Abstract
Background
There are ongoing clinical trials exploring the efficacy of dopamine receptor D2 (DRD2) inhibition against glioblastomas, the most common primary brain tumor. Here we examine potential molecular determinants of this efficacy.
Methods
The Cancer Genome Atlas glioblastoma database and other published mRNA profiles were used to analyze the DRD2 and epidermal growth factor receptor (EGFR) expression pattern. In vitro and in vivo responses to DRD2 inhibitors were determined using patient-derived xenograft (PDX) glioblastoma models. Immunohistochemical studies were performed on clinically annotated glioblastoma samples derived from patients treated with ONC201.
Results
Analysis of clinical glioblastoma specimens derived from independent patient cohorts revealed an inverse correlation between EGFR and DRD2 mRNA expression, with implication that signaling mediated by these proteins shares overlapping functions. In independent panels of PDX glioblastoma lines, high EGFR expression was associated with poor in vitro and in vivo response to DRD2 inhibitors, including haloperidol and ONC201. Moreover, ectopic expression of a constitutively active EGFR, variant (v)III, suppressed glioblastoma sensitivity to ONC201. DRD2 expression positively correlated with expression of rate-limiting enzymes for dopamine synthesis as well as dopamine secretion, suggesting contribution of autocrine DRD2 signaling. Analysis of specimens from patients treated with ONC201 (n = 15) showed an inverse correlation between the intensity of EGFR staining and clinical response. The median overall survival for patients with high and low EGFR staining was 162 and 373 days, respectively (0.037).
Conclusions
High EGFR expression is a determinant of poor glioblastoma response to DRD2. This finding should inform future clinical trial designs.
Keywords: anti-DRD2 treatment, DRD2, EGFR, efficacy, ONC201
Key Points.
1. EGFR and DRD2 expressions are anti-correlated in glioblastomas.
2. Glioblastoma expression of DRD2 was coupled to the level of dopamine synthesis.
3. Glioblastomas with low EGFR expression are most sensitive to DRD2 inhibition.
Importance of the Study.
There is mounting evidence supporting DRD2 inhibition as a glioblastoma therapy, with an increasing number of durable responses documented on and off clinical trials. However, it is equally clear from this literature that not all glioblastoma patients benefit from this therapy. As such, molecular determinant of response is essential for meaningful translation. Our study suggests that high EGFR expression is associated with poor glioblastoma response to DRD2 inhibition. This finding informs the design of future DRD2-based clinical trials and bears relevance for glioblastoma pathogenesis.
Therapeutic efficacy has been elusive for glioblastomas, the most common form of adult brain cancer.1 Fatality within 2 years of diagnosis remains nearly universal.2 Results from experimental models3 and large-scale genomic profiling of clinical glioblastoma specimens4 converge on the central importance of receptor tyrosine kinase (RTK) activation in many glioblastomas. The prototypical RTK, epidermal growth factor receptor (EGFR), is frequently amplified or mutated in glioblastomas.4,5 Consequent signaling fuels all aspects of glioblastoma biology, including mitogenic signaling,6 anti-apoptotic function,7 and self-renewal.8 While initial studies with small-molecule EGFR inhibitors have not yielded meaningful clinical efficacy,9 ongoing efforts continue to hold considerable potential.10,11
Despite the general importance of RTKs, subsets of glioblastomas rely on alternate means of oncogenic signaling.4,5 Our previous genome-wide short hairpin RNA screen unveiled critical roles for dopamine receptor D2 (DRD2) in this RTK-independent signaling.12 DRD2 encodes a 7-transmembrane, G-protein coupled receptor whose function is best characterized in the context of neurotransmission.13 DRD2 hyperactivation contributes to glioblastoma pathogenesis and is essential for a subset of glioblastomas.12 Of note, lower incidence of glioblastoma and long-term glioblastoma survival have been observed in schizophrenia patients undergoing DRD2 antagonist based anti-psychotic therapy.14,15 Importantly, a blood–brain barrier penetrant and DRD2 selective inhibitor, ONC201, has shown promise in early phase glioblastoma trials.16,17 This molecule achieves potent selectivity for DRD2 via an exclusive bitopic interaction.
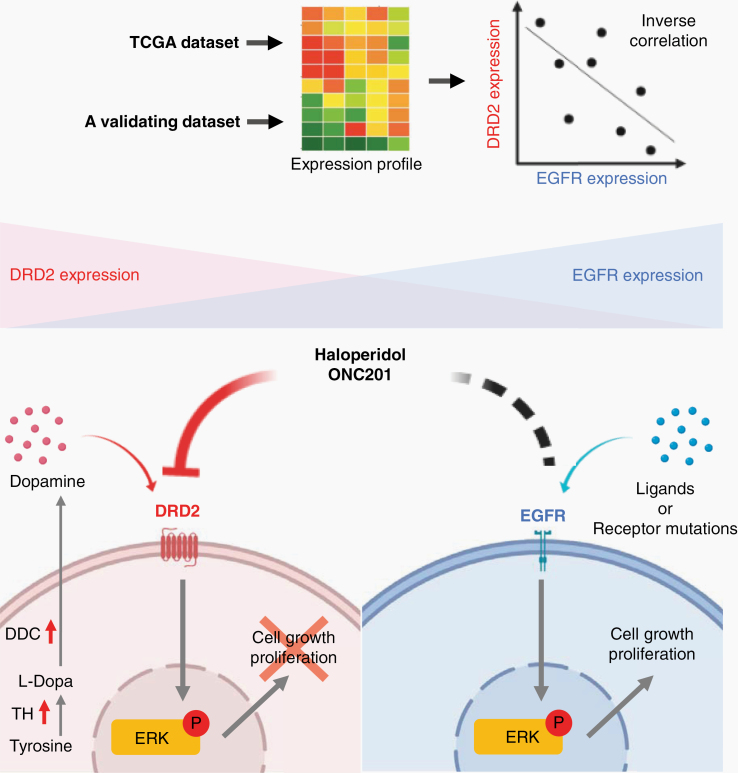
Extracellular signal-regulated kinases (ERKs; also known as mitogen-activated protein kinase) are evolutionary conserved, ubiquitous serine-threonine kinases that regulate cell proliferation, stress response, and self-renewal.18 ERKs receive and coordinate signal transduction from different classes of cardinal transmembrane receptors,19 including EGFR and DRD2. EGFR triggers ERK-mediated signaling through Ras activation.5 In contrast, DRD2 signaling activates canonical heterotrimeric G-protein complex. GNAI2 (guanine nucleotide-binding protein alpha 2), the β-subunit of this heterotrimeric complex, subsequently recruits Rap–GTPase-activating protein II (GAPII), a GTPase that hydrolyzes replication protein A1 (RPA1)-GTP. Because the physiologic role for RPA1-GTP involves inactivation of Ras, DRD2 signaling ultimately culminates in Ras and ERK activation.20,21 Since ERK receives convergent signals from EGFR and DRD2, EGFR suppression can be compensated by DRD2 activation.12 Moreover, tumoricidal activity against glioblastomas with dual DRD2 and EGFR hyperactivation requires simultaneous inhibition of both signaling axes.12
There is an increasing number of durable glioblastoma responses documented on and off clinical trials in response to DRD2 inhibitors,16,17 suggesting that subsets of glioblastomas are uniquely sensitive to this treatment. Understanding the molecular determinant of these responses constitutes an essential next step in clinical translation. Here, we explored how intertumoral heterogeneity in EGFR and DRD2 expression influenced glioblastoma sensitivity to DRD2 inhibitors.
Materials and Methods
Cell Lines and Reagents
Patient-derived glioblastoma cell lines (GBM1–6) were established as previously described.22 An additional 25 glioblastoma cell lines (BT74, G022, G206, G464, G816, GBM6, GBM39, GSC11, GSC23, HK281, HK296, HK301, HK374, HK385, MGG4, MGG6, MGG8, MGG13, MGG18, MGG31, MGG123, MGG152, TS543, TS576, TS600) established by different groups23–28 were included in this study. Those glioblastoma neurosphere cell lines were propagated under neurosphere conditions in NeuroCult medium (Stem Cell Technology) supplemented with B27, Glutamax, epidermal growth factor, fibroblast growth factor, and heparin according to the manufacturer’s instructions. GBM4 cells overexpressing EGFRvIII were generated as previously described.29 Haloperidol was purchased from Sigma-Aldrich, and ONC201 was provided by Oncoceutics. Both were stored based on provided instructions. In vitro cell viability was determined using CellTiter-Blue cell viability assay (Promega) for the 6 neurosphere cultures (GBM1–6).12 For the ONC201 sensitivity assay for an additional 25 neurosphere lines, ATPlite 1step (PerkinElmer) was used according to the manufacturer’s instructions. Dopamine quantitation was performed using the Dopamine ELISA Kit (Enzo Life Sciences) according to the manufacturer’s instructions using condition media derived after 72 hours of culture. For quantitative real-time (qRT)-PCR, total RNA was extracted using a TRIzol-based method30 followed by cDNA synthesis using iScript Supermix (Bio-Rad). Quantitative RT-PCR was performed using gene-specific primers purchased from Qiagen following the manufacturer’s instruction.
Western Blotting and Immunohistochemical Staining
Western blotting was performed to determine the expression of EGFR, DRD2, and phosphorylated (p)ERK protein. Protein lysates were extracted with NP-40 buffer (1% NP-40, 20 mM Tris-HCl [pH 8.0], 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM sodium orthovanadate, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 10 μg/mL pepstatin). Fifty micrograms of the protein lysate was fractionated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis following western blotting using phosphor-specific anti-pERK antibody (Cell Signaling Technology, 1:1000), anti- Ku86 antibody (Sigma, 1:10 000), anti-EGFR antibody (Cell Signaling Technology, 1:1000), and anti-DRD2 antibody (Cell Signaling Technology, 1:1000).
Anti-EGFR monoclonal antibody (Santa Cruz Biotechnology, 1:50) was used for immunohistochemical (IHC) staining. Slides derived from ONC201 clinical trials17 were incubated at 60°C for 60 min in a hybridization oven to get heated. Deparaffinization and rehydration were achieved by following the wash/incubation sequence: Xylene 4 × 5 min, 100% ethanol 2 × 3 min, 90% ethanol 2 × 3 min, 70% ethanol 2 × 3 min, 50% ethanol 1 × 3 min, 30% ethanol 1 × 3 min, Milli-Q water 1 × 10 min, phosphate buffered saline 1 × 10 min. Antigen retrieval was accomplished with incubating the sections for 20 minutes in Tris-EDTA buffer at sub-boiling temperature. Primary antibodies were applied and incubated overnight at 4°C. The reaction was visualized by 3,3′-diaminobenzidine horseradish peroxidase (Vector Laboratories). The sections were counterstained with Mayer’s hematoxylin and mounted with Permount Mounting Medium (Electron Microscopy Sciences). Staining of EGFR was scored based on the criteria as follows: 0 (no cells stained), 1+ (<5% tumor cells stained), 2+ (5–50% cells stained), and 3+ (>50% cells stained). Classified as low expression were 1+ and 2+; 3+ was classified as high expression; 0 and 1+ staining were labeled “low EGFR,” while 2+ and 3+ staining were labeled “high EGFR.” Two independent board-certified neuropathologists scored each sample. No discrepant results were observed in the classification of low and high EGFR staining. Statistical comparisons of average scores were performed using the non-parametric Mann–Whitney test with GraphPad Prism software.
Subcutaneous and Orthotopic Xenograft Models
Both intracranial and subcutaneous experiments were performed using nu/nu mice (~7 wk old) purchased from Charles River Laboratories. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication 80-23). Tested were 8–10 animals for each experiment, repeated at least twice. For each experiment, the mice were randomized into control and treated groups.
For the subcutaneous experiments, 1 × 106 neurosphere cells were injected in 50 μL volume into the flanks. Haloperidol (10 mg/kg) or vehicle was injected intraperitoneally (i.p.) daily once the xenograft reached 20 mm3. Tumor volume was calculated based on the formula: volume = (width)2 × length/2 and was recorded every 5–7 days. Mice were sacrificed when they developed tumors greater than 1.0 cm in diameter or at the termination of the experiment of 60 days.
For the orthotopic models, 1 × 104 cells in 4 μL Hank’s Balanced Salt Solution were stereotactically injected into the brains of the nu/nu mice at age 5–6 weeks old. The coordinates were: 1.8 mm to the right of the bregma and 3 mm deep from the dura. Starting on day 8, mice received administrations of haloperidol (10 mg/kg i.p., daily), ONC201 (100 mg/kg i.p., once a week), or vehicle. Mice with >20% reductions in body weight or neurologic deficit were sacrificed, and brains were processed for paraffin or frozen section. These animal experiments were conducted in compliance with policies established by the University of Minnesota and the University of California San Diego Institutional Animal Care and Use Committee.
Clinical Informatics
The Cancer Genome Atlas (TCGA) glioblastoma dataset was downloaded from the Genomic Data Commons Legacy Archive (https://portal.gdc.cancer.gov/legacy-archive/search/f). The dataset of TCGA contains the expression profiles of 499 glioblastoma patients ascertained through the Agilent 244K Custom Gene Expression G4502A-07-1 and the Agilent 244K Custom Gene Expression G4502A-07-2 platforms. For the cases measured by both platforms, the average expression level was calculated. A non-TCGA glioblastoma dataset was assembled as described by Verhaak et al.5 High and low expression of DRD2, tyrosine hydroxylase (TH), and DOPA decarboxylase (DDC) were classified based on the median value.
Statistical Analysis
Statistical analysis of in vitro and in vivo experiments was performed using GraphPad Prism v5 software. Data of these experiments were shown as means and standard errors. Analysis of clinical glioblastoma survival was performed using SPSS software. Survival analysis was performed by the Kaplan–Meier method, and the difference between survival curves was tested by log-rank test. Independences between categorical variables were tested by chi-square. Analysis and visualization of mRNA expression data were performed using R v3.5.2. Gene expression level higher than the median was defined as “high” or “positive” expression. Pearson’s product-moment correlation was calculated to reflect the linear correlation between 2 variables of glioblastoma patients who were not EGFR and DRD2 double positive.
Results
Expression Pattern of DRD2 and EGFR in Clinical Glioblastoma Specimens
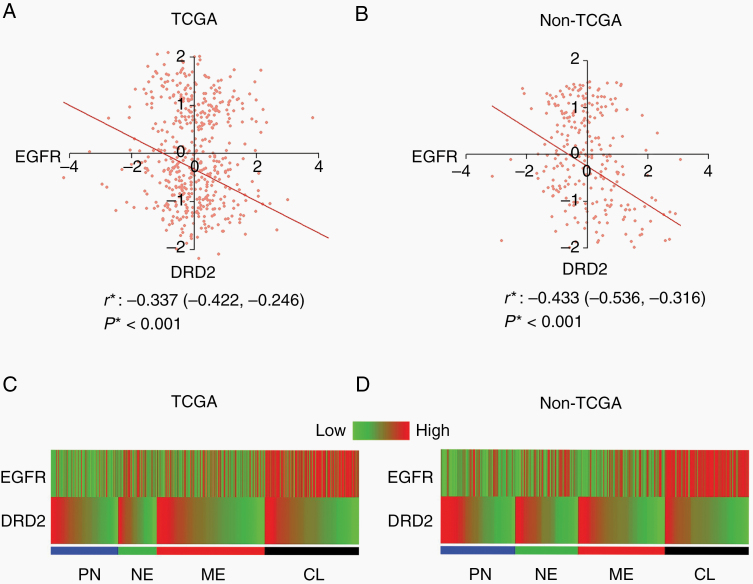
We first analyzed the patterns of DRD2 and EGFR expression in clinical glioblastoma specimens profiled by the dataset of TCGA as well as mRNA expression profiles collated from independent non-TCGA studies.5 In both datasets, we observed that ~25% of clinical glioblastoma samples exhibited high expression of both DRD2 and EGFR. In our previous study, durable responses against these glioblastomas required a combination of DRD2 and EGFR inhibition.12 In the remaining clinical glioblastoma samples, DRD2 expression anti-correlated with EGFR expression (Fig. 1A, B). Such anti-correlation was not observed between DRD2 and other RTKs implicated in glioblastoma pathogenesis, such as platelet-derived growth factor alpha and beta (Supplementary Figure 1). Of note, while the correlation between EGFR and DRD2 is statistically significant in 2 independent patient cohorts, this correlation was imperfect. Glioblastomas with similar DRD2 expression values nevertheless exhibit some level of heterogeneity in EGFR expression. Vice versa was also observed.
Fig. 1.
EGFR and DRD2 expression are inversely correlated in most glioblastoma patients. (A, B) Plots of levels of expression of EGFR and DRD2 in TCGA (A) and non-TCGA (B) datasets. r* and P*: Pearson’s correlation coefficient calculated after exclusion of patients with high EGFR and DRD2 expression. (C, D) EGFR and DRD2 expression among different molecular subtypes of glioblastoma patients in TCGA (C) and non-TCGA (D) datasets. PN: proneural; NE: neural; CL: classical; ME: mesenchymal.
Consistent with the previously published results,4,5 high EGFR expression correlated well with the classical subtype in both expression datasets (Fig. 1C, D). In contrast, the expression pattern of DRD2 appeared comparable across subtypes.
Glioblastoma Response to DRD2 Inhibition In Vitro
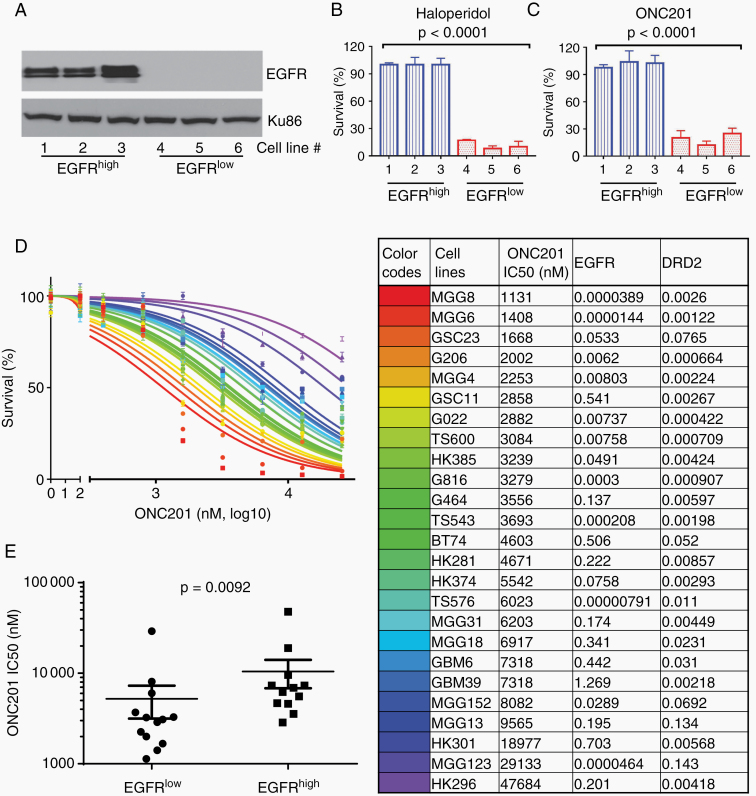
In the context of convergent signaling between DRD2 and EGFR, the anti-correlative relationship between EGFR and DRD2 expression suggests overlapping functions, such that hyperactivation of one pathway obviates the need for the other.31 In this context, we hypothesized that glioblastomas with low or no EGFR expression would be exquisitely dependent on DRD2 signaling. To test this hypothesis, we first tested the sensitivity of a small panel of patient-derived glioblastoma xenograft (PDX) lines with differing EGFR expression (termed EGFRhigh and EGFRlow; Fig. 2A) to independent DRD2 inhibitors. The EGFRhigh glioblastoma lines were insensitive to the 2 DRD2 inhibitors tested (haloperidol and ONC201; Fig. 2B, C). In contrast, the EGFRlow glioblastoma lines were highly sensitive to haloperidol or ONC201 treatment (Fig. 2B, C). At concentrations that did not affect the viability of EGFRhigh lines (20 µM for haloperidol and 10 µM for ONC201), the viability of EGFRlow lines were reduced to <20%.
Fig. 2.
PDX lines with low EGFR expression are sensitive to DRD2 inhibitors in vitro. (A) Western blots showing EGFR expression in 6 different PDX lines. (B, C) Survival fractions of the PDX lines in response to 20 µM of haloperidol (B) and 10 µM of ONC201 (C). (D) Dose response curves of the second panel of 25 glioblastoma neurosphere cell lines to ONC201. The 6 cell lines studied in the first panel were not included in this second panel of 25 lines (left panel). The colors in the graph correspond to the cell lines listed in a table on the right panel. Half-maximal inhibitory concentration (IC50) for ONC201, and relative transcription levels of EGFR and DRD2 measured by qRT-PCR in each cell line (right panel). (E) IC50 for ONC201 in EGFRlow and EGFRhigh glioblastoma PDX lines.
To further explore the interaction between DRD2 and EGFR, we next characterized the levels of EGFR mRNA expression, DRD2 mRNA expression, and ONC201 sensitivity for an additional panel of 25 PDX glioblastoma lines (Fig. 2D, Supplementary Figure 2). Lines were stratified based on median expression value of EGFR or DRD2 expression. In this analysis, higher than median EGFR expression was associated with resistance to ONC201 in vitro (Fig. 2E). Interestingly, higher than median DRD2 expression was also associated with resistance to ONC201 (Supplementary Figure 3A). The subset of glioblastoma lines most sensitive to ONC201 had low EGFR and DRD2 expression (Supplementary Figure 3B). Of note, the level of DRD2 expression in the initial panel of 6 glioblastoma lines tested (Fig. 2A–C) was in the lower range of the DRD2 expression spectrum defined by the 25 additional lines.
Glioblastoma Response to DRD2 Inhibition In Vivo
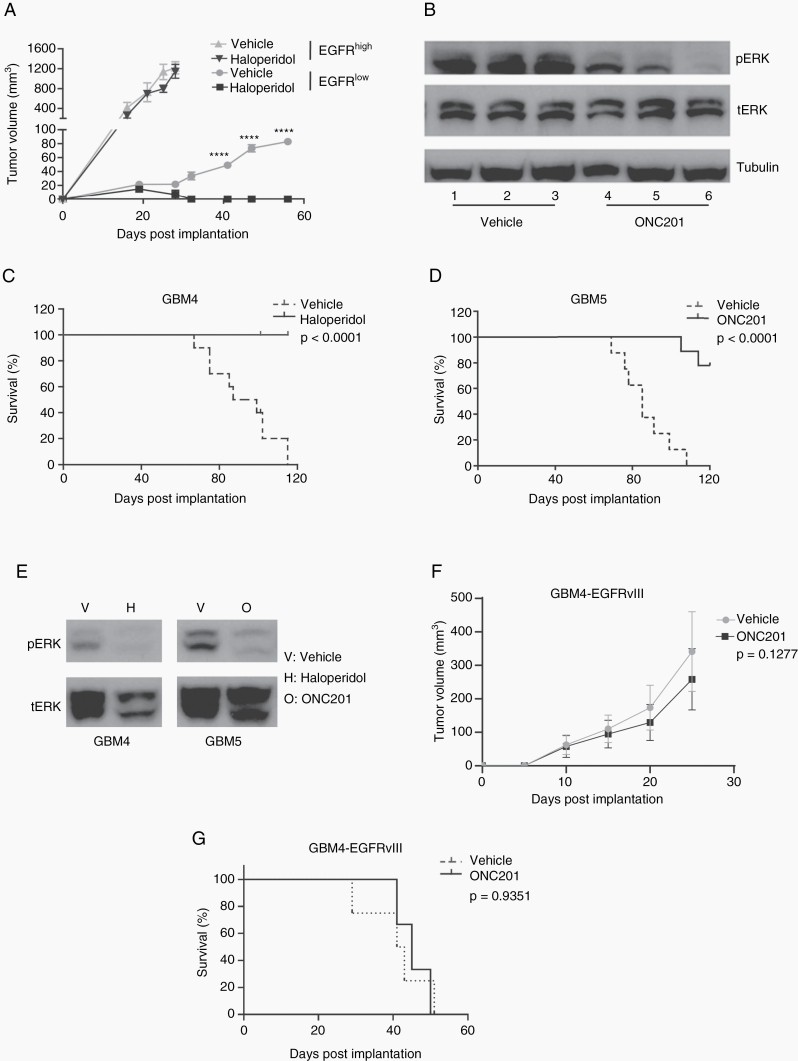
To determine whether the pattern of response observed in vitro is recapitulated in vivo, we first tested the effects of haloperidol on subcutaneous xenograft growth using one EGFRhigh and one EGFRlow glioblastoma PDX line (GBM1 and GBM6, respectively). The EGFRhigh line grew significantly faster than the EGFRlow line, reaching a tumor volume of 100 mm3 by post-implant day 13. In contrast, the DRD2high line grew to a tumor volume of 100 mm3 by post-implant day 43 (Fig. 3A). To control the difference in growth rate, haloperidol (10 mg/kg, i.p. daily) was administered when each xenograft reached 20 mm3. Consistent with our in vitro observations, haloperidol treatment did not affect the growth of the EGFRhigh xenografts. In contrast, haloperidol retarded the growth of the EGFRlow xenografts. By day 30, complete tumor regression was observed in the haloperidol treated xenografts while the untreated tumors continue to enlarge (Fig. 3A).
Fig. 3.
PDX lines with low EGFR expression are sensitive to DRD2 inhibitors in vivo. (A) Growth of EGFRhigh and EGFRlow subcutaneous xenografts in response to treatment with vehicle or haloperidol. The growth of EGFRlow glioblastoma was significantly inhibited. (B) ONC201 treated EGFRlow tumors were collected to evaluate the expression of total ERK (tERK) and pERK. T1–T6: independent tumor samples collected after vehicle (T1–3) or ONC201 treatment (T4–6). (C) Survival of mice orthotopically engrafted with an EGFRlow PDX on treatment with vehicle or haloperidol (n = 9 in the vehicle group, n = 9 in the haloperidol group). (D) Survival of mice orthotopically engrafted with an EGFRlow PDX line on treatment with vehicle or ONC201 (n = 8 in the vehicle group, n = 9 in the ONC201 group). (E) pERK expression at 72 hours after treatment of EGFRlow PDX xenografts with vehicle, haloperidol, or ONC201. (F) Growth of and EGFRlow PDX cell line exogenously overexpressing EGFRvIII as subcutaneous xenografts in response to treatment with vehicle or ONC201. (G) Survival of mice orthotopically engrafted with an EGFRlow PDX line exogenously overexpressing EGFRvIII on treatment with vehicle or ONC201 (n = 10 in both groups).
We previously demonstrated ERK is a downstream effector of DRD2 activation in glioblastomas.12 As such, we tested whether ONC201 and haloperidol treatment suppressed ERK activation using the EGFRlow xenografts. ONC201 or haloperidol was administered after the xenograft reached 100 mm3. Tumors were extracted 2 hours after haloperidol or vehicle infusion and analyzed by pERK immunoblotting. Three independent tumors were tested for the ONC201, haloperidol, or vehicle infused animals. Immunoblotting demonstrated that ONC201 (Fig. 3B) and haloperidol treatment consistently suppressed pERK accumulation (Supplementary Figure 4A).
We next tested the effects of DRD2 inhibition on glioblastoma growth using orthotopic xenograft models. We first selected GBM4 for this purpose. Haloperidol was administered 14 days after tumor implant. In this model, the median survival of the untreated mice was 89 days. All mice treated with haloperidol survived beyond 120 days (P < 0.0001; Fig. 3C). To ensure that the result is robust and not cell line or inhibitor dependent, we next confirmed these results with a second PDX model (GBM5) and a distinct DRD2 inhibitor (ONC201). In this experiment, ONC201 was administered 14 days after tumor implant. The median survival of the untreated mice was 84 days, and 80% of the mice treated with ONC201 survived beyond 120 days (P < 0.0001; Fig. 3D).
Consistent with results reported by our previous study,12 DRD2 inhibition through ONC201 treatment suppressed pERK accumulation in both GBM4 and GBM5 (Fig. 3E). Of note, the pERK suppressive effects of ONC201 and haloperidol were epistatic. The combination of ONC201 and haloperidol suppressed pERK accumulation to the same extent as each individual drug (Supplementary Figure 4B), suggesting that they act on the same target.
To determine if constitutive EGFR activity suppressed glioblastoma sensitivity to ONC201, we next introduced a constitutively active form of EGFR, EGFRvIII,7 into GBM4, a PDX line sensitive to ONC201. Introduction of EGFRvIII accelerated the growth rate of GBM4 as heterotopic and orthotopic implants (Figures 3F, G). In contrast to those observed using the parental GBM5 line (Fig. 3D), growths of GBM4-EGFRvIII as subcutaneous (Fig. 3F) and intracranial xenografts (Fig. 3G) were refractory to DRD2 inhibition. Of note, suppression of ONC201 sensitivity by ectopic EGFR expression has been recapitulated in another recently published study.32
In summary, these results suggest that EGFR hyperactivation suppress glioblastoma sensitivity to ONC201.
Association Between Expression of DRD2, TH, DDC, and Dopamine Secretion
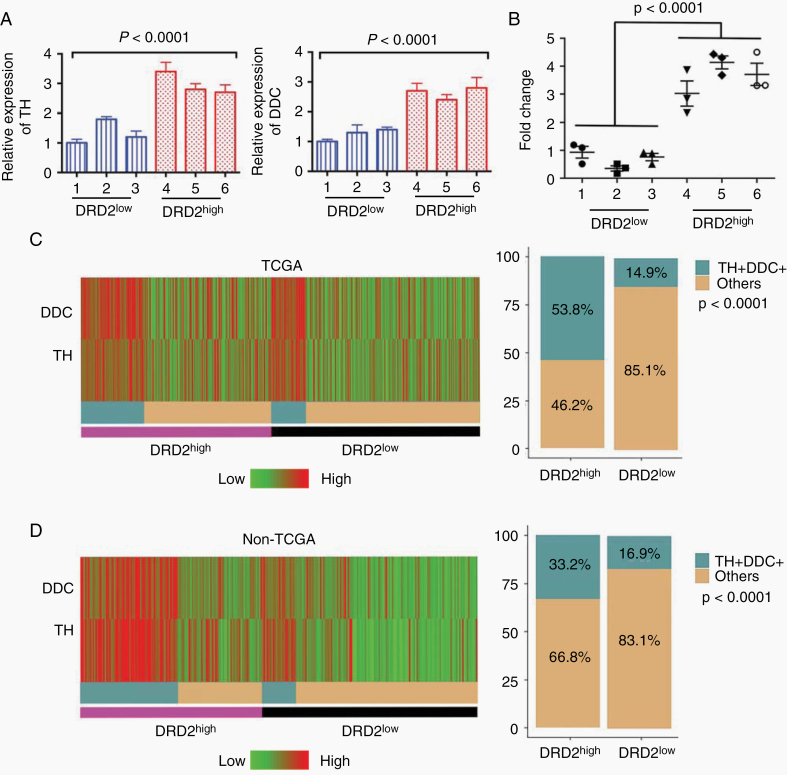
While DRD2 signaling can take place without the cognate ligand, most studies suggest that optimal DRD2 signaling requires dopamine.13 In this context, we tested whether dopamine synthesis and release were increased in high DRD2 expressing glioblastomas. The initial panel of 6 glioblastoma cell lines (Fig. 2A) showed variable expression in DRD2 (Fig. 4A). Using this panel, we tested the expression of the 2 genes that encode enzymes catalyzing rate-limiting steps in dopamine synthesis, TH and DDC.33 The mRNA expressions of TH and DDC were 2- to 3-fold higher in 3 lines with higher DRD2 expression relative to the 3 lines with lower DRD2 expression (Fig. 4A).
Fig. 4.
Dopamine synthesis activity is upregulated in glioblastoma samples. (A) Relative expression of TH and DDC in the 6 PDX lines measured by qRT-PCR. The DRD2high lines expressed 2–3 fold higher TH and DDC relative to the DRD2low lines. (B) Expression of dopamine among the PDX lines measured by ELISA. (C) Heatmap showing the expression of DDC and TH of the glioblastoma patients from the database of TCGA (n = 499, left panel); the proportion of patients with high levels of expression of DDC and TH among patients with high and low DRD2 (right panel). (D) Heatmap showing the expression of DDC and TH of the glioblastoma patients from the non-TCGA dataset (n = 260, left panel); the proportion of patients with high levels of expression of DDC and TH among patients with high and low DRD2 (right panel). TH+DDC+: TH and DDC double positive patients.
To confirm that the expression of TH and DDC correlated with dopamine synthesis, we performed dopamine enzyme-linked immunosorbent assay (ELISA) analysis of supernatants collected from these glioblastoma lines. The dopamine levels in media collected from the 3 lines with higher DRD2 expression were 3- to 4-fold higher than those derived from the 3 lines with lower DRD2 expression (P < 0.001; Fig. 4B). These results suggest that higher DRD2 expression is associated with higher DDC and TH expression, which is in turn associated with increased levels of dopamine release.34
Next, we determined if there is an association between DRD2 expression and the expression of TH and DDC in clinical glioblastoma samples. To this end, we analyzed the glioblastoma dataset of TCGA .4 Each glioblastoma sample was classified as high or low DRD2 expressing based on median expression value. We found that high DRD2 expressing glioblastomas are more likely to exhibit increased expression of TH and DDC (Fig. 4C, D). In the glioblastomas from TCGA, 53.8% of glioblastomas with high DRD2 expression exhibited higher than median expression of TH and DDC (P < 0.001; Fig. 4C). In contrast, only 14.9% of glioblastomas expressing low levels of DRD2 exhibit such TH and DDC expression. We observed similar findings in the non-TCGA dataset (Fig. 4D), where 33.2% of high DRD2 expressing glioblastomas exhibited higher expression of TH and DDC, in comparison to 16.9% of low DRD2 expressing glioblastomas (P < 0.001; Fig. 4D).
Finally, we assessed whether the correlation between DRD2 and TH/DDC expression varied as a function of DRD2 and EGFR expression. To this end, we had stratified the TCGA and non-TCGA glioblastomas into the following subgroups: DRD2low-EGFRlow / DRD2low-EGFRhigh / DRD2high-EGFRlow / DRD2high-EGFRhigh. The correlation between DRD2 and DDC/TH expression remained robust in all subgroups (Supplementary Figure 5).
Clinical Response to ONC201, a Blood–Brain Penetrant Bitopic DRD2 Inhibitor
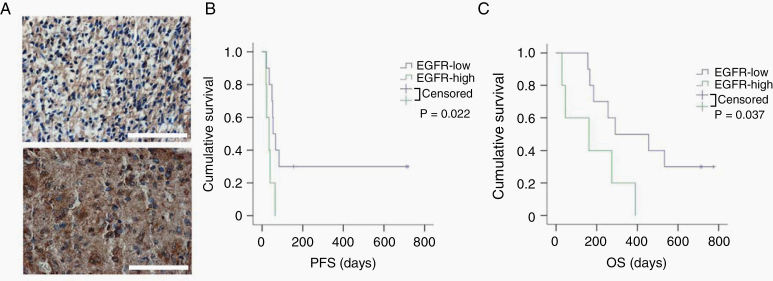
Our results suggest that low EGFR expression in glioblastomas potentially serve as a biomarker of positive response to DRD2 inhibitors. We tested this hypothesis using 15 clinically annotated specimens derived from a phase II clinical trial where glioblastoma patients were treated with ONC201.16 These specimens were procured prior to ONC201 treatment. EGFR expression was assessed by IHC and reviewed by 2 independent board-certified neuropathologists (Fig. 5A). Supporting our hypothesis, all patients who exhibited progression-free survival beyond 200 days showed low to no EGFR expression (P = 0.002; Fig. 5B). The median overall survivals for patients with high and low EGFR expressing glioblastomas were 162 and 373 days, respectively (P = 0.037; Fig. 5C).
Fig. 5.
Glioblastoma patients with low EGFR expression showed significantly extended survival after DRD2 antagonist treatment. (A) Representative images showing EGFR expression in human glioblastoma tumor tissue (low expression and high expression, respectively). Scale bars 100 μm. (B, C) Survival curves of the patients with high or low EGFR expression (progression-free survival [PFS] and overall survival [OS], respectively). All the patients received DRD2 antagonist (ONC201) treatment.
While these results suggest that EGFR expression is a determinant of ONC201 response, an alternative explanation is that high EGFR expression confers poor prognosis independent of ONC201 treatment. To exclude this possibility, we analyzed the datasets of TCGA and non-TCGA sources in search of potential association between EGFR expression and overall survival. We classified glioblastoma EGFR expression as high or low based on median expression. Our analyses revealed no survival association with EGFR expression in the datasets of both TCGA and non-TCGA sources (Supplementary Figure 6).
These results support our hypothesis that high EGFR expression is a determinant of poor glioblastoma response to ONC201.
Discussion
There is mounting interest in the blood–brain barrier penetrant and DRD2 inhibitor ONC201 as a glioblastoma therapy. Here, we explored EGFR expression as a determinant of ONC201 response. The rationale for the underlying hypothesis involves our previous finding of signaling convergence of EGFR and DRD2, such that hyperactivation of one pathway compensates for the inhibition of the other.13 Key findings in this study that support the EGFR expression as a potential biomarker for ONC201 sensitivity include:
1. ONC201 sensitivity correlated with low EGFR expression in independent panels of glioblastoma PDX lines.
2. Ectopic expression of a constitutively active EGFR, EGFRvIII, abolished glioblastoma sensitivity to ONC201.
3. ONC201 efficacy in a phase II glioblastoma trial was inversely associated with low EGFR staining on IHC of clinical samples procured prior to therapy (Fig. 6).
Fig. 6.
Graphical summary. EGFR and DRD2 signaling converge at ERK. Thus, EGFR hyperactivation compensates for DRD2 inhibition.
These observations provide a compelling argument for clinical trial design for the validation of the proposed hypothesis.
While the canonical signaling mechanisms of DRD2 and EGFR differ,6,13 both play key roles in glioblastoma pathogenesis12,35 and share the downstream effector, ERK.12,36,37 Notably, RTK activation can be achieved by “G protein coupled receptor (GPCR) jacking” 38 in the absence of RTK ligand. And increased expression of 7-transmembrane GPCR, including DRD2, is an escape mechanism against EGFR inhibition.39 Despite data supporting the cross-talks and overlapping function between these receptors, it is intriguing that EGFR- and DRD2-driven glioblastomas exhibit distinct phenotypes, including differential growth kinetics (Fig. 3). It is equally puzzling why certain glioblastomas harbor dual hyperactivation of EGFR and DRD2 while others predominantly depend on a single signaling axis. Understanding the basis underlying such difference should yield insights into glioblastoma molecular circuitry and clinical trial design.
The detection of secreted dopamine in media derived from glioblastoma PDX lines suggests autocrine signaling as one potential mechanism of DRD2 activation. This interpretation is largely consistent with findings reported by Caragher et al, where maintenance of stem cell–like states in glioblastoma is dependent on autocrine secretion of dopamine.35 However, the imperfect correlation between DRD2 and DDC/TH expression in clinical glioblastomas (Fig. 4C, D) suggests potential contributions from nonneoplastic cells in the tumor microenvironment. While microglia and astrocytes have not been shown to actively secrete dopamine, they modulate aspects of dopamine metabolism that contribute to the steady-state level in the tumor microenvironment.40,41 Moreover, glioblastoma located in the proximity of dopaminergic synapse may be exposed to higher levels of dopamine.42 As such, both autocrine and paracrine mechanisms are likely to contribute to local dopamine concentrations in the tumor microenvironment.
Oncogenic mutations in neurotransmitters or neurotransmitter receptors have not yet been identified in glioblastomas. This observation is in stark contrast to the frequent oncogenic mutations found in growth factor receptors, such as EGFR.4,5 This observation, coupled with the potent transforming activity of oncogenic EGFR mutants in experimental models, suggests that oncogenic EGFR activation is a more “efficient” means of glioblastoma pathogenesis. The mechanism of autocrine signaling between dopamine and DRD2 is intriguing in this context. While an autocrine DRD2-dopamine circuit affords cell-autonomous signaling to facilitate tumorigenesis, it additionally mediates non-cell-autonomous functions including quorum sensing43 and other populational dynamics44 that influence the heterogeneity within the glioblastoma microenvironment.45 To the extent that physiologic, genetic, and epigenetic heterogeneity form the denominator from which selective pressures (whether imposed by therapy or by intrinsic tumor development) exert their effect,46 the DRD2 circuit may harbor heretofore unappreciated impacts on tumor evolution and therapeutic response.47
In summary, our study suggests that a subset of patients with glioblastomas with low EGFR expression may preferentially benefit from ONC201. This result is congruent with the efficacy of ONC201 against glioblastomas bearing the histone H3 K27M mutation,24 a mutation that is mutually exclusive with EGFR hyperactivation.45 Future clinical trials should consider this and other identified molecular predictors of ONC201 response, including DRD5 expression/mutation,17 levels of TRAIL (tumor necrosis factor–related apoptosis inducing ligand),48 expression of ClpP (caseinolytic mitochondrial matrix peptidase proteolytic subunit),49 and Myc status.32
Supplementary Material
Acknowledgments
The authors thank Drs Jianfang Ning and Ming Li for assistance in arrangement of PDX cell lines.
Funding
This work was supported by 1RO1NS097649-01, NIH 9R44GM128223-02, the Doris Duke Charitable Foundation Clinical Scientist Development Award, The Sontag Foundation Distinguished Scientist Award, the Kimmel Scholar Award, and BWF 1006774.01 (CCC).
Conflict of interest statement. VVP and JEA are employees and stockholders of Oncoceutics, Inc.
Authorship statement. Funding: CCC; study/experimental design: CCC, JL, YH, VVP, JEA, FF; experiment implementation: YH, JL, TK, JM, SD, YS, FF, VVP, JEA; data collection: CCC, YH, FF, VVP, JEA; data analysis and visualization: YH, JL, data interpretation: CCC, YH, JL, JM, FF, VVP; manuscript preparation and review: all authors.
References
- 1. Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97–117. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Dunn GP, Rinne ML, Wykosky J, et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26(8):756–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brennan CW, Verhaak RG, McKenna A, et al. ; TCGA Research Network . The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verhaak RG, Hoadley KA, Purdom E, et al. ; Cancer Genome Atlas Research Network . Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. [DOI] [PubMed] [Google Scholar]
- 7. Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci U S A. 1998;95(10):5724–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liffers K, Lamszus K, Schulte A. EGFR amplification and glioblastoma stem-like cells. Stem Cells Int. 2015;2015:427518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Westphal M, Maire CL, Lamszus K. EGFR as a target for glioblastoma treatment: an unfulfilled promise. CNS Drugs. 2017;31(9):723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu X, Chen X, Shi L, et al. The third-generation EGFR inhibitor AZD9291 overcomes primary resistance by continuously blocking ERK signaling in glioblastoma. J Exp Clin Cancer Res. 2019;38(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwatra MM. A rational approach to target the epidermal growth factor receptor in glioblastoma. Curr Cancer Drug Targets. 2017;17(3):290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J, Zhu S, Kozono D, et al. Genome-wide shRNA screen revealed integrated mitogenic signaling between dopamine receptor D2 (DRD2) and epidermal growth factor receptor (EGFR) in glioblastoma. Oncotarget. 2014;5(4):882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bibb JA. Decoding dopamine signaling. Cell. 2005;122(2):153–155. [DOI] [PubMed] [Google Scholar]
- 14. Faraz S, Pannullo S, Rosenblum M, Smith A, Wernicke AG. Long-term survival in a patient with glioblastoma on antipsychotic therapy for schizophrenia: a case report and literature review. Ther Adv Med Oncol. 2016;8(6):421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grinshpoon A, Barchana M, Ponizovsky A, et al. Cancer in schizophrenia: is the risk higher or lower? Schizophr Res. 2005;73(2-3):333–341. [DOI] [PubMed] [Google Scholar]
- 16. Arrillaga-Romany I, Chi AS, Allen JE, Oster W, Wen PY, Batchelor TT. A phase 2 study of the first imipridone ONC201, a selective DRD2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget. 2017;8(45):79298–79304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prabhu VV, Madhukar NS, Gilvary C, et al. Dopamine receptor D5 is a modulator of tumor response to dopamine receptor D2 antagonism. Clin Cancer Res. 2019;25(7):2305–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eblen ST. Extracellular-regulated kinases: signaling from Ras to ERK substrates to control biological outcomes. Adv Cancer Res. 2018;138:99–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun C, Bernards R. Feedback and redundancy in receptor tyrosine kinase signaling: relevance to cancer therapies. Trends Biochem Sci. 2014;39(10):465–474. [DOI] [PubMed] [Google Scholar]
- 20. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1): 182–217. [DOI] [PubMed] [Google Scholar]
- 21. Gazi L, Nickolls SA, Strange PG. Functional coupling of the human dopamine D2 receptor with G alpha i1, G alpha i2, G alpha i3 and G alpha o G proteins: evidence for agonist regulation of G protein selectivity. Br J Pharmacol. 2003;138(5):775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kozono D, Li J, Nitta M, et al. Dynamic epigenetic regulation of glioblastoma tumorigenicity through LSD1 modulation of MYC expression. Proc Natl Acad Sci U S A. 2015;112(30):E4055–E4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sarkaria JN, Yang L, Grogan PT, et al. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007;6(3):1167–1174. [DOI] [PubMed] [Google Scholar]
- 24. Laks DR, Crisman TJ, Shih MY, et al. Large-scale assessment of the gliomasphere model system. Neuro Oncol. 2016;18(10): 1367–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chakravarty D, Pedraza AM, Cotari J, et al. EGFR and PDGFRA co-expression and heterodimerization in glioblastoma tumor sphere lines. Sci Rep. 2017;7(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Esaki S, Nigim F, Moon E, et al. Blockade of transforming growth factor-β signaling enhances oncolytic herpes simplex virus efficacy in patient-derived recurrent glioblastoma models. Int J Cancer. 2017;141(11):2348–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ning J-F, Stanciu M, Humphrey MR, et al. Myc targeted CDK18 promotes ATR and homologous recombination to mediate PARP inhibitor resistance in glioblastoma. Nat commun. 2019;10(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiao A, Brenneman B, Floyd D, et al. Statins affect human glioblastoma and other cancers through TGF-β inhibition. Oncotarget. 2019;10(18):1716–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Binder ZA, Thorne AH, Bakas S, et al. Epidermal growth factor receptor extracellular domain mutations in glioblastoma present opportunities for clinical imaging and therapeutic development. Cancer Cell. 2018;34(1):163–177.e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc. 2006;1(2):581–585. [DOI] [PubMed] [Google Scholar]
- 31. Ciriello G, Cerami E, Sander C, Schultz N. Mutual exclusivity analysis identifies oncogenic network modules. Genome Res. 2012;22(2):398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ishida CT, Zhang Y, Bianchetti E, et al. Metabolic reprogramming by dual AKT/ERK inhibition through imipridones elicits unique vulnerabilities in glioblastoma. Clin Cancer Res. 2018;24(21):5392–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Commun Signal. 2013;11(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walsh JH, Karnes WE, Cuttitta F, Walker A. Autocrine growth factors and solid tumor malignancy. West J Med. 1991;155(2):152–163. [PMC free article] [PubMed] [Google Scholar]
- 35. Caragher SP, Shireman JM, Huang M, et al. Activation of dopamine receptor 2 prompts transcriptomic and metabolic plasticity in glioblastoma. J Neurosci. 2019;39(11):1982–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beaulieu JM, Del’guidice T, Sotnikova TD, Lemasson M, Gainetdinov RR. Beyond cAMP: the regulation of Akt and GSK3 by dopamine receptors. Front Mol Neurosci. 2011;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261–273. [DOI] [PubMed] [Google Scholar]
- 38. Delcourt N, Bockaert J, Marin P. GPCR-jacking: from a new route in RTK signalling to a new concept in GPCR activation. Trends Pharmacol Sci. 2007;28(12):602–607. [DOI] [PubMed] [Google Scholar]
- 39. Mukasa A, Wykosky J, Ligon KL, Chin L, Cavenee WK, Furnari F. Mutant EGFR is required for maintenance of glioma growth in vivo, and its ablation leads to escape from receptor dependence. Proc Natl Acad Sci U S A. 2010;107(6):2616–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vainchtein ID, Molofsky AV. Astrocytes and microglia: in sickness and in health. Trends Neurosci. 2020;43(3):144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Färber K, Pannasch U, Kettenmann H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol Cell Neurosci. 2005;29(1):128–138. [DOI] [PubMed] [Google Scholar]
- 42. Slaney TR, Mabrouk OS, Porter-Stransky KA, Aragona BJ, Kennedy RT. Chemical gradients within brain extracellular space measured using low flow push-pull perfusion sampling in vivo. ACS Chem Neurosci. 2013;4(2):321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Doğaner BA, Yan LKQ, Youk H. Autocrine signaling and quorum sensing: extreme ends of a common spectrum. Trends Cell Biol. 2016;26(4):262–271. [DOI] [PubMed] [Google Scholar]
- 44. Youk H, Lim WA. Sending mixed messages for cell population control. Cell. 2014;158(5):973–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Podlaha O, Riester M, De S, Michor F. Evolution of the cancer genome. Trends Genet. 2012;28(4):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168(4):613–628. [DOI] [PubMed] [Google Scholar]
- 48. Allen JE, Krigsfeld G, Mayes PA, et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013;5(171): 171ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Graves PR, Aponte-Collazo LJ, Fennell EMJ, et al. Mitochondrial protease ClpP is a target for the anticancer compounds ONC201 and related analogues. ACS Chem Biol. 2019;14(5):1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.