Abstract
Background
The interleukin-13 receptor alpha 2 (IL13RA2) and ephrin type A receptor 2 (EPHA2) are attractive therapeutic targets, being expressed in ~90% of canine and human gliomas, and absent in normal brain. Clinical trials using an earlier generation IL-13 based cytotoxin showed encouraging clinical effects in human glioma, but met with technical barriers associated with the convection-enhanced delivery (CED) method. In this study, IL-13 mutant and ephrin A1 (EFNA1)–based bacterial cytotoxins targeted to IL13RA2 and EPHA2 receptors, respectively, were administered locoregionally by CED to dogs with intracranial gliomas to evaluate their safety and preliminary efficacy.
Methods
In this phase I, 3 + 3 dose escalation trial, cytotoxins were infused by CED in 17 dogs with gliomas expressing IL13RA2 or EPHA2 receptors. CED was performed using a shape-fitting therapeutic planning algorithm, reflux-preventing catheters, and real-time intraoperative MRI monitoring. The primary endpoint was to determine the maximum tolerated dose of the cytotoxic cocktail in dogs with gliomas.
Results
Consistent intratumoral delivery of the cytotoxic cocktail was achieved, with a median target coverage of 70% (range, 40–94%). Cytotoxins were well tolerated over a dose range of 0.012–1.278 μg/mL delivered to the target volume (median, 0.099 μg/mL), with no dose limiting toxicities observed. Objective tumor responses, up to 94% tumor volume reduction, were observed in 50% (8/16) of dogs, including at least one dog in each dosing cohort >0.05 μg/mL.
Conclusions
This study provides preclinical data fundamental to the translation of this multireceptor targeted therapeutic approach to the human clinic.
Keywords: animal models, bacterial cytotoxins, canine, glioblastoma, interstitial drug delivery
Key Points.
Dogs with spontaneous glioma are predictive translational models of human disease.
Cytotoxins were well tolerated at doses up to 6-fold higher than previously given to humans.
A single cytotoxin treatment produced ≥65% tumor volumetric reductions in 50% of dogs.
Importance of the Study.
Using a real-time MRI-monitored convection enhanced delivery technique in a canine model of spontaneous intracranial glioma, we demonstrate that locoregional administration of recombinant bacterial cytotoxins targeting IL13RA2 and EPHA2 tumor-associated receptors is safe and capable of inducing objective and clinically relevant antitumor effects over a range of doses. Given the shared molecular fingerprint of IL13RA2 and EPHA2 overexpression in canine and human malignant glioma, our findings provide evidence that justifies translational investigations of CED platforms that incorporate real-time imaging and evolving technical advancements in conjunction with personalized biomarker screening for rational locoregional delivery of targeted therapeutics to humans with malignant glioma.
High-grade gliomas represent primary brain tumors with unmet medical needs. The most common malignant glioma is glioblastoma (GBM), which is incurable with surgery and chemoradiotherapy.1,2 Obstacles to GBM treatment include tumor heterogeneity, which limits lasting effects from single-factor targeted drugs, and the blood‒brain and blood‒tumor barriers, which limit access of systemically administered drugs.2
One approach to glioma treatment is the use of targeted cytotoxins, which bind to plasma membrane receptors and are subsequently internalized to deliver a lethal toxin load to targeted cells.3 The interleukin-13 receptor alpha 2 (IL13RA2) is one of 2 receptors that bind IL13 ligand and has been widely studied in glioma.3–7 IL13RA2 is overexpressed in canine and human glioma cells, but not normal brain cells.3,5,6 We have modified the IL13 ligand for IL13RA2 to increase target specificity by differentially increasing binding avidity for tumor-expressed IL13RA2, but not for the physiological receptor, IL13RA1/IL-4A.7
To advance the targeted cytotoxin approach, we sought to make therapy more specific and combinatorial and have identified ephrin type A receptor 2 (EPHA2), which is also overexpressed in gliomas, but not in normal brain.8 In this study, we used a superagonist conjugate of ephrin A1 (eA1), a specific ligand for EPHA2 receptor, with PE38QQR.8–10 IL13RA2 and EPHA2 are conjointly present in >90% of glioma patients, with the majority expressing at least one of these proteins in 50–100% of tumor cells, as well as within the tumor vasculature, glioma stemlike cells, and tumor cells infiltrating normal brain.10 By concomitantly delivering IL13- and eA1-based cytotoxins, we address tumor heterogeneity to some extent by killing glioma cells that express either IL13RA2, EPHA2, or both receptors.10
A first-generation IL13-based cytotoxin, huIL13-PE38QQR,3 produced significant clinical responses in early trials, which was the chief reason for moving the candidate drug to the efficacy trial.11 However, the phase III trial design required a 50% extension of overall survival over controls, which made achieving the primary endpoint difficult, since few oncologic drugs demonstrated such efficacy.4 Other flaws of the trial included lack of real-time imaging of the drug distribution during convection-enhanced delivery (CED), use of a catheter with a tendency for infusate reflux and air blockade, and lack of confirmation of target expression.4,12 Regardless, patients with recurrent GBM lived a mean 45 weeks (range, 35 to 53), a result that has not been seen in other subsequent efficacy trials in this patient cohort.4 To address these limitations associated with CED technique, we utilize antireflux catheters and real-time intraoperative imaging, which have been shown to improve CED.13,14
Use of a canine model of spontaneous glioma, which represents the closest translational model to human disease, provides a more realistic assessment of potential efficacy in human trials regarding mechanistic and biological aspects of treatment.14,15 In this study, we demonstrate the tolerability and preliminary efficacy of cytotoxins targeting IL13RA2 and EPHA2 receptors administered with CED in dogs with gliomas. A secondary objective was to characterize the serum cytokine profiles of dogs subjected to cytotoxin treatment. We hypothesized that dogs with glioma would have elevated concentrations of IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) and that a cytokine signature reflecting immunogenic cell death (ICD) would be observed after cytotoxin treatment.16 Given the shared IL13RA2 and EPHA2 expression in canine and human glioma, this trial provides crucial knowledge regarding translation of this therapy to humans.6
Materials and Methods
Canine Trial Design
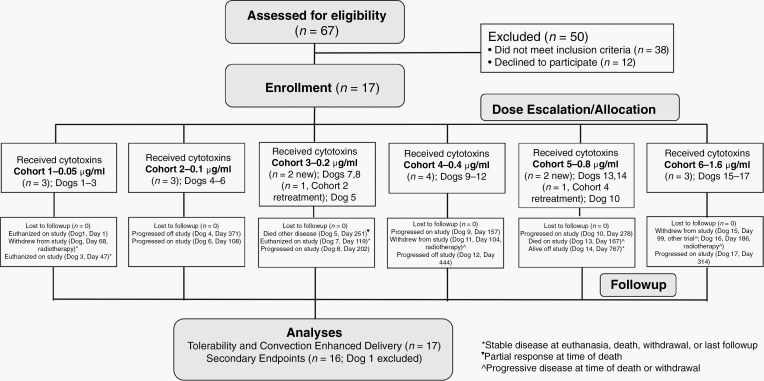
This was a prospective, open-label 3 + 3 dose escalation phase I trial (Fig. 1). Trial procedures were approved by the institutional animal care and use committee (protocols 14–235 and 17–203). Owners provided written, informed consent for their dogs to receive treatment. For inclusion, dogs had to have histopathologically confirmed gliomas that were immunohistochemically positive for IL13RA2 or EPHA2 receptors6,10; a Karnofsky performance score (KPS) ≥60; stable cardiopulmonary functions; and no evidence of concurrent malignancy or significant bone marrow, hepatic, or renal dysfunction. All tumor biopsies were classified using the revised canine glioma system.17 Cases were enrolled from a national veterinary clinical trial registry or the clinical population presented to the study center.18
Fig. 1.
Canine trial workflow.
Study Endpoints
The primary endpoint was to determine the maximum tolerated dose (MTD) of the cytotoxic cocktail when delivered by convection enhancement. MTD was defined as the dose below the one in which dose-limiting toxicities (DLTs) occurred in 2 dogs. DLTs were defined as any of the following observed within 28 days of CED: exacerbation of a preexisting neurologic deficit; new grade 3 or 4 adverse events of the nervous system that did not resolve spontaneously or with corticosteroid treatment within 14 days; or any grade 5 adverse event of the nervous system .19
Secondary endpoints consisted of quantitative analyses of the CED infusion, progression-free survival (PFS), overall survival (OS), and MRI volumetric tumor responses. Therapeutic responses were determined from follow-up T2-weighted MRI volumes, as not all tumors were contrast enhancing, and defined as follows: a complete response (CR) required no evidence of tumor; partial response (PR) if tumor volume had decreased by ≥65%; progressive disease (PD) if tumor volume increased ≥40%; all other responses constituted stable disease (SD).20
Exploratory analyses consisting of KPS, Engel seizure outcome, and owner-reported quality of life (QoL) questionnaires were performed as quality of life surrogates.21
Canine Trial Workflow
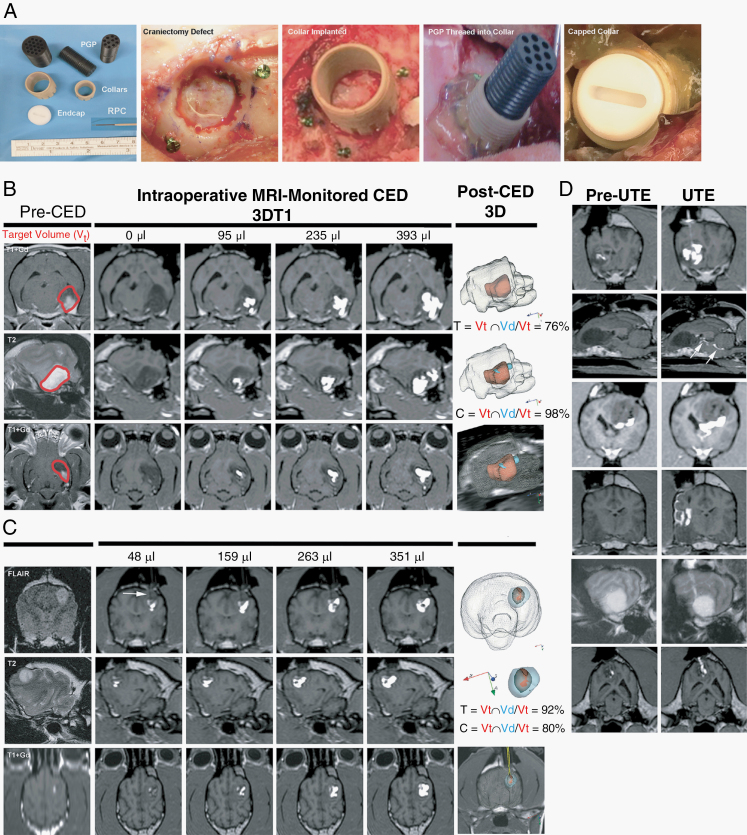
On day −4, dogs underwent clinical examinations, KPS, QoL scoring, and laboratory assessments. On day −3, dogs were anesthetized (Supplementary Methods) and placed in a stereotactic head frame, and pre- and postcontrast CT and MR images (Supplementary Table 1)22 were used for planning and performing tumor biopsy and CED through a burr hole craniectomy (Fig. 2).21,23 Following tumor biopsy, threaded probe guide pedestal (PGP) collars (MRI Interventions; Supplementary Material) were implanted into craniectomy defects.14,21 The pedestal collars could accept threaded multiport PGP (MRI Interventions), through which the CED catheters could be passed (Fig. 2). A CT scan was then obtained to verify PGP positioning, and dogs recovered from anesthesia.
Fig. 2.
CED instrumentation and procedure. Probe guide pedestals (PGP; A) are implanted into the craniectomy defects for CED. MRI monitored CED treatment (Dog 2; B) representing target volume and delivery outcomes that approximate trial medians, with 76% target coverage [T] achieved. Superior CED treatment (Dog 14; C), with the Vd extending beyond the T2/FLAIR lesion volume and reflux-preventing catheter (RPC) delineated by the white arrow. Increasing Vd of the infusate (white) is visible within the Vt as the treatment progresses, and 3D reconstructions illustrate the total Vd spatial distribution (blue) relative to the Vt (red). Unanticipated technical events (UTE; D), from top to bottom: withdrawal reflux; subependymal leakage (arrows); ventricular leakage; subarachnoid leakage; cerebral edema along catheter track; leakage up catheter track.
On days −2 and −1, dogs underwent clinical examinations and KPS while tumor biopsies were processed and CED plans developed using an inverse shape-fitting technique (Supplementary Figure 1).24 On day 0, dogs were anesthetized, the implanted pedestal collar surgically exposed and the pedestal installed, and dogs transported to the radiology suite where the CED treatment was performed using reflux preventing catheters (MRI Interventions) and serial 3D T1-weighted MRI monitoring using described techniques (Fig. 2; Supplementary Methods, Supplementary Table 2).14,24,25 Cytotoxins were manufactured as reported previously6,8 and co-delivered with galbumin (5 mg/mL; BioPal). Following completion of CED, catheters were removed and dogs then transported to the operating theater where the pedestal was removed, the collar sealed with an end-cap, and the cranial wound closed.
On day 1 or 2, dogs underwent clinical examinations and KPS and were discharged to the care of their owners. On days 21–28, dogs underwent clinical examinations, laboratory evaluations, and KPS. Outpatient visits occurred on days 42, 84, 150–180, 240–270, and 330–365 and consisted of clinical examinations, KPS, Engel seizure, QoL scoring, laboratory evaluations, and brain MRI examinations.
Analyses of Convection-Enhanced Delivery
The infusate Vd within the brain was determined using commercial image analysis software (BrainLab). A threshold pixel value was determined for the galbumin signal, with signal above the threshold semi-automatically segmented (iPlan, Smartbrush, BrainLab).14,24 The Vd could be calculated from the galbumin-segmented image mask at any given timepoint by correlating time-stamped MR images with time-registered Vi and infusion rates. Target coverage (T) and infusate containment (C) were calculated using published criteria.24
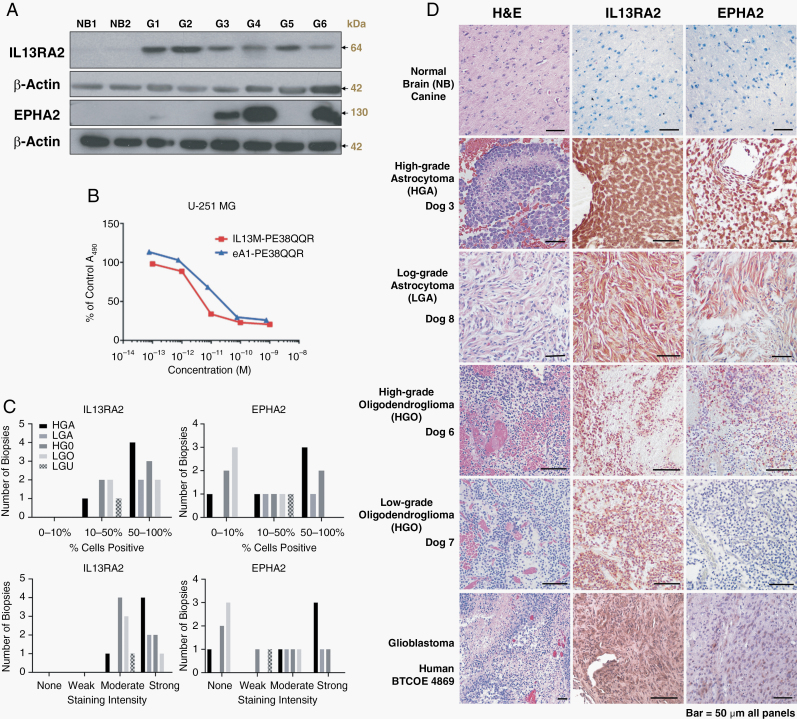
Canine Glioma Cell Western Blots and Cytotoxicity Assays
Western blots were performed on canine primary glioma lines SDT-3G and GO6A, derived from spontaneously occurring canine gliomas and normal brain lysates, prepared from subconfluent cultures.6 Cytotoxicity assays evaluating the effects of the cytotoxins and galbumin vehicle on the human glioblastoma cell line U-251 MG and the canine primary glioma cell lines J3T (Supplementary Figure 2) and GO6-A were performed as described.6
Serum Cytokine Assays
Concentrations of 13 cytokines were measured in duplicate on days 0 and 42 in serum using a commercial canine-specific multiplex immunoassay (Milliplex Map Canine Cytokine Magnetic Bead Panel CCYTOMAG-90K) with an automated analyzer (Luminex 200), and expressed as raw fluorescent intensity values. Serum from 6 healthy adult breed-matched dogs were used as controls. Glioma dogs also had cytokines assayed on day 84–365 visits.
Statistical Analyses
Descriptive statistics were calculated for all quantitative variables. Continuous variables (ie, KPS scores and tumor coverage) were compared between responders and nonresponders using 2-sample t-tests. For categorical variables, Fisher’s exact tests were used to compare group outcomes with 2 levels, and chi-square tests were used to compare groups with 3 (or more) levels. For measures of time-to-event outcomes, medians and ranges were calculated. In addition, tumor characteristics were calculated within response categories (ie, for PR, SD, etc). Pearson’s correlation coefficients were calculated to examine the relationships between quantitative CED treatment variables and survival outcomes.
Longitudinal mixed models were used to compare serum cytokines between groups. For each outcome, pretreatment values were included as covariates and time and treatment by time interactions examined. Dogs were included in models as random effects to account for the repeated outcome measures. Responders were considered those dogs that experienced PR. Prolonged responders were defined as those dogs that demonstrated serial PR over days 42–180. Analyses were performed with SAS v9.4 software.
Results
Canine Subject and Tumor Characteristics
Trial workflow, canine subject, tumor characteristic, CED, and outcome data for the 17 dogs enrolled are summarized in Fig. 1 and Table 1. All dogs had structural epilepsy and 10/17 had additional neurologic deficits referable to the brain region containing the tumor. All 17 tumors demonstrated positive immunohistochemical staining for IL13RA2, and 11/17 were positive for EPHA2 (Fig. 3).
Table 1.
Canine subject demographics, tumor characteristics, quantitative CED treatment parameters, and outcomes
| Dog- Dose Cohorta | Breed | Age (yrs) | Body Weight (kg) | Sex | Baseline KPS | Diagnosis | Total Target Volume (Vt, cm3) | Contrast Enhancing Target Volume (cm3) | Infusion Volume (Vi, µL) | Distribution Volume (Vd, cm3) | Vd:Vi | Number of Catheters | Target Coverage (T)/% T = Vt∩Vd/ Vt | Target Containment (C)/% C = Vt∩Vd/Vd | Infusion Duration (min) | Total Cytotoxin Dose Delivered (µg) | Cytotoxin Dose/ Total Target Volume (µg/mL) | Maximum Objective Tumor Response | PFS (Days) | OS (Days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-0.05 | Boston Terrier | 10 | 13 | M | 65 | HGA | 0.68 | 0 | 493* | 0.74 | 1.5 | 3 | 94 | 86 | 121 | 0.05 | 0.074 | NA | NA | 1 |
| 2-0.05 | Boston Terrier | 8 | 13 | FS | 65 | HGO | 3.47 | 0 | 403* | 2.68 | 6.6 | 2 | 76 | 98 | 132 | 0.04 | 0.012 | SD | 139 | 244 |
| 3-0.05 | Mixed breed |
5 | 42 | MN | 70 | HGA | 2.51 | 0.68 | 374* | 2.53 | 6.8 | 1 | 84 | 94 | 149 | 0.04 | 0.016 | SD | 47 | 47 |
| 4-.01 | Catahoula Leopard | 5 | 22 | F | 75 | HGO | 1.94 | 0.86 | 511* | 1.42 | 2.9 | 2 | 72 | 98 | 125 | 0.10 | 0.052 | PR | 356 | 371 |
| 5.i-0.1 | Boxer | 6 | 22 | FS | 90 | HGO | 3.43 | 0.95 | 570* | 1.94 | 3.4 | 1 | 55 | 97 | 115 | 0.11 | 0.032 | PR | 195 | 251 |
| 5.ii-0.2 | Boxer | 6 | 22 | FS | 90 | HGO | 1.51 | 0.59 | 371* | 1.71 | 4.6 | 2 | 52 | 46 | 179 | 0.15 | 0.099 | PR | NA | NA |
| 6-0.1 | English Bulldog | 7 | 21 | FS | 70 | HGO | 6.49 | 0.41 | 1136* | 3.04 | 2.7 | 5 | 46 | 98 | 390 | 0.23 | 0.035 | SD | 108 | 108 |
| 7-0.2 | Am. Staff Terrier | 6 | 25 | FS | 90 | LGO | 4.97 | 0 | 356* | 2.23 | 6.2 | 5 | 40 | 90 | 126 | 0.14 | 0.028 | SD | 118 | 119 |
| 8-0.2 | Boston Terrier | 5 | 14 | MN | 80 | LGA | 3.59 | 1.65 | 286* | 1.77 | 6.2 | 7 | 43 | 88 | 76 | 0.11 | 0.031 | PR | 189 | 202 |
| 9-0.4 | French Bulldog | 5 | 13 | FS | 80 | HGA | 10.04 | 0 | 1522* | 6.96 | 4.6 | 7 | 66 | 95 | 269 | 1.22 | 0.122 | SD | 106 | 157 |
| 10.i-0.4 | Am. Staff Terrier | 6 | 36 | MN | 90 | LGO | 3.15 | 1.32 | 510* | 2.26 | 4.4 | 1 | 70 | 97 | 139 | 0.41 | 0.130 | PR | 211 | 278 |
| 10.ii-0.8 | Am. Staff Terrier | 6 | 36 | MN | 90 | LGO | 3.22 | 0.57 | 366* | 2.89 | 7.9 | 3 | 86 | 93 | 132 | 0.59 | 0.183 | NA | NA | NA |
| 11-0.4 | Am. Staff Terrier | 7 | 26 | FS | 80 | LGO | 2.26 | 0 | 270* | 1.69 | 6.3 | 2 | 74 | 98 | 94 | 0.22 | 0.097 | SD | 114 | 117 |
| 12-0.4 | Boxer | 6 | 28 | MN | 80 | HGA | 3.10 | 0.28 | 741◊ | 3.71 | 5.0 | 4 | 93 | 76 | 171 | 0.59 | 0.190 | SD | 387 | 444 |
| 13-0.8 | Boxer | 9 | 43 | MN | 90 | LGA | 3.15 | 1.16 | 994◊ | 2.92 | 2.9 | 2 | 85 | 92 | 174 | 1.59 | 0.403 | PR | 167 | 167 |
| 14-0.8 | Portuguese Water Dog | 10 | 21 | FS | 90 | LGU | 1.60 | 0.24 | 396◊ | 1.83 | 4.6 | 2 | 92 | 80 | 71 | 0.31 | 0.194 | PR | >767 | >767 |
| 15-1.6 | Boxer | 8 | 31 | MN | 90 | HGA | 2.61 | 0.13 | 258◊ | 1.57 | 6.1 | 2 | 58 | 95 | 68 | 0.83 | 0.318 | SD | 99 | 224 |
| 16-1.6 | Boxer | 10 | 31 | FS | 90 | HGO | 1.69 | 0.42 | 674◊ | 0.99 | 1.5 | 8 | 52 | 88 | 151 | 2.16 | 1.278 | PR | 323 | 493 |
| 17-1.6 | Boxer | 5 | 43 | FS | 90 | LGO | 4.09 | 0 | 656◊ | 2.70 | 4.1 | 2 | 55 | 84 | 98 | 2.10 | 0.513 | PR | 274 | 314 |
| Median | NA | 7 | 25 | NA | 90 | NA | 3.15 | 0.41 | 493 | 2.28 | 4.6 | 2 | 70 | 92 | 132 | 0.23 | 0.099 | NA | 187 | 224 |
aDose cohort = μg of each cytotoxin/mL of infusate
*CED infusion rates of 1–7.5 μL/min for individual catheters
◊CED infusion rates of 1–20 μL/min for individual catheters
F, female
FS, female, spayed
HGA, high-grade astrocytoma
HGO, high-grade oligodendroglioma
KPS, Karnofsky performance score
LGA, low-grade astrocytoma
LGO, low-grade oligodendroglioma
LGU, low-grade undefined glioma
M, male
MN, male, neutered
NA, not applicable
OS, overall survival
PD, progressive disease
PFS, progression-free survival
PR, partial response
SD, stable disease
Fig. 3.
Expression of IL13RA2 and EPHA2 in western blots (A) of normal canine brain and glioma cultures [G1–G6]. Cytotoxin activity (B) on human GBM cells. Immunohistochemical scoring distribution (C) and images (D) of IL13RA2 and EPHA2 expression by tumor type from dogs in the trial. Bar = 50 µm in all panels.
Convection-Enhanced Delivery Treatment
Nineteen CED procedures were performed (Fig. 2, Table 1), with 17/19 infusions achieving ≥52% tumor coverage. The median total target coverage was 70% (range, 40–94%). The maximum administered cytotoxic cocktail concentration was 3.2 μg/mL infusate (1.6 μg/mL of each cytotoxin), and the median cytotoxic dose delivered was 0.099 μg/mL of target volume (range, 0.012–1.278 μg/mL). Unintended technical events (UTEs) occurred in 13/19 CED infusions (Fig. 2, Supplementary Table 3). In one case each of subarachnoid, subependymal, and ventricular leakage UTE, target infusion could not be achieved despite multiple catheter revisions. Cerebral edema was the only UTE associated with a clinically apparent adverse event.
Trial Endpoints
No DLTs were observed (Supplementary Table 4). Dog 1 was euthanized on day 1, after developing pulmonary thromboembolism, with the remaining 16/17 dogs surviving for >28 days. Systemic hypertension was the only adverse event possibly attributed to the cytotoxin with Dogs 9 and 10 experiencing grade 2 systemic hypertension during CED, which responded to treatment with vasodilators and resolved without sequelae upon anesthetic recovery. In Dog 10, systemic hypertension occurred during both CED infusions.
Five dogs had improved KPS (median Δ+10, range +5 to +15) after treatment, with 2/5 improving in conjunction with PR (Fig. 4). Five dogs had deteriorations in KPS (median Δ−20, range −10 to −50) in proximity to PD. At 42 days, volumetric tumor reductions were observed in 15/16 dogs, with a median reduction of 42% (range, 5–94%; Fig. 4). PRs were observed in 8/16 (50%) dogs (Table 1, Fig. 4). The median tumor volume reduction in PR cases was 79% (range, 65–94%). In 4/8 dogs with PR, the maximum tumor response was documented at 42 days. In the other 4/8 dogs with PR, sequential reductions in tumor volume occurred up to 6 months following treatment.
Fig. 4.
Summary of secondary endpoints and cytokine analysis. Representative MRI of prolonged partial responders (A) and partial responders (B). Tumor volumes in dogs classified as nonresponders (C) and responders (D), with corresponding KPS appearing above each plotted tumor volume. Blue KPS indicates an SAE occurred in proximity; red KPS represents PD; CED repeated where KPS appears in green. ^necropsy performed; *withdrew from trial. Engel seizure outcomes (E) were superior in partial responders compared with dogs with stable or progressive disease. Comparisons of serum cytokines (mean ± SE) between control and glioma dogs (top panels) and between controls, responders, and nonresponders (bottom panels) on day 42.
The median PFS in this study was 187 days (range, 1 to >767 d), and the median OS was 224 days (range, 1 to >767 d). Ten dogs died on study, 3 dogs completed the 365-day follow-up period, and 4 dogs were withdrawn for other treatment (Fig. 1).
Post-Hoc Response Analyses
No differences in breed, sex, age, body weight, KPS, tumor type or grade, presence or type of UTE, or distribution or intensity of IL13RA2 and EPHA2 immunoreactivity among tumors between responders and nonresponders were observed (Supplementary Table 5). Owner-reported QoL survey results were significantly lower in responders at the day 42 evaluation (Supplementary Table 6). Nonresponders had significantly larger mean Vd:Vi ratio (5.4 [95% CI, 4.2–6.5] vs 3.7 [95% CI, 4.2–6.5]) and significantly lower mean contrast-enhancing lesion volumes (0.18 cm3 [95% CI, 0.02–0.3] vs 0.82 cm3 [95% CI, 0.45–1.24]) compared with responders, but other CED variables were not different between groups. None of the quantitative CED variables were significantly correlated with PFS or OS (Supplementary Table 7). Responders had superior Engel seizure outcomes compared with nonresponders (Fig. 4).
Serum Cytokine Assays
Glioma dogs had significantly higher IL-6, IL-8, IL-10, keratinocyte chemoattractant (KC), monocyte chemoattractant protein 1 (MCP-1), and interferon gamma (IFN-δ) than controls (Fig. 4). Baseline cytokine concentrations were not significantly different between tumor types. Significant differences in IL-6, IL-10, IFN-δ, MCP-1, TNF-α, granulocyte-macrophage colony-stimulating factor, IL-2, IL-7, IL-18, and KC were observed between controls, responders, and nonresponders. Responders had significantly higher IL-10, IFN-δ, MCP-1, TNF-α, and IL-18 values than nonresponders, and significantly lower IL-2 and IL-7 concentrations. Longitudinal analyses of cytokines demonstrated that IL-6, IL-8, IL-10, IFN-δ, MCP-1, TNF-α, IL-2, and IL-18 were significantly higher in responders than nonresponders (Supplementary Figure 3). Significant differences in IL-8, IL-10, IFN-δ, MCP-1, and TNF-α were also observed among prolonged responders and other responders.
Necropsy Examinations
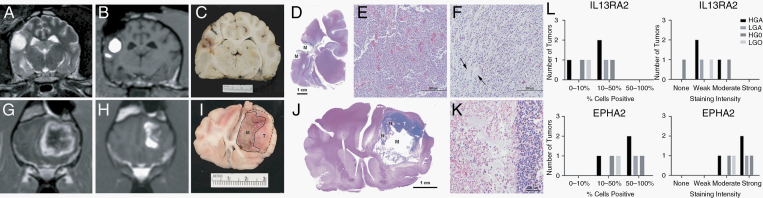
Seven dogs that did not receive other treatment had necropsy examinations performed. In all dogs, geographic regions of cystic malacia and intratumoral necrosis were observed that mapped to MRI infusion image masks (Fig. 5). Hemosiderin-laden macrophages and aggregates of mineral were scattered throughout regions of necrosis, and sharp transitions from treatment-related necrotic regions to viable untreated tumor were present. Minimal changes attributed to catheter placement were observed, consisting of small diameter linear necrotic tracts bordered by localized astrogliosis. No necrosis was observed in normal brain, ventricles, or ependyma that were infused in individual dogs. In 2 dogs, islands of a basophilic acellular homogeneous matrix, which was presumed to be galbumin treatment vehicle, and nondegenerate neutrophils were also observed in treated and necrotic regions. Immunoreactivity to IL13RA2 and EPHA2 was observed in 6/7 and 7/7 necropsy tumor specimens, respectively (Fig. 5).
Fig. 5.
Correlation of CED infusions with necropsy findings from Dogs 1 (A‒F) and 10 (G‒L). Pretreatment (A, G) and post-infusion MR images showing Vd within tumors (B, H). Gross (C, I) and subgross (D, J; hematoxylin and eosin [H&E]) brain sections from the same levels as MR images B and H, with malacia [M] and necrosis [N] within infused regions and viable tumor (D, I, dashed lines and [T]) in untreated areas. Compared with the pretreatment biopsy (E; H&E), a modified tumor phenotype was observed along the infusion margin (D-dashed lines and F; H&E). Untreated tumor [T] is prominent (H, I) where there was poor coverage of the dorsolateral tumor. Necrotic regions (J [N], left side, K; H&E) correspond to distribution of the infusion and are sharply delineated from untreated tumor (right side, K). IL13RA2 and EPHA2 immunoreactivity by tumor type in necropsy samples (L).
In Dog 1, the morphology of remaining neoplastic cells in the margins surrounding the area of malacia was modified compared with the pretreatment phenotype (Fig. 5). The pretreatment biopsy was characterized by pleomorphic glial fibrillary acidic protein (GFAP)–immunoreactive neoplastic cells arranged in dense sheets with regional pseudopalisading and necrosis, and the pretreatment Ki-67 proliferation index was 18%. Neoplastic cells in treated regions from necropsy samples retained GFAP immunoreactivity but were homogeneously spindle shaped and far less dense. The posttreatment Ki-67 proliferative index was 1%, and no mitoses were observed.
Discussion
In dogs with gliomas expressing IL13RA2 and EPHA2 receptors, treatment with targeted cytotoxins was well tolerated and induced clinically relevant responses over a range of doses. Using therapeutic planning, reflux-preventing catheters, and real-time MRI monitoring, we demonstrate that robust volumes of canine brain can be infused with CED. Given the shared expression of IL13RA2 and EPHA2 between canine and human gliomas,6,10 our results provide a framework for future trials of novel targeted cytotoxins in human glioma and highlight the study design and technical delivery limitations that have contributed to non-optimal results of CED as a drug delivery platform.4,12,14
Locoregional cytotoxin delivery was sufficiently safe as to preclude identification of an MTD, although the maximum administered drug concentration given here represents a 6-fold increase over the doses of huIL13-PE38QQR delivered to human patients with glioma.4 We have shown in translational models that tumoricidal concentrations of IL13RA2/EPHA2-targeted cytotoxins are likely orders of magnitude less than MTD defined by neurotoxicity.6,26 Non–dose limiting intraoperative systemic hypertension was the only adverse event possibly attributed to the cytotoxin infusion. Transient systemic hypertension was reported in dogs with gliomas treated using CED with an agent targeted at epidermal growth factor receptor, suggesting that this may be a nonspecific response to brain infusions in dogs.27 Two procedure-related serious adverse events (SAEs) occurred during the 28-day observation window, including an indirectly fatal pulmonary thromboembolic event and transient cerebral edema associated with catheter placement. These SAEs have occurred in canines and humans with gliomas that are subjected to neurosurgical interventions, including CED of huIL13-PE38QQR.4,21
Real-time imaging was essential for confirmation of catheter positioning, quantitative evaluation of surrogates of the quality of infusions such as tumor coverage, assessment of therapeutic efficacy as a function of target coverage, evaluation of the feasibility and efficiency of high-rate CED infusions, and early identification of UTEs. Suboptimal catheter placement has contributed to poor target coverage and therapeutic outcomes in humans and dogs with glioma,12,14 and 25–30% of canine tumors have been inaccurately targeted in previous CED studies that did not utilize intraoperative imaging.27,28 The last 6 dogs were treated at infusion rates of up to 20 μL/min through individual catheters with tumor coverages that were comparable to lower rate infusions, and without any reflux, which supports the utility of the reflux preventing catheter.13 Intraoperative catheter revisions were critical for continued target infusion in this study, as catheter air obstruction, reflux, and ventricular infusate leakages have notoriously impeded drug delivery in previous CED studies.12,14 Intraoperative imaging was also paramount to safety evaluations, as it allowed for characterization of UTE in temporal and clinical contexts, confirmation of the safety of the catheter placement and removal, and allowed assessment of possible toxicity in regions of normal brain exposed to cytotoxins during necropsy.
Our technique resulted in improved delivery metrics, with 100% of gliomas in this study receiving a cytotoxin payload with a median tumor coverage of 70%. Another canine glioma study quantified CED using real-time imaging with a median target coverage of 28%.14 With the exception of the Vd:Vi ratio, which was significantly higher in nonresponders, other differences in quantitative CED variables between responders and nonresponders were not observed, suggesting that limitations of the CED delivery technique were not a major contributor to lesser responses in this trial. This difference in Vd:Vi may be related to the significantly lower contrast-enhancing tumor volumes in nonresponders. In humans undergoing CED treatment of glioma, the Vd of infusates in non-enhancing tumor may be up to 10-fold higher compared with enhancing tumor.29 Despite improvements in delivery metrics, achieving consistent and complete targeting of MRI-defined tumor volumes remains a challenge of CED.4,14
The longest PFS occurred in the two dogs that had ≥92% target coverage with Vd that extended beyond the T2/fluid attenuated inversion recovery (FLAIR)–defined tumor. This supports the need to target the infiltrative, microscopic tumor burden that occurs adjacent to the imaging-defined enhancing tumor mass that often accounts for local treatment failures. Although we could not assess Vd:Vi in the non-enhancing lesion separately from enhancing tumor due to close proximities of catheters, our experience supports routine placement of catheters into specific targeting of non-enhancing tumor regions. The use of multiport catheters that allow for infusion of large volume through a single portal represents one solution for more complete tumor targeting.29,30
Our study provides preliminary evidence of the efficacy of the cytotoxins as a sole treatment modality as assessed with MRI response criteria and demonstration of tumor cell death on necropsy. Objective PR was observed in 8 dogs, and an additional dog experienced SD associated with a >50% tumor reduction for one year. Imaging responses were associated with clinical benefits including improved KPS scores, seizure control, and QoL surveys. Limited survival data in dogs with histologically confirmed gliomas treated with conventional modalities currently exist.31 The PFS (187 days) and OS (224 days) observed in our trial were longer than those of dogs with gliomas treated palliatively (OS, 79 days) or surgically (OS, 66 days), similar to trials in dogs that underwent investigational therapies including surgery followed by dendritic cell vaccination (OS, 185 days), surgery and metronomic chemotherapy (OS, 254 days), repeated CED infusions (OS, 190 days) of liposomal camptothecin-11 (CPT-11), and comparable to dogs treated with radiotherapy (OS range, 225–390 days).14,31
Necropsy examinations allowed for evaluation of tissue level treatment effects and confirmed tumor necrosis in infused regions, providing insight into a range of histologically defined effective cytotoxin doses. Tumor cells with a modified posttreatment phenotype, characterized by less cellular anaplasia and a decrease in the proliferative index, were observed in one dog receiving the lowest concentration of cytotoxins. The mechanism of phenotypic change is unclear but may involve selective targeting of more proliferative tumor cells, leaving residual terminally differentiated tumor cells.8,20,32 We observed similar changes after CED of liposomal CPT-11 treatment in orthotopic murine tumor models with a canine glioma cell line and in dogs with gliomas.14 The presence of essentially nondividing, yet apparently viable, tumor tissue after therapy has important implications for both the prognosis and assessment of therapeutic efficacy if measured by a reduction in tumor volume. Tumor cells at the infusion margin may also have been exposed to sublethal cytotoxin concentrations, or the drug exposure time was insufficient, as data suggest that it can take several hours for internalization of cytotoxin after receptor binding.6 It is unlikely that the 0.5 μg/mL cytotoxin concentration is subtherapeutic, as tumor necrosis in treated regions was observed at necropsy in another dog in this dosing cohort.
Cytokine analyses supported our hypothesis that dogs with glioma have increased concentrations of IL-6 and IL-8, as well as IL-10, KC, MCP-1, and IFN-δ, although no differences in TNF-α were observed between tumors and controls. Similar cytokine patterns occur in humans with glioma.33 However, these findings are not specific to glioma, being seen in other human and canine cancers, and support the coexistence of both local immunostimulation and immunosuppression in cancer patients.33,34 Glioma dogs had simultaneously increased IL-6 and IL-10 concentrations. IL-6 is a potent pro-inflammatory cytokine, and IL-10 generally an anti-inflammatory cytokine produced by tumor cells that can inhibit antigen-presenting properties of dendritic cells and suppress pro-inflammatory cytokines.33 IL-8 and IL-10 promote glioma cellular proliferation, motility, and invasion, and in the case of IL-8, tumor angiogenesis and endothelial permeability. MCP-1 secretion by glioma cells, monocytes, and microglia promote immunosuppression by driving accumulation of regulatory T cells and myeloid-derived suppressor cells.33 This cytokine profile, which likely reflects both inherent features of the canine glioma microenvironment as well as responses to the cytotoxin infusion, could result in dysregulated immunosurveillance in which immune cells are recruited to the tumor, but tumor cells are spared from destruction by infiltrating immune cells by local immunosuppressive factors. Upregulated CCL2 (MCP-1), IFNG, and IL10 gene expression were observed in gliomas from dogs enrolled in this trial.35
We found significant and temporally sustained increases in IL-2, IFN-δ, and TNF-α in responders, which are hallmarks of ICD and antitumor T helper cell 1 responses, coinciding with serial tumor volume reduction in prolonged responders. The small size of our study, as well as the lack of inclusion of control groups precludes us from specifically associating this cytokine signature with the cytotoxin treatment, but this preliminary data are encouraging that cytotoxins may exert an in situ vaccination effect by producing ICD of targeted cells.16 We envision an approach in which a targeted cytotoxin is administered to cause tumor death and release a spectrum of tumor-associated antigens that induce an adaptive immune response coupled with an immune checkpoint inhibitor.26
While surgical resection benefits local disease control in humans, its efficacy in canine glioma has not been established.31 As local tumor control was achieved in several cases, extensions of our work would be to repeat CED, combine it with other approaches, or incorporate new technologies such as multiport catheters that have shown promise for large-volume locoregional infusions.29,30 Although we did treat several canine glioma entities, expression of IL13RA2 or EPHA2 were unifying molecular denominators for all dogs, and we observed tumor responses that were agnostic to tumor phenotype. However, the variable cytotoxin dosing and low numbers of dogs with each type and grade of glioma treated in this study limit our ability to robustly determine effects of tumor phenotype on therapeutic response. This trial serves as a template supporting our parallel development of cytotoxins simultaneously targeting 4 tumor-associated receptors—IL13RA2, EPHA2, EPHA3, and EPHB2—and our own arborizing, multiport CED catheter intended to facilitate locoregional CED infusions.26,30 Expanding targeting to include EPHA3 and EPHB2 receptors will further address intra- and intertumoral heterogeneity and allow treatment of differentiated tumor cells, glioma stemlike cells, infiltrative tumor cells, neovasculature, and tumor-infiltrating cells.26
In conclusion, CED of IL13RA2/EPHA2 cytotoxins at concentrations ranging from 0.05–1.6 μg/mL was safe and resulted in clinically relevant responses in 50% of dogs with gliomas. Further investigation of locoregional delivery of targeted therapeutics by CED are warranted for the treatment of dogs and humans with glioma.
Supplementary Material
Acknowledgments
The authors would like to thank the owners and dogs that participated in the trial, and the Boo Radley Foundation for supporting client travel. This study was presented in part at the 2017 WFNOS, 2017 SNO-SCIDOT, 2018 SNO, and 2019 ECVN meetings.
Funding
This work was supported by grants from the Wake Forest University Translational Sciences Institute, National Cancer Institute (R01CA139099, R01CA74145, P01CA207206), the Comprehensive Cancer Center of Wake Forest University Brain Tumor Center of Excellence, Hearn Fund for Brain Tumor Research, Pratto Fund for Brain Tumor Research, and Dallas Ray Swing Brain Tumor Fund.
Conflict of interest statement. The following patents pertain to results presented in the paper: United States Patent 9,974,830: Molecular signature of cancer; United States Patent 9,868,788: Antibodies against human and canine IL-13RA2; United States Patent 9,290,558: Molecular signature of cancer; United States Patent 8,343,461: Molecular signature of cancer; and United States Patent 6,884,603: Nucleic acids encoding IL13 mutants. All of the above patents have been licensed to industry. WD is a scientific advisor to WPD Pharmaceuticals, Inc.
Authorship statement. Conception and design: JHR, JLR, RBD, SBT, WD. Data collection, analysis, and interpretation: JHR, DH, MQ, TEC, JLR, JH, PJD, RBD, WD. Data management: MQ, JH. Provision of material support for in vitro experiments: JHR, PJD, WD. Drafted the manuscript: JHR, MQ, WD. Final approval of completed manuscript: All authors.
References
- 1. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Qazi MA, Vora P, Venugopal C, et al. Intratumoral heterogeneity: pathways to treatment resistance and relapse in human glioblastoma. Ann Oncol. 2017;28(7):1448–1456. [DOI] [PubMed] [Google Scholar]
- 3. Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res. 1995;1(11):1253–1258. [PubMed] [Google Scholar]
- 4. Sampson JH, Akabani G, Archer GE, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12(8):871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999;5(5):985–990. [PubMed] [Google Scholar]
- 6. Debinski W, Dickinson P, Rossmeisl JH, Robertson J, Gibo DM. New agents for targeting of IL-13RA2 expressed in primary human and canine brain tumors. PLoS One. 2013;8(10):e77719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madhankumar AB, Mintz A, Debinski W. Interleukin 13 mutants of enhanced avidity toward the glioma-associated receptor, IL13Ralpha2. Neoplasia. 2004;6(1):15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wykosky J, Gibo DM, Debinski W. A novel, potent, and specific ephrinA1-based cytotoxin against EphA2 receptor expressing tumor cells. Mol Cancer Ther. 2007;6(12 Pt 1):3208–3218. [DOI] [PubMed] [Google Scholar]
- 9. Lema Tomé CM, Palma E, Ferluga S, et al. Structural and functional characterization of monomeric EphrinA1 binding site to EphA2 receptor. J Biol Chem. 2012;287(17):14012–14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wykosky J, Gibo DM, Stanton C, Debinski W. Interleukin-13 receptor alpha 2, EphA2, and Fos-related antigen 1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin Cancer Res. 2008;14(1):199–208. [DOI] [PubMed] [Google Scholar]
- 11. Kunwar S, Prados MD, Chang SM, et al. Cintredekin besudotox intraparenchymal study group. J Clin Oncol. 2007;25(7):837–844. [DOI] [PubMed] [Google Scholar]
- 12. Sampson JH, Archer G, Pedain C, et al. ; PRECISE Trial Investigators . Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg. 2010;113(2):301–309. [DOI] [PubMed] [Google Scholar]
- 13. Krauze MT, Saito R, Noble C, et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J Neurosurg. 2005;103(5):923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dickinson PJ, LeCouteur RA, Higgins RJ, et al. Canine spontaneous glioma: a translational model system for convection-enhanced delivery. Neuro Oncol. 2010;12(9):928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. LeBlanc AK, Mazcko C, Brown DE, et al. Creation of an NCI comparative brain tumor consortium: informing the translation of new knowledge from canine to human brain tumor patients. Neuro Oncol. 2016;18(9):1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leshem Y, King EM, Mazor R, et al. SS1P immunotoxin induces markers of immunogenic cell death and enhances the effect t of the CTLA4 blockade in AE17M mouse mesothelioma tumors. Toxins. 2018;10(11):E470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koehler JW, Miller AD, Miller CR, et al. A revised diagnostic classification of canine glioma: towards validation of the canine glioma patient as a naturally occurring preclinical model for human glioma. J Neuropathol Exp Neurol. 2018;77(11):1039–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Veterinary Medical Association. Animal Health Studies Database (2018) Trial Number AAHSD000005. 2018. https://ebusiness.avma.org/aahsd/study_search.aspx. Accessed June 25, 2018.
- 19. Veterinary Comparative Oncology Group. Veterinary Cooperative Oncology Group‒Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. 2016;14(4):417–466. [DOI] [PubMed] [Google Scholar]
- 20. Rossmeisl JH Jr, Garcia PA, Daniel GB, et al. Invited review–neuroimaging response assessment criteria for brain tumors in veterinary patients. Vet Radiol Ultrasound. 2014;55(2):115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossmeisl JH Jr, Garcia PA, Pancotto TE, et al. Safety and feasibility of the NanoKnife system for irreversible electroporation ablative treatment of canine spontaneous intracranial gliomas. J Neurosurg. 2015;123(4):1008–1025. [DOI] [PubMed] [Google Scholar]
- 22. Packer RA, Rossmeisl JH, Kent MS, Griffin JF 4th, Mazcko C, LeBlanc AK. Consensus recommendations on standardized magnetic resonance imaging protocols for multicenter canine brain tumor clinical trials. Vet Radiol Ultrasound. 2018;59(3):261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rossmeisl JH, Andriani RT, Cecere TE, et al. Frame-based stereotactic biopsy of canine brain masses: technique and clinical results in 26 cases. Front Vet Sci. 2015;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenbluth KH, Martin AJ, Mittermeyer S, Eschermann J, Dickinson PJ, Bankiewicz KS. Rapid inverse planning for pressure-driven drug infusions in the brain. PLoS One. 2013;8(2):e56397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han SJ, Bankiewicz K, Butowski NA, Larson PS, Aghi MK. Interventional MRI-guided catheter placement and real time drug delivery to the central nervous system. Expert Rev Neurother. 2016;16(6):635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharma P, Roberts C, Herpai D, et al. Drug conjugates for targeting Eph receptors in glioblastoma. Pharmaceuticals (Basel). 2020;13(4):pii E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freeman AC, Platt SR, Holmes S, et al. Convection-enhanced delivery of cetuximab conjugated iron-oxide nanoparticles for treatment of spontaneous canine intracranial gliomas. J Neurooncol. 2018;137(3):653–663. [DOI] [PubMed] [Google Scholar]
- 28. Young JS, Bernal G, Polster SP, et al. Convection-enhanced delivery of polymeric nanoparticles encapsulating chemotherapy in canines with spontaneous supratentorial tumors. World Neurosurg. 2018;117:e698–e704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vogelbaum MA, Brewer C, Mohammadi AM, et al. First-in-human evaluation of the Cleveland Multiport Catheter for convection-enhanced delivery of topotecan in recurrent high-grade glioma: results of pilot trial 1. J Neurosurg. 2018;1:1–10. [DOI] [PubMed] [Google Scholar]
- 30. Elenes EY, Rylander C. Maximizing local access to therapeutic deliveries in glioblastoma. Part II: arborizing catheter for convection-enhanced delivery in tissue phantoms. In: DeVlesschouwer S, ed. Glioblastoma. Brisbane, Australia: Codon; 2017:359–372. [PubMed] [Google Scholar]
- 31. Miller AD, Miller CR, Rossmeisl JH. Canine primary intracranial cancer: a clinicopathologic and comparative review of glioma, meningioma, and choroid plexus tumors. Front Oncol. 2019;9:1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown CE, Warden CD, Starr R, et al. Glioma IL13Rα2 is associated with mesenchymal signature gene expression and poor patient prognosis. PLoS One. 2013;8(10):e77769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nijaguna MB, Patil V, Hegde AS, et al. An eighteen serum cytokine signature for discriminating glioma from normal healthy individuals. PLoS One. 2015;10(9):e0137524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Irac SE, Oksa A, Jackson K, et al. Cytokine expression in canine lymphoma, osteosarcoma, mammary gland tumor, and melanoma: comparative aspects. Vet Sci. 2019;6:6020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Connolly NP, Shetty AC, Stokum JA, et al. Cross-species transcriptional analysis reveals conserved and host-specific neoplastic processes in mammalian glioma. Sci Rep. 2018;8(1):1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.