Abstract
Background
The objective of this study was to evaluate the contribution of radiation dose to different intracranial structures on changes in intellectual function for children with brain tumors.
Methods
We evaluated children with brain tumors treated in 2005–2017 who had longitudinal neuropsychological assessments and available photon dosimetric data (if radiation therapy [RT] given). Full Scale Intelligence Quotient (FSIQ) and index scores were evaluated (perceptual reasoning index [PRI], processing speed index [PSI], verbal comprehension index [VCI], and working memory index [WMI]). Multivariable linear mixed effects models were used to model endpoints, with age at RT and dose to different brain regions as fixed effects and patient-specific random intercepts. P-values (P*) were adjusted for multiple comparisons.
Results
Sixty-nine patients were included, 56 of whom received RT. Median neuropsychological follow-up was 3.2 years. Right temporal lobe mean dose was strongly associated with decline in FSIQ (P* = 0.005); with each gray increase in mean dose, there was a decrease of 0.052 FSIQ points per year. Dose to 50% (D50) of the supratentorial brain was associated with decline in PSI (P* = 0.006) and WMI (P* = 0.001). Right and left hippocampus D50 were individually strongly associated with declines in VCI (P* = 0.009 for each). Presence of a ventriculoperitoneal shunt decreased FSIQ by 10 points.
Conclusions
We reported associations between dosimetry to specific brain regions and intellectual outcomes, with suggested avoidance structures during RT planning. These models can help clinicians anticipate changes in neurocognition post-RT and guide selection of an optimal RT plan.
Keywords: brain neoplasms, cognitive dysfunction, intelligence tests, pediatrics, radiation
Key Points.
Radiation to the right temporal lobe is associated with IQ decline.
The supratentorial brain and hippocampi are important for processing speed, working memory, and verbal comprehension.
Radiation dose to these structures should be minimized when possible.
Importance of the Study.
Radiation therapy for children with brain tumors can result in intellectual changes over time. However, the brain structures most vulnerable to RT are not well understood. In this study, we found that mean dose to the right temporal lobe was most strongly associated with decline in full scale IQ, while dose to 50% (D50) of the supratentorial brain was associated with declines in processing speed and working memory. Furthermore, left and right hippocampus D50 were individually associated with verbal comprehension. These findings have important implications for neuro-oncologists, radiation oncologists, and neuropsychologists, and will guide clinicians in treatment decision making, RT planning, and further research to minimize the burden of late effects for children undergoing treatment for brain tumors.
Radiation therapy (RT) plays an important curative role in many pediatric brain tumors, such as medulloblastoma, ependymoma, and craniopharyngioma.1 However, it is now well known that exposure of developing brain tissue to therapeutic radiation can cause long-term neurocognitive sequelae.2,3 In a Childhood Cancer Survivor Study of patients who had a childhood brain tumor, use of RT or ventriculoperitoneal (VP) shunt or presence of hearing impairment were associated with challenges in patient-reported measures of cognitive function later in life.4
Improvements in RT planning and delivery have allowed for precise treatment of tumor targets and quantitative evaluation of dose to specific substructures within the brain. Few studies have employed volumetric radiation dosimetry to specific brain substructures to model change in intellectual function following RT. Availability of 3-dimensional dose data allows delineation and quantification of the effects of irradiating different parts of the brain, such as the hippocampus, supratentorial brain, or temporal lobes. Furthermore, volumetric dosimetry allows for calculation of dose-volume metrics, such as mean brain dose or dose to 50% of the structure (D50),5 to more clearly define parameters that are associated with intellectual changes.
Comprehensive neuropsychological assessment is recommended for pediatric patients who have had RT to the brain or certain types of chemotherapy.6,7 An age-appropriate standardized test of intelligence is one of the measures that may be used. The most commonly used tools are the Wechsler Intelligence Scales, which provide the Full Scale Intelligence Quotient (FSIQ).8 The FSIQ comprises multiple, specific cognitive processes; analysis of only broad measures such as FSIQ can obfuscate more specific brain–behavior relationships. Instead, core cognitive functions, such as processing speed and working memory, have been shown to be more sensitive to specific treatment effects (such as RT) to different brain structures than FSIQ in pediatric brain tumor patients.9
The contribution of quantitative radiation dosimetry of individual intracranial structures to intellectual variables is not clear. Radiation to specific brain regions may lead to localized cognitive changes. It is not known which structures are most important for each domain of intellectual function after RT and how changes in dose to those structures can affect global and specific intellectual functions.
The goal of this study was to create models of intellectual function after RT based on quantitative dosimetric metrics to brain substructures, as well as clinical and treatment factors. This will allow us to better understand which brain structures are most sensitive to RT with respect to changes in full scale IQ and index scores of global intellectual function (perceptual reasoning index [PRI], processing speed index [PSI], verbal comprehension index [VCI], and working memory index [WMI]). We hypothesize that higher radiation doses to specific brain structures are associated with lower intelligence domain scores.
Materials and Methods
This was a retrospective study of patients who received neuro-oncology care at the Hospital for Sick Children and radiation treatment at Princess Margaret Cancer Centre in Toronto, Canada. Patients were eligible if they were age 18 or under at the time of diagnosis, had a primary brain tumor treated between 2005 and 2017, and had longitudinal (2 or more) neuropsychological assessments after their brain tumor diagnosis (see Supplementary Figure 1). Patients who did not receive RT were eligible for inclusion to create a group of patients who had zero dose to any brain structures to ensure robust modeling of baseline function and non-RT variables (including the model intercepts and fixed effect coefficients). A direct comparison of the patients treated with or without RT was not made because of different indications for RT in our population. Neuropsychology evaluations done after tumor recurrence were excluded. All patients were covered by the government-funded Ontario Health Insurance Plan. This study was approved by the research ethics boards of the Hospital for Sick Children and University Health Network.
Clinical, pathologic and intellectual data were retrieved from patient records. Radiation was delivered using photon linear accelerators; no patient received proton therapy. All focal and boost RT plans were delivered using intensity modulated RT or volumetric modulated RT. Composite radiation dosimetry, radiation planning CT datasets, and fused and registered MR T1/T2/fluid attenuated inversion recovery images were retrieved from the treatment planning system (TPS). Brain regions and substructures (whole brain, supratentorial brain, infratentorial brain, cerebellum, right and left temporal lobes, right and left hippocampi) were manually contoured in the TPS. Hippocampal contours were done using the Radiation Therapy Oncology Group 0933 atlas.10 Normal brain was defined as the whole brain minus the gross tumor volume contour. Mean dose11 and dose to 50% of the structure (D50)12 were recorded.
Neuropsychology Testing
Prospective neuropsychological surveillance was offered to families as standard of care for children treated with upfront, curative-intent radiation for brain tumors. Patients were then seen for repeat psychology testing every 2–3 years, concurrent with routine neuro-oncology care. Due to the longitudinal nature of this study, different test versions were used; tests that were administered included the Wechsler Preschool and Primary Scale of Intelligence version III or IV,13 Wechsler Intelligence Scale for Children version III, IV, or V,8 or Wechsler Adult Intelligence Scale version III or IV,14 depending on the child’s age. Two assessments for 2 different patients were performed using the Wechsler Abbreviated Scale of Intelligence.15 One patient had 2 of 4 assessments done using the Stanford-Binet Intelligence Scale16 due to marked cognitive deficit; these 2 evaluations reported only FSIQ and VCI. A list of all evaluations and test versions is provided in Supplementary Table 1. The measures have good convergent validity, with correlations for composite scores ranging from 0.80 to 0.92 across measures and test versions administered in this study.16–21
For the Wechsler Intelligence scales, FSIQ, PRI, PSI, VCI, and WMI were analyzed to capture global and specific intellectual functions.8 FSIQ is a reliable measure of overall cognitive functioning; the VCI measures verbal reasoning; the PRI evaluates interpretation and organization of visual and nonverbal information; the WMI measures attentional and concentration abilities as well as mental manipulation ability; and the PSI evaluates the speed of graphomotor and mental processing. Each score has a mean of 100 and standard deviation of 15. Higher values indicate greater intelligence scores.
Statistical Analysis
Baseline characteristics were reported descriptively. Multivariable linear mixed effects analyses were used to model each longitudinal intellectual endpoint, with age at RT and dose to different brain regions as fixed effects and patient-specific random intercepts. This method is able to model baseline function (the intercept, β 0) even in patients whose first neuropsychology evaluation was done after RT had started. To create models, we first evaluated the following variables in Model 1:
where age at RT and time since RT are in years, and RT is binary (0 for no and 1 for yes).
The Age × Time term was used because all the endpoints are age standardized and are expected to share the same value at time zero.11 Age was included as a continuous variable. The RT variable in term β 1 is present because this term dropped out in patients treated without RT. Other covariates that were individually evaluated included: presence of any hydrocephalus; VP shunt; any use of: VP shunt, endoscopic third ventriculostomy (ETV), or Ommaya reservoir; posterior fossa syndrome; number of surgeries at diagnosis (ie, if second-look surgery was done); use of any chemotherapy; and provision of craniospinal irradiation (CSI). Hydrocephalus was quantified using the frontal and occipital horn ratio, with a normal threshold of <0.37.22 Only the VP shunt covariate contributed significantly to the model and was subsequently included as a fixed effect; this is described further in the Results section.
Dosimetry to individual brain substructures was then evaluated in Model 2:
where age at RT and time since RT are in years, RT and VP shunt are binary (0 for no and 1 for yes), and RT dose (to the specified brain structure) is in gray (Gy).
The dose to brain substructures were individually evaluated in the above model. Dosimetric variables chosen a priori included: supratentorial brain mean and D50, infratentorial brain mean and D50, cerebellum mean and D50, left temporal mean and D50, right temporal mean and D50, left hippocampus mean and D50, right hippocampus mean and D50, whole brain mean, normal brain mean). Correlation between dosimetric measures were reported using Pearson’s correlation coefficient. The structure and metric with the strongest association with an intellectual endpoint (ie, smallest P-values) was selected for inclusion in the final model. P-values for all coefficients, including the dosimetry coefficient term (β 3), were obtained through the likelihood ratio test by assessing the goodness of fit of 2 nested models, with and without the β 3 term. Where indicated with an asterisk, P-values (P*) were adjusted for multiple comparisons using the false discovery rate (FDR) method to account for the number of dosimetric variables tested. This is also known as a q-value; a threshold of <0.05 was used for statistical significance. Subsequently, we created plots of each endpoint at 3-year post-RT follow-up while varying RT dose to brain substructures, age at RT, and presence or absence of VP shunt. Three-year follow-up was chosen because it was the approximate median neuropsychological follow-up time. Statistical analyses were performed using R v3.5.2.
Results
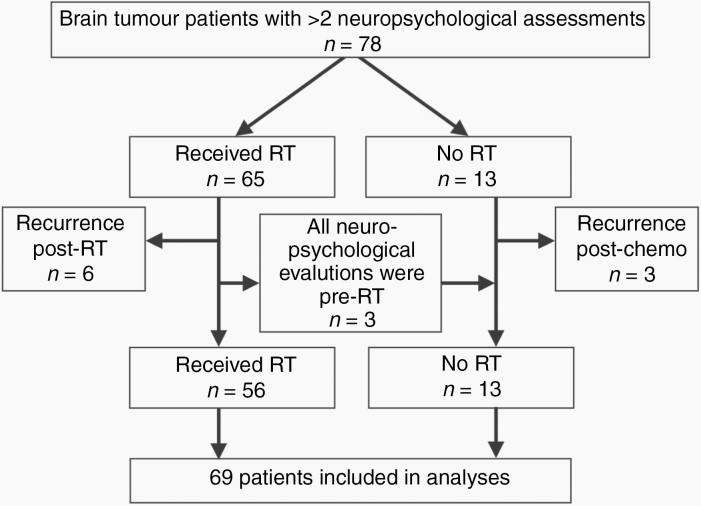
Seventy-eight patients met initial eligibility criteria of having a primary brain tumor diagnosis and for whom longitudinal neuropsychological assessment (2 or more assessments) was available. Nine patients were excluded because they had developed a tumor recurrence prior to their second neurocognitive evaluation. The final analysis cohort included 69 patients (Figure 1).
Fig. 1.
Flow diagram of patients included in this study. Patients were excluded if they had a recurrence after treatment but before their second neurocognitive evaluation.
Baseline characteristics of the study cohort are listed in Table 1. Among 56 patients in the final RT cohort, the median total prescription was 55.8 Gy (range, 23.4–59.4 Gy). The median CSI dose was 23.4 Gy (range, 18.0–39.6 Gy; n = 22). Radiation doses to organs at risk for all patients in the cohort are listed in Supplementary Table 2. Correlation between the dosimetry variables is shown in Supplementary Figure 2.
Table 1.
Baseline characteristics of all patients
| Variable | N = 69 |
|---|---|
| Sex | |
| Female | 31 (45%) |
| Male | 38 (55%) |
| Age, y, at tumor diagnosis, median (range) | 6 (1–16) |
| Age, y, at radiation,a median (range) | 8 (1–17) |
| Diagnosis | |
| Atypical teratoid/rhabdoid tumor | 4 (6%) |
| Craniopharyngioma | 12 (17%) |
| Embryonal tumorb | 22 (32%) |
| Ependymoma | 13 (19%) |
| Germ cell tumor | 8 (12%) |
| Low-grade glioma | 7 (10%) |
| Otherc | 3 (4%) |
| Infratentorial tumor involvement | 34 (49%) |
| Hydrocephalus at diagnosis | 50 (72%) |
| VP shunt | 16 (23%) |
| VP shunt, ETV, or Ommaya | 23 (33%) |
| Right-handed | 62 (90%) |
| Posterior fossa syndrome | 7 (10%) |
| Baseline index scores at first assessment, median (range) | |
| Full scale IQ | 91 (47–127) |
| Perceptual reasoning | 91 (45–128) |
| Processing speed | 88 (50–127) |
| Verbal comprehension | 95 (45–132) |
| Working memory | 95 (56–127) |
| Treatments | |
| Number of tumor-directed surgeries, median (range) | 1 (0–3) |
| No adjuvant treatment, surgery only | 2 (3%) |
| Chemotherapy | 53 (77%) |
| Radiation, any field | 56 (81%) |
| Craniospinal irradiationd | 22 (32%) |
| Radiotherapy techniquee | |
| 3DCRT | 5 (9%) |
| IMRT | 45 (80%) |
| VMAT | 6 (11%) |
| Daily general anesthetic during radiation | 11 (16%) |
3DCRT, 3-dimensional conformal radiation therapy; IMRT, intensity modulated radiation therapy (step-and-shoot); VMAT, volumetric modulated arc therapy.
a n = 56.
bIncludes one of each of pineoblastoma and primitive neuroectodermal tumor; otherwise medulloblastoma (n = 20).
cIncludes one each of pleomorphic sarcoma, anaplastic astrocytoma, and meningioma.
dCraniospinal irradiation includes RT to the whole brain using a 3DCRT technique
eFor patients who had craniospinal irradiation followed by focal tumor boost, the focal boost technique was recorded.
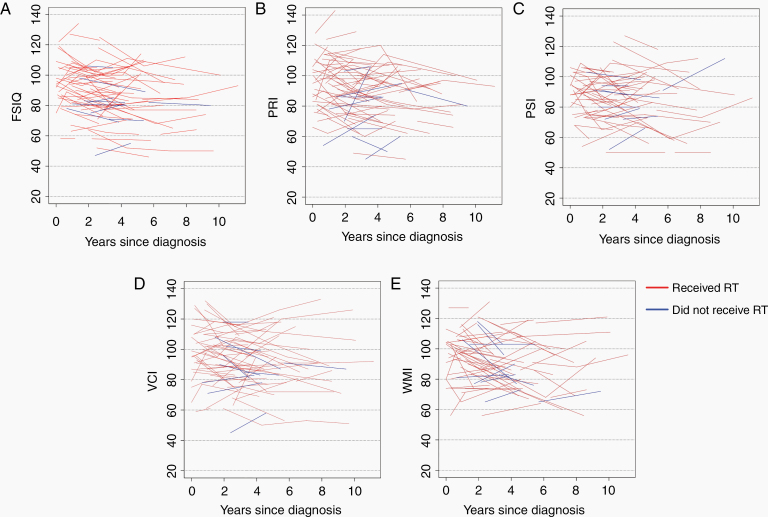
Median neuropsychological follow-up was 3.2 years from first to last assessment. Median time from diagnosis to last assessment was 4.6 years. Median times from RT to first and last assessments were 0.4 and 4.2 years, respectively. A total of 165 assessments were included; all patients had 2 or more evaluations, while 22 had 3 evaluations, 4 patients had 4 evaluations, and a single patient had 5 evaluations. Median time between first and second evaluation was 2.3 years, between second and third was 3.1 years, and between third and fourth was 2.9 years. Spaghetti plots of all intellectual scores are shown in Figure 2, stratified by receipt of RT. Improving and declining intellectual trajectories were both seen in patients treated with and without RT, indicating variability in an individual child’s longitudinal changes. Spaghetti plots for intellectual scores, with index time at the start of RT, are shown in Supplementary Figure 3. Correlation between endpoints is shown in Supplementary Figure 4.
Fig. 2.
Spaghetti plots of intelligence endpoints since time of diagnosis. Colors represent patients who did (red) or did not (blue) receive RT. (A) Full scale IQ. (B) Perceptual reasoning. (C) Processing speed. (D) Verbal comprehension. (E) Working memory. Because this study was not intended to compare those who did or did not receive RT (due to different indications for RT), regression lines were not created to compare these 2 groups.
Full-Scale IQ
Patient age at RT and presence of VP shunt (P = 0.02) were associated with change in FSIQ. Presence of a VP shunt decreased FSIQ by 10.2 points. Mean radiation dose to the right temporal lobe was also strongly associated with change in FSIQ; inclusion of the dose term improved the model fit (P = 0.0007). After adjusting for these variables, posterior fossa syndrome (P = 0.42), number of surgeries (P = 0.29), chemotherapy (P = 0.12), and CSI (P = 0.71) were not associated with FSIQ, and were not included in the final model. The model coefficients for Model 2 are listed in Table 2. Each Gy increase in mean dose to the right temporal lobe led to a 0.052-point decrease in FSIQ per year. Table 3 shows FSIQ values, modeled across various clinical scenarios.
Table 2.
Coefficients for longitudinal models evaluating intelligence endpoints*
| Endpoint | RT Dose Metric | n a | Variable | Estimate | Standard Error | P-value | q-Valuec |
|---|---|---|---|---|---|---|---|
| FSIQ | Right temporal lobe mean | 68 | β 0 (intercept) | 92.08 | 2.22 | <0.0001 | |
| β 1 (ageb × time) | 0.09 | 0.06 | 0.18 | ||||
| β 2 (VP shunt) | −10.18 | 4.39 | 0.023 | ||||
| β 3 (dose × time) | −0.05 | 0.02 | 0.00073 | 0.0048 | |||
| PRI | Right temporal lobe mean | 69 | β 0 (intercept) | 93.89 | 2.32 | <0.0001 | |
| β 1 (ageb × time) | 0.03 | 0.08 | 0.71 | ||||
| β 2 (VP shunt) | −9.40 | 4.56 | 0.043 | ||||
| β 3 (dose × time) | −0.04 | 0.02 | 0.036 | 0.26 | |||
| PSI | Supratentorial brain D50 | 67 | β 0 (intercept) | 88.10 | 2.15 | <0.0001 | |
| β 1 (ageb × time) | 0.16 | 0.07 | 0.029 | ||||
| β 2 (VP shunt) | −9.21 | 4.14 | 0.03 | ||||
| β 3 (dose × time) | −0.10 | 0.03 | 0.00032 | 0.0059 | |||
| VCI (model A) | Right hippocampus D50 | 69 | β 0 (intercept) | 97.47 | 2.23 | <0.0001 | |
| β 1 (ageb × time) | 0.09 | 0.08 | 0.23 | ||||
| β 2 (VP shunt) | −9.09 | 4.39 | 0.042 | ||||
| β 3 (dose × time) | −0.04 | 0.01 | 0.0019 | 0.0095 | |||
| VCI (model B) | Left hippocampus D50 | 69 | β 0 (intercept) | 97.45 | 2.24 | <0.0001 | |
| β 1 (ageb × time) | 0.10 | 0.08 | 0.22 | ||||
| β 2 (VP shunt) | −8.91 | 4.42 | 0.048 | ||||
| β 3 (dose × time) | −0.04 | 0.01 | 0.0021 | 0.0095 | |||
| WMI | Supratentorial brain D50 | 68 | β 0 (intercept) | 95.32 | 2.15 | <0.0001 | |
| β 1 (ageb × time) | 0.14 | 0.07 | 0.055 | ||||
| β 2 (VP shunt) | −7.05 | 4.20 | 0.098 | ||||
| β 3 (dose × time) | −0.10 | 0.03 | 0.00012 | 0.0014 |
*Negative estimates indicate a decrease in the intelligence score. The equation for calculating endpoints is presented as Model 2 in the Materials and Methods section.
a Number of patients evaluable in each model.
b Age at RT, in years.
c P-value adjusted for multiple comparisons.
Table 3.
Full scale IQ modeled across different clinical scenarios*
| Age at RT, y | VP Shunt | Right Temporal Lobe, Mean Dose, Gy | Predicted Full Scale IQ | ||
|---|---|---|---|---|---|
| Baseline pre-RTa | 3 years post-RT | 5 years post-RT | |||
| 4 | No | 15 | 92.1 | 90.8 | 90.0 |
| 4 | No | 30 | 92.1 | 88.5 | 86.1 |
| 4 | Yes | 30 | 81.9 | 78.3 | 75.9 |
| 8 | No | 15 | 92.1 | 91.9 | 91.7 |
| 8 | No | 30 | 92.1 | 89.5 | 87.8 |
| 8 | Yes | 30 | 81.9 | 79.4 | 77.7 |
| 16 | No | 15 | 92.1 | 94.0 | 95.3 |
| 16 | No | 30 | 92.1 | 91.7 | 91.4 |
| 16 | Yes | 30 | 81.9 | 81.5 | 81.2 |
*Intellectual endpoint values for any clinical scenario may be calculated using Model 2 (in the text) and the coefficient values in Table 2.
aValues are unchanged with age because full scale IQ is age standardized.
Intelligence Index Scores
We subsequently evaluated the effect of dosimetry on index scores. Mean dose to the right temporal lobe was associated with declines in PRI (P = 0.036), but this effect was lost after FDR adjustment for multiple comparisons (P* = 0.26). No other clinical variable was associated with PRI after adjusting for age at RT and VP shunt (P = 0.035). P-value was 0.2 for each of posterior fossa syndrome, number of surgeries, chemotherapy, and CSI (in separate models).
Processing speed index was most strongly associated with D50 of the supratentorial brain; each Gy increase in D50 led to a 0.097-point decrease in PSI per year. As compared with a model without dose to the supratentorial brain, inclusion of the dose term significantly improved the model fit (P = 0.0004). Again, no other clinical variable (tested in separate models) was associated with PSI after adjusting for age at RT and VP shunt (P = 0.021): posterior fossa syndrome (P = 0.080), number of surgeries (P = 0.81), chemotherapy (P = 0.41), and use of CSI (P = 0.28). Therefore, the variables in the final model are the same as those for FSIQ (Model 2).
Similar to PSI, D50 of the supratentorial brain was also associated with WMI. Each Gy increase in D50 led to a 0.1 decrease in WMI points per year. As compared with a model without a dose term, inclusion of dose to the supratentorial brain significantly improved the model fit (P = 0.0001). No other clinical variable was associated with WMI after adjusting for age at RT and VP shunt (P = 0.069). P-values were >0.3 for each of posterior fossa syndrome, number of surgeries, chemotherapy and CSI. Again, the variables in the final model are the same as for FSIQ and PSI (Model 2).
Because the D50 to each hippocampus was strongly co-linear (Supplementary Figure 2), 2 models for VCI were created and reported separately in Table 2. D50 values to the left and right hippocampi were both strongly associated with VCI, with each Gy increase in D50 leading to a 0.04-point decrease in VCI per year. Again, inclusion of the doses to the hippocampi separately improved model fit compared with no dose term (P = 0.009 for each hippocampus). No other clinical variable was associated with VCI after adjusting for dose to the right hippocampus, age at RT, and VP shunt (P = 0.032): posterior fossa syndrome (P = 1.0), number of surgeries (P = 0.19), chemotherapy (P = 0.12), and use of CSI (P = 0.43).
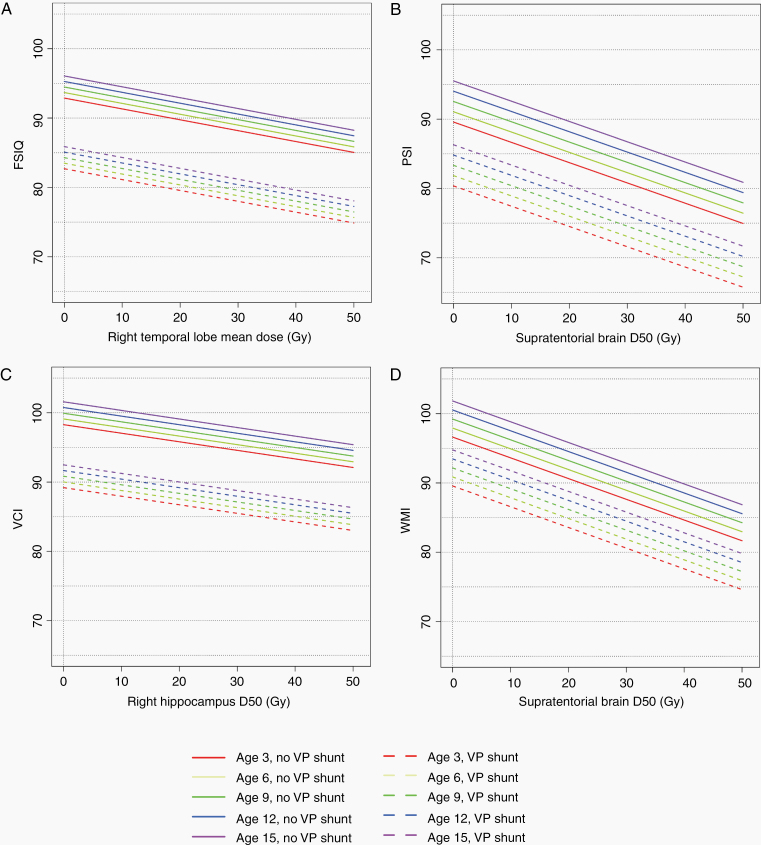
Intelligence domain scores at 3-year follow-up, by dose to brain substructure and stratified by age at RT and VP shunt, are shown in Figure 3. VP shunt, age at RT, and dose were all associated with changes in intellectual scores post-RT.
Fig. 3.
Modeled intelligence endpoints at 3 years post-RT, by dose to brain substructure, age at RT, and presence of VP shunt (D50 = dose to 50% of the structure). Solid lines represent patients without VP shunt, while dotted lines represent patients with a VP shunt. Line colors represent age at RT: 3 years (red), 6 years (yellow-green), 9 years (dark green), 12 years (blue), 15 years (purple). (A) Full scale IQ. (B) Processing speed. (C) Verbal comprehension. (D) Working memory. Perceptual reasoning was not included because its relationship with dose was not statistically significant.
For each intellectual domain, the statistical significance of VP shunt was the strongest of the 3 hydrocephalus-related variables (also examined were presence of any hydrocephalus or any use of VP shunt, ETV, or Ommaya); therefore, VP shunt was retained in all models as a fixed effect. None of the other covariates evaluated (as described in Methods) were significant, either as a covariate alone (as a β 2 term in Model 1) or as a covariate in addition to VP shunt. Distribution of hydrocephalus and treatments thereof are listed in Supplementary Table 3.
Discussion
In this study, we evaluated the association between clinical variables, radiation dosimetry to brain substructures, and neuropsychological outcomes across multiple intelligence domains. We found significant associations between mean dose to the right temporal lobe and decline in FSIQ, dose to 50% of the supratentorial brain with declines in PSI and WMI, and dose to 50% of both hippocampi separately with decline in VCI. These findings will guide oncologists during RT treatment planning, allow practitioners to anticipate expected changes in cognition following RT, and direct application of early educational interventions for at-risk patients.
To our knowledge, this is the first study to report the results of longitudinal intelligence testing in a large cohort of pediatric patients with different diagnoses, correlated with radiation dosimetry to a variety of different brain substructures. The availability of intelligence metrics, including FSIQ, and of specific index scores and their association with volumetric RT dosimetry is relatively unique to the pediatric radiation oncology literature. Some prior studies have evaluated the association with radiation dosimetry and specific neuropsychological subdomains (rather than a comprehensive, broad evaluation of intelligence and index scores). For example, Merchant et al studied the effect of dose on brain substructures in 39 and 58 patients with medulloblastoma and 76 patients with ependymoma.11,23,24 Dose to cerebellar structures was important for estimated IQ (EIQ) and academic achievement tests for reading, mathematics, and spelling evaluated with the Wechsler Individual Achievement Tests.
Importantly, 2 domains were most strongly associated with RT dosimetry to large regions of the brain (supratentorial compartment): processing speed and working memory. This contrasts with verbal comprehension, which was associated with dose to a more localized region of the brain (hippocampus). Processing speed is known to be a vulnerable domain after wide-field RT, including cranial RT for leukemia and CSI for primary brain tumors.25–27 Working memory is also known to decline after photon CSI,28 though this effect may be mitigated by proton CSI followed by proton boost radiotherapy.29 Minimizing dose to broad regions of the brain is possible and important in patients with localized brain tumors receiving focal irradiation, but more challenging in tumors requiring CSI. Nonetheless, any reduction in dose to the brain, such as with highly conformal boost RT, may be helpful to maintain intelligence function over time, even after proton CSI.30
Our finding that the temporal lobe and hippocampus were important for some cognitive domains is consistent with prior works. Acharya et al evaluated 80 patients aged 6 to 21 years treated on a prospective phase II study of radiation for brain tumors (RT1).31 In that study, memory was evaluated using the California Verbal Learning Test‒Children’s Version, and both right and left hippocampal dosimetry were associated with various memory recall endpoints. In a study of 70 patients with varied diagnoses and RT fields, investigators from Boston found that the volume of the left hippocampus receiving 20 Gy was associated with delayed memory scores, both visual and verbal, after proton therapy.32 A multi-institutional study of pediatric brain tumor patients evaluated 14 patients using the Children’s Memory Scale; performance on some memory subtests was associated with dose to the whole brain and right hippocampus.33 In a study by Goda et al, mean doses of >30 Gy to the left hippocampus were associated with FSIQ declines of >10% in older children with mainly craniopharyngioma and suprasellar gliomas.34
A few studies from the literature did evaluate RT to other brain regions and neurocognitive change. In a study of posterior fossa tumors treated with CSI (n = 17) or no RT (n = 13), orbitofrontal RT was associated with declines in working memory, while declines in processing speed were associated with RT to the posterior fossa and temporal lobe.35 Notably, all patients who received RT were treated with CSI. Another study, which compared 20 patients who received RT to 55 healthy controls, found that RT dose to components of the corpus callosum was associated with declines in dexterity and processing speed.36
The left and right hippocampi are related but distinct structures37,38; however, it is unclear why some studies found stronger associations with one side. The right hippocampus is classically associated with nonverbal processing.39 However, it is known that lateralization advances with brain maturation over time.40 Thus, in children, lateralization of specific brain functions to one side or the other may not be well developed. This may explain our finding that both left and right hippocampi were associated with verbal comprehension. Furthermore, doses to left and right structures were correlated (r = 0.89 and 0.93 for the temporal lobes and hippocampi, respectively; see Supplementary Material). When patients are treated with conformal RT to a midline target, such as in patients with ependymoma, medulloblastoma, or craniopharyngioma, the doses to paired structures are also expected to be similar. The lack in variation of dose between right and left increases the difficulty of finding specific associations between neuropsychological change and paired, lateralized structures without large datasets. Therefore, the laterality of the hippocampi, temporal lobes (which contain the hippocampi), and other brain structures and their relationships with RT with respect to intelligence change require further evaluation.
This study found, consistent with other studies, that presence of VP shunt had a detrimental effect on multiple neuropsychological domains. Baseline EIQ can decrease up to 13 points in children with a shunt and diagnosis of medulloblastoma or ependymoma.23,24 The negative effect of a shunt is also maintained in studies that included brain tumors beyond these 2 histologies.41,42 However, it should be noted that a shunt is often unavoidable in these patients. It is likely that VP shunt is an indicator of severe hydrocephalus requiring surgical treatment. The contribution of non-RT factors is an important point because surgeries,41,43 systemic therapies,44,45 and direct tumor effects all likely contribute to neuropsychological change at baseline and over time.46
We found strong co-linearity between different supratentorial brain structures. There are several reasons for this. First, approximately one-third of patients in this study received CSI, which intentionally delivers a homogeneous dose to all intracranial structures. Second, all patients were treated with photon RT, which delivers some dose to adjacent brain structures even with focal treatment. Although our models found strong associations between specific brain structures and intelligence index scores, it does not necessarily mean that total avoidance of one structure with irradiation of the rest of the brain would result in no cognitive change, due to the co-linearity observed. Rather, our data describe brain structures that are more vulnerable to the effects of RT than others, and reveal sensitive areas that should be carefully evaluated during RT planning. It may not be possible to completely avoid certain brain regions, particularly regions of tumor involvement; oncologic control should be prioritized in RT planning.
Strengths of this study include the availability of comprehensive intelligence testing in our patient cohort, including use of tests that provide FSIQ (rather than EIQ). There is evidence that EIQ is less sensitive to changes in children who have received RT for brain tumors.42 All patients receiving focal RT had modern, conformal RT planning, ensuring relevance of our findings to patients receiving contemporary photon RT techniques. Future work should evaluate longitudinal white matter changes and their association with RT dosimetry.
Our findings are limited by an inability to provide diagnosis- or histology-specific models due to insufficient patient numbers. This was a retrospective study, and many eligible patients were unable to complete neuropsychology follow-up visits. Different test versions were used; although they have very good correlation with each other, this is an inherent limitation to all longitudinal neuropsychology research.16–21 There is also a need for longer psychological follow-up, ideally into adulthood. It is known that hearing loss plays an important role in neurocognitive decline47–49; however, our dataset was unable to formally examine hearing loss as a mediator of neuropsychological change due to insufficient follow-up, as hearing loss can take more than 3 years to occur.50 Not all evaluations were performed before RT; however, the latent time between treatment and first neuropsychological assessment was accounted for in the linear mixed effect models. Finally, our data were derived from a single photon-treated cohort only. Further study is required to determine whether our models are valid in patients treated with proton therapy or other photon-treated cohorts. Although the availability of proton therapy is increasing globally, many countries still do not have access to this advanced treatment modality51; thus, our dose-risk models are still widely applicable.
Conclusions
We found an association between FSIQ and mean dose to the right temporal lobe, processing speed, and working memory with dose to the supratentorial brain, and verbal comprehension and dose to the left and right hippocampi. Models to quantify changes across IQ and 4 intelligence domains were created. These models help oncologists anticipate how intelligence can change over time with age at RT, use of a VP shunt, and RT dose. Therefore, we propose that these brain structures should be routinely segmented and considered during RT planning. Efforts should be made to minimize dose to these vulnerable brain substructures to reduce the risk of long-term neurocognitive changes post-RT.
Supplementary Material
Supplementary data are available at Neuro-Oncology online (http://neuro-oncology.oxfordjournals.org/).
Funding
LK and MK received salary support from the Princess Margaret Cancer Foundation.
Biostatistics support was provided by the Academic Enrichment Fund, Department of Radiation Oncology, University of Toronto. This work was also partially supported by the Mount Sinai Hospital-University Health Network Academic Medical Organization Innovation Fund.
Acknowledgments
Accepted for oral presentation at ASTRO, Miami (October 2020) and poster presentation at ISPNO, Japan (December 2020).
Conflict of interest statement. None to declare.
Authorship statement. Conception: DST, EB, DCH, DM. Data collection: LK, LJ, MK, HD, DK, TC, DM. Data analysis: DST, LK, ZAL, MK, DK, DCH, DM. Data interpretation: DST, ZAL, LJ, MK, EB, NL, VR, DCH, DM. Manuscript writing: All authors. Final approval of manuscript: All authors.
References
- 1. Halperin EC, Constine LS, Tarbell NJ, Kun LE. Pediatric Radiation Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 2. Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. [DOI] [PubMed] [Google Scholar]
- 3. Krull KR, Hardy KK, Kahalley LS, Schuitema I, Kesler SR. Neurocognitive outcomes and interventions in long-term survivors of childhood cancer. J Clin Oncol. 2018;36(21):2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23(6):705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma TM, Grimm J, McIntyre R, et al. A prospective evaluation of hippocampal radiation dose volume effects and memory deficits following cranial irradiation. Radiother Oncol. 2017;125(2):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Group CsO. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers, Version 3.0. Arcadia, CA; October 2008. [Google Scholar]
- 7. Annett RD, Patel SK, Phipps S. Monitoring and assessment of neuropsychological outcomes as a standard of care in pediatric oncology. Pediatr Blood Cancer. 2015; 62 Suppl 5:S460–S513. [DOI] [PubMed] [Google Scholar]
- 8. Wechsler D Wechsler Intelligence Scale for Children. San Antonio, TX: Psychological Corporation; 1949. [Google Scholar]
- 9. Mabbott DJ, Penkman L, Witol A, Strother D, Bouffet E. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22(2):159–168. [DOI] [PubMed] [Google Scholar]
- 10. Gondi VT, Tolakanahalli R, Rowley HA, Mehta MP. Hippocampal contouring: a contouring atlas for RTOG 0933. [PowerPoint].https://www.rtog.org/CoreLab/ContouringAtlases/HippocampalSparing.aspx. Accessed April 16, 2020.
- 11. Merchant TE, Kiehna EN, Li C, et al. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. Int J Radiat Oncol Biol Phys. 2006;65(1):210–221. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong CL, Fisher MJ, Li Y, et al. Neuroplastic response after radiation therapy for pediatric brain tumors: a pilot study. Int J Radiat Oncol Biol Phys. 2016;95(3):991–998. [DOI] [PubMed] [Google Scholar]
- 13. Raiford SE, Coalson DL, Tong SA. WPPSI-IV clinical applications. Essentials of WPPSI™-IV Assessment. Hoboken, NJ: John Wiley & Sons Inc; 2014:237–268. [Google Scholar]
- 14. Wechsler D WAIS-R: Wechsler Adult Intelligence Scale-Revised. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- 15. McCrimmon AW, Smith AD. Review of the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II). J Psychoeduc Assess. 2012; 31(3):337–341. [Google Scholar]
- 16. Gale HR Stanford Binet Intelligence Scales. 5th ed. Itasca, IL: Riverside Pub; 2003. [Google Scholar]
- 17. Wechsler D Wechsler Preschool and Primary Scale of Intelligence—Third Edition Technical Manual. San Antonio, TX: NCS Pearson; 2002. [Google Scholar]
- 18. Wechsler D Wechsler Intelligence Scale for Children—Fourth Edition Technical and Interpretive Manual. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 19. Wechsler D Wechsler Adult Intelligence Scale—Fourth Edition Technical and Interpretive Manual. San Antonio, TX: NCS Pearson; 2008. [Google Scholar]
- 20. Wechsler D Wechsler Preschool and Primary Scale of Intelligence—Fourth Edition Technical and Interpretive Manual. San Antonio, TX: NCS Pearson; 2012. [Google Scholar]
- 21. Wechsler D Wechsler Intelligence Scale for Children—Fifth Edition Technical and Interpretive Manual. Bloomington, MN: NCS Pearson; 2014. [Google Scholar]
- 22. Kulkarni AV, Drake JM, Armstrong DC, Dirks PB. Measurement of ventricular size: reliability of the frontal and occipital horn ratio compared to subjective assessment. Pediatr Neurosurg. 1999;31(2):65–70. [DOI] [PubMed] [Google Scholar]
- 23. Merchant TE, Schreiber JE, Wu S, Lukose R, Xiong X, Gajjar A. Critical combinations of radiation dose and volume predict intelligence quotient and academic achievement scores after craniospinal irradiation in children with medulloblastoma. Int J Radiat Oncol Biol Phys. 2014;90(3):554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merchant TE, Sharma S, Xiong X, Wu S, Conklin H. Effect of cerebellum radiation dosimetry on cognitive outcomes in children with infratentorial ependymoma. Int J Radiat Oncol Biol Phys. 2014;90(3):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kahalley LS, Conklin HM, Tyc VL, et al. Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psychooncology. 2013;22(9):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Antonini TN, Ris MD, Grosshans DR, et al. Attention, processing speed, and executive functioning in pediatric brain tumor survivors treated with proton beam radiation therapy. Radiother Oncol. 2017;124(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scantlebury N, Bouffet E, Laughlin S, et al. White matter and information processing speed following treatment with cranial-spinal radiation for pediatric brain tumor. Neuropsychology. 2016;30(4):425–438. [DOI] [PubMed] [Google Scholar]
- 28. Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32(17):1760–1768. [DOI] [PubMed] [Google Scholar]
- 29. Kahalley LS, Peterson R, Ris MD, et al. Superior intellectual outcomes after proton radiotherapy compared with photon radiotherapy for pediatric medulloblastoma. J Clin Oncol. 2020;38(5):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kahalley LS, Peterson R, Ris MD, et al. Reply to S.A. Milgrom et al. J Clin Oncol. 2020;38(19):2212–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Acharya S, Wu S, Ashford JM, et al. Association between hippocampal dose and memory in survivors of childhood or adolescent low-grade glioma: a 10-year neurocognitive longitudinal study. Neuro Oncol. 2019;21(9):1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zureick AH, Evans CL, Niemierko A, et al. Left hippocampal dosimetry correlates with visual and verbal memory outcomes in survivors of pediatric brain tumors. Cancer. 2018;124(10):2238–2245. [DOI] [PubMed] [Google Scholar]
- 33. Raghubar KP, Lamba M, Cecil KM, et al. Dose-volume metrics and their relation to memory performance in pediatric brain tumor patients: a preliminary study. Pediatr Blood Cancer. 2018; 65(9):e27245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gupta T, Maitre M, Sastri GJ, et al. Outcomes of salvage re-irradiation in recurrent medulloblastoma correlate with age at initial diagnosis, primary risk-stratification, and molecular subgrouping. J Neurooncol. 2019;144(2):283–291. [DOI] [PubMed] [Google Scholar]
- 35. Doger de Speville E, Robert C, Perez-Guevara M, et al. Relationships between regional radiation doses and cognitive decline in children treated with cranio-spinal irradiation for posterior fossa tumors. Front Oncol. 2017; 7:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Redmond KJ, Hildreth M, Sair HI, et al. Association of neuronal injury in the genu and body of corpus callosum after cranial irradiation in children with impaired cognitive control: a prospective study. Int J Radiat Oncol Biol Phys. 2018; 101(5):1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ezzati A, Katz MJ, Zammit AR, et al. Differential association of left and right hippocampal volumes with verbal episodic and spatial memory in older adults. Neuropsychologia. 2016; 93(Pt B):380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shipton OA, El-Gaby M, Apergis-Schoute J, et al. Left-right dissociation of hippocampal memory processes in mice. Proc Natl Acad Sci U S A. 2014; 111(42):15238–15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Katz JS, Wright AA. The hippocampus and memory of verbal and pictorial material. Learn Mem. 2002; 9(3):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hopf L, Quraan MA, Cheung MJ, Taylor MJ, Ryan JD, Moses SN. Hippocampal lateralization and memory in children and adults. J Int Neuropsychol Soc. 2013; 19(10):1042–1052. [DOI] [PubMed] [Google Scholar]
- 41. Willard VW, Berlin KS, Conklin HM, Merchant TE. Trajectories of psychosocial and cognitive functioning in pediatric patients with brain tumors treated with radiation therapy. Neuro Oncol. 2019;21(5):678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burgess L, Pulsifer MB, Grieco JA, et al. Estimated IQ systematically underestimates neurocognitive sequelae in irradiated pediatric brain tumor survivors. Int J Radiat Oncol Biol Phys. 2018; 101(3):541–549. [DOI] [PubMed] [Google Scholar]
- 43. Heitzer AM, Raghubar K, Ris MD, et al. Neuropsychological functioning following surgery for pediatric low-grade glioma: a prospective longitudinal study. J Neurosurg Pediatr. 2020;25:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peterson RK, Tabori U, Bouffet E, et al. Predictors of neuropsychological late effects and white matter correlates in children treated for a brain tumor without radiation therapy. Pediatr Blood Cancer. 2019; 66(10):e27924. [DOI] [PubMed] [Google Scholar]
- 45. de Ruiter MA, van Mourik R, Schouten-van Meeteren AY, Grootenhuis MA, Oosterlaan J. Neurocognitive consequences of a paediatric brain tumour and its treatment: a meta-analysis. Dev Med Child Neurol. 2013; 55(5):408–417. [DOI] [PubMed] [Google Scholar]
- 46. Tsang DS, Edelstein K. Late effects after childhood brain tumor treatment: it’s not just about the radiation. Neuro Oncol. 2019; 21(5):565–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heitzer AM, Villagran AM, Raghubar K, et al. Effect of sensorineural hearing loss on neurocognitive and adaptive functioning in survivors of pediatric embryonal brain tumor. J Neurooncol. 2020; 146(1):147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Olivier TW, Bass JK, Ashford JM, et al. Cognitive implications of ototoxicity in pediatric patients with embryonal brain tumors. J Clin Oncol. 2019; 37(18):1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Orgel E, O’Neil SH, Kayser K, et al. Effect of sensorineural hearing loss on neurocognitive functioning in pediatric brain tumor survivors. Pediatr Blood Cancer. 2016; 63(3):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bass JK, Hua CH, Huang J, et al. Hearing loss in patients who received cranial radiation therapy for childhood cancer. J Clin Oncol. 2016; 34(11):1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tsang DS, Patel S. Proton beam therapy for cancer. CMAJ. 2019; 191(24):E664–E666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.