Abstract
Background
No standard medical treatment exists for adult patients with recurrent ependymoma, and prospective clinical trials in this population have not succeeded because of its rarity and challenges in accruing patients. The Collaborative Ependymoma Research Network conducted a prospective phase II clinical trial of dose-dense temozolomide (TMZ) and lapatinib, targeting the unmethylated O6-methylguanine-DNA methyltransferase (MGMT) promoter status and increased expression of ErbB2 (human epidermal growth factor receptor 2) and ErbB1 (epidermal growth factor receptor) in ependymomas.
Methods
Patients age 18 or older with histologically proven and progressive ependymoma or anaplastic ependymoma were eligible and received dose-dense TMZ and daily lapatinib. The primary outcome measure was median progression-free survival (PFS). Landmark 6- and 12-month PFS and objective response were measured. Serial assessments of symptom burden using the MD Anderson Symptom Inventory Brain Tumor (MDASI-BT)/MDASI–Spine Tumor modules were collected.
Results
The 50 patients enrolled had a median age of 43.5 years, median Karnofsky performance status of 90, and a median of 2 prior relapses. Twenty patients had grade III, 16 grade II, and 8 grade I ependymoma. Half had spinal cord tumors; 15 had a supratentorial tumor, 8 infratentorial, and 2 had disseminated disease. Treatment was well tolerated. The median PFS was 7.8 months (95% CI: 5.5,12.2); the 6- and 12-month PFS rates were 55% and 38%, with 2 complete and 6 partial responses. Measures of symptom burden showed reduction in moderate-severe pain and other disease-related symptoms in most patients.
Conclusions
This treatment, with demonstrated clinical activity with objective responses and prolonged disease control associated with disease-related symptom improvements, is an option as a salvage regimen for adult patients with recurrent ependymoma.
Keywords: combination chemotherapy, ependymoma, lapatinib, progression-free survival, temozolomide
Key Points.
1. This is the first prospective clinical trial for adults with ependymoma.
2. The study reached its therapeutic goal with tumor response and prolonged control of growth.
3. Many patients experienced improvement in disease-related symptoms.
Importance of the Study.
Ependymoma is a rare cancer of the central nervous system. Although the incidence in adults is greater than pediatric ependymoma, there have been no prospective clinical trials in the adult population. While surgical removal often followed by radiation is standard treatment for newly diagnosed adult ependymoma, no standard of care has been established for recurrent disease. This clinical trial combined 2 oral agents, TMZ and lapatinib, targeting potential TMZ resistance with a dose-dense schedule and increased tumor ErbB2 and ErbB1 expression with lapatinib. The disease control rate complemented by marked improvement in disease-related symptoms and objective radiographic responses support the efficacy of this well-tolerated regimen, which should be considered as a treatment option for recurrent ependymoma.
Ependymoma is a rare primary brain tumor in adults. The Central Brain Tumor Registry of the United States compiled incidence of both ependymoma and anaplastic ependymoma and found a population rate of approximately 0.38 per 100 000.1 Ependymomas are found throughout the central nervous system (CNS) in the supratentorial, posterior fossa, and spinal compartments, and they affect pediatric and adult populations. The grading of ependymomas is based on the degree of pleomorphism, tumor cells proliferation, cellularity, and tumor infiltration into surrounding brain tissue, leading to the designation of either low-grade (World Health Organization [WHO] grade II ependymoma) or the more malignant designation of anaplastic ependymoma (WHO grade III ependymoma).2 Grade I ependymomas in adults are restricted to the distinctive histologic subtype, myxopapillary ependymoma. Importantly, despite the grade I designation, failure to remove the myxopapillary tumor en bloc with an intact surrounding soft-tissue capsule frequently leads to tumor recurrence from spilled tumor cells, resulting in a prognosis that is similar to grade II spinal cord ependymomas.3 In light of this, a recent consensus from the cIMPACT group suggests that myxopapillary ependymoma be given a grade II designation.4
Tumors of the posterior fossa are much more common in the pediatric population, and spinal cord tumors are more common in young to middle age adults. Overall, grade III ependymomas are more common in adults. A series of studies elucidated at least 9 molecular subtypes of ependymoma that vary by tumor location, age, and prognosis.5 Recently, a new variant of spinal cord ependymoma was described molecularly by amplification of MYCN and clinically has a very aggressive course of rapid dissemination and resistance to known treatments.6,7
Currently, the standard therapy for newly diagnosed low-grade ependymoma is total surgical excision typically followed by radiation therapy. In some instances, radiation treatment is deferred if the tumor resection of the low grade (WHO grade I or II) is felt to be complete.8 However, a retrospective analysis of posterior fossa ependymomas in adults found a significant improvement in 10-year disease-free survival if postoperative radiation was administered to patients who underwent a gross total tumor resection compared with resection alone.9 Often, complete surgical resection is not possible because of the location of the tumor and concern for potential damage to surrounding brain. In these situations, postsurgical radiation to the region of tumor is now considered standard of care; craniospinal radiation is reserved for patients with proven dissemination either by imaging or positive cerebrospinal fluid cytopathology.8 Patients with anaplastic ependymoma receive postoperative radiation even in the setting of a complete resection because of the higher proliferative rate and greater propensity for tumor infiltration into surrounding normal brain. Neoadjuvant or post-radiation adjuvant chemotherapy has not been shown to be of benefit for any grade or subtype of ependymoma (reviewed by Sartor and Wen10).
There are no established treatments for recurrent ependymoma, although frequently tumors undergo a repeat resection. Re-irradiation has been evaluated in pediatric ependymoma with some evidence of efficacy, but comparable studies have not been performed in adults with recurrent ependymoma.11 Additionally, there have been no established chemotherapy regimens with proven efficacy in recurrent disease. However, there have been anecdotal reports of response of ependymoma to TMZ, an oral alkylating agent with good penetration of the CNS. A study by Brandes included 4 patients who received this either as single agent or in combination with cisplatin.12 All patients were reported to have stable disease as their best response. A single case report describes a patient with recurrent anaplastic ependymoma with a greater than 10-year response to TMZ therapy.13 A larger retrospective series from Rudà involving 18 patients treated with standard dose TMZ reported a median progression-free survival (PFS) of 9.6 months, with 4 objective responses and functional improvement in 2 patients.14
Although a randomized clinical trial failed to demonstrate improved outcomes in newly diagnosed glioblastoma with a dose-dense schedule, studies in recurrent glioblastoma using a dose-dense schedule of TMZ had encouraging results.15,16 Additionally, correlative studies have demonstrated that dose-dense administration of TMZ decreased peripheral blood mononuclear cell levels of O6-methylguanine-DNA methyltransferase (MGMT).17 In this context, many ependymomas have an unmethylated MGMT promoter, although there has not been extensive analysis. One study of 10 tumor samples demonstrated that nearly all ependymomas have an unmethylated MGMT promoter, whereas another study reported that nearly half of the ependymomas evaluated had an MGMT methylated promoter.14,18 However, since prior use of TMZ was allowed for this study, an alternate treatment schedule of TMZ was felt to be the best choice.
Molecular targeting of ependymoma has proven challenging, further complicated by the recently uncovered distinct molecular subtypes. Studies have shown increased expression of ErbB2 and ErbB4 in pediatric intracranial ependymomas.19 Additionally, studies have demonstrated that a high percentage of ependymomas have increased expression of epidermal growth factor receptor (EGFR) (ErbB1), suggesting that EGFR may be a suitable target for treating intracranial ependymomas.20
Temozolomide has an established track record in treating primary brain tumors and a good safety profile. Recent data suggest that certain dosing schedules may modulate tumor cell resistance via the MGMT mechanism.17 Lapatinib was selected for the combination regimen to target the frequent alterations in ErB1(EGFR) and ErB2 (human epidermal growth factor receptor 2 [HER2]) in ependymoma, with the potential additive or synergistic benefit of combining a cytotoxic agent with a cytostatic agent.21
Methods
This was a prospective multicenter phase II study (NCT00826241) designed to determine the efficacy of the combination of TMZ and lapatinib in recurrent brain and spinal cord ependymoma and anaplastic ependymoma as measured by median PFS. This clinical trial was performed in the Collaborative Ependymoma Research Network (CERN) Adult Clinical Trials Network and the protocol was approved by the institutional review board at each of the participating institutions. Patients recruited to the trial had histologically proven ependymoma or anaplastic ependymoma with pathologic or imaging confirmation of tumor progression. Histologic diagnosis was confirmed by central pathology review prior to registration. All patients were required to sign the study informed consent. Patients had to be able to undergo brain or spine MRI scans and were required to be on stable steroid doses and recovered from the toxic effects of prior therapy. Patients with severe active comorbidities were excluded, as were those with a prior invasive malignancy that was not ependymoma (except non-melanomatous skin cancer or carcinoma in situ of the cervix) unless the patient had been disease free for a minimum of 3 years. Additional inclusion criteria were normal bone marrow, hepatic and renal function, and electrolytes. Baseline cardiac testing was performed within 14 days prior to registration. Toxicity grade was assessed using Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
Treatment
Initially, eligible patients received one cycle of the combination of TMZ and lapatinib. The TMZ was administered at a dose of 125 mg/m2 as a single daily dose on days 1–7 and 15–21 of a 28-day cycle. The lapatinib was given as a single daily dose of 1250 mg orally. Patients who did not experience any grade 1 or greater myelotoxicity were eligible to have the TMZ dose increased to 150 mg/m2 as a single daily dose on days 1–7 and 15–21 of a 28-day cycle. The lapatinib dose was not increased. After 4 of the initial 6 patients developed grade 3 or 4 myelotoxicity with the increase of TMZ dosing after 1 cycle, subsequent patients received 2 cycles of the combination of TMZ and lapatinib with the TMZ dosing at 125 mg/m2 days 1–7 and 15–28 before consideration of dose escalation. This dramatically reduced the subsequent rate of myelotoxicity.
During active treatment, a complete blood count was performed on days 14 and 28 (±72 hours) after the first daily dose of each treatment cycle. Physical exams and MRIs were conducted every 8 weeks prior to odd-numbered cycles and when therapy was discontinued. Patient treatment diaries were collected, and pill counts were performed. Hepatic function testing was done with each cycle and cardiac monitoring was performed every 3 months during and continued in the posttreatment period to ensure that no late cardiotoxicity developed.
Patient-reported outcomes
The MD Anderson Symptom Inventory–Spine Tumor Module (MDASI-SP) and the MD Anderson Symptom Inventory–Brain Tumor Module (MDASI-BT) symptom instruments were used along with the Karnofsky performance status (KPS) to assess patient clinical outcomes.22,23 The instruments consist of 22 symptoms (MDASI-BT) or 18 symptoms (MDASI-SP) rated on an 11-point scale (0 to 10) to indicate the presence and severity of the symptom, with 0 being “not present” and 10 being “as bad as you can imagine.” Each symptom is rated at its worst in the last 24 hours. Factor groupings of symptoms associated with the disease or treatment have been identified and were used for this analysis. Also included are ratings of how much symptoms interfere with different aspects of a patient’s life in the last 24 hours. These interference items are general activity, mood, work (both outside the home and housework), relations with other people, walking, and enjoyment of life. The interference items are also measured on 0–10 scales.
The primary endpoint for this trial was median PFS. A combination of a neurological examination and MRI brain scan were used to define progression. Progression was defined by either a >25% increase in tumor area or worsening of neurologic function based on the clinical examination. Due to improvements in neuroimaging and the fact that tumor growth in certain regions of the CNS is without neurologic signs and symptoms, greater reliance was placed on neuroimaging to define progression. One of the secondary endpoints of this trial was antitumor activity as determined by overall response rate. Response was determined using bidimensional measurement of postcontrast enhanced MRI using the established Macdonald criteria.24 All determinations of objective response or absence of progression required stable or decreasing corticosteroid dosing. Patients with no measurable disease were not eligible for response assessment, only determination of PFS. Complete response (CR) required complete resolution of all lesions, whereas partial response (PR) required a >50% reduction of total tumor area in the absence of any enlargement of a tumor in the case of disseminated disease. Additional secondary endpoints included overall survival (OS), adverse event profile and tolerability, and longitudinal changes in MDASI (BT or SP) and KPS scores. Demographic and tumor data collected included age, sex, race, histology disease, and histology grade.
Statistical Methods
Survival and Cox regression analysis
Data were summarized using standard descriptive statistics such as mean, standard deviation, median, and range for continuous variables; and frequency and proportion for categorical variables. OS was defined as the time from first treatment until death from any cause. PFS was defined as the time from first treatment until objective tumor progression or death, whichever happened first. A patient would be censored if he/she was progression free without an event occurrence at last follow-up. OS and PFS times were estimated using the Kaplan–Meier method, and the comparison between or among patients’ characteristics groups was evaluated by log-rank test. Both univariate and multivariate Cox regression models were applied to assess the effect of covariates of interest, which include preselected symptom subscales, on OS and PFS. All computations were carried out in SAS 9.3 and R 3.4.0.
Analysis of Patient-Reported Outcomes
Received MDASI-BT and MDASI-SP forms were considered valid if they fell within 1 week of the scheduled assessment. Descriptive statistics were used to describe how patients rate symptom severity and interference with function at each timepoint. Mean severity of the MDASI-BT or MDASI-SP and mean symptom interference were calculated at the time of clinical evaluation. Brain symptom factors included affective, cognitive, neurologic, treatment-related, related to general disease, or gastrointestinal effects. Six categories of individual symptoms included: affective (fatigue, disturbed sleep, feeling distressed, feeling sad, irritability); cognitive (difficulty remembering, difficulty understanding, difficulty speaking, difficulty concentrating); neurologic (pain, numbness/tingling, weakness on one side of body, seizure); treatment-related (lack of appetite, feeling drowsy, dry mouth); related to general disease (shortness of breath, vision, change in appearance, change in bowel pattern); or related to gastrointestinal effects (nausea, vomiting).
Spine categories of symptoms included: disease-related (pain, fatigue, disturbed sleep, feeling drowsy, numbness/tingling, radiating spine pain, weakness in arms/legs/trunk); related to autonomic function (loss of control of bladder or bowel, change in bowel pattern, sexual dysfunction); constitutional (nausea, shortness of breath, difficulty remembering, lack of appetite, dry mouth, vomiting); and emotional (feeling distressed, feeling sad). Interference scales were activity related (general activity, work, walking) or mood related (mood, relations with others, enjoyment of life). For the univariate Cox regression analysis, baseline activity-related interference for all patients, baseline cognitive and neurologic subscales for brain tumor patients, and baseline disease and autonomic subscales for spine patients were included as covariates of interest.
All patients with at least one valid questionnaire were included in the analyses. Differences of at least 1 point were classified as the minimum clinically meaningful change in the symptom severity and symptom interference measures. For example, a decrease of 2 points or more would mean a moderate improvement, whereas an increase of 2 points or more would be interpreted as moderate worsening. For individual symptoms, a rise in a symptom score means deterioration, whereas a reduced score means improvement of the specific symptom. The percentage of patients reporting each symptom as moderate-severe (rating ≥5) was found at each timepoint. The percent change between baseline and subsequent cycles was calculated and graphed.
KPS was assessed by the examiner at each timepoint. Change in KPS was categorized as “improved” (≥10-point increase), “no change” (remained the same), and “worsened” (≥10-point decrease).
Results
Patient Population
A total of 58 patients were recruited. Eight patients who were not treated under the protocol were excluded from the evaluation. All 8 withdrew consent before therapy was initiated. The 50 evaluable patients included 28 females and 22 males; the majority of the patients were white (91.5%). Characteristics of the treated patient population are provided in Table 1. In brief, the median age of the treated patients was 43.5 years with a range of 18 to 78. A majority of patients had a histology grade of II or III (36.4% and 45.5%, respectively). Fifty-one percent of patients’ tumor location was the spinal cord and 30.6% was supratentorial, and anatomic location by grade is provided in Supplementary Table 1. Baseline KPS ranged from 60 to 100, with the majority of patients (58%) having a good KPS (90–100). As shown in Table 1, most (94%) of the patients had at least 2 prior treatment regimens. Fourteen patients had prior chemotherapy treatments, as shown in Supplementary Table 2.
Table 1.
Summary of patient characteristics
| Category | Levels | N (%) |
|---|---|---|
| Patients | 50 (100) | |
| Age | <45 | 26 (52) |
| ≥45 | 24 (48) | |
| Sex | Female | 28 (56) |
| Male | 22 (44) | |
| Race | White | 43 (91.5) |
| American Indian/Alaskan Native | 1 (2.1) | |
| Black | 2 (4.3) | |
| Other | 1 (2.1) | |
| Unknown | 3 | |
| Prior treatment of any modality | Yes | 50 (100) |
| Prior radiotherapy | No | 3 (6) |
| Yes | 47 (94) | |
| Prior systemic therapy | No | 36 (72) |
| Yes | 14 (28) | |
| Prior surgery | Yes | 50 (100) |
| Number of prior treatments | 1 | 3 (6) |
| 2 | 33 (66) | |
| ≥3 | 14 (28) | |
| Histology grade | Grade I | 8 (18.2) |
| Grade II | 16 (36.4) | |
| Grade III | 20 (45.5) | |
| Unknown | 6 | |
| Tumor location | Spinal cord | 25 (51) |
| Supratentorial | 15 (30.6) | |
| Infratentorial | 8 (16.3) | |
| Multiple regionsa | 2 (4) | |
| Performance status | 60–70 | 11 (22) |
| 80 | 10 (20) | |
| 90 | 19 (38) | |
| 100 | 10 (20) |
aMultiple locations indicates that there were tumors located in multiple compartments at the time of diagnosis preventing the determination of the site of origin
Response
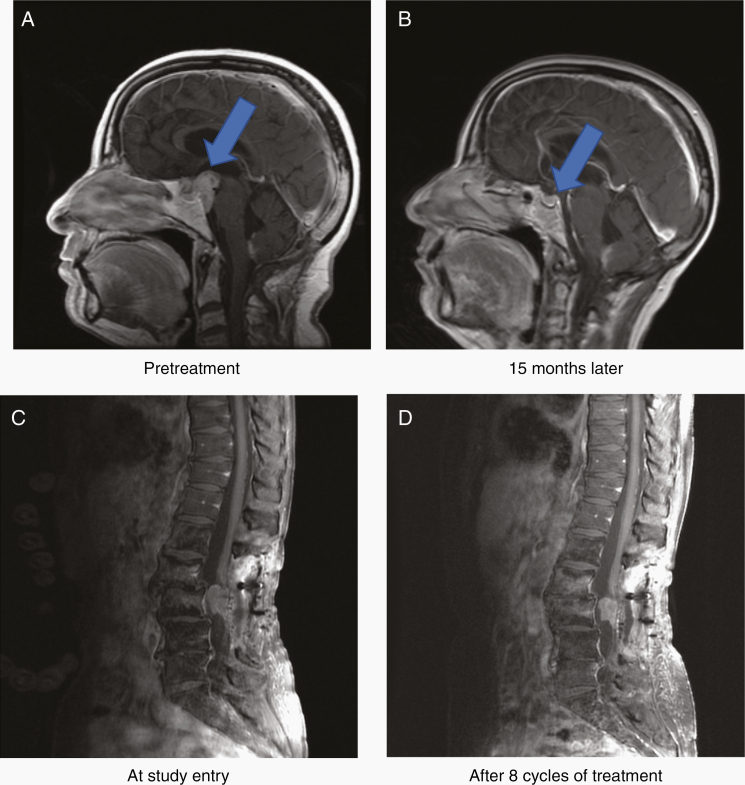
At the time of this analysis, the overall median follow-up time was 4.41 years, 42 patients had documented disease progression, and 30 of 50 patients had died. The median PFS time was 7.8 months (95% CI: 5.5, 12.2) and the median OS time was 2.25 years (95% CI: 1.70, 3.97). As shown in Table 2 and Supplementary Figure 1, median PFS varied by tumor grade but did not reach statistical significance. A summary of median PFS by tumor grade and tumor location is provided in Supplementary Table 3 and by prior therapies (radiation or systemic therapy) in Supplementary Table 4. The 6-month and 12-month PFS rates were 55% and 38%, respectively. Objective responses were seen in 8 patients (16% rate) with 2 complete responses (CRs) and 6 partial responses (PRs). Additional data describing responses by both grade and tumor location is provided in Supplementary Table 5. Figures 1A, B show a PR in a patient with disseminated grade III ependymoma; although the tumor at the clival region has achieved a CR, small spinal cord nodules remained. Figure 1C, D show a patient who achieved a modest reduction in a spinal cord grade III ependymoma. Although the decrease in tumor area did not reach the required 50% reduction in area required for a PR, the patient experienced dramatic improvement in neurologic function and pain reduction.
Table 2.
Summary of PFS and OS
| Survival Analysis | Levels | Total | Failed | Censored | Median Time, y | 95% CI | |
|---|---|---|---|---|---|---|---|
| PFS | Overall | 50 | 42 | 8 | 0.65 | 0.46 | 1.02 |
| Histologic grade | I | 8 | 6 | 2 | 1.02 | 0.46 | 3.82 |
| II | 16 | 12 | 4 | 0.90 | 0.34 | 2.19 | |
| III | 20 | 18 | 2 | 0.47 | 0.15 | 1.36 | |
| Tumor location | Infratentorial | 8 | 7 | 1 | 0.43 | 0.14 | 1.70 |
| Spinal cord | 25 | 18 | 7 | 0.90 | 0.46 | 1.84 | |
| Supratentorial | 15 | 15 | 0 | 0.47 | 0.15 | 1.87 | |
| Multiple regions | 2 | 2 | 0 | 0.46 | . | . | |
| OS | Overall | 50 | 30 | 20 | 2.25 | 1.70 | 3.97 |
| Histologic Grade | I | 8 | 4 | 4 | 3.97 | 1.50 | . |
| II | 16 | 8 | 8 | 2.37 | 0.60 | . | |
| III | 20 | 15 | 5 | 1.76 | 0.85 | 2.92 | |
| Tumor Location | Infratentorial | 8 | 5 | 3 | 2.04 | 0.40 | . |
| Spinal cord | 25 | 12 | 13 | 3.63 | 1.76 | . | |
| Supratentorial | 15 | 11 | 4 | 2.02 | 0.74 | 2.92 | |
| Multiple regions | 2 | 2 | 0 | 1.22 | . | . |
Fig. 1.
Examples of imaging response.
Univariate Cox modeling using covariates of interest, including baseline symptom subscales, were performed for both PFS and OS. As shown in Table 2, with the exception of preselected symptom subscales, none of the covariates, including age, sex, tumor grade, location or number of prior therapies predicted PFS or OS. (The results of these analyses are summarized in Tables 3 and 4, respectively.)
Table 3.
Summary of univariate Cox modeling of covariates for PFS
| Covariate | Level | Hazard Ratio | 95% CI | P-value | |
|---|---|---|---|---|---|
| Age | < 45 vs ≥45 | 0.97 | 0.52 | 1.79 | 0.9186 |
| Sex | Female vs male | 0.72 | 0.39 | 1.35 | 0.3051 |
| Race | Nonwhite vs white | 0.82 | 0.29 | 2.32 | 0.7033 |
| Histology grade | Grade I vs grade III/IV | 0.60 | 0.24 | 1.52 | 0.2803 |
| Grade II vs grade III/IV | 0.70 | 0.34 | 1.47 | 0.3484 | |
| Histology grade | Grade I/II vs grade III/IV | 0.67 | 0.35 | 1.28 | 0.2241 |
| Tumor location | Infratentorial vs spinal cord | 1.77 | 0.73 | 4.27 | 0.2067 |
| Multiple regionsa vs spinal cord | 2.60 | 0.34 | 20.11 | 0.3606 | |
| Supratentorial vs spinal cord | 1.77 | 0.88 | 3.55 | 0.1099 | |
| Karnofsky Performance Status | 60–70 vs 100 | 0.52 | 0.20 | 1.32 | 0.1669 |
| 80 vs 100 | 0.89 | 0.36 | 2.17 | 0.7968 | |
| 90 vs 100 | 0.41 | 0.18 | 0.95 | 0.0368 | |
| Karnofsky Performance Status | 60–80 vs 90–100 | 1.20 | 0.64 | 2.25 | 0.5617 |
| Number of prior treatments | 1 vs 3 | 1.92 | 0.53 | 7.03 | 0.3234 |
| 2 vs 3 | 1.18 | 0.58 | 2.38 | 0.6467 | |
| Age | 0.99 | 0.97 | 1.01 | 0.4115 | |
| MDASI-BT/SP | |||||
| Baseline activity-related interference score | 0–10 | 0.98 | 0.88 | 1.11 | 0.8056 |
| Baseline cognitive score | 0–10 | 1.34 | 0.93 | 1.95 | 0.1171 |
| Baseline neurologic score | 0–10 | 1.20 | 0.77 | 1.87 | 0.4097 |
| Baseline disease score | 0–10 | 0.72 | 0.55 | 0.94 | 0.0178 |
| Baseline autonomic score | 0–10 | 0.88 | 0.71 | 1.10 | 0.2628 |
aMultiple locations indicates that there were tumors located in multiple compartments at the time of diagnosis preventing the determination of the site of origin
Table 4.
Summary of univariate Cox modeling of covariates for OS
| Covariate | Level | Hazard Ratio | 95% CI | P-value | |
|---|---|---|---|---|---|
| Age | <45 vs ≥45 | 0.47 | 0.22 | 1.01 | 0.0531 |
| Sex | Female vs male | 1.10 | 0.53 | 2.29 | 0.7916 |
| Race | Nonwhite vs white | 0.37 | 0.05 | 2.72 | 0.3272 |
| Histology grade | Grade I vs grade III | 0.48 | 0.16 | 1.46 | 0.1936 |
| Grade II vs grade III | 0.54 | 0.23 | 1.29 | 0.1682 | |
| Histology grade | Grade I/II vs grade III | 0.52 | 0.24 | 1.12 | 0.0958 |
| Tumor location | Infratentorial vs spinal cord | 1.81 | 0.63 | 5.21 | 0.2739 |
| Multiple regionsa vs spinal cord | 4.93 | 0.60 | 40.16 | 0.1364 | |
| Supratentorial vs spinal cord | 1.89 | 0.83 | 4.32 | 0.1321 | |
| Karnofsky Performance Status | 60–70 vs 100 | 1.53 | 0.49 | 4.85 | 0.4663 |
| 80 vs 100 | 1.90 | 0.62 | 5.83 | 0.2625 | |
| 90 vs 100 | 1.02 | 0.35 | 2.98 | 0.9735 | |
| Karnofsky Performance Status | 60–80 vs 90–100 | 1.69 | 0.82 | 3.46 | 0.1534 |
| Number of prior treatments | 1 vs 3 | 0.92 | 0.12 | 7.41 | 0.9405 |
| 2 vs 3 | 0.86 | 0.39 | 1.90 | 0.7063 | |
| Age | 1.01 | 0.99 | 1.04 | 0.2598 | |
| MDASI-BT/SP | |||||
| Baseline activity-related interference score | 0–10 | 1.09 | 0.95 | 1.24 | 0.2112 |
| Baseline cognitive score | 0–10 | 1.54 | 1.07 | 2.22 | 0.0212 |
| Baseline neurologic score | 0–10 | 2.14 | 1.22 | 3.76 | 0.0081 |
| Baseline disease score | 0–10 | 0.87 | 0.63 | 1.20 | 0.4052 |
| Baseline autonomic score | 0–10 | 1.00 | 0.78 | 1.29 | 0.9936 |
aMultiple locations indicates that there were tumors located in multiple compartments at the time of diagnosis preventing the determination of the site of origin
Clinical Outcomes Assessments
Fifty patients completed at least one MDASI: 25 patients completed MDASI-BT and 25 completed MDASI-SP. Forty-six completed a MDASI-BT/SP at baseline. Completion rates ranged from 84% (cycle 8) to 92% (baseline and cycle 4) for the entire sample (Supplementary Table 6). Among brain patients, 92% completed an assessment at baseline and ranged from 71% (cycle 10) to 100% (cycles 6 and 8). Among spine patients, 92% completed an assessment at baseline and ranged from 75% (cycle 8) to 100% (cycle 10).
Among spine tumor patients, overall symptom burden was rated, on average, 2.3 (SD = 1.5) among 23 patients at baseline. Of the 4 symptom factors, the disease-related factor was rated the highest (mean = 3.5; SD = 2.1) and constitutional/treatment factor the lowest (mean = 0.9; SD = 0.9). Overall interference was rated, on average, 3.4 (SD = 2.6), with activity-related interference rated as 4.6 (SD = 3.1) and mood-related interference as 2.2 (SD = 2.3). The disease-related symptom factor was ranked first and remained so throughout the treatment cycles. Weakness in arms/legs/trunk was ranked first among the symptoms at baseline. For the remaining treatment cycles, fatigue was ranked first. Interference with walking was ranked first at 4 of the 7 treatment cycles, including baseline.
In the sample of 23 spine patients, the percent who were reporting moderate-severe symptoms decreased, most notably in symptoms pertaining to the disease-related and autonomic factors. The percentage of patients reporting the following moderate to severe symptoms decreased from baseline to cycle 6: pain (44% to 17%; 62% decrease), numbness/tingling (39% to 17%; 57% decrease), radiating spine pain (29% to 8%; 71% decrease), and loss of control of bladder and/or bowel (30% to 8%; 73% decrease) (Supplementary Table 7 and Supplementary Figure 2).
Eight of the patients with spinal cord ependymoma had a diagnosis of myxopapillary ependymoma. Treatment of this group of patients with WHO grade I tumors resulted in one partial response and stable disease in the remainder (Supplementary Table 5). Importantly, treatment led to decreases in the percent of patients who reported moderate-severe symptoms, most notably in symptoms pertaining to the disease-related and autonomic factors. The percentage of patients reporting the following moderate to severe symptoms decreased from baseline to cycle 6: pain (62% to 33%; 47% decrease), numbness/tingling (50% to 0%; 100% decrease), radiating spine pain (43% to 17%; 60% decrease), and loss of control of bladder and/or bowel (50% to 17%; 66% decrease).
Similarly, in the sample of 11 spine patients who received at least 6 cycles of treatment, the percent who were reporting moderate-severe symptoms decreased from baseline to cycle 6 for pain (46% to 18%; 60% decrease), numbness/tingling (36% to 18%; 50% decrease), radiating spine pain (33% to 9%; 73% decrease), and loss of control of bladder and/or bowel (27% to 9%; 67% decrease). Among brain patients, the percent who were reporting moderate-severe symptoms also decreased from baseline to cycle 6 for pain (17% to 8%; 53% decrease) and weakness on one side of body (9% to 8%; 11% decrease) (Supplementary Table 8 and Supplementary Figure 3).
Among brain tumor patients, at baseline overall symptom burden was rated, on average, 1.4 (SD = 1.3) among 23 patients. Of the 6 symptom factors, the affective factor was rated the highest (mean = 2.4; SD = 2.2) and gastrointestinal factor the lowest (mean = 0.5; SD = 1.8). Overall interference was rated, on average, 2.5 (SD = 2.7), with activity-related interference rated as 2.6 (SD = 2.8) and mood-related interference as 2.4 (SD = 2.7). The affective symptom factor was ranked first and remained so throughout the treatment cycles, except at cycle 6 where it was second to the treatment-related symptom factor. Feeling distressed was ranked first among the symptoms at baseline. For the remaining treatment cycles, fatigue was ranked first. Interference with mood was ranked first at baseline; however, interference with work ranked first at 4 of the 7 treatment cycles.
There were longitudinal changes in KPS. Among spine patients, by cycle 6, 4/8 (50%) spine patients had an improved KPS, and the remainder had an unchanged KPS. By cycle 6, 3/7 (43%) brain patients had an improved KPS, 3/7 (43%) had an unchanged KPS, and 1/7 (14%) had a worsened KPS.
Cox Regression Analysis
As shown in Table 3, only the MDASI-SP disease symptom subscale score was statistically significantly associated with PFS. The results suggest that a 1-point increase in the disease symptom subscale indicates a 28% lower risk of progression. Table 3 also shows that brain tumor patients who had a 1-point increase in the MDASI-BT cognitive symptom subscale or the neurologic symptom subscale had approximately a 1.5 or 2 times higher risk of death, respectively.
Toxicity and Adverse Events
CTCAE grades 2–4 treatment-related adverse events are summarized in Table 5 and a complete listing of all adverse events, regardless of attribution, is provided in Supplementary Table 9. There were no treatment-related deaths. As expected, myelotoxicity with neutropenia, leukopenia, and thrombocytopenia were collectively the most common grades 3 and 4 adverse events. The rate of myelotoxicity was markedly reduced after altering the protocol to consider escalating the TMZ dosing after 2 cycles of treatment. As summarized in Supplementary Table 10, disease progression was the most common cause of early treatment cessation (62%). Among 7 patients who stopped treatment because of treatment-related toxicities, rash was the most common reason (6/7).
Table 5.
Serious treatment-related adverse events
| CTCAEa Category | Grade II | Grade III | Grade IV |
|---|---|---|---|
| Anorexia | 5 | 0 | 0 |
| Constipation | 4 | 0 | 0 |
| Diarrhea | 2 | 0 | 0 |
| Nausea/vomiting | 9 | 0 | 0 |
| Fatigue | 8 | 1 | 0 |
| Rash | 18 | 6 | 0 |
| Anemia | 18 | 3 | 0 |
| Leukopenia | 61 | 11 | 0 |
| Neutropenia | 31 | 7 | 0 |
| Thrombocytopenia | 15 | 5 | 2 |
| Infection | 11 | 3 | 0 |
| Thrombosis | 0 | 1 | 1 |
aCommon Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Discussion
This study combined dose-dense TMZ to modulate MGMT and lapatinib to target EGFR and HER2. Both treatments are orally administered, not only providing flexibility of dosing schedules, but also, importantly, minimizing the number of visits to the participating centers, a key concern for rare disease studies where the geographic distribution of patients may be extensive and frequent medical visits impractical.
The early discovery of cycle 2 TMZ-related myelotoxicity after cycle 1 dose escalation led to a protocol amendment that only escalated if the first 2 cycles showed no hematologic toxicity. This reduced the number of hematologic adverse events. Although most patients had some side effects, these uncommonly led to treatment cessation (7 patients), but most of this was due to lapatinib-associated skin rash. Importantly, hepatic toxicity was uncommon and routine monitoring of cardiac function using echocardiography showed no evidence of cardiac toxicity, a potential concern with the extended use of lapatinib. Interestingly, diarrhea that is common with lapatinib was infrequent, a toxicity that may have been modulated by the constipating effects of TMZ.
The treatment regimen had clear antitumor activity. This was evident from both the objective responses in 16% of patients including 2 CRs as well as disease stabilization that led to notable 6- and 12-month PFS rates of 62% and 38%, respectively. This disease control was associated with either stabilization or improvement of both KPS and, more precisely, measures of symptom burden using the MDASI-BT or SP modules. Not only did the majority of patients have stable symptoms, many had a reduction in several disease-related symptoms. Notably, while disease-related symptoms improved, these patients frequently had some worsening in treatment-related symptoms, indicating that the improved disease symptom scores were accurate assessments of individual symptoms and not related to a “global” sense of well-being. Therefore, the combination of objective responses with the symptom stabilizing or improving disease control rate strongly suggests that the combination regimen of dose-dense TMZ and lapatinib is an effective regimen and should be considered as a standard treatment for adult patients with recurrent ependymoma. Although comparable results were reported by Rudà et al from a retrospective series of patients with recurrent ependymoma treated with single agent TMZ, comparison is difficult as their series included only patients without prior chemotherapy treatment.14 In the current study, nearly one third of the patients had prior chemotherapy and most had 2 or more prior disease recurrences.
As mentioned previously, there have been several seminal discoveries in ependymoma that have led to a new classification scheme that contains at least 9 subtypes. The protocol included collection of archived tumor tissue with the plan to look for predictive markers of response such as HER2 and EGFR expression. Unfortunately, although all enrolled patients had central review of histopathology to confirm the diagnosis of ependymoma, additional tumor tissue was available for only 26 patients. Attempts were made to assess HER2 expression by immunohistochemistry, which was uninterpretable as this protein remains intracellular rather than the classic membrane location in other cancers, notably breast cancer. Furthermore, attempts to isolate RNA for gene array analysis were unsuccessful for technical reasons and did not yield adequate amounts for gene expression studies.
In summary, the combination of TMZ and lapatinib showed efficacy in a diverse group of patients with ependymoma. All tumor locations, histologies, and grades appeared to have some benefit, although the higher-grade tumors, as expected, had a shorter duration of disease control. Importantly, this clinical trial clearly demonstrates the importance of routine and mandatory collection of Clinical Outcomes Assessments (COAs), as these demonstrated that the achievement of disease stability, such as PFS, was associated with clinical benefit. The determination of improvement in patient outcomes measures, particularly in indolent tumors (grade I or II), helps to demonstrate that the disease stability was likely related to treatment efficacy. Future studies of CNS tumors, particularly those that are indolent and with variable growth rates, should include comparable COAs to enable better interpretation of stable disease.
While the designation of ependymoma is increasingly more complex, our results support the use of the TMZ-lapatinib combination for recurrent disease. However, at the current time there are no data supporting the use of this regimen either as an adjuvant treatment after radiation or alone after surgical resection. Studies are planned to conduct comprehensive molecular analysis of tumors in future prospective clinical trials to test the hypothesis that tumor expression of HER2 and/or EGFR predict response to the combination of TMZ plus a dual HER2/EGFR inhibitor.
Supplementary Material
Acknowledgments
We would like to thank the patients and their families for their participation on this clinical trial. We are grateful for the support from the CERN Foundation for creating the clinical trial network and creating awareness for this study. We also thank the clinical research staff at the participating institutions for their outstanding effort.
Funding
This work was supported by an unrestricted grant from Collaborative Ependymoma Research Network and Glaxo Smith Kline. This research was supported [in part] by the Intramural Research Program of the National Institutes of Health, NCI, Cancer for Cancer Research and by Cancer Moonshot funds.
Conflict of interest statement. YY reports serving as a consultant to Abbvie, Amgen, Boehringer Ingelheim Pharmaceuticals, Bristol Myers Squibb, Midas Medical Technologies, Servier Pharmaceuticals, Starpax, and Vertex Pharmaceuticals. AO reports advisory board with Merck. FL reports research support from Inovio. PYW reports consulting/advisory board agreements with: Agios, Astra Zeneca, Bayer, Blue Earth Diagnostics, Boston Pharmaceuticals, Immunomic Therapeutics, Karyopharm, Integral Health, Vascular Biogenics, VBI Vaccines, Tocagen, Voyager, Novocure, QED, and Imvax and clinical trial support from the following companies: Agios, Astra Zeneca/Medimmune, Bayer, Beigene, Celgene, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Oncoceutics, Vascular Biogenics, and VBI Vaccines. No other authors report conflict.
Authorship statement. Analysis and interpretation: MRG, YY, JW, TM, EV, AO, FL, HIR, ERG, JW, PYW, TM, KA, TSA. Data analysis: MRG, YY, JW, TM, EV, TSA. Experimental design: MRG, YY, JW, TM, TSA.
References
- 1. Truitt G, Gittleman H, Leece R, et al. Partnership for defining the impact of 12 selected rare CNS tumors: a report from the CBTRUS and the NCI-CONNECT. J Neurooncol. 2019;144(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3. Vera-Bolanos E, Aldape K, Yuan Y, et al. ; CERN Foundation . Clinical course and progression-free survival of adult intracranial and spinal ependymoma patients. Neuro Oncol. 2015;17(3):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ellison DW, Aldape KD, Capper D, et al. cIMPACT-NOW update 7: advancing the molecular classification of ependymal tumors. Brain Pathol. 2020;30(5):863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghasemi DR, Sill M, Okonechnikov K, et al. MYCN amplification drives an aggressive form of spinal ependymoma. Acta Neuropathol. 2019;138(6):1075–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raffeld M, Abdullaev Z, Pack SD, et al. High level MYCN amplification and distinct methylation signature define an aggressive subtype of spinal cord ependymoma. Acta Neuropathol Commun. 2020;8(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruda R, Reifenberger G, Frappaz D, et al. EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro Oncol. 2018;20:445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rogers L, Pueschel J, Spetzler R, et al. Is gross-total resection sufficient treatment for posterior fossa ependymomas? J Neurosurg. 2005;102(4):629–636. [DOI] [PubMed] [Google Scholar]
- 10. Sartor EA, Wen PY. Adjuvant treatments for ependymomas. J Neurosurg Sci. 2018;62(1):71–77. [DOI] [PubMed] [Google Scholar]
- 11. Tsang DS, Burghen E, Klimo P Jr, Boop FA, Ellison DW, Merchant TE. Outcomes after reirradiation for recurrent pediatric intracranial ependymoma. Int J Radiat Oncol Biol Phys. 2018;100(2):507–515. [DOI] [PubMed] [Google Scholar]
- 12. Brandes AA, Cavallo G, Reni M, et al. A multicenter retrospective study of chemotherapy for recurrent intracranial ependymal tumors in adults by the Gruppo Italiano Cooperativo di Neuro-Oncologia. Cancer. 2005;104(1):143–148. [DOI] [PubMed] [Google Scholar]
- 13. Rehman S, Brock C, Newlands ES. A case report of a recurrent intracranial ependymoma treated with temozolomide in remission 10 years after completing chemotherapy. Am J Clin Oncol. 2006;29(1):106–107. [DOI] [PubMed] [Google Scholar]
- 14. Rudà R, Bosa C, Magistrello M, et al. Temozolomide as salvage treatment for recurrent intracranial ependymomas of the adult: a retrospective study. Neuro Oncol. 2016;18(2):261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wick W, Platten M, Weller M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol. 2009;11(1):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tolcher AW, Gerson SL, Denis L, et al. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer. 2003;88(7):1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buccoliero AM, Castiglione F, Rossi Degl’Innocenti D, et al. O6-methylguanine-DNA-methyltransferase in recurring anaplastic ependymomas: PCR and immunohistochemistry. J Chemother. 2008;20(2):263–268. [DOI] [PubMed] [Google Scholar]
- 19. Gilbertson RJ, Bentley L, Hernan R, et al. ERBB receptor signaling promotes ependymoma cell proliferation and represents a potential novel therapeutic target for this disease. Clin Cancer Res. 2002;8(10):3054–3064. [PubMed] [Google Scholar]
- 20. Friedrich C, von Bueren AO, Kolevatova L, et al. Epidermal growth factor receptor overexpression is common and not correlated to gene copy number in ependymoma. Childs Nerv Syst. 2016;32(2): 281–290. [DOI] [PubMed] [Google Scholar]
- 21. Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21(41): 6255–6263. [DOI] [PubMed] [Google Scholar]
- 22. Armstrong TS, Mendoza T, Gning I, et al. Validation of the M.D. Anderson symptom inventory brain tumor module (MDASI-BT). J Neurooncol. 2006;80(1):27–35. [DOI] [PubMed] [Google Scholar]
- 23. Armstrong TS, Gning I, Mendoza TR, et al. Reliability and validity of the M. D. Anderson symptom inventory-spine tumor module. J Neurosurg Spine. 2010;12(4):421–430. [DOI] [PubMed] [Google Scholar]
- 24. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.