-state deaths might not have been recorded, death rates could have been underestimated. However, our paper has been the best exercise you can do to try to answer the role of uncontrolled diabetes mellitus in cryptococcal mortality with limited available data. A low prevalence of these patients hampers investigations designed to establish the role of glucose levels in mortality in patients with cryptococcosis. A definitive understanding of glucose levels with mortality in these patients necessitates large prospective studies. Collecting sufficient patients for this kind of definitive study is not feasible since it would require excessively prolonged periods of subject accrual and multi-institution enrollment. Therefore, single-institution retrospective studies can generate new hypotheses and unveil or propose new associations.
Diabetes alone is an uncommon but possible risk factor by itself for acquiring Cryptococcus infection. It often accompanies additional risk factors, and it is commonly complicated by chronic kidney disease. Uncontrolled diabetes in cryptococcosis—especially pulmonary infection—may worsen outcomes from infection, leading to increased mortality. It remains to be determined whether glucose control interventions can improve clinical outcomes in patients with cryptococcal infection, and we need follow-up studies for validation of the findings in additional external cohorts.
Footnotes
Author contributions: Conceptualization and design: SA, SS, AHM; data acquisition: SA, PC, KS, AHM; data analysis and interpretation: AHM, CFP, IS; original draft writing: SA, AHM; original draft review and editing: AG, PC, SC, WM, MB, JO, EM, DC, KD, LS, IS, CFP; statistical analysis: SS, AHM; supervision: CFP, AHM.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics statement: The present investigation is in the Health Insurance Portability and Accountability Act (HIPAA) compliance and was approved by the Colorado Multiple Institutional Review Board (COMIRB) at the University of Colorado Denver. Analysis of clinical data has been performed under an approved protocol (COMIRB Protocol 15-1340) and an exemption of informed consent was granted.
ORCID iDs: Sindhu Chadalawada  https://orcid.org/0000-0002-6669-8363
https://orcid.org/0000-0002-6669-8363
Daniel B. Chastain  https://orcid.org/0000-0002-4018-0195
https://orcid.org/0000-0002-4018-0195
Carlos Franco-Paredes  https://orcid.org/0000-0001-8757-643X
https://orcid.org/0000-0001-8757-643X
Andrés F. Henao-Martínez  https://orcid.org/0000-0001-7363-8652
https://orcid.org/0000-0001-7363-8652
Contributor Information
Solana Archuleta, School of Medicine, Division of Infectious Diseases, University of Colorado Denver, Aurora, CO, USA.
Amal A. Gharamti, Department of Internal Medicine, American University of Beirut, Beirut, Lebanon
Stefan Sillau, Department of Neurology, Division of Infectious Diseases, University of Colorado Denver, Aurora, CO, USA.
Paula Castellanos, Universidad de Manizales, Manizales, Colombia.
Sindhu Chadalawada, NRI General Hospital, Guntur, India.
William Mundo, School of Medicine, Division of Infectious Diseases, University of Colorado Denver, Aurora, CO, USA.
Mehdi Bandali, School of Medicine, Division of Infectious Diseases, University of Colorado Denver, Aurora, CO, USA.
Jose Oñate, Universidad del Valle, Cali, Colombia.
Ernesto Martínez, Universidad del Valle, Cali, Colombia.
Daniel B. Chastain, Department of Clinical and Administrative Pharmacy, University of Georgia College of Pharmacy, Albany, GA, USA
Kristen DeSanto, Health Sciences Library, University of Colorado Denver, Aurora, Colorado, USA.
Leland Shapiro, Department of Medicine, Division of Infectious Diseases, University of Colorado Denver, Aurora, CO, USA; Rocky Mountain Regional Veterans Affairs Medical Center, Aurora, CO, USA; The Emily Foundation for Medical Research, Boston, MA, USA.
Ilan S. Schwartz, Division of Infectious Diseases, Department of Medicine, Faculty of Medicine & Dentistry, University of Alberta, Edmonton, AB, Canada
Carlos Franco-Paredes, Department of Medicine, Division of Infectious Diseases, University of Colorado Denver, Aurora, CO, USA; Hospital Infantil de México, Federico Gómez, México City, México.
Andrés F. Henao-Martínez, Department of Medicine, Division of Infectious Diseases, University of Colorado Denver, 12700 E. 19th Avenue, Mail Stop B168, Aurora, CO 80045, USA.
References
- 1. Center for Disease Control and Prevention. National diabetes statistics report 2020, https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. (accessed, November 1, 2020)
- 2. Bertoni AG, Saydah S, Brancati FL. Diabetes and the risk of infection-related mortality in the U.S. Diabetes Care 2001; 24: 1044–1049. [DOI] [PubMed] [Google Scholar]
- 3. Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab 2012; 16(Suppl. 1): S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alba-Loureiro TC, Hirabara SM, Mendonca JR, et al. Diabetes causes marked changes in function and metabolism of rat neutrophils. J Endocrinol 2006; 188: 295–303. [DOI] [PubMed] [Google Scholar]
- 5. Ibrahim AS, Spellberg B, Walsh TJ, et al. Pathogenesis of mucormycosis. Clin Infect Dis 2012; 54(Suppl. 1): S16–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with cryptococcosis according to immune status. PLoS One 2013; 8: e60431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. George IA, Spec A, Powderly WG, et al. Comparative epidemiology and outcomes of Human Immunodeficiency virus (HIV), non-HIV non-transplant, and solid organ transplant associated cryptococcosis: a population-based study. Clin Infect Dis 2018; 66: 608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kashef Hamadani BH, Franco-Paredes C, McCollister B, et al. Cryptococcosis and cryptococcal meningitis: new predictors and clinical outcomes at a United States academic medical centre. Mycoses 2018; 61: 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baddley JW, Perfect JR, Oster RA, et al. Pulmonary cryptococcosis in patients without HIV infection: factors associated with disseminated disease. Eur J Clin Microbiol Infect Dis 2008; 27: 937–943. [DOI] [PubMed] [Google Scholar]
- 11. Wang LR, Barber CE, Johnson AS, et al. Invasive fungal disease in systemic lupus erythematosus: a systematic review of disease characteristics, risk factors, and prognosis. Semin Arthritis Rheum 2014; 44: 325–330. [DOI] [PubMed] [Google Scholar]
- 12. Cancelli I, Merlino G, Serafini A, et al. Sarcoidosis as risk factor for cryptococcal meningitis in an apparently immunocompetent patient. Neurol Sci 2008; 29: 33–35. [DOI] [PubMed] [Google Scholar]
- 13. Lin YY, Shiau S, Fang CT. Risk factors for invasive Cryptococcus neoformans diseases: a case-control study. PLoS One 2015; 10: e0119090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henao-Martinez AF, Beckham JD. Cryptococcosis in solid organ transplant recipients. Curr Opin Infect Dis 2015; 28: 300–307. [DOI] [PubMed] [Google Scholar]
- 15. Henao-Martinez AF, Gross L, McNair B, et al. Risk factors for cryptococcal meningitis: a single United States center experience. Mycopathologia 2016; 181: 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Setianingrum F, Rautemaa-Richardson R, Denning DW. Pulmonary cryptococcosis: a review of pathobiology and clinical aspects. Med Mycol 2019; 57: 133–150. [DOI] [PubMed] [Google Scholar]
- 17. Kohno S, Kakeya H, Izumikawa K, et al. Clinical features of pulmonary cryptococcosis in non-HIV patients in Japan. J Infect Chemother 2015; 21: 23–30. [DOI] [PubMed] [Google Scholar]
- 18. Segal BH, Herbrecht R, Stevens DA, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for research and treatment of cancer consensus criteria. Clin Infect Dis 2008; 47: 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Critchley JA, Carey IM, Harris T, et al. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care 2018; 41: 2127–2135. [DOI] [PubMed] [Google Scholar]
- 20. Hu D, Zhang Q, Jiang W, et al. Cryptococcal meningitis: a rare complication in HIV-negative patients with nephrotic syndrome in a Chinese teaching hospital. Mycopathologia 2020; 185: 959–969. [DOI] [PubMed] [Google Scholar]
- 21. Messina FA, Negroni R, Maiolo EI, et al. Criptococosis meníngea en pacientes con diabetes y sida. Enferm Infecc Microbiol 2014; 32: 643–646. [DOI] [PubMed] [Google Scholar]
- 22. Abu-Ashour W, Twells LK, Valcour JE, et al. Diabetes and the occurrence of infection in primary care: a matched cohort study. BMC Infect Dis 2018; 18: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumar NP, Sridhar R, Nair D, et al. Type 2 diabetes mellitus is associated with altered CD8+ T and natural killer cell function in pulmonary tuberculosis. Immunology 2015; 144: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tan KS, Lee KO, Low KC, et al. Glutathione deficiency in type 2 diabetes impairs cytokine responses and control of intracellular bacteria. J Clin Invest 2012; 122: 2289–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopez-Lopez N, Martinez AGR, Garcia-Hernandez MH, et al. Type-2 diabetes alters the basal phenotype of human macrophages and diminishes their capacity to respond, internalise, and control Mycobacterium tuberculosis. Mem Inst Oswaldo Cruz 2018; 113: e170326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Syed-Ahmed M, Narayanan M. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis 2019; 26: 8–15. [DOI] [PubMed] [Google Scholar]
- 27. Brinkkoetter P-T, Marinaki S, Gottmann U, et al. Altered CD46-mediated T cell co-stimulation in haemodialysis patients. Clin Exp Immunol 2005; 139: 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stachowski J, Pollok M, Burrichter H, et al. Signalling via the TCR/CD3 antigen receptor complex in uremia is limited by the receptors number. Nephron 1993; 64: 369–375. [DOI] [PubMed] [Google Scholar]
- 29. Magliano DJ, Harding JL, Cohen K, et al. Excess risk of dying from infectious causes in those with type 1 and type 2 diabetes. Diabetes Care 2015; 38: 1274–1280. [DOI] [PubMed] [Google Scholar]
- 30. Lin Y-Y, Shiau S, Fang C-T. Risk factors for invasive Cryptococcus neoformans diseases: a case-control study. PLoS One 2015; 10: e0119090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, Fang W, Jiang W, et al. Cryptococcosis in patients with diabetes mellitus II in mainland China: 1993–2015. Mycoses 2017; 60: 706–713. [DOI] [PubMed] [Google Scholar]
- 32. Lin KH, Chen CM, Chen TL, et al. Diabetes mellitus is associated with acquisition and increased mortality in HIV-uninfected patients with cryptococcosis: a population-based study. J Infect 2016; 72: 608–614. [DOI] [PubMed] [Google Scholar]
- 33. Guo W, Zhou Q, Jia Y, et al. Increased levels of glycated hemoglobin A1c and iron deficiency anemia: a review. Med Sci Monit 2019; 25: 8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. English E, Idris I, Smith G, et al. The effect of anaemia and abnormalities of erythrocyte indices on HbA 1c analysis: a systematic review. Diabetologia 2015; 58: 1409–1421. [DOI] [PubMed] [Google Scholar]
- 35. Kalil AC, Syed A, Rupp ME, et al. Is bacteremic sepsis associated with higher mortality in transplant recipients than in nontransplant patients? A matched case-control propensity-adjusted study. Clin Infect Dis 2015; 60: 216–222. [DOI] [PubMed] [Google Scholar]
- 36. Bratton EW, El Husseini N, Chastain CA, et al. Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PLoS One 2012; 7: e43582. [DOI] [PMC free article] [PubMed] [Google Scholar]