Abstract
In this article, we aim to provide general principles as well as personal views for colonic capsule endoscopy. To allow an in-depth understanding of the recommendations, we also present basic technological characteristics and specifications, with emphasis on the current as well as the previous version of colonic capsule endoscopy and relevant software. To date, there is no scientific proof to support the optimal way of reading a colonic capsule endoscopy video, or any standards or guidelines exist. Hence, any advice is a mixture of recommendations by the capsule manufacturer and experts’ opinion. Furthermore, there is a paucity of data regarding the use of term(s) (pre-reader/reader-validator) in colonic capsule endoscopy. We also include a couple of handy tables in order to get info at a glance.
Keywords: capsule colonoscopy, capsule endoscopy, colon, pre-readers, software
Introduction
Capsule endoscopy (CE) has become the mainstream diagnostic modality for the small bowel (SB), particularly in the context of occult intestinal bleeding.1 However, branding CE non-invasive and patient-friendly, should neither translate to ease of interpretation nor overlook the importance and labour intensity of CE reading.2,3 The American Society of Gastrointestinal Endoscopy (ASGE) swiftly suggested credentialing for CE reading,4 while Sidhu and colleagues5 showed that prior endoscopic experience enables trainees to interpret CE images more accurately than those with no relevant experience. The same group also pointed out a lag in accepted standardised credentials.6 The reported lesion missed rate, in early CE works, has been quoted as high as 10%.2,7 Recently, we alluded to the fact that CE is open to a high level of scrutiny, as the recorded video data can be readily accessible for further on-demand review.2 It also provides a unique learning platform for new generations of CE readers as well as unique database potential for the application of artificial intelligence (AI) and computer-aided diagnosis (CADx) with machine-learning techniques.8 Based on previous paragons,3,9,10 we recently published a practical guide for daily use on how one should read single-dome, SB CE video recordings.2
Completing the effort, in this article, we aim to provide general principles as well as personal views for colonic capsule endoscopy (CCE) reading which can also be applied for other double-headed CE reading such as the Crohn’s capsule. One important thing to remember is that any type of currently available endoscopic capsule is still behind the performance of a conventional endoscope. There is substantial evidence confirming that polyp and adenoma detection rates have increased following the introduction and wider use of high-definition (HD) white light imaging (WLI).11 The colon is a challenging environment, further compounded by the lack of steerability and capsule control. This review will not focus on colon preparation or the procedural steps for CCE; the reader is advised to consult relevant pieces of work in addition to highly informative free-access videos clips.12,13 Non-double headed models are currently tested; a pilot study has been designed to evaluate the safety and performance of the CapsoCam®Colon CE with four side-viewing cameras at in patients who meet relevant eligibility criteria and are scheduled for a conventional colonoscopy.14 Therefore, there is no relevant guidance in this work concerning reading this type of capsule.
Before reading CCE videos
A pre-requisite for CCE video reading, similar to reporting any other imaging modality, is to be aware of or – at least – able to swiftly acquire vital info such as clinical presentation details, comorbidities, medications and mostly the referrer’s query.2 Eventually, one must strive to provide a thorough, yet focused and informative, CCE report which should be considered the attainable outcome of this process. Anecdotal evidence suggests that the availability of clinical info and referrer’s request could introduce ‘anticipation’ bias leading therefore to misinterpretation of the clinical significance of some findings. This stands true not only in SB CE, where the incidence of some findings such mucosal erythema, red spots or chylous cysts have only become evident after the advent of CE, but also in CCE where both the significance, detection, and the clinical impact of, for example, diminutive polyps remain under ongoing review. In the following sections, the majority of recommendations derive from SB CE reading experience; there are very few data about CCE reading at this point.
Software interface and challenges of colon capsule endoscopy
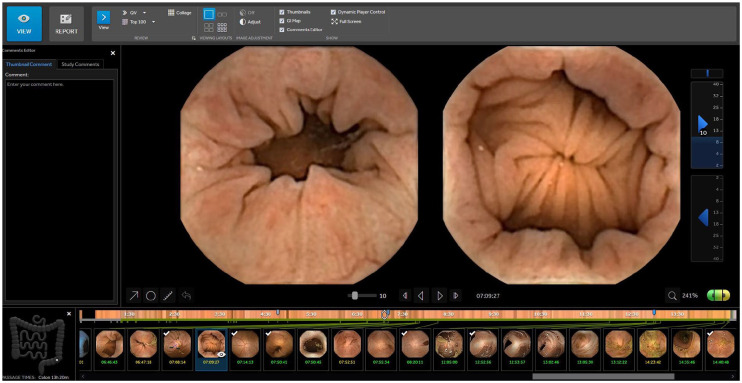
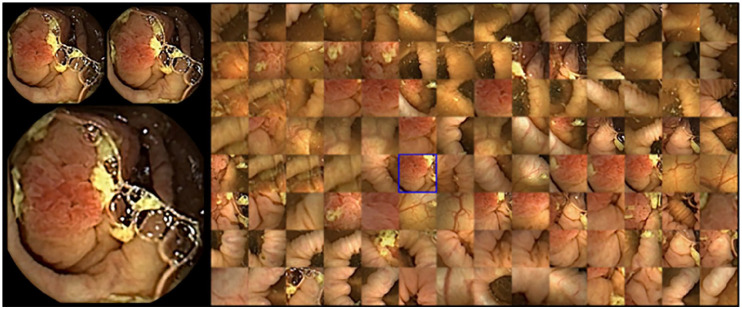
The current proprietary software version of RAPID allows single view (one of the two cameras per time); double view with both cameras simultaneously (Figure 1); FICE I (Figure 2); Blue Mode (Figure 10(b)); suspected blood indicator (Figure 3); Quick view, collage mode (Figure 4); top 100 mode (Figure 5); polyp size estimation (Figure 6); atlas consultation (Figure 7) and capsule localisation (Figure 8). Although there is limited evidence that FICE improves detection and/or lesion localisation in conventional colonoscopy, as compared to other modalities such as narrow-band imaging (NBI) or even blue-laser imaging (BLI), there is certainly no clinical evidence regarding the use of FICE in CCE. Extrapolating from SB CE data, FICE 1 (the only available FICE mode in CCE) was the only one that performed better in lesion delineation and detection.15
Figure 1.
Rapid software: screen with images from both camera heads (green/yellow) and marked thumbnails.
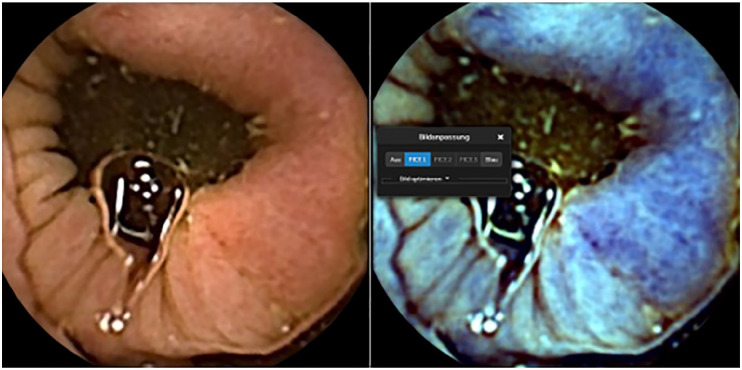
Figure 2.
Semi-circumferential colon cancer with white light (left image) and FICE 1(right image).
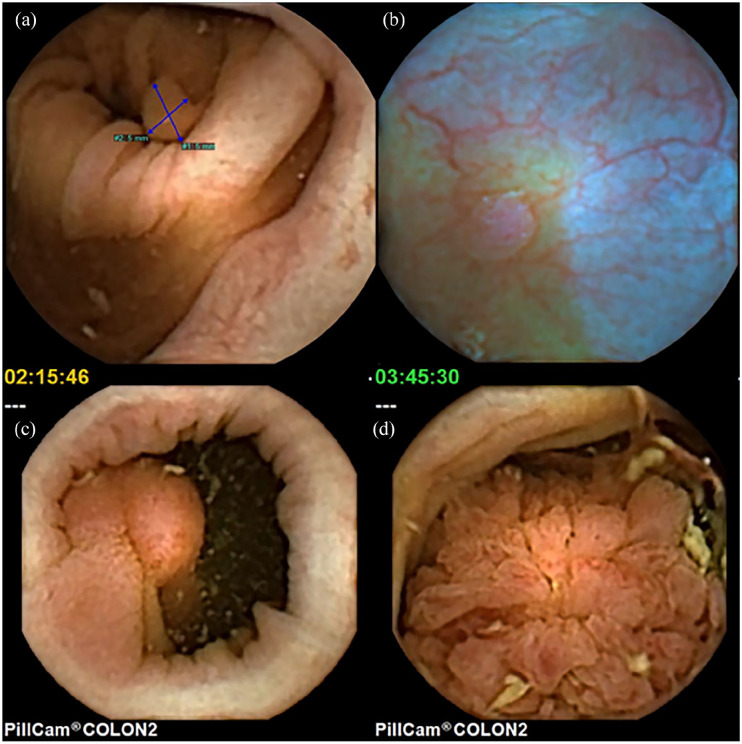
Figure 10.
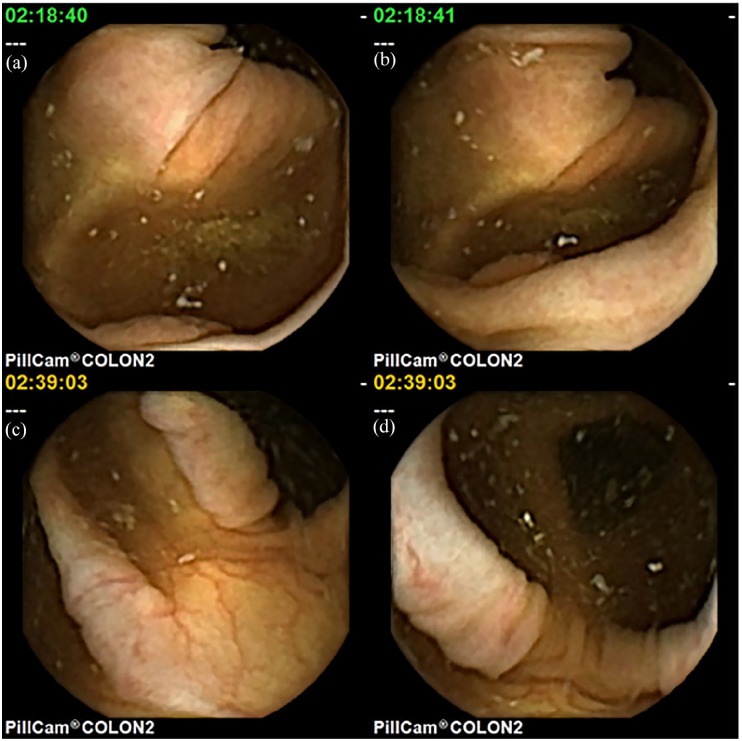
Polyps (a) small sessile polyp with PSE measuring 5 mm, (b) small sessile polyp seen in blue mode showing a subtle surrounding rim of liquid, disruption of the vessels running towards the polyp and more reddish surface colour, (c) large pedunculated polyp, and (d) large lumen-filling polyp with a villous surface.
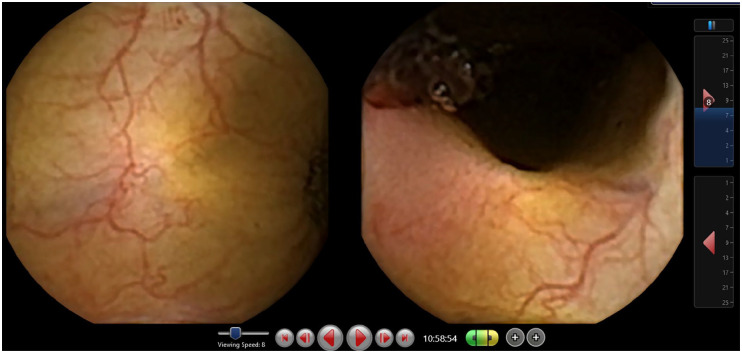
Figure 3.
Suspected blood indicator (SBI): only images with suspected blood are displayed.
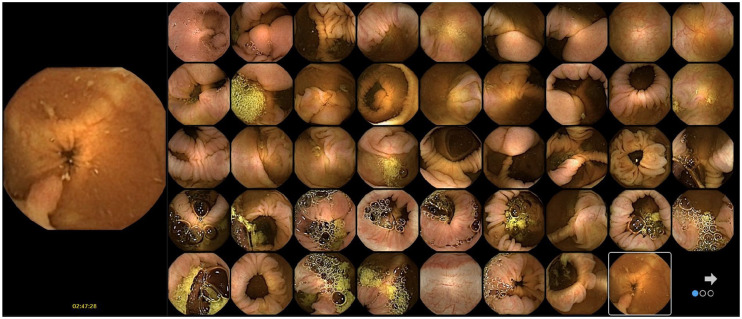
Figure 4.
Collage mode: only suspicious frames selected by AI are displayed. When hoovering with the cursor over an area, the complete frame and the one before and after are additionally displayed (left side of image).
Figure 5.
Top 100 modes with small polyp.
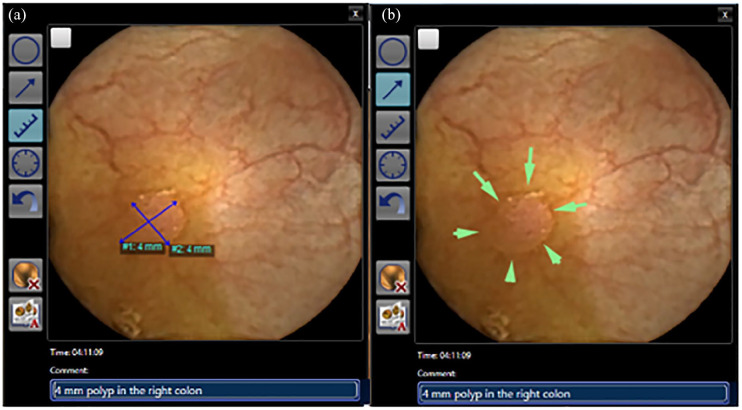
Figure 6.
Thumbnail with annotation, polyp size estimation tool (a) and marking with arrows (b).
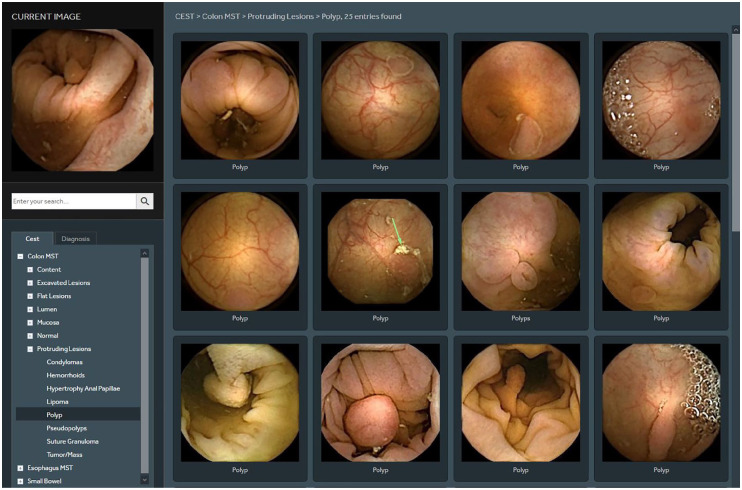
Figure 7.
Comparison of CCE finding (polyp) with images from the atlas incorporated within the software.
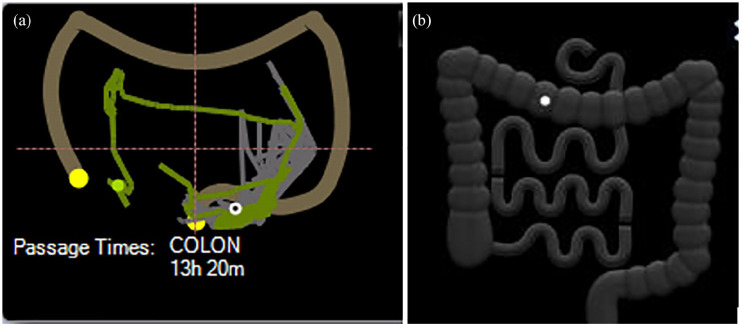
Figure 8.
Localisation trace: real image of the measured capsule’s path through the colon (a) and icon with projection of the capsule position estimated by transit times between landmarks defined by the physician according to endoscopic images (first caecum image, hepatic flexure, and splenic flexure) (b).
Reviewing a CCE video pauses challenges that are not present in SB CE review; prolonged segmental delays compound by the to-and-froth, spiralling move of the capsule in the caecum and proximal ascending colon (AC), and capsule’s bullet-type propulsion in more ‘muscular’ colonic segments, requires time, focused attention and accurate landmarking for proper evaluation and video review. The feeling one gets when reading an SB CE video stream is comparable to taking a long dive into featureless tube; there are indeed times when the diving head goes first and others that rolls into a bottom-first move but this is not particularly disconcerting. In CCE though, the feeling one gets is that of a bouncing ball in a pinball arcade, bumping unpredictably and often spinning around, in the wider space of the colonic lumen.
Furthermore, procedural factors such as difficulties associated with the amount/type of bowel prep and – often – suboptimal cleansing create significant reporting challenges as bowel prep outcome if one of the pillars for a high-quality colonoscopy, also a key quality indicator even in SB CE.16 For those of us who routinely read CCE, observing the amount of ink spilled to confirm the validity (or not) of laxative preparation in SB CE is puzzling. As often happens, data extrapolation from other techniques can resolve an issue more effectively than several well-constructed meta-analyses.17,18 In the vast majority of CCE videos, the SB appears clear and well-distended (even at the distal segment of the ileum), a likely outcome of fluid loading. More fluid washes out and distends the SB lumen consequently gives you better views. Alas, the situation is not the same in the colon, as one may expect.
When facing heavy workload or situations where high-output reporting is required, sessions should be adjusted to include short breaks, ideally every couple of cases, for the reader to rest, move the eyes away from the computer screen to a more natural light, and stretch their legs and neck or simply fetch a cup of coffee.10 It may sound trivial but we occasionally recommend either that people wet their face or use if more convenient refreshing/moisturising eye drops. Moreover, even when the sitting position in front of the reading workstation has been optimised to reduce neck flexion, basic head and neck exercises or even neck/upper back brace & clavicle support devices19 for overloaded readers may relieve the stress caused by eye fixation and concentration into the screen and allow for optimal outcomes by maintaining the readers’ attention during the rest of reviewing session.2
Environment for reading
Just like its SB counterpart, CCE reading should be performed during protected time slots to maintain high-standards and remain a thorough and diligent process just like any other endoscopic activity. Admittedly, amassing reading experience can reduce one’s reading times; however, the official time allocated for review/landmarking of a CCE video should be at least 45–65 min for the first/pre-readers and at least 25–35 min for the validators on average. Similar to SBCE,2,3,9,10 this remains highly dependent to the complexity of each case. The review time needed is based on the cleansing level, colon anatomy, and transit times. Unfortunately, neither factor is predictable.
Whether you read CCE as pre-reader (first reader) or validator (second reader/responsible for the final vetting of the report) in your office or remotely from home – where setting and/or infrastructure allow – it is our firm belief that a neutral background – devoid of noise distractions, activities or ongoing duties – is essential to optimise outcomes. For SB CE reading, we conceded to a quiet room, with adequate ventilation, adjustable/dimmable lights and possibly soft/relaxing music to assist concentration and minimise remaining external distraction.2,10 However, unlike SB CE where the main reading risk is promoting drowsiness, especially in monotonous and tedious clips – with no pathology – due to the limited cylindrical space of the SB lumen that does not allow pronounced or continuous capsule tumbling, in CCE the situation is different.
The see-saw swirl of the capsule in the caecum and the frequently long forward–backward travel due to haustral shuttling/contractions causes a significant degree of reading anxiety and often lead to repeated reads of big segments of the video recording. Of course, what we want is continuous and controllable video pictures of the colonic lumen. Instead, what we get is an illusion of continuity; even with the adaptable frame rate, we are seeing (at the best of times) no more than 35 frames per sec(fps) with both lenses together. Furthermore, despite four white light emitting diodes (LEDs) on each side and 336° total (168° from each head), we are still seeing what the colon is willing to allow us.17,20
It is our anecdotal experience and strong belief that in CE reading the subconscious brain takes over to maximise performance, picking up subtle colour and/or contour changes as it is working in a flight or fight overdrive.2 Perhaps one of the things to use as base built one’s knowledge when start reporting any type of CE is the common pathology expected per organ. For instance, in the SB, the vast majority of identified lesions will be angiectasias, inflammatory and/or polypoid. In the colon, as one would expect, polyps are the bread and butter of CCE findings. However, colonic polyps are a rather diverse category. Big ‘juicy’ pedunculated polyps are not the only (or common) finding. Instead, it is all those small, sessile and inconspicuous lesions that are noted and become a real ‘headache’ of reporting.
Practical process or reading
To date, there is no scientific proof to support the optimal way of reading a CCE video, nor any standards or guidelines exist. Hence, it is a mixture of recommendations by the capsule manufacturer and experts’ opinion. There is a strong need for a validated and structured reading process.
As in SBCE reading, the first step should be a quick preview of the entire video (Table 1). This can be done using a fast reading (Quick view) mode with both camera heads simultaneously. One should look at the total length of time that the capsule needed to go through the colon. Then, they need to identify the landmarks (caecum, hepatic and splenic flexures and rectum/anus/excretion of capsule). Then, a proper review of the images from one camera alone, followed by the other camera, is performed. If the transit is very short or too long, a different approach is advisable. Often the capsule tends to stagnate more in colonic segment as the colon muscular propulsive mechanism is most of the time weaker than the SB propulsive mechanism, and if that occurs, the reading speed might be increased. On the contrary, a short video means that the capsule has gone through the colon quickly and there are fewer frames to view so our rate of frames per minute could be decreased. This often happens in the transverse colon where the transit can be quick; a lower (pre)reading speed is advised in the segment to avoid missing lesions.
Table 1.
Capsule endoscopy reading process.
| Step 1: Preview |
| Using QuickView and both yellow and green head simultaneously for an overview Marking landmarks as first gastric and duodenal image, first caecal and last rectal image |
| Step 2: Review |
| Using yellow and green head alone one after the other at a frame rate max 8–15 fps, slow down using scroll wheel if the capsule moving fast Capturing landmarks and marking any suspected lesions, and some normal images for photo-documentation Checking Top 100 and collage mode images for missed findings |
| Step 3: Report |
| Checking the marked suspected lesions using white light and sometimes virtual chromoendoscopy for characterization Measuring polyp size using polyp size estimation tool Checking for duplicate polyp counting Using proprietary software or hospital own documentation system Reporting all findings – colonic and extracolonic, transit times (stomach, SB, colon) Reporting all study characteristics Making endoscopic diagnosis and recommendation |
SB, small bowel.
Landmarking is essential to assess complete exploration. Their relative position in the video would allow us to vary the frame speed per segment. It also allows lesion localisation which is a delicate issue with CCE as there is to-and-fro movement of the capsule plus inadequate bowel preparation sometimes. Thumbnails are annotated and compared to images saved on platforms, that is, in the atlas incorporated in the RAPID software (Figure 7). Finally, a final confirmatory run on the annotated findings is done optionally in reverse mode, that is, from the rectum up to confirm findings and eliminate duplicates.
As opposed to SBCE, CCE often provides info on other, not previously examined, gastrointestinal (GI) segments. Therefore, one needs to look at what the GI tract offers from the mouth to the anus. The stomach may be explored with a speed between 30 and 40 frames in a double view. The same is recommended for the SB. Afterall, the use of double-view or multi-view is based on reader’s confidence and preference. After identifying the colon, the first and last colon image is to be marked. Further on, localisation of the flexures is required (Figure 8(a)). This may be particularly challenging in a belt-based procedure without the pictogram of the capsule movement (Figure 8(b)). The software provides an AI-assisted suggestion for flexure identification. However, this has to be counterchecked and corrected where appropriate by considering last images of the caecum, appendix, or ileo-cecal valve before the hepatic flexure, which should be followed by the triangular aspect of the transverse colon. The more roundish lumen of the left colon may be helpful as well as the more frequent appearance of diverticula in the sigmoid colon.
To deliver a fast and high-quality reading process, we need to use computer-guided aid. However, presently, there is no clear guidance yet on how to use readily available computed assistance (collage mode, Quick view). In a study with 65 CCEs, Quick view selected patients with a significant polyp in 89%–98%, depending on the Quick view setting.21 The collage mode (Figure 4) gives a quick overview with an acceptable sensitivity for polyps (73.3%–93.3% for experienced readers) by selecting suspicious areas within images.22 A conservative approach to CCE reading is shown in Table 1, where AI is used to reassure that no findings have been missed. With increasing accuracy of AI, these software modes might be applied as a first reading and complete reading with two camera heads separately at slow speed in future only in case AI did not detect pathology.
Pre-readers and CCE
There is a paucity of data regarding the use of team(s) (pre-reader/reader-validator) in CCE. Due to the type of pathology encountered in the human colon, we favour the approach of a close-knitted team in which one reviewer acts as a pre-reader and one as a validator. The pre-reader, who is usually an experienced nurse, reviews the CCE recording adding the landmarks and any obvious pathology. Not only diagnostic yield in SBCE23,24 but also polyp detection rate by trained nurses in CCE also approximated the rate by experts.25 Pre-readers would also highlight areas of ambiguity where a second opinion, that of the validator, is needed. Moreover, as the first responders they are responsible to flag up any CCE that requires immediate attention, especially when there is a significant reading workload. In most European countries though, responsibility for reporting is up to the physician and cannot be delegated. So, physicians acting as validators should go through the study, at a speed of his or her preference, to obtain a bird’s eye inspection and form a first opinion about the significance of any major findings; this way, they can quickly identify landmarks and obvious pathology.
Some centres prefer this approach, not only because two pairs of eyes are better than one but also because it allows the validator to move through more colonic videos per session, as the existing numbers of validators are small, especially in settings where the workload is heavy. A number of multiplying and reliable AI solutions, which in the fullness of time – but only after further stringent safety studies26 – can remove the bottleneck of human pre-reading resource and allow for a combination of AI-validator pairs. This also could lead to fully AI-read capsule studies reducing the need for any human input in CE reporting. One could also concur for experienced pre-readers aware of colon pathology to do both AI-assisted pre-reading and validation with only a few cases going to consultant-level validators (more expensive resource). In future, fast AI-assisted pre-reading immediately after downloading the data might select patients who could profit from consecutive colonoscopy on the same day without repeating bowel prep.27
Diagnosis and reporting
Once pathologies are tracked, the reading process should be optimised to the point. As many images capturing the area of interest as possible should be evaluated by scrolling the mouse wheel forward and backward to get a better impression about the extent of a lesion, and of its morphologic features. Elevated tissue, a rim around a polyp, debris surrounding a polyp, small vessels interrupted by the polyp tissue, differences in surface structure and colour can help differentiate small polyps from normal mucosa (Figure 9). A constant more roundish elevation of a polyp (Figure 9(a) and (b)) should be distinguished from the more longitudinal stretching of a fold while the capsule is moving (Figure 9(c) and (d)). Sometimes virtual chromoendoscopy, for example, FICE 1 (Figure 2), or blue mode (Figure 10(b)) enables further delineation.
Figure 9.
Polyp with a slightly elevated, more roundish mucosa with a rim around it and a discretely rougher surface appearance, constant over more images (a, b) polypoid elevation of a normal mucosa (c), stretching to a normal fold (d) during movement of the capsule.
Describing the correct number of polyps is another issue. Presently, reporting three or more polyps will trigger a therapeutic colonoscopy.22 Hence, duplicate counting should be avoided. Looking at a polyp with one camera head should be accomplished with the polyp appearance in the other camera head shortly before or after, depending on the capsule’s actual orientation. In addition, size and morphology of the polyp images should be comparable for both cameras, forward and back movement of the capsule should be traced as precise as possible to detect repeated approaches of the capsule to the same polyp, especially in a segment with a long transit. The second, more important trigger for colonoscopy is detection of at least one polyp of ⩾6 mm.28 Therefore, exact measurement of size with the implemented polyp size estimation tool is crucial (Figure 6(a)). However, there is limited validating the value of the size estimation tool.
The reading speed is dictated by the overall circumstances. If the capsule runs fast and the preparation is suboptimal, the video has to be reviewed at low speed or even frame-by-frame. Speeding up the video is also possible; for example, if the capsule stagnates in colonic segment maximum viewing speed is possible. The realistic overall average reading time is between 30 and 60 min. A second opinion is always wise and welcome for difficult frames. As suggested in SBCE quality measures,29 the reading speed is best stated in the report. Furthermore, colon transit time is included in the report. Optionally, transit times are noted separately for the three segments in between the landmarks (right/transverse/left colon). Slow transit poses a risk of falsely counting duplicate views of the same lesions, especially polyps. On the contrary, a very fast transit may be a risk factor for missing polyps. For this reason, the Food and Drug Administration (FDA) CCE approval study excluded CCE with a colon transit <40 min.30 In clinical practice, this does not seem feasible. Nevertheless, a very short transit should be stated and discussed in the report, as it may influence the interval for follow-up examination if no polyp was detected.
Possibilities for optical classification of polyps by CCE are far from those provided by flexible colonoscopy with washing, suction, insufflation, moving of the camera, zoom, virtual chromo – endoscopy and dye spray. Hence, optical biopsy enabling detailed description is not applicable. Furthermore, there is no accepted standard terminology for CCE yet and description has to use an analogy to the minimal standard terminology (MST) of World Endoscopy Organisation (WEO)31 presently being updated, and the capsule endoscopy standard terminology (CEST) for SBCE.32 A possible standard terminology for CCE should include all those terms differentiable by CE (Table 2). For polyps, size as measured by PSE, type as sessile, flat, or pedunculated are provided in Figure 10. Bleeding stigmata (Figure 3) and any suspicion of malignancy (Figure 2) should be reported, as well as localisation as exact as possible.
Table 2.
Content of a CCE report.
| What to include in the CCE report | ||
|---|---|---|
| Reason for CCE | Incomplete colonoscopy Colo-rectal cancer (CRC) screening Pos. iFOBT Inflammatory bowel disease (suspected/follow-up) others |
Reason, deepest point reached Risk factors, preceding CRC screening test Accompanying anaemia? Previous tests, therapy, symptoms Specify . . . |
| Clinical data | Surgery Relevant medication Bleeding Complaints Risk factors |
Type, anastomosis Anticoagulants, thrombocyte aggregation inhibitors, opioids . . . Overt, occult, iron deficiency anemia Constipation, diarrhoea, overt bleeding Personal/family history of CRC, polyposis, known colitis |
| Characteristics of study | Bowel Prep | Liquid diet from . . . Lavage: type, amount, timing) Booster (type, amount, timing), additional supp? |
| Equipment | Capsule (type/charge), Recorder (sensor array/belt) | |
| Procedure | Time Institution and staff performing procedure, Complications? |
|
| Reading and reporting | Physician/pre-reader Speed and software mode used for reading |
|
| Completeness | Complete CCE | Visualisation of terminal ileum, caecum, haemorrhoidal plexus Excretion of transmitting capsule |
| Complementation of incomplete optical colonoscopy (OC) | Visualisation of the colon segment reached by OC Visualisation of a marking (biopsy/polypectomy site/clip/ink mark) |
|
| Transit times | Colon transit Gastric/small bowel transit |
Optional: segmental transit: Caecum/right colon, transverse colon, left colon/recto-sigmoid If first duodenal image recorded |
| Visualisation of colon mucosa | Adequate (excellent/good) |
Inadequate (poor/fair) |
| Optional for right, transverse and left colon separately | ||
| Findings | Protruding lesions - Polyp - Tumour - Varix |
Type (sessile, pedunculated, flat and unknown) Size (⩽5 mm, 6–9 mm, >10 mm) Size (small/medium/large) Type (Subepithelial/ulcerated/stenosing/bleeding/exophytic) |
| Excavated lesions Diverticula Ulcers |
Inflammation? Stenosis? Bleeding? Size? Number? Distribution? |
|
| Flat lesions - Angiectasia(s) - Erythema |
Size small/medium/large Number (single/few/multiple) Distribution (focal/diffuse) Extension (short segment, long segment and entire colon) |
|
| Extra-colonic findings | Oesophagus Stomach, small bowel |
Suspected Barrett’s, Reflux esophagitis Ulcer, gastritis, polyps, bleeding Angiectasia, ulcers, tumour, polyp, stenosis, lymphangiectasia, bleeding |
| Significance of findings | Significant polyps Size: ⩾6 mm or Number: ⩾3 polyps Bleeding potential Angiectasia(s) Large ulcers Haemorrhagic mucosa Tumours/large polyps Cause of abdominal pain Ulcers (large/deep/extended) Stenosis with (temporary) capsuleretention |
Non-significant polyps Size: <6 mm and Number: <3 polyps No/uncertain bleeding potential Red spots Diverticula without bleeding stigmata No/uncertain cause of abdominal pain Single small superficial ulcers Narrowing of lumen without delay of capsule transit |
| Endoscopic diagnosis | Polyp(s) Tumour/mass Colitis Diverticulosis/diverticulitis Angiectasia(s) Hemorrhoid(s) Others |
Specify |
| Photo-documentation | Landmarks Relevant findings |
Terminal ileum, IC-valve, caecum, appendix, ascending colon, right flexure, transverse colon, left flexure, left colon (descending colon/sigmoid/rectum), last rectal image/haemorrhoidal plexus As described above, optional also variants of normal |
| Recommendations | Referral for other tests Referral for therapy Repeating CCE Follow-up |
Standard colonoscopy for completion/histology/confirmation/exclusion Colonoscopy/surgery/medical Modification of bowel prep? Consider alternative test Time interval |
CCE, colonic capsule endoscopy; OC, optical colonoscopy.
The overall adequateness of bowel cleanliness must be documented in the report as the polyp, and cancer miss rate have been shown to increase in patients with inadequate visualisation.33 A first judgement can be made during the first quick view. A four-point scale (excellent, good, fair or poor) based on assessment of fluid and debris, bubbles, bile/chyme staining and reduction of brightness, each as none to minimally, mildly, moderate or severe has been described. Brotz and colleagues34 had suggested a two-point scale of adequate visualisation of mucosa (⩾90%) for excellent or good cleanliness versus inadequate with <90% visualisation of mucosa in fair prep and <80% in poor prep. Minimum requirement is the overall assessment of cleanliness by a two-point scale. A more detailed description will provide a four-point scale separately for the right, transverse and left colon.35
In case the haemorrhoidal plexus is visualised definitely, a complete CCE can be assumed. If not, the patient and referring physician must be informed by a timely report to take care for documentation of capsule excretion by the patient, or an X-ray respectively to rule out retention. In case CCE was indicated to complement an incomplete colonoscopy,36 and CCE is also incomplete, all available information has to be included in the decision if the furthest point reached by both investigations has been passed by the complementary procedure.
Recommendation on further management is based on the judgement of the significance of detected polyps or the ability of other findings to explain symptoms. Therefore, a detailed description of findings is as important as a proper knowledge of patient’s history and situation. There is little evidence on appropriate follow-up intervals, especially in patients with insignificant polyps, positive iFOBT, or partially in case of impaired visualisation of the mucosa, or very short transit. Future studies might modify management recommendations,33,37 but they will always have to rely on a standardised reading and reporting.
Presently, there is no guidance for accessing competency and for credentialing in CCE reading
For SB CE, a recent European Society of Gastrointestinal Endoscopy (ESGE) curriculum has been published. Here, a structured course is recommended. Furthermore, direct observation of procedural skills (DOPS) is considered preferable. However, in the shortage of DOPS outside the United Kingdom, a minimum of 30 supervised SBCE reading is suggested as a surrogate parameter for credentialing.1 For the United States, the American Society for Gastrointestinal Endoscopy ASGE recommends a minimum of 20 supervised SBCE readings.38 However, later research showed evidence for 25 supervised SBCE readings being appropriate.39 There are multiple similarities between SBCE and CCE, especially concerning the software. Experience in SBCE seems helpful for training in CCE but cannot replace training in CCE. An early study demonstrated that very experienced experts in SBCE performed worse in CCE reading than technicians specifically trained in CCE.40 This clearly shows the need for dedicated training in CCE.
Details on reporting CCE are provided in the ESGE guideline on CCE published in 2012. However, there is no statement on achieving of competency or on credentialing.28 A curriculum for dedicated hands-on training courses in CE including CCE has been proposed by a group of experts and trainers in Europe.41 For CCE, a 1.5-day course of 12 h is proposed with a distribution of 2.5 h for theoretical lessons, 3.5 h for procedure lessons and 6 h for hands-on training. For the latter, 1–2 h training and discussion per video case is assumed. Assessment of competency is suggested by written examination (recommended), or by oral examination, quiz, web-based approach, or e-learning. For trainers in formal CCE courses, reporting of 200 CCE videos is demanded being considered an expert. However, no minimum number of supervised CCEs reported has been defined for assessment of competency in trainees. In the absence of evidence for CCE, 25–30 cases evaluated under supervision might be considered appropriate in analogy to SBCE.
In CCE, an electronic learning system has been developed for the Japanese Association for Capsule Endoscopy.42 The system includes reading theory, and guided training followed by unguided training. A competence assessment test automatically scoring lesion detection, diagnosis (based on location, size and shape of a lesion), management recommendations and quality of view were performed before and after the training. Performance in this test increased significantly after training in both groups of trainee endoscopists (cohort 1 to train the system; from 42 ± 18% to 79 ± 15%; p = 0.0004, n = 10), and cohort 2 (validation group, from 52 ± 15% to 79 ± 14%; p = 0.0003; n = 11), respectively. Future research needs to provide evidence for a definite number of supervised CCE readings together with structured training courses to achieve competency. The role of e-learning module in clinical practice has to be defined additionally.
Footnotes
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: A.K. and K.D. are co-directors of iCERV; A.K. has received travel support and speaker’s fees from Jinshan; he has also received research support from IntroMedic/SynMed and a grant from GivenImaging Ltd (2011); E.T. and M.K. are consultants for Medtronic; M.K. has received speaker’s fees from Olympus.
ORCID iD: Martin Keuchel  https://orcid.org/0000-0001-5288-4993
https://orcid.org/0000-0001-5288-4993
Contributor Information
Anastasios Koulaouzidis, Department of Social Medicine and Public Health, Faculty of Health Sciences, Pomeranian Medical University, Szczecin, Poland.
Konstantinos Dabos, Gastroenterology Department, St John’s Hospital, Livingston, UK.
Michael Philipper, Private Gastroenterology Office, Duesseldorf, Germany.
Ervin Toth, Department of Gastroenterology, Skåne University Hospital, Lund University, Malmö, Sweden.
Martin Keuchel, Clinic for Internal Medicine, Agaplesion Bethesda Krankenhaus Bergedorf, 21029 Hamburg, Germany.
References
- 1. Sidhu R, Chetcuti Zammit S, Baltes P, et al. Curriculum for small-bowel capsule endoscopy and device-assisted enteroscopy training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2020; 52: 669–686. [DOI] [PubMed] [Google Scholar]
- 2. Rondonotti E, Pennazio M, Toth E, et al. How to read small bowel capsule endoscopy: a practical guide for everyday use. Endosc Int Open 2020; 8: E1220–E1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewis BS. How to read wireless capsule endoscopic images: tips of the trade. Gastrointest Endosc Clin N Am 2004; 14: 11–16. [DOI] [PubMed] [Google Scholar]
- 4. Faigel DO, Baron TH, Adler DG, et al. ASGE guideline: guidelines for credentialing and granting privileges for capsule endoscopy. Gastrointest Endosc 2005; 61: 503–505. [DOI] [PubMed] [Google Scholar]
- 5. Sidhu R, Sakellariou P, McAlindon ME, et al. Is formal training necessary for capsule endoscopy? The largest gastroenterology trainee study with controls. Dig Liver Dis 2008; 40: 298–302. [DOI] [PubMed] [Google Scholar]
- 6. Sidhu R, McAlindon ME, Davison C, et al. Training in capsule endoscopy: are we lagging behind? Gastroenterol Res Pract 2012; 2012: 175248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis BS, Eisen GM, Friedman S. A pooled analysis to evaluate results of capsule endoscopy trials. Endoscopy 2005; 37: 960–965. [DOI] [PubMed] [Google Scholar]
- 8. Koulaouzidis A, Iakovidis DK, Karargyris A, et al. Optimizing lesion detection in small-bowel capsule endoscopy: from present problems to future solutions. Expert Rev Gastroenterol Hepatol 2015; 9: 217–235. [DOI] [PubMed] [Google Scholar]
- 9. Barkin JA, Barkin JS. Video capsule endoscopy: technology, reading, and troubleshooting. Gastrointest Endosc Clin N Am 2017; 27: 15–27. [DOI] [PubMed] [Google Scholar]
- 10. Lo S. How should we do capsule reading? Tech Gastrointest Endosc 2006; 8: 146–148. [Google Scholar]
- 11. Tziatzios G, Gkolfakis P, Lazaridis LD, et al. High-definition colonoscopy for improving adenoma detection: a systematic review and meta-analysis of randomized controlled studies. Gastrointest Endosc 2020; 91: 1027–1036.e9. [DOI] [PubMed] [Google Scholar]
- 12. Singhal S, Nigar S, Paleti V, et al. Bowel preparation regimens for colon capsule endoscopy: a review. Therap Adv Gastroenterol 2014; 7: 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. https://www.youtube.com/watch?v=WvG7akyfD8s
- 14. https://www.clinicaltrials.gov/ct2/show/NCT04246632?cond=capsoCam&draw=2&rank=1
- 15. Yung DE, Boal Carvalho P, Giannakou A, et al. Clinical validity of flexible spectral imaging color enhancement (FICE) in small-bowel capsule endoscopy: a systematic review and meta-analysis. Endoscopy 2017; 49: 258–269. [DOI] [PubMed] [Google Scholar]
- 16. Spada C, McNamara D, Despott EJ, et al. Performance measures for small-bowel endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. United European Gastroenterol J 2019; 7: 614–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koulaouzidis A, Rondonotti E, Karargyris A. Small-bowel capsule endoscopy: a ten-point contemporary review. World J Gastroenterol 2013; 19: 3726–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yung DE, Rondonotti E, Sykes C, et al. Systematic review and meta-analysis: is bowel preparation still necessary in small bowel capsule endoscopy. Expert Rev Gastroenterol Hepatol 2017; 11: 979–993. [DOI] [PubMed] [Google Scholar]
- 19. Green BN. A literature review of neck pain associated with computer use: public health implications. J Can Chiropr Assoc 2008; 52: 161–167. [PMC free article] [PubMed] [Google Scholar]
- 20. Cass OW. Is half-knowledge worse than ignorance? Gastrointest Endosc 2006; 64: 542–543. [DOI] [PubMed] [Google Scholar]
- 21. Farnbacher MJ, Krause HH, Hagel AF. QuickView video preview software of colon capsule endoscopy: reliability in presenting colorectal polyps as compared to normal mode reading. Scand J Gastroenterol 2014; 49: 339–346. [DOI] [PubMed] [Google Scholar]
- 22. Hausmann J, Linke JP, Albert JG, et al. Time-saving polyp detection in colon capsule endoscopy: evaluation of a novel software algorithm. Int J Colorectal Dis 2019; 34: 1857–1863. [DOI] [PubMed] [Google Scholar]
- 23. Brock AS, Freeman J, Roberts J, et al. A resource efficient tool for training novices in wirelees capsule endoscopy. Gastroenterol Nurs 2012; 35: 317–321. [DOI] [PubMed] [Google Scholar]
- 24. Yung DE, Fernandez-Urien I, Douglas S, et al. Systematic review and meta-analysis of the performance of nurses in small bowel capsule endoscopy reading. United European Gastroenterol J 2017; 5: 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pfeifer UG. RCT zur Prüfung der Auswertungsqualität der PillCam Colon Kapselendoskopie von Ärzten und Pflegepersonen im Vergleich zur Expertenauswertung unter Einbezug der beruflichen Identität von Pflegepersonen in der Endoskopie [RCT comparing quality of reading PillCamColon capsule endocopy by physicians and care givers versus experts regarding professional identity of care givers in endoscopy]. Vallendar: Philosophisch-Theologische Hochschule Vallendar, Hochschulbibliothek, 2012. [Google Scholar]
- 26. Dray X, Iakovidis D, Houdeville C, et al. Artificial intelligence in small bowel capsule endoscopy – current status, challenges and future promise. J Gastroenterol Hepatol 2021; 36: 12–19. [DOI] [PubMed] [Google Scholar]
- 27. Yang YJ. The future of capsule endoscopy: the role of artificial intelligence and other technical advancements. Clin Endosc 202; 53: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spada C, Hassan C, Galmiche JP, et al. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2012; 44: 527–536. [DOI] [PubMed] [Google Scholar]
- 29. Rondonotti E, Spada C, Adler S, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) technical review. Endoscopy 2018; 50: 423–446. [DOI] [PubMed] [Google Scholar]
- 30. Rex DK, Adler SN, Aisenberg J, et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology 2015; 148: 948–957. [DOI] [PubMed] [Google Scholar]
- 31. Minimal standard terminology (MST) of World Endoscopy Organisation (WEO), https://www.worldendo.org/wp-content/uploads/2016/08/160803_MST30.pdf
- 32. Korman LY, Delvaux M, Gay G, et al. Capsule endoscopy structured terminology (CEST): proposal of a standardized and structured terminology for reporting capsule endoscopy procedures. Endoscopy 2005; 37: 951–959. [DOI] [PubMed] [Google Scholar]
- 33. Spada C, Hassan C, Bellini D, et al. Imaging alternatives to colonoscopy: CT colonography and colon capsule. European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) guideline – update 2020. Endoscopy 2020; 52: 1127–1141. [DOI] [PubMed] [Google Scholar]
- 34. Brotz C, Nandi N, Conn M, et al. A validation study of 3 grading systems to evaluate small-bowel cleansing for wireless capsule endoscopy: a quantitative index, a qualitative evaluation, and an overall adequacy assessment. Gastrointest Endosc 2009; 69: 262–270. [DOI] [PubMed] [Google Scholar]
- 35. Leighton JA, Rex DK. A grading scale to evaluate colon cleansing for the PillCam COLON capsule: a reliability study. Endoscopy 2011; 43: 123–127. [DOI] [PubMed] [Google Scholar]
- 36. Deding U, Kaalby L, Bøggild H, et al. Colon capsule endoscopy vs. CT colonography following incomplete colonoscopy: a systematic review with meta-analysis. Cancers 2020; 12: 3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cash BD, Fleisher MR, Fern S, et al. Multicentre, prospective, randomised study comparing the diagnostic yield of colon capsule endoscopy versus CT colonography in a screening population (the TOPAZ study). Gut. Epub ahead of print 18 December 2020. DOI: 10.1136/gutjnl-2020-322578. [DOI] [PubMed] [Google Scholar]
- 38. Rajan EA, Pais SA, Degregorio BT, et al. ASGE training committee – small-bowel endoscopy core curriculum. Gastrointest Endosc 2013; 77: 1–6. [DOI] [PubMed] [Google Scholar]
- 39. Rajan E, Martinez M, Gorospe E, et al. Prospective multicenter study to evaluate capsule endoscopy competency using a validated assessment tool. Gastrointest Endosc 2020; 91: 1140–1145. [DOI] [PubMed] [Google Scholar]
- 40. Eliakim R, Fireman Z, Gralnek IM, et al. Evaluation of the PillCam colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy 2006; 38: 963–970. [DOI] [PubMed] [Google Scholar]
- 41. Fernandez-Urien I, Panter S, Carretero C, et al. International core curriculum for capsule endoscopy training courses. Endosc Int Open 2017; 5: E526–E538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Watabe H, Nakamura T, Yamada A, et al. Assessment of an electronic learning system for colon capsule endoscopy: a pilot study. J Gastroenterol 2016; 51: 579–585. [DOI] [PubMed] [Google Scholar]