Abstract
Background
Pulsed radiation therapy (PRT) has shown effective tumor control and superior normal-tissue sparing ability compared with standard radiotherapy (SRT) in preclinical models and retrospective clinical series. This is the first prospective trial to investigate PRT in the treatment of patients with newly diagnosed glioblastoma (GBM).
Methods
This is a single-arm, prospective study. Patients with newly diagnosed GBM underwent surgery, followed by 60 Gy of PRT with concurrent temozolomide (TMZ). Each day, a 2-Gy fraction was divided into ten 0.2-Gy pulses, separated by 3-minute intervals. Patients received maintenance TMZ. Neurocognitive function (NCF) and quality of life (QoL) were monitored for 2 years using the Hopkins Verbal Learning Test‒Revised and the European Organisation for Research and Treatment of Cancer QLQ-C30 QoL questionnaire. Change in NCF was evaluated based on a minimal clinically important difference (MCID) threshold of 0.5 standard deviation.
Results
Twenty patients were enrolled with a median follow-up of 21 months. Median age was 60 years. Forty percent underwent subtotal resection, and 60% underwent gross total resection. One patient had an isocitrate dehydrogenase (IDH)–mutated tumor. Median progression-free survival (PFS) and overall survival (OS) were 10.7 and 20.9 months, respectively. In a post-hoc comparison, median OS for the prospective cohort was longer, compared with a matched cohort receiving SRT (20.9 vs 14 mo, P = 0.042). There was no decline in QoL, and changes in NCF scores did not meet the threshold of an MCID.
Conclusions
Treatment of newly diagnosed GBM with PRT is feasible and produces promising effectiveness while maintaining neurocognitive function and QoL. Validation of our results in a larger prospective trial warrants consideration.
Keywords: glioblastoma, neurocognitive function, pulsed radiation therapy, quality of life
Key Points.
Treatment of newly diagnosed GBM with pulsed irradiation is feasible and produces promising effectiveness.
GBM patients receiving pulsed RT showed no decline in quality of life or neurocognitive function.
Importance of the Study.
PRT has shown effective tumor control and superior normal-tissue sparing ability compared with SRT in preclinical models. However, no prospective trials have studied PRT, and the available retrospective clinical series were mainly limited to cases of re-irradiation. In this pilot study, we prospectively investigated PRT in the treatment of patients with newly diagnosed GBM.
Glioblastoma (GBM) is the most common primary malignant central nervous system tumor in adults and carries a dismal prognosis.1 The current standard of care involves maximal safe resection of the tumor and adjuvant radiotherapy (RT) with concurrent temozolomide (TMZ), followed by maintenance TMZ. This treatment strategy, however, yields a modest median overall survival (mOS) of approximately 15 months.2 Recently, the addition of tumor treating fields (TTF) after completion of adjuvant RT was shown to improve outcomes and resulted in a mOS of approximately 21 months.3 The poor prognosis of GBM is largely explained by the infiltrative nature of these tumors, which makes eliminating clinically occult disease with surgery virtually impossible and highlights the need to improve the efficacy of adjuvant RT. Despite drastic improvements in imaging and our ability to identify and accurately target the resection cavity and potential areas of microscopic disease, patterns of failure have not changed significantly over the past three decades, and 80–90% of patients fail within RT volumes.4–6
RT dose escalation is one potential strategy to improve local control. A randomized clinical trial showed that compared with 45 Gy, 60 Gy improved progression-free survival (PFS) and OS.7 However, subsequent trials reported no additional survival benefit for doses beyond 60 Gy.8,9 Doses as high as 90 Gy did not alter failure patterns, with >90% of the recurrent tumors failing within the treatment fields.9 The lack of improved outcomes with RT dose escalation may reflect a therapeutic ceiling for standard RT (SRT), in which RT is delivered as continuous, daily 2-Gy fractions. Additionally, the risk of normal tissue damage with doses of SRT is particularly prominent in the context of GBM treatment, since large volumes of normal brain parenchyma are typically included in radiation treatment fields to account for microscopic residual disease.
Pulsed RT (PRT), also referred to as low-dose rate therapy, divides each 2-Gy fraction into ten 0.2-Gy pulses, separated by 3-minute intervals. PRT represents a novel approach to delivering RT in a manner that may bypass the limitations of SRT and has proven to be efficacious in preclinical studies.10–14 Furthermore, PRT, while enhancing tumor kill, may also enhance the therapeutic index as it allows more time for repair of RT-induced damage within nondividing normal cells compared with SRT. PRT was compared with SRT in an orthotopic GBM mouse model.13 PRT resulted in significantly better tumor control and increased mOS by approximately 18% while reducing neuronal degeneration.
Several retrospective studies have reported favorable outcomes and toxicity profiles with PRT, mainly in the context of re-irradiation for multiple cancers.15–18 In one series, 22 patients with diagnoses of a variety of cancers with either poor performance status or recurrent treatment-refractory tumors received PRT with a 1-year OS of approximately 70%.16 In a more recent study, 43 patients with recurrent breast cancer received PRT with a 74% control rate with no late grade 4+ toxicities despite a median cumulative dose of approximately 110 Gy.18 In another study, 103 patients with recurrent GBM were treated with pulsed re-irradiation with a median dose of 50 Gy to large volumes of brain tissue with good palliative benefit and minimal toxicity.17 These retrospective data have sparked interest in PRT, and ongoing phase I/II trials are currently investigating PRT in gastric and lung cancers (NCT03061162 and NCT03094884). However, no prospective clinical studies have been published on the use of PRT, and thus far the study of PRT has been mainly limited to the context of re-irradiation. Here we report the results of a prospective study investigating the feasibility, effectiveness, and impact on quality of life (QoL) and neurocognitive function (NCF) of PRT in the treatment of newly diagnosed GBM.
Materials and Methods
Study Design and Population
This is a single-arm, prospective study. The study was approved by our institutional review board, and all patients provided written informed consent before entering the study. All patients were treated between 2013 and 2017. Eligible patients were between 18 and 70 years of age, had a Karnofsky performance status ≥70, and had newly diagnosed GBM. Patients underwent maximal safe surgical resection or biopsy. Patients had to be eligible for concurrent chemoradiotherapy and able to undergo MRI with contrast. Patients were excluded if they had brainstem involvement, prior intracranial irradiation, or other synchronous primary cancers. Tumors were tested for O6-methylguanine-DNA methyltransferase (MGMT) gene promoter methylation status and isocitrate dehydrogenase 1 and 2 (IDH1/2) mutation status.
Treatment Plan
Within 72 hours following surgical resection, patients had contrast enhanced MRI imaging, including T1- and T2‒fluid attenuated inversion recovery (FLAIR) sequences to determine extent of resection. Adjuvant RT had to begin no more than 5 weeks postoperatively. Patients received concurrent TMZ (75 mg/m2), followed by maintenance TMZ (150–200 mg/m2). The protocol specified a minimum of six 28-day cycles of maintenance TMZ when tolerable and in the absence of progression. Additional cycles of maintenance TMZ were administered at the discretion of the treating medical oncologist. The protocol allowed the use of TTF and other salvage therapies.
Patients received 60 Gy PRT utilizing volumetric modulated arc therapy and a single arc. PRT was delivered in daily 2-Gy fractions, given in ten 0.2-Gy pulses. Each pulse was delivered with the same arc, covered the entire planning target volume (PTV), and was separated by 3-minute “beam-off” intervals. Each pulse was delivered in an average of 44 seconds (range, 26–74 sec), and the average arc rotation speed was 5.4 degrees/second (range, 4.0–6.7 degrees/sec). Target volumes were defined per Radiotherapy Oncology Group guidelines. Briefly, patients were treated with 2 treatment volumes. An initial PTV encompassing the T2-FLAIR signal with a 2 cm expansion received 46 Gy; then a boost PTV encompassing the resection cavity and any T1-contrast signal with a 2.5 cm expansion received 14 Gy. Daily cone-beam CT performed prior to the first and sixth pulses was used to make interfraction and interpulse adjustments in patient position if needed. Daily treatment time was approximately 40 minutes.
Follow-up and Surveillance
Acute and chronic treatment toxicities were graded per Common Terminology Criteria for Adverse Events v4.0 weekly during RT and at each follow-up visit. Adverse events were considered acute if they occurred during RT or within 90 days of treatment completion; otherwise they were classified as chronic. Patients completed the European Organisation for Research and Treatment of Cancer (EORTC) 30-item core QoL questionnaire (QLQ-C30) and its 20-item brain neoplasm module (BN20) and underwent neurocognitive testing with the Hopkins Verbal Learning Test–Revised (HVLT-R)19 at baseline, at the end of RT, and at each follow-up visit for the first 2 years or until tumor progression. MR imaging with and without contrast was obtained prior to each follow-up visit to assess for tumor progression. Follow-up visits occurred at 6 weeks post-RT, then every 3 months for the first 24 months, then biannually thereafter. Increasing the frequency of follow-up and incorporating spectroscopy and perfusion studies were done if progression was suspected. Complete blood count with differential was performed weekly during RT and maintenance chemotherapy.
Statistical Analysis
The primary endpoint of this study was PFS, defined as time from surgery to tumor progression or patient death. Progression was defined per Response Assessment in Neuro-Oncology criteria and the clinical judgment of the treating radiation oncologist and neuroradiologist.20 In cases where radiographic changes were equivocal and progression was not confirmed until further follow-up or spectroscopy and perfusion studies, progression was back-dated to the first time radiographic changes were noted. OS was defined as time from surgery to death. PFS and OS were estimated by Kaplan–Meier curves. Survival of the trial cohort was compared with a similar retrospective cohort treated at our institution with SRT using the log-rank test, and a P-value <0.05 was considered significant. This retrospective cohort included all patients receiving SRT to 60 Gy who matched the study’s eligibility criteria and timeframe but declined participation in the trial. Baseline patient characteristics were compared between the trial and retrospective cohorts using the chi-squared test.
HVLT-R scores at each follow-up were compared with baseline, and changes were considered significant if they met the minimal clinically important difference (MCID) threshold. MCID was defined using a distribution-based approach using 0.5 standard deviation (SD) as the significance threshold. An MCID of 0.5 SD is a commonly employed cutpoint as it has been shown to represent the threshold of discrimination for clinically meaningful changes in health-related QoL in most circumstances.21,22
We designed this trial as a demonstration-of-concept pilot study as there are no clinical studies evaluating the combined use of PRT and TMZ.23 Using the calculations suggested by Schoenfeld24 and historical PFS estimates based on the findings of Stupp et al,2 an observed PFS of 8 months or better would qualify as a substantial improvement, and a sample size of 21 would ensure a 90% chance of accepting this improvement as substantially better, warranting a larger trial. The prespecified timepoint for final analysis was death of all patients or a minimum of 3 years follow-up for all surviving patients. All statistics were completed using RStudio (v1.2.5033). The following R packages were used: dplyr, Rmisc, QoLR, PROscorer, tableone, survminer, survival, and ggplot2.
Results
Patients
Twenty-one eligible patients were enrolled. One patient withdrew consent before the start of radiotherapy and was not included in the final analysis. Median follow-up was 21 months (range, 5–70 mo). Baseline patient, treatment, and tumor characteristics are listed in Table 1. Most notably, surgical extent was biopsy only, subtotal resection, and gross total resection in 1 (5%), 12 (60%), and 7 (35%) patients, respectively. One patient had an IDH1 mutated tumor, and 3 had MGMT methylated tumors. All patients completed planned RT and concurrent TMZ. Patients completed a median of 6 cycles of maintenance TMZ (range, 1–14 cycles). Five patients (25%) were treated with TTF following completion of adjuvant RT. A summary of salvage treatments for patients who developed tumor progression is detailed in Supplementary Table 1.
Table 1.
Patient and treatment characteristics1
| Characteristic | N = 20 |
|---|---|
| Sex | |
| Female | 7 (35%) |
| Male | 13 (65%) |
| Median Age at Diagnosis, y | 60 (48, 64) |
| Race | |
| Asian | 1 (5.0%) |
| Black | 1 (5.0%) |
| White | 18 (90%) |
| Karnofsky Performance Status | |
| 70–80 | 8 (40.0%) |
| 90–100 | 12 (60%) |
| Recursive Partitioning Analysis Class | |
| III | 5 (25%) |
| IV | 15 (75%) |
| Resection Extent | |
| Gross total resection | 7 (35%) |
| Subtotal resection | 12 (60%) |
| Biopsy only | 1 (5.0%) |
| IDH 1/2 Status | |
| Wildtype | 19 (95%) |
| Mutated | 1 (5.0%) |
| MGMT Methylation | |
| No | 17 (75%) |
| Yes | 3 (15%) |
| Tumor Maximum Dimension, cm | 4.35 (3.95, 5.05) |
| Maintenance Temozolomide, cycles | 6 (1,14)2 |
| Use of TTF | |
| No | 15 (75%) |
| Yes | 5 (25%) |
| Time from Surgery to RT | 4.21 (3.36, 4.46) |
1Statistics presented: n (%); median (IQR).
2Median (range).
Clinical Outcomes
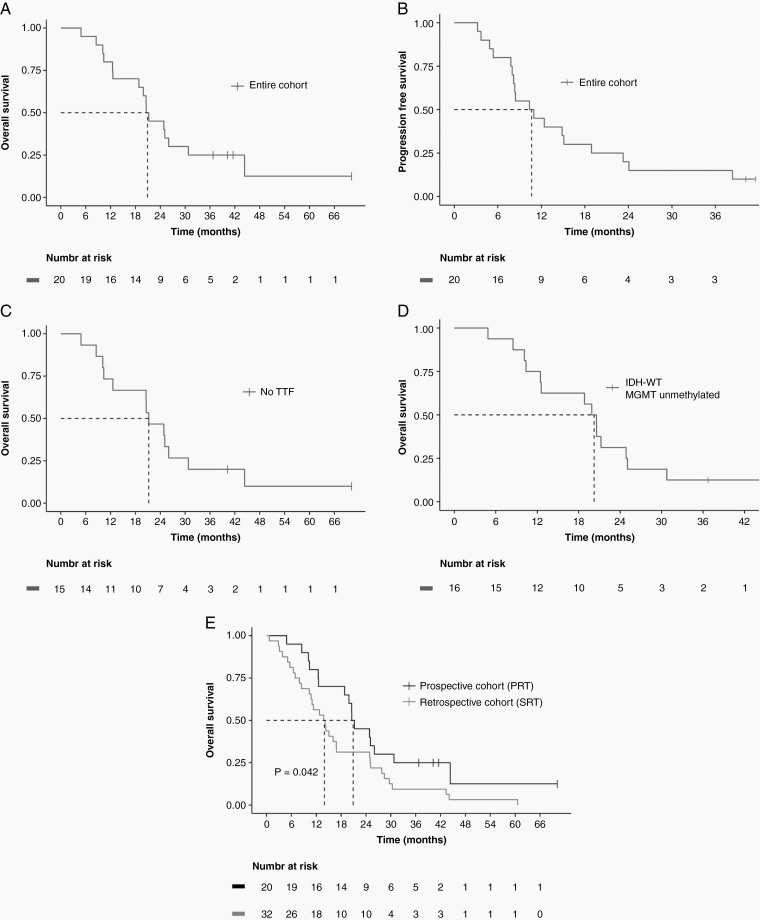
Median OS for the entire cohort was 20.9 months (95% CI: 18.8–upper limit not reached), and estimated 2-year OS was 45% (95% CI: 28%–73%) (Fig. 1A). Median PFS was 10.7 months (95% CI: 8.2–23.3) (Fig. 1B). All but 2 patients progressed within the high-dose radiation field. We performed a sensitivity analysis excluding the 5 patients who received TTF therapy to see if the survival outcomes were largely driven by this subset of patients. However, survival for patients who did not receive TTF did not change significantly, with mOS of 21.2 months (95% CI: 12.6–upper limit not reached) (Fig. 1C). Similarly, survival for patients with IDH wildtype (WT) and MGMT unmethylated tumors remained high, with mOS of 20.2 months (95% CI: 12.5–30.8) (Fig. 1D). For patients who received TTF therapy, 2 out of 5 remain alive, and for patients with either IDH-mutated of MGMT promoter-methylated tumors, 3 out of 4 remain alive. Additionally, in a post-hoc analysis, we compared the study’s patients with 32 patients receiving SRT at our institution within the same timeframe and who met the study’s eligibility criteria. There were no significant differences between the 2 groups in baseline characteristics (Supplementary Table 2). Survival was significantly higher in the PRT group (mOS 20.9 vs 14.0 mo, P = 0.042) (Fig. 1E). We also performed a multivariable Cox regression analysis correlating age, performance status, extent of resection, IDH status, MGMT unmethylation status, use of TTF, and study cohort with OS. Treatment per the PRT protocol was associated with significantly better survival (hazard ratio [HR], 0.39; 95% CI: 0.18–0.83) as shown in Supplementary Table 3. As expected, older age correlated with worse survival, while gross total resection and MGMT methylation correlated with better survival. Use of TTF and IDH mutation appeared to correlate with better survival, but the correlation was not statistically significant, likely due to the small number of patients in these groups.
Fig. 1.
Kaplan–Meier survival curves. (A) mOS was 20.9 months with an estimated 2-year OS of 45% for the entire cohort. (B) Median PFS was 10.7 months for the entire cohort. (C) mOS was 21.2 months for patients not treated with tumor-treating fields. (D) mOS was 20.2 months for patients with IDH-WT and MGMT unmethylated tumors. (E) Survival was significantly higher in the prospective cohort patients treated with PRT compared with a matched retrospective cohort treated with SRT (mOS 20.9 vs 14.0 months, P = 0.042). Dashed lines indicate mOS or mPFS.
Neurocognitive and QoL Outcomes
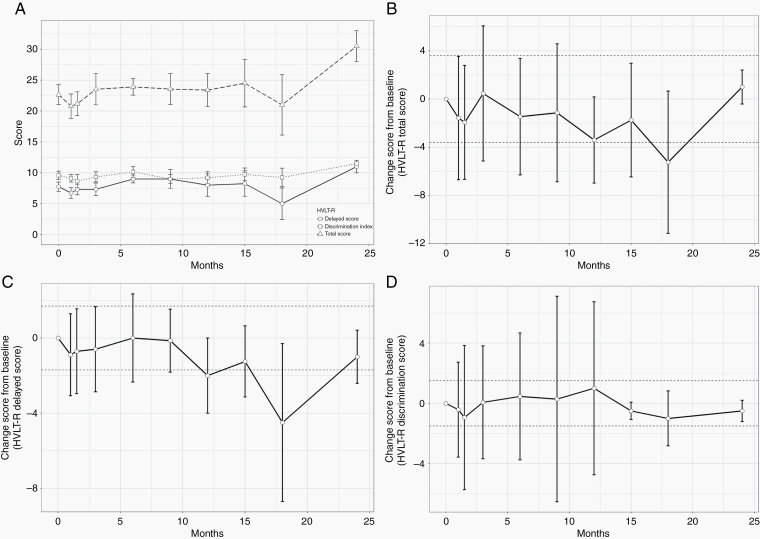
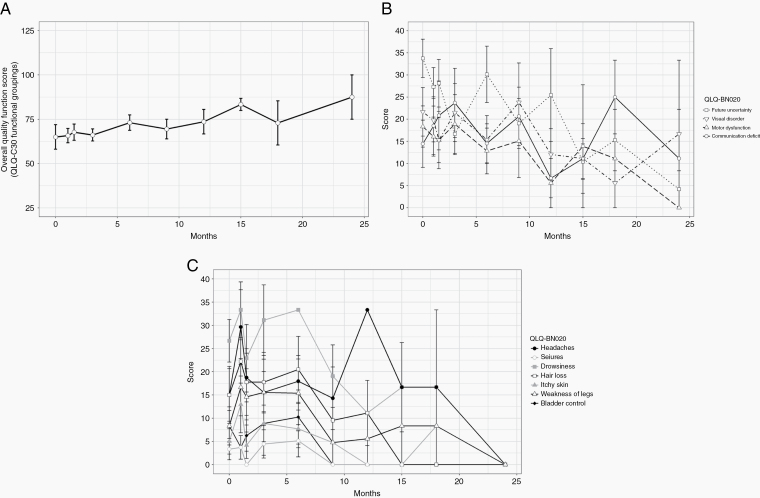
All patients completed HVLT-R testing and the EORTC QLQ-C30 questionnaire and its brain-specific BN20 module at baseline. The majority of patients who were eligible for testing, defined as patients with no disease progression, also completed both tests at subsequent follow-up visits for the first 2 years, as detailed in Supplementary Table 4. The average raw HVLT-R total recall, delayed recall, and recognition discrimination index scores are shown in Fig. 2A. There was no significant deterioration in any of the HVLT-R scores. Additionally, we calculated change in score from each time point to baseline, and HVLT-R scores remained stable throughout the testing period for all eligible patients. The average change in the HVLT-R total recall, delayed recall, and recognition discrimination index scores did not meet the definition of an MCID at any of the tested time points (Fig. 2B–D). Similarly, QoL testing showed no deterioration at any of the tested time points, with a trend toward improvement in QoL near the end of the testing period in the overall quality function score (Fig. 3A), BN20 multisystem score (Fig. 3B), and BN20 single-system score (Fig. 3C). A lower score on the BN20 module indicates improvement in QoL.
Fig. 2.
Results of Hopkins Verbal Learning Test-Revised testing. Results are presented at baseline and at each follow-up for the first 2 years for all patients who had not developed tumor progression at each time point. All results are presented as mean ± standard deviation. (A) Raw values of the HVLT-R scores. (B–D) Changes in the HVLT-R total recall, delayed recall, and recognition discrimination index scores, respectively. The dashed lines represent the MCID threshold. Error bars crossing the MCID line indicate no significant change from baseline.
Fig. 3.
Results of the EORTC QLQ-C30 quality-of-life questionnaire and its brain-specific module (BN20). Results are presented at baseline, end of treatment, and each follow-up for the first 2 years for all patients who had not developed tumor progression at each time point. All results are presented as mean ± SEM. (A) Overall quality score. A higher score indicates improved QoL. (B) Multisystem score on the BN20 module. Lower score indicates improved QoL. (C) Single-system score on the BN20 module. Lower score indicates improved QoL.
Adverse Events
Treatment with PRT was well tolerated with a favorable toxicity profile, as shown in Table 2. Only one patient experienced an acute grade 3 RT-related toxicity, which was fatigue. No other acute grade 3+ toxicities were noted. The most common acute grade 2 toxicities included nausea (15%), alopecia (15%), and cognitive disturbance (15%). There were no chronic grade 3+ RT-related adverse events. All chronic grade 2 adverse events were ≤10%. Hematologic toxicities during concurrent chemoradiotherapy and adjuvant TMZ alone are summarized in Table 3. During concurrent chemoradiotherapy, the highest grade hematologic toxicity was grade 2 thrombocytopenia (5%). During maintenance TMZ therapy, the most notable hematologic toxicities included grade 4 thrombocytopenia (5%) and grade 3 leukopenia (25%), with the remainder of toxicities being grade ≤2.
Table 2.
Acute and chronic radiotherapy related adverse events1
| Adverse Event (AE) | Acute AE | Chronic AE |
|---|---|---|
| N = 20 | N = 20 | |
| Motor Neuropathy | ||
| Grade 1 | – | 1 (5%) |
| Grade 2 | 2 (10%) | 2 (10%) |
| Sensory Neuropathy | ||
| Grade 1 | 3 (15%) | 1 (5%) |
| Grade 2 | 2 (10%) | 2 (10%) |
| Radiation Necrosis | ||
| Grade 1 | – | 1 (5%) |
| Xerostomia | ||
| Grade 1 | 4 (20%) | – |
| Nausea | ||
| Grade 1 | 3 (15%) | 3 (15%) |
| Grade 2 | 3 (15%) | – |
| Hearing Loss | ||
| Grade 1 | 1 (5%) | – |
| Grade 2 | – | 1 (5%) |
| Radiation Dermatitis | ||
| Grade 1 | 10 (50%) | – |
| Grade 2 | 1 (5%) | – |
| Fatigue | ||
| Grade 1 | 10 (50%) | 9 (45%) |
| Grade 2 | 3 (15) | 1 (5%) |
| Grade 3 | 1 (5%) | – |
| Headache | ||
| Grade 1 | 4 (20%) | 3 (15%) |
| Grade 2 | 1 (5%) | 2 (10%) |
| Gait Disturbance | ||
| Grade 1 | 3 (15%) | 2 (10%) |
| Grade 2 | 1 (5%) | 1 (5%) |
| Cognitive Disturbance | ||
| Grade 1 | 2 (10%) | 2 (10%) |
| Grade 2 | 3 (15%) | 1 (5%) |
| Memory Impairment | ||
| Grade 1 | 5 (20%) | 2 (10%) |
| Grade 2 | 1 (5%) | 1 (5%) |
| Alopecia | ||
| Grade 1 | 10 (50%) | 4 (20%) |
| Grade 2 | 3 (15%) | 2 (10%) |
1Statistics presented: n (%).
AEs are defined as events experienced within 90 days of the start of RT; otherwise considered chronic maximum toxicities are presented.
Table 3.
Hematologic toxicity during chemoradiotherapy and maintenance TMZ1
| Hematologic Toxicity | Chemoradiotherapy N = 20 |
Maintenance TMZ N = 20 |
|---|---|---|
| Neutropenia | ||
| Grade 2 | 1 (5%) | 1 (5.0%) |
| Grade 3 | – | 2 (10%) |
| Anemia | ||
| Grade 1 | 6 (30%) | 11 (55%) |
| Grade 2 | – | 1 (5.0%) |
| Leukopenia | ||
| Grade 1 | 1 (5.0%) | 1 (5.0%) |
| Grade 2 | 3 (15%) | 6 (30%) |
| Grade 3 | – | 5 (25%) |
| Thrombocytopenia | ||
| Grade 1 | 2 (10%) | 5 (25%) |
| Grade 2 | 1 (5.0%) | 1 (5.0%) |
| Grade 3 | – | 1 (5.0%) |
| Grade 4 | – | 1 (5.0%) |
1 Statistics presented: n (%).
Maximum toxicities are presented.
Discussion
The results of this prospective study demonstrate the feasibility and safety of PRT for the treatment of newly diagnosed GBM patients. To our knowledge, this is the first study to investigate the use of PRT prospectively and in the definitive management of this patient population. The observed clinical outcomes are promising and compare favorably with those of well-matched patients treated at our institution with SRT as well as to historic controls. The 4 largest randomized trials investigating various treatments for newly diagnosed GBM reported median PFS of 4 to 7.3 months and mOS of 14.6 to 16.1 months in patients receiving concurrent SRT and TMZ, compared with 10.7 and 20.9 months for patients receiving PRT and TMZ on our study.2,3,25,26 Notably, the patient cohort in our study has a slightly higher median age and a higher percentage of patients with subtotal tumor resection compared with all of the aforementioned trials.
All but one of the patients in this study had IDH-WT tumors, and only 3 patients had MGMT methylated tumors. These tumor characteristics portend a poor prognosis. The reported mOS in patients with IDH-WT tumors is in the range of 12 to 15 months.27,28 In a recent randomized trial, which investigated the addition of TTF to maintenance TMZ, patients with MGMT unmethylated tumors receiving SRT and TMZ had mOS of 14.7 months.3 Even the addition of TTF, which has resulted in the highest mOS in GBM patients reported by any major randomized trial to date, did not drastically improve survival in patients with MGMT unmethylated tumors (mOS of 16.9 mo).
Maintenance of QoL and preservation of NCF are important endpoints of treatment, especially in patients with GBM given their poor prognosis and relatively short life span. There are a paucity of longitudinal data on QoL and NCF after definitive treatment in GBM patients. Nonetheless, the existing evidence shows NCF to be an independent predictor of survival in high-grade gliomas and has a strong direct correlation with QoL and the ability to maintain independence and perform daily living activities.29–32 There has been a concerted effort to minimize RT-related NCF, and recent studies have shown promising results in this regard with the addition of memantine and employment of RT techniques that deliberately avoid the hippocampus, which is central to preservation of NCF.33,34 Unfortunately, cognitive decline remains a common complication in long-term GBM survivors. While tumor progression leads to cognitive dysfunction, the treatment of GBM involves irradiating large volumes of normal brain parenchyma, which per se can negatively impact NCF.29 In a study that analyzed approximately 1200 patients who received RT for high-grade gliomas, nearly 20% of patients who did not experience tumor progression showed significant cognitive decline based on the Mini-Mental State Examination (MMSE).35 In our study, patients without tumor progression were able to preserve performance on the HVLT-R test and QoL long term, and intriguingly, QoL appeared to improve with long-term follow-up. Importantly, we employed the brain tumor specific questionnaire EORTC QLQ-BN20 for QoL testing and the HVLT-R test, which is a highly sensitive tool for cognitive examination and can show cognitive decline in more than half of patients with a normal score on the MMSE.36
Possible mechanisms underlying the apparent improved effectiveness with PRT have been proposed. Preclinical studies have demonstrated that proliferating cells are more sensitive per unit dose of radiation to treatments below approximately 0.3 Gy.11 This effect was termed low-dose hyper-radiosensitivity (HRS). At doses below this threshold, insufficient numbers of DNA double strand breaks are produced to induce checkpoints that arrest cell cycle progression and permit cellular repair processes.10,12 Consequently, cells continue to proliferate with unrepaired DNA damage until they reach the critical cell cycle phase of mitosis, in which unrepaired DNA double strand breaks are invariably lethal.37 On the other hand, doses exceeding 0.2 Gy can produce sufficient levels of DNA damage to activate DNA repair and cell cycle checkpoint processes. In the setting of fractionated PRT, interpulse “beam-off” intervals allow passage of tumor cells through the cell cycle and into mitosis, where undetected DNA damage may contribute to lethal events. In the referenced preclinical models, a 3-minute interval allowed sufficient time for cell cycle progression. Various intervals longer than 3 minutes produced similar biological responses. The 3-minute interval was adopted to limit the overall treatment time while preserving low dose HRS. Additionally, preclinical evidence shows that PRT produces less vascular injury compared with SRT and consequently maintains both tumor and normal tissue oxygenation, potentially leading to better TMZ delivery to the tumor while enhancing the impact of RT and sparing normal tissue.13 Concordant with this hypothesis is the finding of higher tumor vascular density and lower rates of neuronal damage with PRT compared with SRT.13
Another treatment technique that utilizes a similar principle to external beam PRT is pulsed dose rate brachytherapy (PDR-BT), in which a high dose rate radioactive source like iridium-192 is used to deliver 0.4–1.0 Gy hourly. Several preclinical and retrospective studies have suggested an improved therapeutic ratio with PDR-BT.38,39 It is notable, however, that with PDR-BT, radiation delivery is constant while the radioactive source is in place. Radiation-free (“beam-off”) intervals are achieved by withdrawing the radioactive source and reapplying it at a later time point. While the interval and magnitude of the delivered pulses in PDR-BT are different from those employed in external beam PRT, the observation that both techniques can change the therapeutic index highlights the importance of dose rates and lends credence to the aforementioned mechanisms for the efficacy of PRT.
An interesting possibility is the utilization of PRT for RT dose escalation in the treatment of GBM. As discussed previously, early results showed dose escalation did not alter failure patterns or improve outcomes significantly. Indeed, dose escalation to 90 Gy with SRT and old RT techniques resulted in worse survival, presumably secondary to treatment-related toxicity.9 However, a recent single institution trial demonstrated the safety of dose escalation up to 75 Gy when utilizing intensity-modulated RT (IMRT) and reported mOS of 20 months.40 As such, there has been renewed interest in the subject and there is an ongoing phase III trial investigating dose escalation using IMRT or proton therapy (NCT02179086). Dose escalation with PRT might mitigate the potential increased treatment-related toxicity and allow for the delivery of very high doses and inclusion of a larger target compared with SRT.
This study has several limitations. Most importantly, the small number of patients limits the generalizability of our findings. Furthermore, 25% of our cohort was treated with TTF, which has been shown to improve survival significantly in GBM patients,3 so the relatively long median survival in our cohort should be interpreted with caution. We performed a sensitivity analysis excluding patients treated with TTF with no change in survival outcomes. It is important to note that our comparison to the retrospective cohort is meant to provide an internal control of patients treated within the same period at our institution. While we attempted to minimize bias by including all patients who matched the protocol’s eligibility criteria and were treated with SRT, the comparison was done post-hoc, and selection bias remains possible. In particular, a higher percentage of patients in the PRT cohort received TTF. As such, our results should be interpreted judiciously, and corroboration with larger studies is needed. The outcomes presented on preservation of QoL and NCF should also be interpreted cautiously. While the HVLT-R test is an excellent tool for assessing NCF, it does not assess the entirety of NCF, and patients’ performance can improve simply with frequent repetition of the test. Furthermore, as with most longitudinal studies, we had a significant drop in compliance on completing the HVLT-R test and QoL questionnaires in the latter half of the study. Hence, the QoL and NCF data may be artificially enhanced if a disproportionate number of patients dropped out due to clinical deterioration. Finally, the delivery of PRT requires vigorous quality assurance and long treatment times (approximately 40 minutes), which may hinder the feasibility of such treatment in some clinics.
In conclusion, the results of this prospective pilot study demonstrate that PRT is feasible and safe for the treatment of patients with newly diagnosed GBM and produces promising effectiveness while maintaining several aspects of NCF. The favorable clinical outcomes were noted in a cohort of patients with mostly IDH-WT and MGMT unmethylated tumors, which carry a dismal prognosis and have shown little response to the currently available therapeutic options. Validation of our findings in a larger prospective trial warrants consideration.
Supplementary Material
Funding
This study was funded by a seed grant from the Zafarana Fund Group.
Conflict of interest statement. No such conflicts reported.
Authorship statement
Muayad F. Almahariq: contributed substantially to data collection, data analysis, data interpretation, and writing of the manuscript.
Thomas J. Quinn: contributed substantially to data collection, data analysis, data interpretation, and revision of the manuscript.
Jessica D. Arden: contributed substantially to data collection, data interpretation, and revision of the manuscript.
Roskos, P. Tyler: contributed substantially to data collection, data analysis, and revision of the manuscript.
George D. Wilson: contributed substantially to the study design, data interpretation, and revision of the manuscript.
Brian Marples: contributed substantially to the study design, data interpretation, and revision of the manuscript.
Inga S. Grills: contributed substantially to the study design, data interpretation, and revision of the manuscript.
Peter Y. Chen: contributed substantially to the study design, data interpretation, and revision of the manuscript.
Daniel J. Krauss: contributed substantially to the study design, data interpretation, and revision of the manuscript.
Prakash Chinnaiyan: contributed substantially to the study design, data analysis, data interpretation, and revision of the manuscript.
Joshua T. Dilworth: contributed substantially to the study’s conception and design, data collection, data interpretation, and revision of the manuscript.
References
- 1. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16(6):1405–1409. [DOI] [PubMed] [Google Scholar]
- 5. Garden AS, Maor MH, Yung WK, et al. Outcome and patterns of failure following limited-volume irradiation for malignant astrocytomas. Radiother Oncol. 1991;20(2):99–110. [DOI] [PubMed] [Google Scholar]
- 6. Gebhardt BJ, Dobelbower MC, Ennis WH, Bag AK, Markert JM, Fiveash JB. Patterns of failure for glioblastoma multiforme following limited-margin radiation and concurrent temozolomide. Radiat Oncol. 2014;9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bleehen NM, Stenning SP. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. Br J Cancer. 1991;64(4):769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laramore GE, Martz KL, Nelson JS, Griffin TW, Chang CH, Horton J. Radiation Therapy Oncology Group (RTOG) survival data on anaplastic astrocytomas of the brain: does a more aggressive form of treatment adversely impact survival? Int J Radiat Oncol Biol Phys. 1989;17(6):1351–1356. [DOI] [PubMed] [Google Scholar]
- 9. Chan JL, Lee SW, Fraass BA, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20(6):1635–1642. [DOI] [PubMed] [Google Scholar]
- 10. Krueger SA, Collis SJ, Joiner MC, Wilson GD, Marples B. Transition in survival from low-dose hyper-radiosensitivity to increased radioresistance is independent of activation of ATM Ser1981 activity. Int J Radiat Oncol Biol Phys. 2007;69(4):1262–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marples B, Collis SJ. Low-dose hyper-radiosensitivity: past, present, and future. Int J Radiat Oncol Biol Phys. 2008;70(5):1310–1318. [DOI] [PubMed] [Google Scholar]
- 12. Krueger SA, Wilson GD, Piasentin E, Joiner MC, Marples B. The effects of G2-phase enrichment and checkpoint abrogation on low-dose hyper-radiosensitivity. Int J Radiat Oncol Biol Phys. 2010;77(5):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dilworth JT, Krueger SA, Dabjan M, et al. Pulsed low-dose irradiation of orthotopic glioblastoma multiforme (GBM) in a pre-clinical model: effects on vascularization and tumor control. Radiother Oncol. 2013;108(1):149–154. [DOI] [PubMed] [Google Scholar]
- 14. Tomé WA, Howard SP. On the possible increase in local tumour control probability for gliomas exhibiting low dose hyper-radiosensitivity using a pulsed schedule. Br J Radiol. 2007;80(949):32–37. [DOI] [PubMed] [Google Scholar]
- 15. Richards GM, Tomé WA, Robins HI, et al. Pulsed reduced dose-rate radiotherapy: a novel locoregional retreatment strategy for breast cancer recurrence in the previously irradiated chest wall, axilla, or supraclavicular region. Breast Cancer Res Treat. 2009;114(2):307–313. [DOI] [PubMed] [Google Scholar]
- 16. Yan J, Yang J, Yang Y, et al. Use of pulsed low-dose rate radiotherapy in refractory malignancies. Transl Oncol. 2018;11(1):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adkison JB, Tomé W, Seo S, et al. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(3):835–841. [DOI] [PubMed] [Google Scholar]
- 18. Burr AR, Robins HI, Bayliss RA, Howard SP. Pulsed reduced dose rate for reirradiation of recurrent breast cancer. Pract Radiat Oncol. 2020;10(2):e61–e70. [DOI] [PubMed] [Google Scholar]
- 19. Brandt J, Benedict RHB.. Hopkins Verbal Learning Test‒Revised. Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- 20. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in Neuro-Oncology Working Group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 21. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. [DOI] [PubMed] [Google Scholar]
- 22. Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546. [DOI] [PubMed] [Google Scholar]
- 23. Moore GC, Carter RE, Nietert PJ, Stewart PW. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci. 2011;4(5):332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schoenfeld D. Statistical considerations for pilot studies. Int J Radiat Oncol Biol Phys. 1980;6(3):371–374. [DOI] [PubMed] [Google Scholar]
- 25. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 27. Hartmann C, Hentschel B, Simon M, et al. ; German Glioma Network . Long-term survival in primary glioblastoma with versus without isocitrate dehydrogenase mutations. Clin Cancer Res. 2013;19(18):5146–5157. [DOI] [PubMed] [Google Scholar]
- 28. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Henriksson R, Asklund T, Poulsen HS. Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: a review. J Neurooncol. 2011;104(3):639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heimans JJ, Taphoorn MJ. Impact of brain tumour treatment on quality of life. J Neurol. 2002;249(8):955–960. [DOI] [PubMed] [Google Scholar]
- 31. Giovagnoli AR, Silvani A, Colombo E, Boiardi A. Facets and determinants of quality of life in patients with recurrent high grade glioma. J Neurol Neurosurg Psychiatry. 2005;76(4):562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Correa DD. Neurocognitive function in brain tumors. Curr Neurol Neurosci Rep. 2010;10(3):232–239. [DOI] [PubMed] [Google Scholar]
- 33. Brown PD, Pugh S, Laack NN, et al. ; Radiation Therapy Oncology Group (RTOG) . Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown PD, Gondi V, Pugh S, et al. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol. 2020:JCO1902767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown PD, Jensen AW, Felten SJ, et al. Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol. 2006;24(34):5427–5433. [DOI] [PubMed] [Google Scholar]
- 36. Lacy M, Kaemmerer T, Czipri S. Standardized Mini-Mental State Examination scores and verbal memory performance at a memory center: implications for cognitive screening. Am J Alzheimers Dis Other Demen. 2015;30(2):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Enns L, Bogen KT, Wizniak J, Murtha AD, Weinfeld M. Low-dose radiation hypersensitivity is associated with p53-dependent apoptosis. Mol Cancer Res. 2004;2(10):557–566. [PubMed] [Google Scholar]
- 38. Skowronek J. Pulsed dose rate brachytherapy—is it the right way? J Contemp Brachytherapy. 2010;2(3):107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Polo A. Pulsed dose rate brachytherapy. Clin Transl Oncol. 2008;10(6):324–333. [DOI] [PubMed] [Google Scholar]
- 40. Tsien CI, Brown D, Normolle D, et al. Concurrent temozolomide and dose-escalated intensity-modulated radiation therapy in newly diagnosed glioblastoma. Clin Cancer Res. 2012;18(1):273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.