Abstract
Background
Hippocampal avoidance whole-brain radiotherapy (HA-WBRT) shows potential for neurocognitive preservation. This study aimed to evaluate whether HA-WBRT or conformal WBRT (C-WBRT) is better for preserving neurocognitive function.
Methods
This single-blinded randomized phase II trial enrolled patients with brain metastases and randomly assigned them to receive HA-WBRT or C-WBRT. Primary endpoint is decline of the Hopkins Verbal Learning Test–Revised (HVLT-R) delayed recall at 4 months after treatment. Neurocognitive function tests were analyzed with a mixed effect model. Brain progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan–Meier method.
Results
From March 2015 to December 2018, seventy patients were randomized to yield a total cohort of 65 evaluable patients (33 in the HA-WBRT arm and 32 in the C-WBRT arm) with a median follow-up of 12.4 months. No differences in baseline neurocognitive function existed between the 2 arms. The mean change of HVLT-R delayed recall at 4 months was −8.8% in the HA-WBRT arm and +3.8% in the C-WBRT arm (P = 0.31). At 6 months, patients receiving HA-WBRT showed favorable perpetuation of HVLT-R total recall (mean difference = 2.60, P = 0.079) and significantly better preservation of the HVLT-R recognition-discrimination index (mean difference = 1.78, P = 0.019) and memory score (mean difference = 4.38, P = 0.020) compared with patients undergoing C-WBRT. There were no differences in Trail Making Test Part A or Part B or the Controlled Oral Word Association test between the 2 arms at any time point. There were no differences in brain PFS or OS between arms as well.
Conclusion
Patients receiving HA-WBRT without memantine showed better preservation in memory at 6-month follow-up, but not in verbal fluency or executive function.
Keywords: brain metastasis, hippocampus, neuropsychological tests, single blind method, whole brain radiotherapy
Key Points.
1. Hippocampal avoidance WBRT without memantine perpetuated memory at 6 months.
2. Hippocampal avoidance WBRT without memantine did not preserve execute function or verbal fluency.
3. Hippocampal avoidance has no impact on survival or intracranial control.
Importance of the Study.
WBRT remained a standard of care for multiple brain metastases with considerable risk of neurocognitive decline. The hippocampus is a neurogenic region in adults, and brain irradiation has been associated with hippocampal dysfunction, which results in memory declines. To validate the hypothesis of cognitive preservation by hippocampal avoidance WBRT, we designed and conducted the first and only blinded randomized trial assessing the effectiveness of hippocampal sparing. With an unbiased blinded design (both testers and testees were blinded to treatment assignment), we found less deterioration in verbal learning and memory, assessed by HVLT-R total recall, recognition index, and the memory scores, for patients receiving hippocampus avoidant WBRT at 6-month follow-up. We conclude that HA-WBRT without memantine provides better preservation of memory at 6-month follow-up without compromising clinical outcomes including OS and intracranial progression.
Brain metastases are the most common brain tumors in adults. A total of 10–30% of cancer patients develop brain metastases during the course of their illness.1 Its incidence continues to increase with advances in diagnostic modalities, effective systemic therapies, and improved survival of cancer patients.1,2
Historically, brain metastasis patients show poor survival, with a median of 1 month if left untreated and 3–6 months3,4 after treatment. Over several decades, whole-brain radiotherapy (WBRT) has become the standard of care for treating brain metastasis,5 with an estimated response rate of 27–56%.6,7 Several prognostic models have been developed to predict clinical outcomes. Recursive partitioning analysis8 proposed by the Radiation Therapy Oncology Group (RTOG) has been the most widely used. Recently, the Graded Prognostic Assessment (GPA) became the most common index for assessing brain metastasis outcomes.9 Patients with a good prognostic score from either index have a predicted overall survival (OS) of around 1 year. Some specific subgroups of patients, such as those with oncogene-driven lung cancer or luminal-A breast cancer, can show an estimated survival of around 2 years.10
Despite improved survival in certain patients with brain metastasis, the toxicity of brain irradiation raises concerns. Late effects of WBRT generally appear ≥3 months after irradiation and could be irreversible and progressive; they are considered secondary to vascular injury and demyelination.11 Symptoms range from mild lassitude to progressive memory loss and dementia.12–14 The hippocampus is a known neurogenesis region15 in adults, and brain irradiation causes hippocampal dysfunction, which results in memory defects and depression-like behavior.16 Radiation dose and volume to the hippocampus can correlate with memory deficit.17,18 Improved novel radiotherapy techniques make it possible to avoid the hippocampus while treating the entire brain.19,20
RTOG 0933 is a single-arm prospective phase II trial to evaluate the effect of hippocampus avoidance WBRT (HA-WBRT) on neurocognitive function.21 It demonstrated the potential value in neurocognitive preservation of HA-WBRT using the Hopkins Verbal Learning Test–Revised (HVLT-R) tests. The HVLT-R delayed recall decline at 4 months from baseline was reduced to 7% compared with 30% decline in historical control.22 Here, we present a single-blinded, phase II randomized trial to compare neurocognitive function outcomes in patients with brain metastasis treated by either HA-WBRT or conformal (C-)WBRT.
Methods
This study was approved by the institutional research ethics committee and was independently monitored by the institutional clinical trial center. Informed consent was obtained from each patient in written form. (This randomized trial is registered in ClinicalTrials.gov with identifier NCT02393131.)
Study Design and Participants
Eligible were patients with histologically proved non-hematological malignancy and radiographic evidence of brain metastasis outside a 5 mm margin around either hippocampus on gadolinium contrast-enhanced MRI obtained within 30 days prior to registration. Eligibility criteria included age 20 years or older, Karnofsky performance status ≥60, and life expectancy of at least 4 months. Patients with the following conditions were excluded: prior brain radiotherapy or radiosurgery to >5 intracranial metastatic lesions or a biologically equivalent dose in 2-Gy fractions >7.3 Gy to 40% of the volume of bilateral hippocampus from prior radiosurgery.17 Other exclusion criteria included serum creatinine >2.0 mg/dL within 30 days prior to registration, leptomeningeal seeding, contraindication to MR imaging, and severe active comorbidities as judged by investigators. Patients were not allowed to receive investigational systemic therapy during WBRT. Patients who met all eligibility criteria were randomly assigned to receive either HA-WBRT or C-WBRT.
Treatments
WBRT treatment was 3 Gy per fraction once per workday for continuous workdays (Monday to Friday) every week for 10 days, to a total dose of 30 Gy. A noncontrast treatment-planning CT scan of the entire head region was required to define planning target volumes and hippocampal avoidance zones. The pretreatment brain MRI was fused semi-automatically with treatment-planning CT for hippocampal contouring. Contouring was carried out in accordance with the RTOG atlas based on RTOG 093321 with the assistance of experienced neuroradiologists. Treatment plans were designed with volumetric modulated arc therapy (VMAT or RapidArc) using 6 MV photon for both HA-WBRT and C-WBRT arms.
Dosimetry criteria for radiotherapy planning are described in the Supplementary Methods. Treatment was delivered with daily image guidance using online cone beam CT for 3D corrections.
The VMAT or RapidArc technique significantly increases treatment delivery time and substantially reduced radiation dose to the scalp compared with the conventional WBRT technique. By using the VMAT or RapidArc techniques, all patients underwent similar treatment delivery procedures and received comparable dosimetry to normal structures other than hippocampus, which ensured the success of blinding.
Assessment
All patients were evaluated at entry, during treatment, and after treatment at 1, 2, 4, and 6 months, then every 3 months until death or brain progression. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.
Neurocognitive function tests, self-reported neurocognitive function, and self-reported health-related quality of life were assessed at baseline and at 1, 2, 4, and 6 months, followed by every 3 months up to 24 months after WBRT unless brain progression or death occurred. The neuropsychological test battery included tests of memory, processing speed, executive function, and verbal fluency. HVLT-R, Trail Making Test Part A (TMT-A), Trail Making Test Part B (TMT-B), and Controlled Oral Word Association (COWA) were conducted by blinded independent health professionals, and data were recorded as raw scores, time, and word counts without normalization. Self-reported cognitive outcomes were assessed using the European Organisation for Research and Treatment of Cancer (EORTC) core 30-item quality of life questionnaire (QLQ-C30) and Functional Assessment of Cancer Therapy–Cognitive Function v3. We assessed self-reported health-related quality of life specific to brain metastasis using the EORTC QLQ 20-item Brain Neoplasm (BN20) questionnaire. All tests and questionnaires used were Traditional Mandarin versions and certified by a board-certified neurologist and psychologist.
Gadolinium contrast-enhanced MRI was used to assess intracranial failure and was obtained prior to treatment and at 4, 9, and 12 months after WBRT until intracranial disease progression, upon new onset of neurological signs or upon symptoms suggestive of progressive brain metastasis.
Statistical Analysis
We conducted a single-blinded randomized trial with a randomization ratio of 1:1. The actual treatment given to individual patients was determined by randomization with permuted-block design stratified by prior cranial radiosurgery. The primary endpoint was neurocognitive function as determined by a decline in HVLT-R delayed recall score from baseline to 4 months after WBRT. Previous results of standard conventional WBRT resulted in a 40% mean decline in cognitive loss at 3 to 6 months.22 We hypothesized that using C-WBRT with or without hippocampal avoidance would reduce the decline from baseline to 20% at 4 months. A Simon’s randomized phase II design was used to calculate sample size.23 We required 42 evaluable subjects (21 in the HA-WBRT group and 21 in the C-WBRT group) for a 90% probability of correctly selecting the best intervention group. We anticipated that up to 35% of patients would drop out prior to the 4-month assessment and would not be included in the final analysis. The target sample size was 64 registered subjects. Neurocognitive failure was defined as a drop in raw scores from baseline more than 2 SD for any HVLT-R category (total recall, delayed recall, and recognition index).
Secondary endpoints included self-reported cognitive functioning, health-related quality of life, progression of brain metastasis, OS, and acute or late treatment related toxicity. Intracranial progression was defined as radiographic evidence of enlarged brain tumor according to RECIST (Response Evaluation Criteria in Solid Tumors) 1.1 or confirmed leptomeningeal seeding from cerebrospinal fluid studies. Brain progression-free survival (PFS) was calculated from randomization until brain progression or death. Hippocampal failure was documented and defined as the presence of new brain metastasis within a 5 mm margin around either hippocampus. OS was defined as time from randomization to death. Self-reported cognitive functioning and health-related quality of life are under analysis and not reported in this article.
Descriptive data were reported and compared between the 2 arms. An independent t-test for continuous variables and chi-square or Fisher’s exact test were performed for categorical variable comparison. A mixed effect model was used for neurocognitive function tests to assess the time effects within patients during serial follow-up and the hippocampus avoidance effect between the 2 arms. Survival was estimated using the Kaplan–Meier method and differences between patients or treatment characteristics were assessed using log-rank tests. A 2-sided P-value of less than 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism software 8.31.
Results
Patient Characteristics
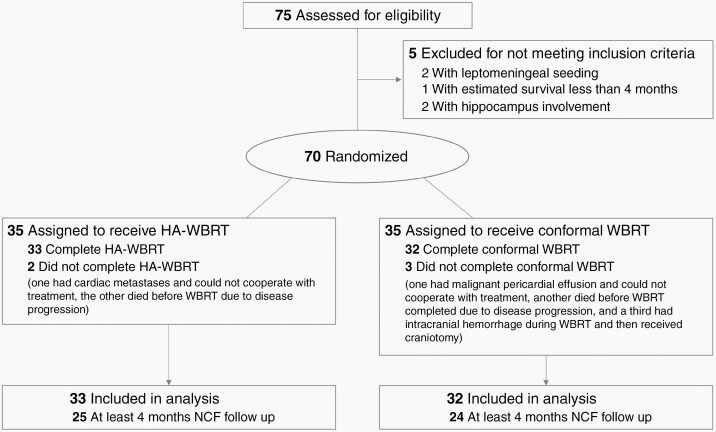
From March 2015 to December 2018, we enrolled 70 eligible and evaluated 65 analyzable patients. The CONSORT (Consolidated Standards of Reporting Trials) diagram is shown in Figure 1. The median follow-up time for all evaluated patients was 12.4 months (range, 0.9‒54.8 mo). Patient characteristics are summarized in Table 1. There were no significant differences between the 2 arms in terms of age, performance status, GPA, and education level, except that more patients in the HA-WBRT arm had prior brain surgery. The representative radiotherapy plans are shown in Supplementary Figures 1 and 2. Despite equivalent prescription dose, patients in the HA-WBRT group received a higher integral dose compared with those in the C-WBRT group (1158.6 monitor units [MUs] vs 727.6 MUs; P < 0.001). The summary of dosimetry results for the 2 arms is shown in Supplementary Table 1.
Fig. 1.
CONSORT flow diagram.
Table 1.
Patient characteristics
| HA-WBRT (N = 33) | Conformal WBRT (N = 32) | P-value | |
|---|---|---|---|
| Mean age, y | 58.4 | 58.3 | 0.977 |
| Sex | Male 42.4% (14) Female 57.6% (19) |
Male 40.6% (13) Female 59.4% (19) |
0.883 |
| KPS (median) | 90 | 90 | 0.988 |
| GPA (median) | 1.5 | 1.25 | 0.656 |
| Pretreatment neurologic symptoms | None: 69.7% (23) Minor: 30.3% (10) |
None: 75% (24) Minor: 25% (9) |
0.633 |
| Primary cancer | Lung: 97.0% (32) Breast: 0% (0) Others: 3.0% (1) |
Lung: 90.6% (29) Breast: 6.2% (2) Others: 3.1% (1) |
0.331 |
| High school education | 84.8% (28) | 84.4% (27) | 0.958 |
| Prior brain surgery | 15.2% (5) | 3.1% (1) | 0.105 |
| Prior cranial SRS | 9.1% (3) | 9.4% (3) | 0.649 |
| Brain only metastasis | 21.2% (7) | 21.9% (7) | 0.948 |
Abbreviations: KPS, Karnofsky performance score; GPA, Graded Prognostic Assessment in brain metastases; SRS, stereotactic radiosurgery.
Adverse Events
Overall, 90.1% of patients in the HA-WBRT arm and 87.5% of patients in the C-WBRT arm experienced some grade of toxicity. The most common toxicities were nausea (n = 36), fatigue (n = 36), vomiting (n = 27), and dizziness (n = 9). The rate of any grade 2 toxicity was 60.6% in the HA-WART arm and 46.9% in the C-WBRT arm (P = 0.74). One patient in each arm experienced complications with grade 3 toxicity after WBRT. One had progressive brain lesions during WBRT and received a craniotomy, while the other had severe symptomatic cerebral edema, which was resolved following bevacizumab and steroid treatment. No patients experienced grade 4 or 5 toxicity.
Neurocognitive Outcomes
All tests were performed using the Traditional Mandarin version. There were no differences in baseline neurocognitive functions between arms as shown in Table 2. The 4-month follow-up showed an average of −8.8% and +3.8% change in HVLT-R delayed recall from baseline in the HA-WBRT arm and C-WBRT arm, respectively. Both were better than expected and without significant differences (P = 0.31). Overall, there were no differences in any neurocognitive assessments between the 2 arms at the 4-month follow-up (Table 2).
Table 2.
Neurocognitive function test at baseline and changes from baseline at 4 and 6 months *
| HA-WBRT | C-WBRT | P-value | |
|---|---|---|---|
| Baseline neurocognitive function | |||
| N = 33 | N = 32 | ||
| HVLT-R Total Recall | 18.61 (16.98 to 20.33) | 19.00 (17.11 to 20.89) | 0.754 |
| HVLT-R Delayed Recall | 5.88 (4.86 to 6.80) | 6.06 (5.06 to 7.06) | 0.794 |
| HVLT-R Recognition Index | 10.30 (9.48 to 11.12) | 10.47 (9.87 to 11.07) | 0.742 |
| TMT-A | 49.52 (42.55 to 56.50) | 54.28 (39.51 to 69.05) | 0.551 |
| TMT-B | 79.18 (68.89 to 89.48) | 86.59 (63.55 to 109.64) | 0.547 |
| COWA | 18.73 (16.49 to 20.97) | 17.10 (14.44 to 19.75) | 0.340 |
| Changes from baseline to 4 months after WBRT | |||
| N = 25 | N = 24 | ||
| HVLT-R Total Recall | +0.84 (−1.68 to +3.36) | +1.67 (−0.73 to +4.06) | 0.626 |
| HVLT-R Delayed Recall | −0.52 (−1.62 to +0.58) | +0.25 (−0.86 to +1.36) | 0.313 |
| HVLT-R Recognition Index | −1.72 (−3.32 to −0.12) | −0.96 (−1.83 to −0.09) | 0.392 |
| TMT-A | +15.67 (−4.77 to +36.11) | +1.56 (−3.30 to +6.41) | 0.177 |
| TMT-B | +24.36 (−2.89 to +51.61) | +9.71 (−8.40 to 27.83) | 0.361 |
| COWA | −0.48 (−2.87 to +1.91) | +1.65 (−0.81 to +4.12) | 0.205 |
| Changes from baseline to 6 months after WBRT | |||
| N = 17 | N = 20 | ||
| HVLT-R Total Recall | +1.65 (−1.68 to +3.36) | −0.95 (−2.75 to +0.85) | 0.079 |
| HVLT-R Delayed Recall | +0.35 (−0.74 to +1.44) | −0.65 (−1.64 to +0.34) | 0.160 |
| HVLT-R Recognition Index | +0.53 (−0.49 to 1.54) | −1.25 (−2.39 to −0.12) | 0.019 |
| TMT-A | −0.53 (−12.57 to +11.51) | +4.10 (−5.38 to +13.58) | 0.528 |
| TMT-B | −3.18 (−22.47 to +16.12) | +9.15 (−14.41 to +32.71) | 0.400 |
| COWA | +1.47 (−1.35 to +4.29) | +1.00 (−2.18 to +4.18) | 0.817 |
* Raw scores (HVLT-R), time (TMT-A/B), and word counts (COWA) were recorded, and all the tests were done in Mandarin version. Data are presented as means plus lower and upper limit of 95% CI.
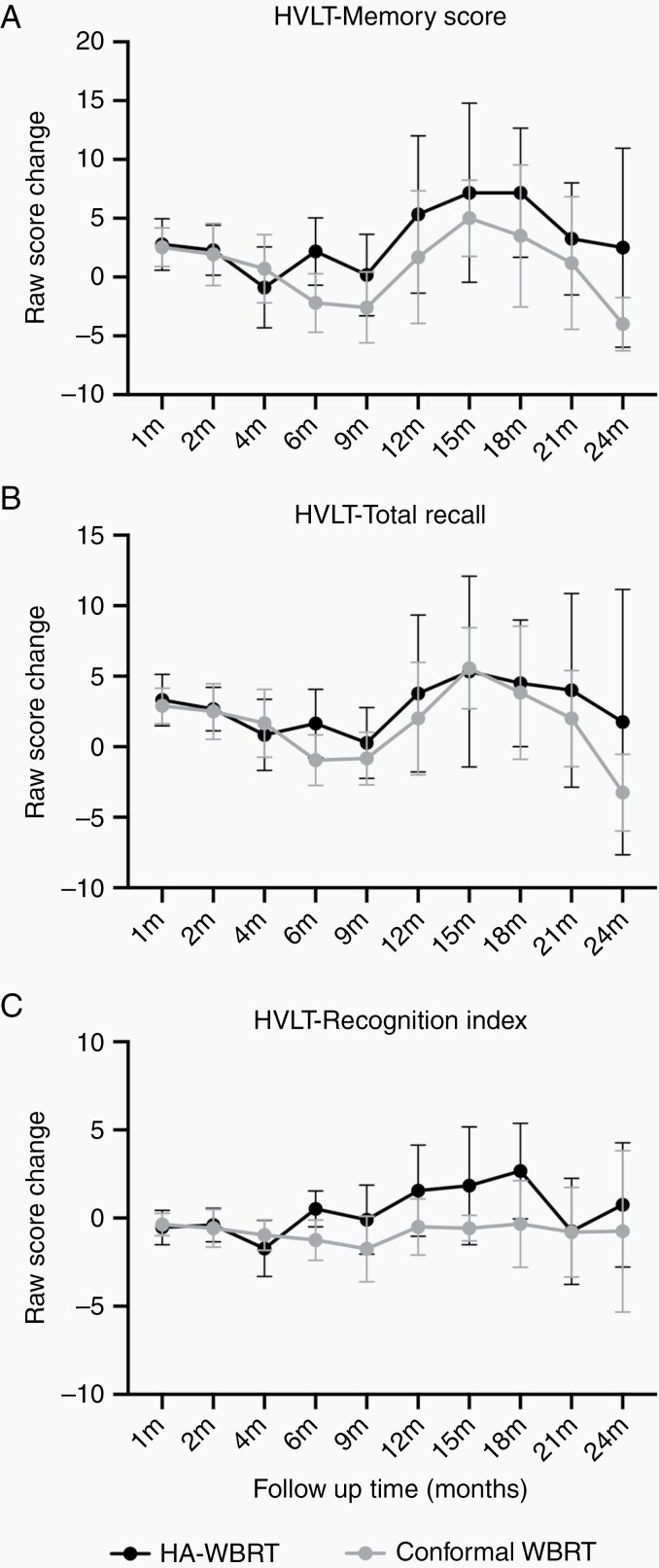
By analyzing all neurocognitive function tests for all timepoints, we found a significant perpetuation of neurocognitive function in the HVLT-R recognition index (mean difference = 1.78, 95% CI: 0.31‒3.25, P = 0.019) and a trend of preservation in HVLT-R total recall (mean difference = 2.60, 95% CI: –0.32 to 5.52, P = 0.079) at the 6-month follow-up in the HA-WBRT arm (Figure 2 and Table 2). By using the memory score24 (the sum of HVLT-R total recall and recognition index), the preservation of verbal learning and memory was significantly superior in the HA-WBRT arm at 6-month follow-up (mean difference = 4.38, 95% CI: 0.72‒8.03, P = 0.020), and in favor of the HA-WBRT arm thereafter till the completion of follow-up at 24 months (Figure 2). Despite a lack of significant differences in HVLT-R delayed recall, TMT-A, TMT-B, or COWA tests at any timepoints, patients receiving HA-WBRT outperformed in all aspects of the neurocognitive function tests at 6-month follow-up (Table 2). There was also no difference in cumulative incidence of neurocognitive failure between the 2 arms after adjusting competing risks (Gray’s test P = 0.93; Supplementary Figure 3). The 4- and 6-month cumulative incidence of neurocognitive failure was 15.2% and 18.2%, respectively, in the HA-WBRT arm and 12.5% and 15.6%, respectively, in the C-WBRT arm.
Fig. 2.
Changes in raw scores of HVLT-R (A) memory score, the sum of (B) total recall and (C) recognition index from baseline between the HA-WBRT arm and the C-WBRT arm. Data were plotted as means and 95% CIs.
Intracranial Progression and Survival
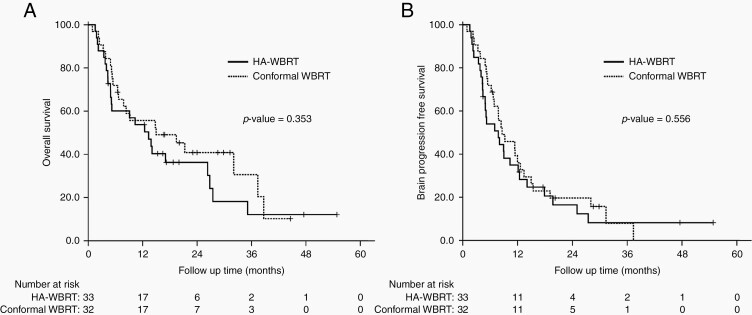
The median OS was 13.3 months for patients in the HA-WBRT arm and 15.0 months in the C-WBRT arm (hazard ratio [HR] = 1.32, 95% CI: 0.73‒2.38, P = 0.355). No significant differences in brain PFS existed between the HA-WBRT and C-WBRT arms (HR = 1.17, 95% CI: 0.69‒1.98, P = 0.557). Figure 3 shows survival curves for OS and brain PFS. Adjusting the competing risk showed that there was also no difference in cumulative incidence for intracranial failure (Supplementary Figure 4). In total, 4 patients developed hippocampal failures, of whom 3 were assigned to intervention of hippocampal avoidance (crude incidence 9.1%).
Fig. 3.
Kaplan–Meier survival curves of (A) OS and (B) brain PFS for analyzable patients between the HA-WBRT arm and the C-WBRT arm.
Discussion
This phase II randomized trial compared the effectiveness of HA-WBRT and C-WBRT in neurocognitive function preservation. The results failed to demonstrate any benefit of hippocampal avoidance in neurocognitive preservation by HVLT-R delayed recall at 4 months after treatment. However, we observed a marginal benefit in perpetuation of HVLT-R total recall and significant protection in HVLT-R recognition index and memory score after HA-WBRT. To our knowledge, this is the first and only blinded randomized trial assessing the clinical benefit of hippocampal sparing performed in a non-English-speaking Asian cohort.
A recently published phase III randomized study, NRG-CC001,25 which was a multicenter, open-label trial comparing HA-WBRT with C-WBRT together with memantine use, demonstrated that HA-WBRT with memantine showed significantly lower risk of neurocognitive failure (adjusted HR, 0.74; P = 0.02). Further, patients in the HA-WBRT arm showed less deterioration of executive function at 4 months (P = 0.01) and learning memory at 6 months (P = 0.02). In our trial, we also found less deterioration in verbal memory by HVLT-R total recall (P = 0.079) and HVLT-R recognition index (P = 0.019) at 6 months after WBRT. Memory score,24 which was reported to have higher sensitivity than HVLT-R total recall in detecting dementia, was also less declined in patients receiving HA-WBRT at 6 months (P = 0.020) and preferably preserved with longer follow-up (though nonsignificant). In contrast, no differences in executive function were noticed. The clinical impact of HA-WBRT seemed to be less beneficial in our study than in the NRG trial. There were some differences in trial design, which may have influenced neurocognitive outcomes.
The present study was a blinded randomized trial. Although only patients and not investigators were blinded to treatment arms, our trial could be considered a double-blind randomized trial, since the neurocognitive functions were assessed independently by trained health professionals with no knowledge of the patients’ assigned group. The patient’s expectation may have some placebo or psychotherapeutic effects on neurocognitive outcomes. Open-label placebo effects are discussed extensively in the neurologic and psychiatric fields.26,27 Studies addressing the placebo effect on neurocognitive function have been consistently presented in various diseases, such as traumatic brain injury and Alzheimer’s disease.28,29 The neurobiological basis mainly involves a brain-rewarding system, where cognitive and affective functions, including awareness, insight, expectation modulation, learning, and memory, all contributed.29 The placebo effect is also evident when treating cognitive function after radiation-induced brain injury.30
In our comparison arm, we chose C-WBRT rather than traditional bilateral opposing-fields WBRT. The C-WBRT was delivered using VMAT or RapidArc techniques. The procedures for radiotherapy delivery were identical for patients in both arms, which makes the blindness more reliable. Further, C-WBRT provided a dose distribution similar to the HA-WBRT compared with bilateral opposing-fields WBRT (Supplementary Figure 2). We assumed that the bias of acute toxicity to normal tissues other than the hippocampus could be minimized. The blinded testers and placebo/psychotherapeutic effects of C-WBRT may be reasons why the difference between intervention arms was trivial in the present trial.
Memantine, an N-methyl-D-aspartate receptor antagonist, is effective in neuroprotection when in concurrent and adjuvant use with WBRT.31 Despite its proven efficacy, the drug is not widely prescribed during WBRT in the US.32 Only 11% of radiation oncologists in the survey considered memantine for use, with fewer than 10% of their patients. Memantine was also not widely used in our society and not reimbursed by the health care system when developing the present clinical trial. Recent preclinical data revealed that memantine may protect against radiation injury. Duman et al33 discovered remodeling of the hippocampal excitatory synapse following radiation treatment. Pre-administration of memantine can revert this radiation-induced phenomenon. This interaction may imply a synergistic effect in neuroprotection from simultaneous use of HA-WBRT and memantine. The lack of memantine use in the present study may be another reason why patients in our cohort benefited less from HA-WBRT than those in the NRG-CC001 trial.
Importantly, current available evidence for HA-WBRT was mainly from Western countries such as the United States, especially for English-speaking cohorts. Despite these neurocognitive function tests in the neuropsychiatric fields being translated into other languages, including Chinese Mandarin with validation reports,34 it is uncommon in clinical oncology trials in Asia. Effectively evaluating neurocognitive function preservation with a language barrier raises concerns. A dosimetry study of HA-WBRT showed that dose volume of the irradiated hippocampus correlated well with neurocognitive function using word list learning tests in the Mandarin version.35 They also reported no significant deterioration in memory function after HA-WBRT for patients with brain oligo-metastases or prophylactic cranial irradiation in a single arm phase II trial.36 Our study is the first randomized trial evaluating HA-WBRT in a Mandarin-speaking cohort. The major results are in accordance with the NRG-CC001 trial, which will help reinforce the confidence of radiation oncologists in Mandarin-speaking areas to treat suitable patients with highly complex and time-consuming HA-WBRT techniques.19
The limitations in our trial include a small patient number, unconventional phase II design, and a single institutional study. Our study is likely underpowered to corroborate all the findings of the much larger trials conducted by the cooperative groups. Despite those limitations, more than three quarters and one half of enrolled patients followed and completed the protocol tests at follow-ups of 4 months and 6 months, respectively, after WBRT. The blinded testers and testees, as well as the high compliance rate of the present study, greatly reduced biases and make our results more reliable.
Combing the NRG-CC001 and present trial, both studies demonstrated that preserved verbal memory measured by HVLT-R was more prominent at 6 months rather than 4 months follow-up after HA-WBRT. This is compatible with previous clinical observations37 and preclinical studies of hippocampal dysfunction.16 Long-term follow-ups later than 6 months may be more appropriate to evaluate effects on verbal memory function following radiation treatment. This also suggests that patients with good prognoses are more likely to benefit from HA-WBRT. Future trials should adapt late timepoints to determine neurocognitive outcomes.
Conclusions
Patients receiving hippocampus-avoidant conformal WBRT without memantine for brain metastases show better preservation of memory at 6-month follow-up, but not of verbal fluency or executive function. Patients with favorable prognoses for longer life expectancy may benefit more from the HA-WBRT treatment.
Supplementary Material
Acknowledgments
We appreciate the assistance of statistician Dr Chin-Hao Chang, PhD at the Department of Medical Research, National Taiwan University Hospital, Taipei, Taiwan, in trial design. We acknowledge the efforts of Chueh-Hui Lin, RN and Tsai-Tsan Wu, RN in trial management and data collection. We would like to thank Anthony Abram at Uni-edit for editing and proofreading this manuscript.
Funding
This work was supported by the Ministry of Science and Technology, Executive Yuan, Taipei, Taiwan; award number: MOST 107–2314-B-002–098 to Feng-Ming Hsu, and National Taiwan University Hospital Yunlin Branch, Yunlin, Taiwan; award number NTUHYL 108.S004 to Wen-Chi Yang.
Conflict of interest statement. All authors declare no conflict of interests.
Authorship statement. Concept and study design: F-M.H. Investigation: F-M.H., W-C.Y., Y-F.C., P-F.W., J.L-Y.C. Methodology: F-M.H., Y-F.C., C-C.Y., P-F.W., H-M.C. Data curation: W-C.Y., Y-F.C., G-Y.C. Formal analysis: W-C.Y., F-M.H., G-Y.C. Validation: F-M.H., C-C.Y. Resource: J.C-H.C., S-H.K. Supervision: F-M.H., J.C-H.C., S-H.K. Writing—original draft: W-C.Y. Writing—review, editing: F-M.H, Y-F.C.
References
- 1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. [DOI] [PubMed] [Google Scholar]
- 2. Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8(6):344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24(8):1295–1304. [DOI] [PubMed] [Google Scholar]
- 4. Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48(2):384–394. [DOI] [PubMed] [Google Scholar]
- 5. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7(4):682–689. [DOI] [PubMed] [Google Scholar]
- 6. Pease NJ, Edwards A, Moss LJ. Effectiveness of whole brain radiotherapy in the treatment of brain metastases: a systematic review. Palliat Med. 2005;19(4):288–299. [DOI] [PubMed] [Google Scholar]
- 7. van der Steen-Banasik E, Hermans J, Tjho-Heslinga R, Caspers R, Leer JW. The objective response of brain metastases on radiotherapy. A prospective study using computer tomography. Acta Oncol. 1992;31(7):777–780. [DOI] [PubMed] [Google Scholar]
- 8. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. [DOI] [PubMed] [Google Scholar]
- 9. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70(2):510–514. [DOI] [PubMed] [Google Scholar]
- 10. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res. 2013;19(9):2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laack NN, Brown PD. Cognitive sequelae of brain radiation in adults. Semin Oncol. 2004;31(5):702–713. [DOI] [PubMed] [Google Scholar]
- 13. Greene-Schloesser D, Robbins ME. Radiation-induced cognitive impairment—from bench to bedside. Neuro Oncol. 2012;14Suppl 4:iv37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saad S, Wang TJ. Neurocognitive deficits after radiation therapy for brain malignancies. Am J Clin Oncol. 2015;38(6):634–640. [DOI] [PubMed] [Google Scholar]
- 15. Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16(2):129–134. [DOI] [PubMed] [Google Scholar]
- 16. Son Y, Yang M, Wang H, Moon C. Hippocampal dysfunctions caused by cranial irradiation: a review of the experimental evidence. Brain Behav Immun. 2015;45:287–296. [DOI] [PubMed] [Google Scholar]
- 17. Gondi V, Hermann BP, Mehta MP, Tomé WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2012;83(4):e487–e493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma TM, Grimm J, McIntyre R, et al. A prospective evaluation of hippocampal radiation dose volume effects and memory deficits following cranial irradiation. Radiother Oncol. 2017;125(2):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(4):1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97(3):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22(1):157–165. [DOI] [PubMed] [Google Scholar]
- 23. Simon R, Wittes RE, Ellenberg SS. Randomized phase II clinical trials. Cancer Treat Rep. 1985;69(12):1375–1381. [PubMed] [Google Scholar]
- 24. Hogervorst E, Combrinck M, Lapuerta P, Rue J, Swales K, Budge M. The Hopkins Verbal Learning Test and screening for dementia. Dement Geriatr Cogn Disord. 2002;13(1):13–20. [DOI] [PubMed] [Google Scholar]
- 25. Brown PD, Gondi V, Pugh S, et al. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG Oncology CC001. J Clin Oncol. 2020;38(10):1019–1029. doi: 10.1200/JCO.19.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Linde K, Witt CM, Streng A, et al. The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain. Pain. 2007;128(3):264–271. [DOI] [PubMed] [Google Scholar]
- 27. Diederich NJ, Goetz CG. The placebo treatments in neurosciences: new insights from clinical and neuroimaging studies. Neurology. 2008;71(9):677–684. [DOI] [PubMed] [Google Scholar]
- 28. Curie A, Yang K, Kirsch I, et al. Placebo responses in genetically determined intellectual disability: a meta-analysis. PLoS One. 2015;10(7):e0133316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Polich G, Iaccarino MA, Kaptchuk TJ, Morales-Quezada L, Zafonte R. Placebo effects in traumatic brain injury. J Neurotrauma. 2018;35(11):1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rapp SR, Case LD, Peiffer A, et al. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015;33(15):1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown PD, Pugh S, Laack NN, et al. ; Radiation Therapy Oncology Group (RTOG) . Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Slade AN, Stanic S. The impact of RTOG 0614 and RTOG 0933 trials in routine clinical practice: the US Survey of Utilization of Memantine and IMRT planning for hippocampus sparing in patients receiving whole brain radiotherapy for brain metastases. Contemp Clin Trials. 2016;47:74–77. [DOI] [PubMed] [Google Scholar]
- 33. Duman JG, Dinh J, Zhou W, et al. Memantine prevents acute radiation-induced toxicities at hippocampal excitatory synapses. Neuro Oncol. 2018;20(5):655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu CC, Hua MS, Hwang TJ, et al. Neurocognitive functioning of subjects with putative pre-psychotic states and early psychosis. Schizophr Res. 2015;164(1-3):40–46. [DOI] [PubMed] [Google Scholar]
- 35. Tsai PF, Yang CC, Chuang CC, et al. Hippocampal dosimetry correlates with the change in neurocognitive function after hippocampal sparing during whole brain radiotherapy: a prospective study. Radiat Oncol. 2015;10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin SY, Yang CC, Wu YM, et al. Evaluating the impact of hippocampal sparing during whole brain radiotherapy on neurocognitive functions: a preliminary report of a prospective phase II study. Biomed J. 2015;38(5):439–449. [DOI] [PubMed] [Google Scholar]
- 37. Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: a review. Front Oncol. 2012;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.