Abstract
Background:
The association between rectal bleeding (RB) and increased stool frequency (SF) with endoscopy in patients with mild-to-moderate ulcerative colitis (UC) is unclear. We evaluated the correlation between RB and SF with endoscopic appearance in this population of UC patients.
Methods:
Post-hoc analysis of a phase III randomized controlled non-inferiority trial of 817 adults with mild-to-moderate UC treated with mesalazine was conducted. RB/SF and central reading of the Mayo endoscopic subscore (MES) were performed at weeks 0, 8, and 38. Sensitivity, specificity, and positive/negative predictive value of RB/SF for MES=0 and MES=0/1 were calculated at weeks 8 and 38. The association between change in RB/SF and change in MES after treatment was quantified using the Spearman’s rank correlation coefficient.
Results:
Among patients with MES=0, 9% (7/82) and 4% (6/167) had RB≥1, and 49% (40/82) and 38% (63/167) had SF≥1 at weeks 8 and 38, respectively. Among patients with MES=0/1, 16% (50/310) and 5% (18/363) had RB≥1, and 52% (162/310) and 39% (141/363) had SF≥1 at weeks 8 and 38, respectively. The Spearman’s rank correlation coefficient for change in RB and SF with change in MES at week 8 was 0.39 [95% confidence interval (CI): 0.32-0.45] and 0.34 [95% CI: 0.27-0.40], respectively. In patients with improved MES at week 8, 10% (39/389) and 21% (81/389) had unchanged/worsening RB and SF, respectively.
Conclusions:
While absence of RB is sensitive for endoscopic remission, increased SF often persists, indicating that it might not be a sensitive marker of disease activity in clinical practice or drug development for mild-to-moderate UC.
Keywords: patient-reported outcomes, endoscopy, ulcerative colitis, mucosal healing
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory disease characterized by symptoms of rectal bleeding (RB) and increased stool frequency (SF).1 Historically, the aim of medical therapy in UC has been to ameliorate patient symptoms, although cumulative evidence that achievement of endoscopic remission is associated with improved clinical outcomes has resulted in consensus recommendations endorsing a Mayo endoscopic subscore (MES) of 0 or 1 in addition to resolution of RB and normalization of bowel habit as therapeutic targets.2-4 The evolution of treatment endpoints in UC has been reflected in both clinical practice and clinical trials. Formal guidance from the United States Food and Drug Administration for evaluation of mucosal healing (MH) claims for product labeling require co-primary endpoints assessed by both a patient-reported outcome (PRO) instrument as well as objective endoscopic and histologic remission.5 RB and SF are patient-reported components of the Mayo Clinic Score (MCS)6 that have been incorporated in an interim two-item PRO tool (PRO2) for use in UC clinical trials.7
Therefore, accurately defining the performance characteristics of RB and SF for predicting endoscopic remission is needed to integrate PROs into clinical management paradigms for UC.8 The correlation between these symptoms and endoscopic remission (defined by a MES=0/1) has recently been evaluated in a meta-analysis of five studies by Narula et al.9 The authors concluded that a RB subscore of 0 was sensitive for endoscopic remission (pooled sensitivity 81% [95% confidence interval (CI): 73%-86%], pooled specificity 68% [95% CI: 61%-75%]) and a SF subscore of 0 was highly specific (pooled sensitivity 40% [95% CI: 25%-58%], pooled specificity 93% [95% CI: 86%-97%]). Conversely, only 40% of patients in endoscopic remission had normal SF. However, these conclusions may not be generalizable to patients with milder UC as the analysis was primarily informed by clinical trials of biologic therapies enrolling patients with moderate-to-severe disease.
The correlation between PROs and endoscopic activity in patients with milder UC has not been well studied. Understanding the diagnostic accuracy of PROs in the milder disease population is particularly important because these patients may be less accepting of a monitoring strategy dependent on serial endoscopy in the absence of severe symptoms. Second, the longitudinal association between changes in PROs and endoscopic activity with treatment is unclear. Finally, there is increased interest in drug development for mild-to-moderate UC and thus further information on the operating properties of endpoints traditionally used for moderate-to-severe disease is required. Therefore, we evaluated the association between RB and SF with endoscopic remission in a post-hoc analysis of a large, phase III, randomized non-inferiority trial of patients with mild-to-moderate UC treated with mesalazine (TP0503).10 We evaluated the sensitivity, specificity, and positive (PPV) and negative predictive value (NPV) of PROs for endoscopic remission and correlated the change in RB/SF with changes in MES over time. In addition, we evaluated predictors of discrepant change in RB/SF compared to endoscopy.
METHODS
Study design and patients
Data were analyzed from a previously reported phase III, active-controlled, double-dummy, multi-center randomized non-inferiority induction trial with an open-label extension, performed at 179 centers in Europe and Canada (Clinicaltrials.gov NCT01903252, TP0503). The study design and patient dispositions have been previously published.10 Briefly, adults with mild-to-moderate UC extending ≥15cm from the anal verge, defined by a total MCS ≥5, RB subscore ≥1, and MES ≥2 at baseline determined by a blinded central reader, were randomized to receive 3.2g of oral mesalazine, administered as either two 1600mg once-daily or four 400mg tablets twice-daily for 8 weeks. Following induction, patients were eligible to participate in an open-label extension study to week 38: patients in clinical remission received mesalazine 1.6 g/day, patients with clinical response received mesalazine 3.2 g/day, and non-responders at week 12 received mesalazine 4.8 g/day. The MCS was evaluated at weeks 0, 8 and 38.
Patient-reported and endoscopic outcomes
Patients kept a diary for at least one week prior to each visit of their SF and amount of blood seen in each stool. SF was scored as 0 (normal number of stools), +1 (1-2 stools/day more than normal), +2 (3-4 stools/day more than normal), or +3 (≥5 stools/day more than normal). RB was scored as 0 (none), +1 (visible blood with stool less than half the time), +2 (visible blood with stool half of the time or more), or +3 (passing blood alone). Endoscopic assessment by video-recorded flexible sigmoidoscopy was performed at screening (week -1) and weeks 8 and 38 and evaluated by a single blinded central reader. The MES is a 4-point scale ranging from 0-3, incorporating mucosal erythema, vascular pattern, friability, erosions, spontaneous bleeding, and ulceration. A modification of the original MES that includes any friability as a MES=2 was used to eliminate the previous ambiguity of ‘mild friability’.11 Two definitions of endoscopic remission were used: MES=0 and MES=0/1.
Statistical analysis
The sensitivity, specificity, PPV, and NPV of RB and SF for each definition of endoscopic remission at weeks 8 and 38 were calculated separately for PROs with RB=0, SF=0, and RB+SF=0 as criteria. Sensitivity was defined as the proportion of patients with the criterion among all those with endoscopic remission, while specificity was defined as the proportion of patients without the criterion among all those without endoscopic remission. The PPV was defined as the proportion of patients with endoscopic remission among all those with the criterion, i.e., the PRO subscore was 0, while the NPV was defined as the proportion of patients without endoscopic remission among all those who’s PRO subscores were ≥1. The association between change in RB and SF and change in MES at week 8 was quantified using the Spearman’s rank correlation coefficient with 95% CI obtained using the Fisher z-transformation. We chose to evaluate the correlation in changes of PROs and endoscopy at week 8 to capture the period of blinded treatment at a consistent mesalazine dose and avoid selection bias because mesalazine dosing was subsequently adjusted after week 12 based on week 8 clinical and endoscopic response. In subgroup analysis, we evaluated the additive effect of incorporating fecal calprotectin concentration (<250μg/g vs. ≥250μg/g) at weeks 8 and 38 when available with RB and SF for determination of endoscopic remission.
Stepwise logistic regression by backwards elimination was used to evaluate predictors of discordant endoscopy/PRO response. The dependent variable was discordance, defined as patients with improved MES but unchanged or worsened RB/SF as compared to patients with improved RB/SF but unchanged or worsened endoscopy. Independent variables were chosen a priori and included gender, age, smoking status, disease duration, disease extent, baseline MCS, RHI, fecal calprotectin, Short Form Survey (SF-36) score (including physical and mental component subscores), European Quality of Life-5 Dimensions (EQ-5D) score, baseline patient and physician subjective global assessment, and Work Productivity and Activity Impairment (WPAI)-UC questionnaire scores). Independent variables were retained if p<0.10. Adjusted association between each covariate and discordance are expressed as odds ratios (OR) with 95% CI.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, United States).
RESULTS
Baseline Patient Demographics
Baseline patient demographics in the TP0503 trial have been previously reported 10. In the intent-to-treat population, 817 patients with a mean UC disease duration of 65.4 months (± standard deviation SD 80.4 months) were randomized to receive 3.2 g/day mesalazine in one of two pill formulations. Most patients had proctosigmoiditis (44.2%) or left-sided colitis (35.1%). The mean MCS at induction was 7.7 (±1.3). Mean fecal calprotectin was 1116.4 μg/g (±1532.5 μg/g) and mean RHI score was 15.8 (±7.6) prior to treatment. RB, SF, and centrally-read MES scores were available for 763 at week 8 and for 657 patients at week 38.
Correlation of PROs and Endoscopic Appearance
At week 8, 82/763 (10.7%) patients had MES=0 and 310/763 (40.6%) patients had MES=0/1. At week 38, the corresponding number of patients with MES=0 and MES=0/1 were 167/657 (25.4%) and 363/657 (55.3%), respectively. Figure 1 summarizes the distribution of RB and SF subscores by MES score at weeks 8 and 38. At week 8, among 310 patients with MES=0/1, 16.1% (50/310) had RB≥1 and 52.3% (162/310) had SF≥1. Among 82 patients with MES=0, 8.5% (7/82) had RB≥1 and 48.8% (40/82) had SF≥1. At week 38, 3.6% (6/167) and 5.0% (18/363) of patients had RB≥1 if they achieved MES=0 or 0/1, respectively. In comparison, 37.7% (63/167) and 38.8% (141/363) of patients with MES=0 and MES=0/1 had SF≥1. The proportion of patients in endoscopic remission with persistent RB or SF ≥1 is summarized in Figure 2.
Figure 1.
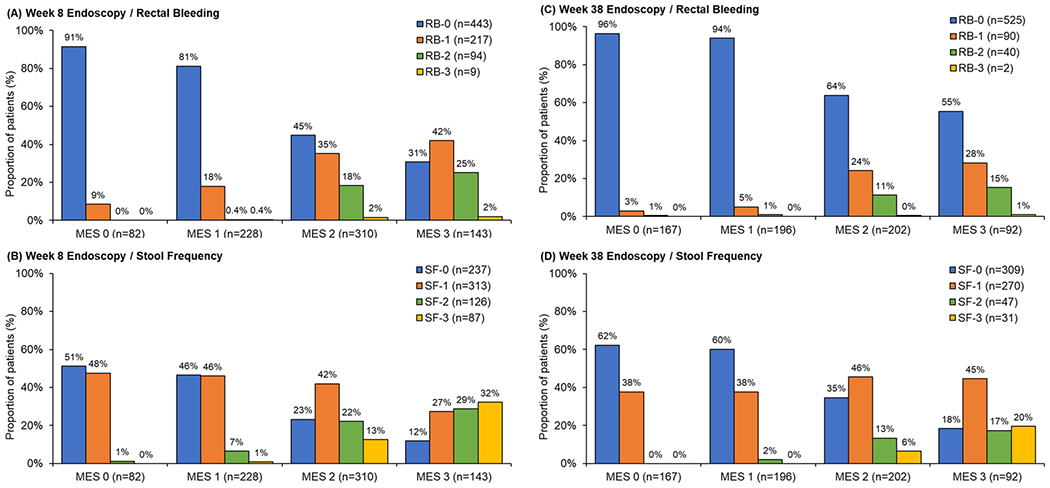
Proportion of patients with rectal bleeding (RB) and stool frequency (SB) subscores at week 8 and 38 by Mayo endoscopic subscore (MES).
Figure 2.
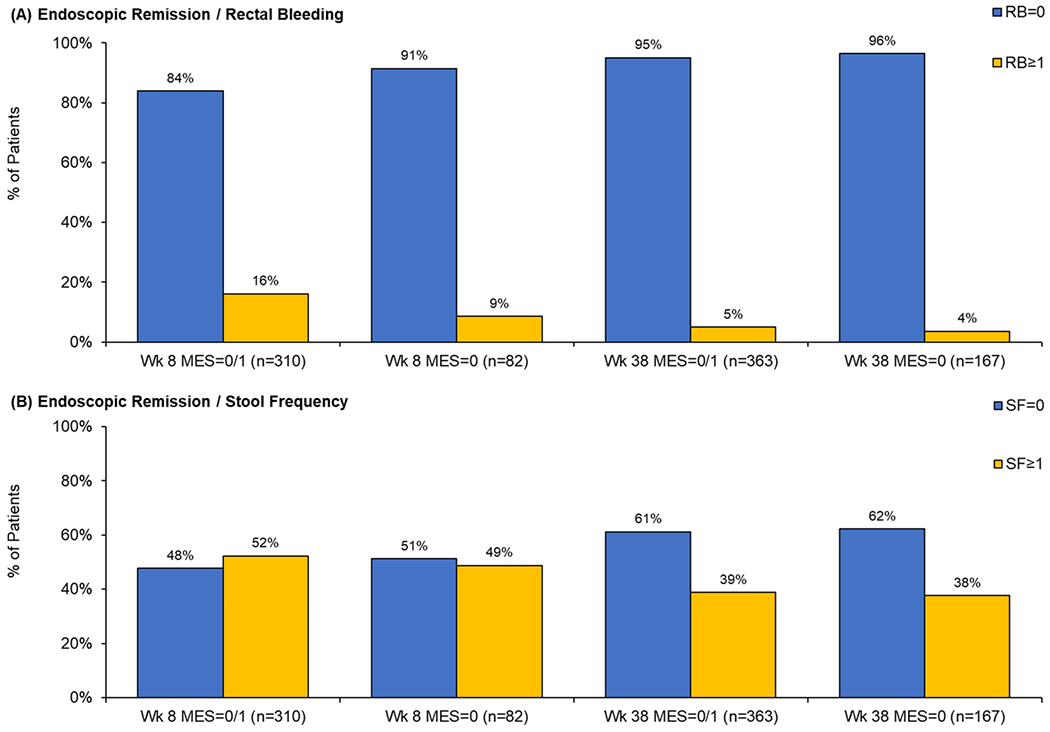
Proportion of patients in endoscopic remission (MES=0 or MES=0/1) with rectal bleeding (RB) and stool frequency (SB) subscores ≥1 at week 8 and 38.
Prediction of Endoscopic Remission by PROs
The sensitivity, specificity, PPV, and NPV of PROs for endoscopic remission is summarized in Table 1. For endoscopic remission defined as MES=0/1, the sensitivity of RB=0 ranged from 83.8%-95.0% and specificity ranged from 57.7%-62.1%. The sensitivity of SF=0 ranged from 47.7%-61.2% and specificity ranged from 70.7%-80.4%. For both RB and SF, the sensitivity increased although specificity decreased when considering endoscopic remission defined by MES=0. For both definitions of endoscopic remission, the PPV of RB=0 or SF=0 were similar and increased from week 8 to week 38. The PPV was lower for identifying MES=0 compared to MES=0/1 for both RB and SF. At week 38, the PPV of SF=0 for MES=0/1 was 70.3% and 32.9% for MES=0; the corresponding PPV of RB=0 was 64.2% and 30.0%. PPV increased slightly when both RB=0 and SF=0 were combined (72.7% for MES=0/1 and 34.7% for MES=0 at week 38). RB had a high NPV for endoscopic remission (98.1% at week 8, 97.9% at week 38 for MES=0 and 86.3% at week 8, 93.6% at week 38 for MES=0/1). In comparison, the NPV of SF=0 for MES=0 was 92.8% and 82.9% at weeks 8 and 38, respectively. The NPV of SF=0 for MES=0/1 was 70.9% and 61.7% at weeks 8 and 38, respectively. The combination of RB=0 and SF=0 did not have a higher NPV compared to absence of RB alone.
Table 1.
Sensitivity, specificity, positive and negative predictive value of rectal bleeding (RB) and stool frequency (SF) for endoscopic remission.
| Symptom Score | Time | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Mayo Endoscopic Subscore = 0 or 1 | |||||
| RB | Week 8 | 83.9% (260/310) |
62.1% (315/365) |
57.5% (260/452) |
86.3% (315/365) |
| Week 38 | 95.0% (345/363) |
57.7% (262/454) |
64.2% (345/537) |
93.6% (262/280) |
|
| SF | Week 8 | 47.7% (148/310) |
80.4% (394/490) |
60.7% (148/244) |
70.9% (394/556) |
| Week 38 | 61.2% (222/363) |
70.7% (227/321) |
70.3% (222/316) |
61.7% (227/368) |
|
| RB+SF | Week 8 | 44.5% (148/310) |
86.4% (438/507) |
66.7% (138/207) |
71.8% (438/610) |
| Week 38 | 59.5% (216/363) |
82.2% (373/454) |
72.7% (216/297) |
71.7% (373/520) |
|
| Mayo Endoscopic Subscore = 0 | |||||
| RB | Week 8 | 91.5% (75/82) |
48.7% (358/735) |
16.6% (75/452) |
98.1% (358/365) |
| Week 38 | 96.4% (161/167) |
42.2% (274/650) |
30.0% (161/537) |
97.9% (274/280) |
|
| SF | Week 8 | 51.2% (42/82) |
71.9% (516/718) |
17.2% (42/244) |
92.8% (516/556) |
| Week 38 | 62.3% (104/167) |
59.0% (305/517) |
32.9% (104/316) |
82.9% (305/368) |
|
| RB+SF | Week 8 | 51.2% (42/82) |
77.6% (570/735) |
20.3% (42/207) |
93.4% (570/610) |
| Week 38 | 61.7% (103/167) |
70.2% (456/650) |
34.7% (103/297) |
87.7% (456/520) |
|
Sensitivity was defined as the proportion of patients with the criterion among all those with endoscopic remission. Specificity was defined as the proportion of patients without the criterion among all those without endoscopic remission. NPV negative predictive value was defined as the proportion of patients without endoscopic remission among all those with PRO subscores (RB, SF, or RB+SF) greater than 0; PPV positive predictive value was defined as the proportion of patients with endoscopic remission among all those with PRO subscores of 0.
Prediction of Endoscopic Remission by PROs and Fecal Calprotectin
In subgroup analysis, we evaluated the predictive value of fecal calprotectin in patients with persistent RB or SF (subscore ≥1) despite endoscopic remission at weeks 8 and 38 (Table 2). Among patients with MES=0 at week 8, 87.2% (34/39) of patients with SF≥1 and 66.7% (4/6) of patients with RB≥1 had fecal calprotectin <250 μg/g. Among patients with MES=0/1, 82.1% (128/156) of patients with SF≥1 and 70.8% (34/48) patients with RB≥1 had fecal calprotectin <250 µg/g. Among patients with MES=0 at week 38, 93.0% (53/57) of patients with SF≥1 and 83.3% (5/6) of patients with RB≥1 had fecal calprotectin <250 μg/g. Among patients with MES=0/1 at week 38, 89.5% (119/133) of patients with SF≥1 and 72.2% (13/18) of patients with RB≥1 had fecal calprotectin <250 μg/g.
Table 2.
Sensitivity, specificity, positive and negative predictive value of fecal calprotectin <250 μg/g for endoscopic remission among patients with persistent rectal bleeding (RB) or stool frequency (SF) at weeks 8 and 38.
| Definition of Endoscopic Remission | Time | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Among patients with persistent RB | |||||
| MES=0/1 | Week 8 | 70.8% (34/48) |
68.0% (174/256) |
29.3% (34/116) |
92.6% (174/188) |
| Week 38 | 72.2% (13/18) |
59.4% (63/106) |
23.2% (13/56) |
92.6% (63/68) |
|
| MES=0 | Week 8 | 66.7% (4/6) |
62.4% (186/298) |
3.4% (4/116) |
98.9% (186/188) |
| Week 38 | 83.3% (5/6) |
56.8% (67/118) |
8.9% (5/56) |
98.5% (67/68) |
|
| Among patients with persistent SF | |||||
| MES=0/1 | Week 8 | 82.1% (128/156) |
62.5% (215/344) |
49.8% (128/257) |
88.5% (215/243) |
| Week 38 | 89.5% (119/133) |
56.9% (112/197) |
58.3% (119/204) |
88.9% (112/126) |
|
| MES=0 | Week 8 | 87.2% (34/39) |
51.6% (238/461) |
13.2% (34/257) |
97.9% (238/243) |
| Week 38 | 93.0% (53/57) |
44.7% (122/273) |
26.0% (53/204) |
96.8% (122/126) |
|
MES Mayo Endoscopic Subscore
Sensitivity was defined as the proportion of patients with fecal calprotectin concentration <250μg/g among all those with endoscopic remission, while specificity was defined as the proportion of patients with fecal calprotectin concentration ≥250μg/g among all those without endoscopic remission. PPV positive predictive value was defined as the proportion of patients with endoscopic remission among all those with fecal calprotectin concentration <250μg/g; NPV negative predictive value was defined as proportion of patients without endoscopic remission among all those with fecal calprotectin concentration ≥250μg/g.
The positive likelihood ratio of fecal calprotectin <250 μg/g for MES=0 ranged from 1.68-1.80 for patients with SF≥1 and 1.78-1.93 for patients with RB≥1. The positive likelihood ratio of fecal calprotectin <250 μg/g for MES=0/1 ranged from 2.07-2.19 for patients with SF≥1 and 1.78-2.21 for patients with SF≥1.
Correlation Between Changes in PROs and MES
Correlations between changes in RB, SF, and MES after 8 weeks of treatment with 3.2g mesalazine are summarized in Figure 3. The Spearman’s rank correlation coefficient for change in RB and SF with MES at week 8 was 0.39 [95% CI: 0.32, 0.45] (p<0.001) and 0.34 [95% CI: 0.27, 0.40] (p<0.001), respectively. Amongst 389 patients with improved MES compared to baseline, 90.0% (350/389) had improved RB and 79.2% (308/389) had improved SF. In contrast, over half of patients with no change or worsening MES experienced improvement in SF (51.1%, 191/374) or RB (55.6%, 208/374).
Figure 3.
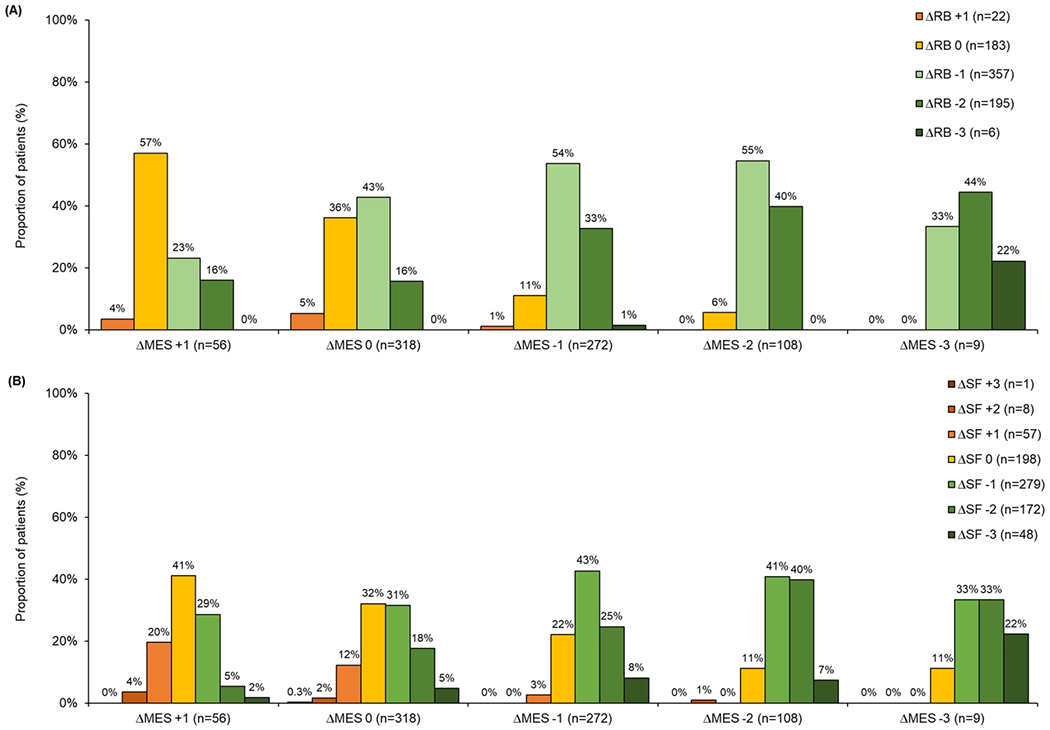
Change in rectal bleeding (RB) and stool frequency (SF) at week 8 after treatment with 3.2g mesalazine compared to change in Mayo endoscopic subscore (MES).
To evaluate potential predictors of discrepant PRO and MES change with treatment, we compared patients with improved MES but unchanged/worsened PRO (n=39 for RB, n=81 for SF) to patients with improved PRO but unchanged/worsened MES (n=208 for RB, n=191 for SF). In a backwards elimination logistic regression analysis, patients reporting no change/worsening SF despite improved MES were more likely to have a higher baseline MCS (OR 1.67 [95% CI: 1.30, 2.13], p<0.0001) and worse baseline subjective global assessment rating (OR 1.40 [95% CI: 0.96, 2.05], p=0.08). Patients reporting no change/worsening RB despite improved MES were more likely to have longer disease duration (OR 1.51 per 5 years [95% CI: 1.00, 2.27], p=0.05). All other covariables were not statistically significant at the p<0.10 level (summary of model fits in backwards elimination summarized in Supplemental Table 1).
DISCUSSION
Resolving RB, normalizing increased stool frequency, and achieving endoscopic remission (defined as a MES=0/1) has been recommended as a therapeutic target in UC.2, 4 However, considerations of cost, invasiveness, and practicality may limit the ability to perform serial endoscopy in routine practice, particularly for patients with mild-to-moderate UC who may be less willing to undergo repeated procedures in the absence of severe symptoms. Therefore, an understanding of the operating properties of PROs for predicting endoscopic remission in this population is necessary. In this post-hoc analysis of a recently conducted large phase III randomized non-inferiority trial of patients with mild-to-moderate UC, we demonstrated that normalization of RB and SF is associated with endoscopic remission. However, there is only a weak-to-modest correlation between changes in PRO items with changes in MES, underscoring the limitations of using PROs alone to determine treatment response in mild-to-moderate UC.
In a meta-analysis of 2,132 UC patients with predominantly moderate-to-severe disease, Narula et al. determined that the pooled sensitivity and specificity of RB=0 for endoscopic remission (MES=0/1) was 81% [95% CI: 73%-86%] and 68% [95% CI: 61%-75%], respectively.9 In comparison, the pooled sensitivity and specificity of SF=0 for endoscopic remission was 40% [95% CI: 25%-58%] and 93% [95% CI: 86%-97%], respectively. In our analysis, normalization of SF was less specific for endoscopic remission (70.7%-80.4%). Although this may reflect differences in disease activity, our findings reinforce that many patients will continue to have abnormal bowel habits, specifically increased SF, despite achieving MES subscores of 0 or 1. Conversely, only 5% of patients with MES=0/1 had persistent RB.
This discrepancy between PRO- and MES-defined remission has important implications for outcome assessment if normalization of SF is set as a therapeutic target in clinical practice or incorporated as a co-primary study endpoint in clinical trials.7 Inflammatory biomarkers may be used to help differentiate patients with persistent symptoms despite endoscopic remission: more than 80% of patients with endoscopic remission but abnormal SF had fecal calprotectin <250 μg/g at both weeks 8 and 38. The improved sensitivity using combined clinical and biomarker evaluation has been recently demonstrated in phase II trials of filgotinib12 and brazikumab.13 However, although a fecal calprotectin <250 μg/g was associated with twice the positive likelihood of endoscopic remission in symptomatic patients in our study, this finding was not specific as approximately half of patients with fecal calprotectin <250 μg/g were not in endoscopic remission by either MES=0 or MES=0/1 definitions.
The correlation between PROs and MES in a similar population of patients with milder UC was evaluated by Colombel et al. using the Emerging Biomarkers in Inflammatory Bowel Disease (EMBARK) cohort.14 In a cross-sectional analysis of 103 UC patients, the authors showed that 39% of patients with MES=0/1 and 25% of patients with MES=0 had abnormal SF. However, the PPV of RB=0, SF=0, and combined RB+SF=0 for identifying endoscopic remission in the EMBARK cohort was substantially higher than described in the TP0503 trial, ranging from 85.5%-95.0% for MES=0/1 and 60.0%-74.3% for MES=0. These differences may be explained by a substantially higher proportion of patients enrolled in EMBARK who had normal endoscopic findings whereas a baseline MES ≥2 was an entry criterion for the TP0503 trial. Second, the EMBARK study was cross-sectional and may not capture the time lag for symptom resolution once endoscopic remission has been achieved. In our study, the sensitivity of both RB=0 and SF=0 for endoscopic remission increased between weeks 8 and 38. Similar findings have been reported by Jharap et al. who evaluated the relationship between RB and SF with endoscopic remission in two phase 3 trials of adalimumab in moderate-to-severe UC (ULTRA 1 and 2).15 Comparing week 8 and 52, normalization of SF increased from 29% to 41% and resolution of RB increased from 87% to 94%. Collectively, these findings suggest that therapeutic optimization based solely on PROs early in the disease course may result in overtreatment of some patients.
Several reasons for the misclassification between PROs and MES have been hypothesized including concurrent functional gastrointestinal disorders such as irritable bowel syndrome (IBS)16, 17; prolonged disease resulting in structural bowel damage with colonic shortening, impaired absorption and decreased rectal compliance18, or persistent histologic inflammation with impaired intestinal permeability despite endoscopic healing.19, 20 Although a history of IBS was not specifically evaluated in the TP0503 study, patients with a high baseline subjective global disease rating were more likely to experience a discrepant PRO/SF response at week 8, while other factors such as quality of life, workplace productivity, and disease duration were not significantly associated. There is inherently more subjective evaluation of SF as compared to RB as patients with UC may report passage of mucous or tenesmus and “normal” bowel habits may be difficult to establish, whereas visualization of blood is subject to less variability. We did not evaluate the role of persistent histologic inflammation as these data were not available in the TP0503 study. However, the addition of histologic assessment by the RHI and Nancy Index in the EMBARK cohort did not significantly change the proportion of patients reporting persistent symptoms.14 Previous studies have suggested that better long-term outcomes can be achieved with histologic as compared to endoscopic remission21 although a prospective randomized controlled trial evaluating the incremental benefit of treating to histologic targets is needed and will clarify the diagnostic accuracy of remission defined by PROs, endoscopy, and histology.
Our study has several strengths. We included over 650 patients and analyzed centrally read blinded endoscopy and PRO data that was rigorously recorded in the context of a randomized controlled trial at baseline as well as follow-up at weeks 8 and 38. The study population of mild-to-moderate UC patients was relatively homogeneous and well-defined. However, our study also has some important limitations. First, mesalazine dosing from weeks 8-38 was variable and dependent on clinical and endoscopic response. Second, not all patients completed 38 weeks of treatment and endoscopic data was not available for some patients at the end of follow-up. This may represent a potential source of selection bias as symptomatic patients are more likely to withdraw from the trial. Third, centrally-read histology data was only available at baseline and therefore, changes in histology could not be correlated with changes in endoscopy or PROs. Fourth, we did not present data evaluating change in PROs versus endoscopy between Weeks 8 and 38 because clinical non-responders were withdrawn from the trial at Week 16. Finally, it is recognized that patients with MES=1 likely have persistent mild inflammation rather than true endoscopic remission: however, this remains an accepted outcome definition used in both clinical trials as well as practice guidelines.
In conclusion, changes in patient-reported SF and RB are only modestly correlated with changes in endoscopic disease activity in patients with mild-to-moderate UC treated with mesalazine. While the absence of RB or SF is associated with endoscopic remission, a substantial proportion of patients will continue to have residual increased SF despite achieving a MES of 0 or 1, particularly during induction. In patients with persistent symptoms, fecal calprotectin is a sensitive marker for endoscopic remission although specificity is limited. Therefore, it may play an adjunctive role in triaging patients who are most likely to benefit from endoscopy. Overall, our findings highlight the limitations of using PROs alone for assessment of treatment response and the importance of objective endoscopic evaluation even in patients with mild-to-moderate UC.
Supplementary Material
Supplemental Table 1. Summary of model fit in backwards elimination
Acknowledgments
Funding Support: Christopher Ma is supported in part by a Clinician Fellowship from the Canadian Institutes of Health Research, Crohn’s and Colitis Canada, and the Canadian Association of Gastroenterology.
The randomized trial was funded in full by Tillotts Pharma, AG. Tillotts Pharma, AG had no role in the design, analysis, or writing of this manuscript.
Disclosures:
Christopher Ma has received consulting fees from Robarts Clinical Trials.
William Sandborn has received research grant support from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, AbbVie, Janssen, Takeda, Lilly, Celgene/Receptos; consulting fees from Abbvie, Allergan, Amgen, Boehringer Ingelheim, Celgene, Conatus, Cosmo, Escalier Biosciences, Ferring, Genentech, Gilead, Gossamer Bio, Janssen, Lilly, Miraca Life Sciences, Nivalis Therapeutics, Novartis Nutrition Science Partners, Oppilan Pharma, Otsuka, Paul Hastings, Pfizer, Precision IBD, Progenity, Prometheus Laboratories, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust or HART), Salix, Shire, Seres Therapeutics, Sigmoid Biotechnologies, Takeda, Tigenix, Tillotts Pharma, UCB Pharma, Vivelix; and stock options from Ritter Pharmaceuticals, Oppilan Pharma, Escalier Biosciences, Gossamer Bio, Precision IBD, Progenity.
Geert D’Haens has received consulting fees from AbbVie, Ablynx, Amakem, AM Pharma, Avaxia, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Cosmo, Covidien/Medtronics, Dr. Falk Pharma, Engene, Ferring, Galapagos, Gilead, GlaxoSmithKline, Hospira, Immunic, Johnson and Johnson, Lycera, Medimetrics, Millennium/Takeda, Mitsubishi Pharma, MSD, Mundipharma, Novo Nordisk, Pfizer Inc, Prometheus Laboratories/Nestle, Receptos, Robarts Clinical Trials, Salix, Sandoz, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, Versant, and Vifor; research grants from AbbVie, Falk, Ferring, MSD, Mundipharma, and Takeda; and lecture and/or speaker bureau fees from AbbVie, Ferring, Johnson and Johnson, Millennium/Takeda, MSD, Mundipharma, Norgine, Pfizer Inc, Shire, Tillotts, and Vifor.
Guangyong Zou has no conflicts of interest to declare.
Larry Stitt has no conflicts of interest to declare.
Siddharth Singh has received research support from Pfizer and AbbVie, consulting fees from AbbVie, Takeda, Pfizer, AMAG Pharmaceuticals.
Ashwin Ananthakrishnan has received funding from the US National Institutes of Health, Crohn’s and Colitis Foundation, Pfizer, and the Chleck Family Foundation.
Parambir Dulai has received research grant support from Takeda, Pfizer, Janssen, Prometheus, Polymedco, and ALPCO; has served as a consultant for Takeda, Janssen, and Abbvie; and received speaker honorariums from Takeda.
Reena Khanna has received scientific advisory board fees from AbbVie, Janssen, Pfizer, Takeda; consulting fees from AbbVie, Janssen, Takeda, Robarts Clinical Trials; payments for lectures/speakers’ bureau fees from AbbVie, Janssen, Shire, and Takeda.
Vipul Jairath has received consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena pharmaceuticals, Genetech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials, Topivert, Celltrion; speaker’s fees from Takeda, Janssen, Shire, Ferring, Abbvie, Pfizer
Brian Feagan has received grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma AG, AbbVie, Novartis Pharmaceuticals, Centocor Inc., Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGenix, and Wyeth Pharmaceuticals Inc.; consulting fees from Millennium Pharmaceuticals, Merck, Centocor Inc., Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, UCB Pharma, AbbVie, Astra Zeneca, Serono, Genentech, Tillotts Pharma AG, Unity Pharmaceuticals, Albireo Pharma, Given Imaging Inc., Salix Pharmaceuticals, Novonordisk, GSK, Actogenix, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand Pharma, Zyngenia, GiCare Pharma Inc., and Sigmoid Pharma; and speaker’s fees from UCB, AbbVie, and J&J/Janssen.
Acronyms and Abbreviations:
- CI
confidence interval
- EMBARK
Emerging Biomarkers in Inflammatory Bowel Disease
- EQ-5D
European Quality of Life-5 Dimensions
- IBS
irritable bowel syndrome
- MCS
Mayo Clinic Score
- MES
Mayo Endoscopic subscore
- MH
mucosal healing
- NPV
negative predictive value
- OR
odds ratio
- PPV
positive predictive value
- PRO
patient-reported outcome
- RB
rectal bleeding
- RHI
Robarts Histopathologic Index
- SF
stool frequency
- SF-36
Short Form Survey
- UC
ulcerative colitis
- WPAI
Work Productivity and Activity Impairment
Footnotes
ClinicalTrials.gov Registration: NCT01903252
REFERENCES
- 1.Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389(10080):1756–70. Epub 2016/12/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110(9):1324–38. Epub 2015/08/26. [DOI] [PubMed] [Google Scholar]
- 3.Shah SC, Colombel JF, Sands BE, et al. Mucosal Healing Is Associated With Improved Long-term Outcomes of Patients With Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14(9):1245–55 e8. Epub 2016/02/02. [DOI] [PubMed] [Google Scholar]
- 4.Bressler B, Marshall JK, Bernstein CN, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015;148(5):1035–58 e3. Epub 2015/03/10. [DOI] [PubMed] [Google Scholar]
- 5.Reinisch W, Gottlieb K, Colombel J-F, et al. Comparison of the EMA and FDA Guidelines on Ulcerative Colitis Drug Development. Clinical Gastroenterology and Hepatology. In press. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–9. Epub 1987/12/24. [DOI] [PubMed] [Google Scholar]
- 7.Jairath V, Khanna R, Zou GY, et al. Development of interim patient-reported outcome measures for the assessment of ulcerative colitis disease activity in clinical trials. Aliment Pharmacol Ther. 2015;42(10):1200–10. Epub 2015/09/22. [DOI] [PubMed] [Google Scholar]
- 8.Dulai PS, Peyrin-Biroulet L. Integrating Patient-Reported Outcomes Into Treat-to-Target Monitoring Algorithms. Clin Gastroenterol Hepatol. 2019;17(3):395–6. Epub 2018/08/29. [DOI] [PubMed] [Google Scholar]
- 9.Narula N, Alshahrani AA, Yuan Y, et al. Patient-Reported Outcomes and Endoscopic Appearance of Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2019;17(3):411–8 e3. Epub 2018/06/19. [DOI] [PubMed] [Google Scholar]
- 10.D’Haens GR, Sandborn WJ, Zou G, et al. Randomised non-inferiority trial: 1600 mg versus 400 mg tablets of mesalazine for the treatment of mild-to-moderate ulcerative colitis. Aliment Pharmacol Ther. 2017;46(3):292–302. Epub 2017/06/02. [DOI] [PubMed] [Google Scholar]
- 11.Scherl EJ, Pruitt R, Gordon GL, et al. Safety and efficacy of a new 3.3 g b.i.d. tablet formulation in patients with mild-to-moderately-active ulcerative colitis: a multicenter, randomized, double-blind, placebo-controlled study. Am J Gastroenterol. 2009;104(6):1452–9. [DOI] [PubMed] [Google Scholar]
- 12.Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389(10066):266–75. Epub 2016/12/19. [DOI] [PubMed] [Google Scholar]
- 13.Sands BE, Chen J, Feagan BG, et al. Efficacy and Safety of MEDI2070, an Antibody Against Interleukin 23, in Patients With Moderate to Severe Crohn’s Disease: A Phase 2a Study. Gastroenterology. 2017;153(1):77–86 e6. Epub 2017/04/10. [DOI] [PubMed] [Google Scholar]
- 14.Colombel JF, Keir ME, Scherl A, et al. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut. 2017;66(12):2063–8. Epub 2016/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jharap B, Sandborn WJ, Reinisch W, et al. Randomised clinical study: discrepancies between patient-reported outcomes and endoscopic appearance in moderate to severe ulcerative colitis. Aliment Pharmacol Ther. 2015;42(9):1082–92. Epub 2015/09/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henriksen M, Hoivik ML, Jelsness-Jorgensen LP, et al. Irritable Bowel-like Symptoms in Ulcerative Colitis are as Common in Patients in Deep Remission as in Inflammation: Results From a Population-based Study [the IBSEN Study]. J Crohns Colitis. 2018;12(4):389–93. Epub 2017/12/01. [DOI] [PubMed] [Google Scholar]
- 17.Fukuba N, Ishihara S, Tada Y, et al. Prevalence of irritable bowel syndrome-like symptoms in ulcerative colitis patients with clinical and endoscopic evidence of remission: prospective multicenter study. Scand J Gastroenterol. 2014;49(6):674–80. Epub 2014/03/22. [DOI] [PubMed] [Google Scholar]
- 18.de Bruyn JR, Meijer SL, Wildenberg ME, et al. Development of Fibrosis in Acute and Longstanding Ulcerative Colitis. J Crohns Colitis. 2015;9(11):966–72. Epub 2015/08/08. [DOI] [PubMed] [Google Scholar]
- 19.Chang J, Leong RW, Wasinger VC, et al. Impaired Intestinal Permeability Contributes to Ongoing Bowel Symptoms in Patients With Inflammatory Bowel Disease and Mucosal Healing. Gastroenterology. 2017;153(3):723–31 e1. Epub 2017/06/12. [DOI] [PubMed] [Google Scholar]
- 20.Pai RK, Jairath V, Vande Casteele N, et al. The emerging role of histologic disease activity assessment in ulcerative colitis. Gastrointest Endosc. 2018;88(6):887–98. Epub 2018/08/25. [DOI] [PubMed] [Google Scholar]
- 21.Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut. 2016;65(3):408–14. Epub 2015/05/20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Summary of model fit in backwards elimination


