Abstract
Dementia is becoming increasingly prevalent in Latin America, contrasting with stable or declining rates in North America and Europe. This scenario places unprecedented clinical, social, and economic burden upon patients, families, and health systems. The challenges prove particularly pressing for conditions with highly specific diagnostic and management demands, such as frontotemporal dementia. Here we introduce a research and networking initiative designed to tackle these ensuing hurdles, the Multi-partner consortium to expand dementia research in Latin America (ReDLat). First, we present ReDLat's regional research framework, aimed at identifying the unique genetic, social, and economic factors driving the presentation of frontotemporal dementia and Alzheimer's disease in Latin America relative to the US. We describe ongoing ReDLat studies in various fields and ongoing research extensions. Then, we introduce actions coordinated by ReDLat and the Latin America and Caribbean Consortium on Dementia (LAC-CD) to develop culturally appropriate diagnostic tools, regional visibility and capacity building, diplomatic coordination in local priority areas, and a knowledge-to-action framework toward a regional action plan. Together, these research and networking initiatives will help to establish strong cross-national bonds, support the implementation of regional dementia plans, enhance health systems' infrastructure, and increase translational research collaborations across the continent.
Keywords: dementia, fronto-temporal dementia, SES, SDOH, genetics, Alzheimer's disease, implementation science, Latin America
Dementia Research in Latin America: Toward Unraveling the Unique Genetic and Environmental Factors
The prevalence and incidence of dementia appears to be stable or declining in the US and other high income countries (HIC) (1–3), where cohorts being studied typically consist of relatively homogeneous populations with middle/high social determinants of health (SDH), including socioeconomic status (SES) (4, 5). Latin American countries (LAC) are marked by an opposite scenario (2, 3, 6–10), with increased dementia prevalence amidst a fast demographic shift (3, 11). Together, residents of Argentina, Brazil, Chile, Colombia, Mexico, and Peru make up more than 75% of the region's population. This rise may be driven by unique genetic factors and unfavorable SDH in the region which may influence the prevalence and presentation of dementia (4, 9, 11–16). Across the region, the case of frontotemporal dementia (FTD) is even more challenging than Alzheimer's dementia (AD).
Environmental factors seem to be critical for dementia presentation in the region. SDH may selectively impact dementia in LAC (11, 12) by modulating cognitive progression and brain health burden. However, available reports on SDH have not used sophisticated cognitive and imaging measures, and scant evidence comes from LAC (2, 11). The region presents an important opportunity to study these questions because of the greater disparities in SES and SDH compared to HIC (2, 11). To address these pressing needs without overlooking the region's heterogeneity, harmonized data must be collected from several countries with different SDH levels. This poses a unique challenge for clinical characterization, as these factors will strongly influence dementia presentation (17). Traditional markers of disease severity, including informant ratings, cognitive performance (executive, memory, and social cognition), and neuroimaging features, should be interpreted in the context of SDH factors. Our consortium has developed core composite measures of SDH capturing the heterogeneity of different factors, including SES (food & housing insecurity, access to foods that support health eating habits), education (Early childhood development, language and literacy, higher education), health and health care (access to health services, health literacy), occupation (lifetime employment history, employment status), and social and community context (discrimination, social cohesion, crime and violence).
At the same time, genetic factors also seem to drive dementia presentation in LAC, with apparent stronger familial aggregation of dementia compared with HIC. The region hosts some of the largest populations of familial dementing disorders, and some populations may harbor unique genetic influences conferring increased risk of dementia (4, 9, 11, 15, 18–20). Long isolation periods, endogamy, and the admixture of different ancient populations provide unique opportunity to assess genetic-environmental influences in heterogeneous samples (11, 19). Genetic studies in Latin-American immigrants have shown large effect sizes (14) but these studies have not been explored at a regional level (21). Large consortia have assessed genetic susceptibility mostly in HIC, but other regions, including LAC (4, 11) still remain understudied. The recent development of polygenic risk scores (PRS) to identify individuals at risk for dementia in developed countries are very promising, but they lack validation in more heterogeneous samples (22–25). Our group has found multiple genetic influences of dementia (16, 19, 26–53). The identification of new families may have a long-term impact on therapeutic initiatives (19). Assessing genetic markers, combining common and novel variants, as well as future development of PRS in LAC populations, will bring valuable knowledge about neurogenetic determinants of dementia.
The ways in which the combination of genetic and SDH-related risks interact in the dementia presentation across LAC is not well-understood. New studies in this region are needed to identify novel genetic and gene-environment interactions (i.e., genetic interactions with LAC-specific geography and SDH) leading to dementia, and novel pathways applicable to regional therapies. LAC face a dearth of innovative, harmonized, and cross-regional studies on AD and FTD, and establishing multi-center LAC initiatives is critical for global discovery and research harmonization in these underrepresented populations. Yet, region-specific determinants remain uncharted and, due to different factors (54, 55) the region is still underrepresented in international publications (11). Thus, given that dementia research critically calls for a more global perspective (4), there is an urgent need to compare US and LAC samples via integrative approaches. The development of a more extended regional network, based on multi-institutional harmonized research (11), is crucial for the field.
The ReDLat Approach
The Latin America and the Caribbean Consortium on Dementia [LAC-CD (1)], in association with world-class researchers from the US has developed an agenda to tackle the unique genetic and SDH risk for dementia in LAC (2). In response to this call, the Multi-partner consortium to expand dementia research in Latin America (ReDLat, supported by the NIH-NIA, the Alzheimer's Association, the Rainwater Charitable Foundation, and Global Brain Health Institute) is aimed to identify the unique genetic and SDH factors that drive AD and FTD presentation in LAC relative to the US, including risk factors, cognitive profiles and brain imaging (Figure 1). To this end, we are establishing a first-in-class cohort anchored in six LAC (Argentina, Chile, Colombia, Brazil, Mexico, and Peru), compared to US samples (totaling > 4,200 participants, including 2,100 controls, 1,050 AD patients, and 1,050 FTD patients), led by world-renowned leaders in dementia research. We couple standardized clinical assessments with innovative analytical techniques to account for heterogeneity in these diverse populations. By combining standardized genetic, neuroimaging, and behavioral (cognitive and SDH) measures, we will investigate whether there are unique risk factors for AD and FTD in LAC (e.g., genetic risk factors enriched in LAC populations; underlying cognitive and neural vulnerability due to SDH) compared to US populations. Our plan to recruit large numbers of controls and patients across these diverse populations will provide excellent opportunities to identify new genetic and SDH risks for AD and FTD. In addition, the machine learning strategies we are developing to reduce the impact of background heterogeneity will allow us to refine the accuracy of our association studies.
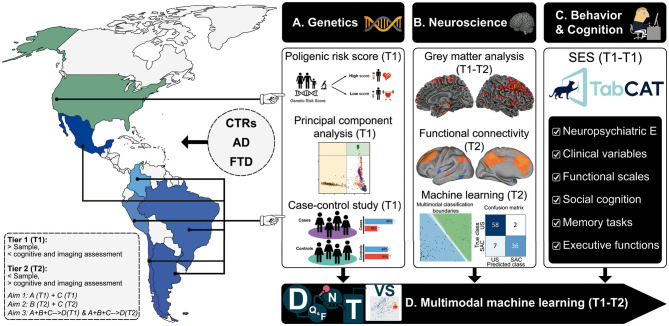
Figure 1.
The ReDLat initiative. Systematic comparisons between LAC and US samples of AD and FTD via a novel, multimodal approach. The multimodal patterns will be assessed with different measures of (A) genetic risk (Aim 1), (B) imaging markers boosted by computational approaches, and (C) harmonized and novel measures of cognitive profiles and SDH (Aim 2). These data sources will be (D) integrated and compared among countries through machine learning (Aim 3) to unveil the main commonalities and differences between US and LAC samples. Tier 1 (T1): Larger study (Aim 1 & 3). Tier 2 (T2, smaller study with deep neurocognitive investigation (Aim 2 & 3). D, Data; Q&F, Quality & feature extraction; N, normalization; T, test; VS, visualization; Neuropsychiatric E, Neuropsychiatric evaluations. Reproduced with authorization from (1).
Our first specific aim is to establish genetic contributions to AD and FTD in diverse LAC cohorts. First, by elucidating the genetic substructure and familial contributions to AD and FTD in LAC relative to the US, we will be able to identify proper populations for replication of our genetic findings. We anticipate that, relative to the US, LAC will have a higher frequency of familial forms of AD and FTD. Discovery of new families with multiple affected individuals will advance efforts to treat AD and FTD in patients with rare mutations. Second, by assembling this large cohort, we will also be well-positioned to establish a preliminary LAC-specific polygenic risk score (PRS) for predicting AD and FTD risk in future samples. We expect that PRS will work best at discriminating patients from controls in the European predominant subpopulation (US and, to a lesser extent, Argentina, Chile) than in the African and Indigenous-majority admixed cohorts (Peru, Brazil, Colombia, Mexico).
In our second specific aim, we will elucidate the impact of SDH on clinical, cognitive, and brain imaging signatures in LAC and the US. To compare patients across regions, we establish standardized neurocognitive measures and harmonization protocols to understand how SDH impacts the manifestations of dementia in LAC. First, we will evaluate how SDH moderates the relationship between age at onset and disease severity in AD and FTD. We anticipate that AD and FTD will emerge at an earlier age in low-SDH vs. high-SDH (dichotomized) patients, and measures of disease severity, including cognitive performance, and multimodal neuroimaging, will be worse in the low-SDH group even after accounting for age. We expect that difference in disease severity ratings, cognition, and multimodal neuroimaging that reflect low vs. high SDH disparities will be greater in LAC patients compared to US patients. Latin America constitute the region with the largest inequalities in the world (56). Moreover, SES/SDH represent a strong influence on dementia risk (2, 57).
Our last specific aim seeks to determine whether genetic risk and SDH yield better discrimination between LAC and US patients as compared with other cognitive, neuroimaging, and clinical variables. To our knowledge, no study has sought to establish which potential predictors prove more sensitive to discriminate between LAC and US patients. In particular, although genetic risk and SDH have the potential to robustly differentiate between such samples, no study has explored their combined role, let alone as compared to other multimodal factors. To address this issue, we will apply data-driven machine-learning pipelines to determine top factors that best discriminate patients in LAC from those in the US (Figure 2). Multimodal measures from controls of each country will be used for population-specific normalization of patient data. We anticipate that the top features, better discriminating LAC from US patients will be related to SDH and genetic risk (e.g., standardized PRS) in comparison to other variables (clinical, cognitive, and imaging measures).
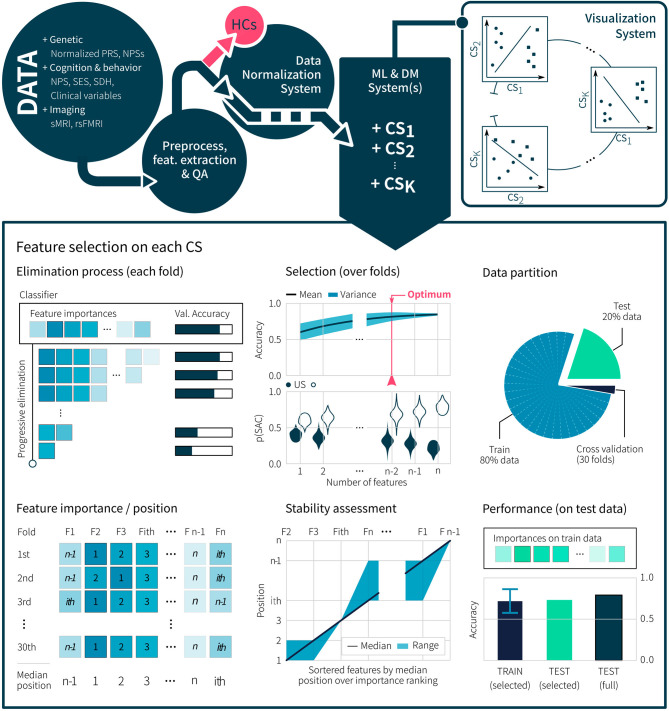
Figure 2.
Machine Learning approach for the discovery of discriminant multidomain features between US and LAC patients with AD and FTD. Genetic, cognitive, SES, SDH, and imaging data are preprocessed with specific normalization methods to extract reliable features. After a feature-based quality assessment (QA), samples are separated in healthy controls (HCs) and patients. HC samples are used to apply normalization and harmonization methods over patient samples, enabling the correction of site-dependent bias in the data. Then, machine learning (ML) and data mining (DM) methods are used for multi-domain classification systems (CS), to find robust features, and to develop visualization dashboards. Each CS performs a progressive feature elimination process to find the most important features and to assess the stability of the model performance using a k-fold cross-validation over the training partition. Finally, performance and generalization in the classification are assessed via test independent partition from the training set.
ReDLat will establish a large LAC cohort of harmonized, well-characterized AD and FTD patients and controls. We anticipate the development of a better understanding of genetic and environmental contributions to neurocognitive manifestations of dementia and the identification of novel targets for risk reduction and disease prevention in LAC. Our large multimodal, cross-sectional study will enable clinical assessment of understudied patient groups, extend and harmonize existing data sets, prompt the development of novel measures, and inform future work on the clinical value of combined multimodal profiles to predict disease presentation and progression in longitudinal studies of diverse populations.
ReDLat Ongoing Progress and Extensions
On January 27, 2020, a kickoff meeting (Figure 3) involved more than 50 leaders in dementia from Latin America and community members from the Global Brain Health Institute (GBHI), Alzheimer's Association, the Tau Consortium, the National Institute of Health (NIH) and private companies at UCSF Mission Bay (58). Since then, ReDLat has been led by an Executive Committee (EC), with working groups (biospecimen handling, cognitive & clinical assessments, neuroimaging, data management, research & publications, and finance), made up of representatives from each site, who meet bi-weekly to review progress, build consensus and address issues as they arise. The ReDLat taskforce (EC and working groups involving more than 90 people) guarantees a shared decision-making process and equal distribution of opportunities for involved centers.
Figure 3.
ReDLat Kickoff meeting at San Francisco, CA. On January 27 2020, regional leaders, local investigators, ReDLat members, as well as authorities from the Global Brain Health Institute (GBHI), Alzheimer's Association, the Tau Consortium, the National Institute of Health (NIH) and other organizations met at UCSF in a US-Latin American Networking on Dementia Symposium. Co-hosted by GBHI and the UCSF Memory and Aging Center, the symposium served to launch ReDLat. Reproduced with authorization from (58).
Substantial effort has been devoted to developing strategies for harmonization of participant enrollment across sites. We created a detailed study-wide protocol (see Supplementary Data 1) and site-specific manuals of operation to ensure accurate and consistent collection. In addition to outlining recruitment procedures and requirements for personnel, the manual provides step-by-step instructions for completing each assessment, processing and shipping specimens, and collecting harmonized neuroimaging data. We adapted the standardized diagnostic assessment used at the UCSF Memory and Aging Center to align with the local sites' procedures (see Supplementary Data 2). The instrument is brief enough to be completed in full for every enrolled participant and will incorporate impressions from the physician who examined the participant, with input from the evaluating neuropsychologist. We also hold in-depth training for personnel at each site, covering neuropsychological testing, clinical assessments, DNA extraction, image acquisition, and genealogy collection procedures, among others. Time is set aside to ensure access to all technological platforms and to teach staff about accurate and timely entry of data into the central database. Videos detailing these instructions are available to each site for ongoing training purposes. Site staff who complete this training are certified to assess participants and this certification will be renewed on an annual basis, either in-person or via video recording, to minimize drift over time. While these trainings were conducted in person for several sites, travel restrictions due to COVID-19 required us to transition to a virtual format.
We have worked with the National Alzheimer's Coordinating Center to obtain permission for adaptation of the Uniform Data Set Cognitive Assessment to Spanish and Portuguese. Based on feedback from investigators, we adapted the language for some tasks to optimize cultural appropriateness at each site. We developed a new instrument to systematically assess SES and SDH. This UCSF-ReDLat questionnaire has been culturally adapted with input from each enrolling site, based on previous reports as well as national censuses from Colombia, Brazil, Chile, and Perú and following cross-cultural implementation recommendations (59–64). The questionnaire captures educational attainment, race and ethnicity, health literacy, financial strain, food insecurity, housing insecurity, childhood trauma, social connections, social isolation, access to healthcare, occupation, and employment status.
Based on the data sharing process detailed above, we performed preliminary analysis. With respect to genetics, we have identified multiple new families with different mutations including PSEN1, PSEN2, TARDBP, GRN, TREM2, MAPT, EPO4, and C9orf72 (see Figure 4C). Regarding the use of machine learning for combination of neuroimaging modalities as well as behavioral/cognitive assessment, we have developed several pipelines with preliminary data and other samples (65–71). We plan to develop a semi-empirical whole-brain multimodal computational approach (MRI, DTI, and fMRI) with mathematical modeling for characterization of global brain dynamics restricted by structural priors (72). This model will also allow data augmentation (73, 74) amplifying the expansion of our machine learning protocol. Regarding SDH and cognitive assessment, we have shown the power of social cognition and SDH (64) in predicting brain health. We have also developed complementary measures of emotion processing (75), and preliminary assessments of naturalistic speech (70), and multi-country validation of our social cognition measures (76) for future assessment of our patients.
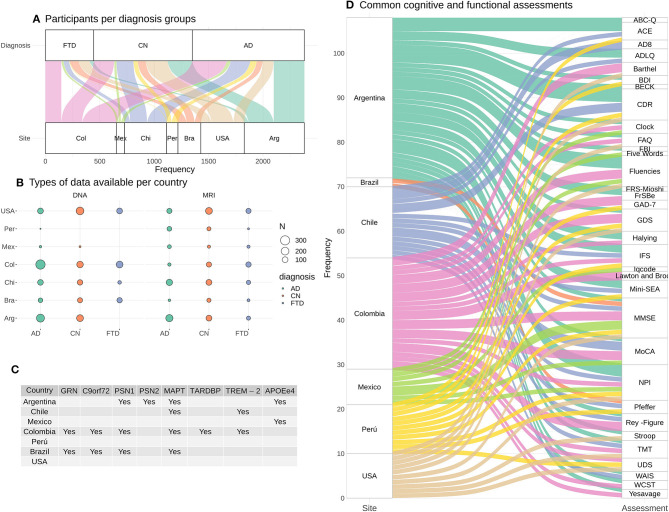
Figure 4.
ReDLat pre-existing data. (A) Estimates of cases with MRI (T1, rs-fMRI, or DTI) and/or DNA per country. (B) Number of participants with DNA and MRI data per diagnosis and per country. (C) Mutations already identified across countries. (D) Summary of the cognitive and functional assessments available in each country.
The assessment of affordable measures such as high-density EEG has emerged as a highly promising transdiagnostic and disease-specific approach for dementia (77–79). EEG provides highly affordable, non-fatiguing, non-invasive measures which can reveal early deficits traceable to well-established neurophysiological processes affected across conditions. Our taskforce has developed expertise in EEG markers, including ERPs, oscillations, connectivity measures, source space analysis, decoding and machine learning approaches, for both active tasks and resting state recordings (29, 35, 80–117) alone or in combination with other technics (35, 81, 82, 87, 89, 89, 112, 118–120). In a regional project based on the ReDLat platform and additionally supported by Takeda, we will extend the protocol to compare multimodal EEG markers. We will explore the robustness of such markers (in comparisons with cognitive and neuroimaging markers) to discriminate between patients (AD and FTD) as well as disease severity and familial status. Also, using multi-feature machine learning, we will combine the ReDLat approaches (using neuroimaging) with EEG features to predict disease subtype, status and severity.
Given the current challenges triggered by the global pandemic, our group has taken advantage of this time to identify new opportunities for expanding the platform use by integrating existing datasets with genetic, cognitive, and imaging analyses of samples in hand. Figure shows an estimate of cases with MRI (4A: T1, rs-fMRI, or DTI) and/or DNA per country. All countries have data from participants that belong to AD, FTD and healthy controls. Figure 4B shows the number of participants with DNA and MRI data per diagnosis and per country. In total, an estimate of 2,208 participants have DNA data, and 1,349 participants have MRI data across diagnosis groups and across countries. Figure 4C provides a summary of the mutations that have already been found in patients and/or patient relatives. Figure 4D highlights a summary of the cognitive and functional assessments used in each country. These preexisting datasets will guarantee the continuity of ReDLat research during total or partial lockdowns.
LAC-CD: Toward Networking, Implementation Science, and Capacity Building
RedLat is also aimed to develop implementation science and capacity building, and several actions has been performed via regional networking, training, and development of educational projects. This is the main goal of the Latin America and the Caribbean Consortium on Dementia [LAC-CD (1)], the regional organization where ReDLat was built. LAC-CD focuses on (a) training health practitioners in dementia field, (b) establishing networks to support multicentric research and clinical practice, (c) harmonizing clinical approaches to diagnosis and post-diagnostic support, (d) validating these approaches in unique populations, (e) increasing the appeal of regional and international grant proposals emerging from LAC networks rather than from individual groups, (f) accelerating access to knowledge and evidence-based decisions via a unified platform, (g) setting up effective communication channels to persuade heads of governments and private agencies of the need for integration and support via national and regional dementia strategies.
Nowadays, the consortium is promoted by the Alzheimer's Association and the Global Brain Health Institute, and holds more than 240 regional members. LAC-CD involves national representatives working in specific priority areas including dementia biomarkers, genetics and epidemiology, a dementia data platform, a clinical trial program, non-pharmacological interventions, and translational research networks. LAC-CD initiatives include (a) empowering local groups, (b) boosting coordinated efforts across the region, and (c) developing a Knowledge-to-Action Framework to develop a regional action plan.
Empowering Local Groups: Education, Visibility, and Capacity Building
A key ambition of our consortium is to create harmonized approaches to dementia diagnosis in order to allow multi-country comparisons. First, we developed diagnostic recommendations (relevant clinical, neuropsychological, and behavioral assessments), for diagnosis of AD and FTD across LAC even where there are no available resources required for classification of dementias (121). Then, supported by the Inter-American Developmental Bank (IDB) and a GBHI pilot funding, we develop a best practice manual for dementia diagnosis1. The manual has been highlighted by the Alzheimer's & Dementia journal (122) and involves more than 40 leaders from expert panels and authors. The manual provides a regional approach to dementia in the region, its epidemiology and different health systems, clinical and neuropsychological assessments and a chapter on carers.
Several initiatives are being created to expand the visibility and dissemination of the consortium activities, including a LAC-CD website (http://lac-cd.org/, in English, Spanish, and Portuguese) providing information on projects, membership, news, opportunities, dissemination products, press releases, and social media. With the Alzheimer's Association, we have launched a LAC-CD – ISTAART webinar including an annual meeting, and periodic webinars focused on different topics.
Regarding capacity building, a Latin American Institute for Brain Health (BrainLat2) will be launched in Chile in 2021 by the University Adolfo Ibanez (UAI). BrainLat will bring together leading national and international institutions to develop support for the ReDLat and LAC-CD expansion and to develop world-class research in brain health. BrainLat will support the Latin American research on dementia with annual seed projects, postdoc positions, infrastructure support, a PhD program, neuroscientific equipment, and permanent support for 16 full research positions.
Boosting Brain Health Coordinated Efforts Across the Region
Albeit the common dementia regional challenges, coordinated multilateral responses are scarce (2, 56). Coordinative efforts such as brain health diplomacy (BHD) and convergence science (123–125) can facilitate the integration of expertise, institutions and strategies between governments and non-governmental organizations (NGOs) in the region. An example of this is a call we develop to raise awareness of the long-term syndemic impact (Figure 5) of coronavirus in aging and dementia across LACs (56). Subsequently, we proposed specific coordinated actions LACs to reduce such impact and new upcoming challenges, including the development of inexpensive mass testing and actions related to telemedicine, care, and research (126).
Figure 5.
Testimonies from Peru highlighting different dimensions of the coronavirus outbreak and their impact on older people, and patients with cognitive decline and their families. The pictures above illustrate the people's vulnerabilities and the unpreparedness of the health system. Top left inset: Enrique (64 years old, Trujillo) suffers from diabetes mellitus but has been unable to get medication for 2 months. He is a shoe repairer with a small mobile stall and, after months of quarantine, he has to go out to work. Top right inset: Juana (64 years old, Trujillo) is a merchant diagnosed with coronavirus 3 months ago, which led to her needing supplemental oxygen and intravenous medications. Given the collapse of the hospitals, she was treated at home by her daughter. She thought she might lose her life, unable to perform simple activities (such as walking and eating) without great effort. Bottom left inset: Enedina (65 years old, Lima) lives with her youngest son who lost his job due to the pandemic restrictions. They live in a precarious room, without electricity, water or drainage. Bottom right Inset: On the other side of Lima, 83-year-old Mrs. Rosita lives with her family in a wealthy district. Her daughter has noted typical dementia symptoms, which have exacerbated since the quarantine. She doesn't understand the isolation, needs constant monitoring and urgently requires a neurological evaluation, but there are no services available due to the pandemic. Photos and testimonies from Peru documented by Alexander Kornhuber and Maritza Pintado Caipa. Individuals and relatives portrayed in the photos have provided written consent for reproduction. Reproduced with authorization from (126).
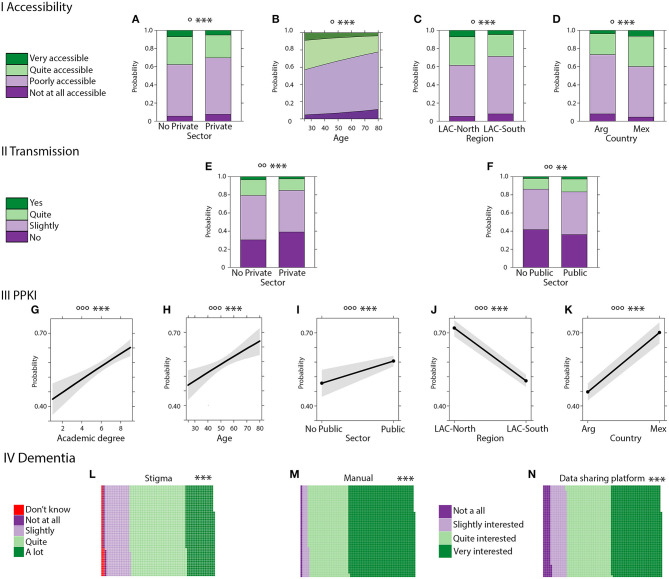
Another example of coordinated actions are related to surveying expert knowledge on dementia across different LACs. We recently assessed multiple dimensions of expert knowledge of health professionals working in aging across LACs (N = 3,365) and its modulation by different factors including expertise-related information (knowledge of public policies), individual differences (work, age, academic degree), and location across LACs (127). Results evidenced a tough knowledge gap of dementia at manifold levels (Figure 6) including lack of access and transmission of public health knowledge, stigma among professionals, and almost complete unawareness of innovative behavioral insights or nudges tools in public health domains. The survey also evidenced a critical need for regional manuals for best practices and data-sharing platforms for both clinical and research initiatives. These specific knowledge gaps and critical needs should be assessed by governmental agencies and NGOs to improve dementia knowledge at regional level.
Figure 6.
Dementia Public policies in Latin America. (I) Public policies accessibility. (A) Probability of response frequency regarding accessibility by sector. (B) Probability of response frequency regarding accessibility by age. (C) Probability of response frequency regarding accessibility by region. (D) Interaction of probability of response frequency of accessibility by country. (II) Public policies transmission. (E) Probability of response frequency regarding transmission by private sector. (F) Probability of response frequency regarding transmission by the public sector. (III) PPKI (public policy knowledge index). (G) Probability of response frequency regarding high PPKI by academic degree. (H) Probability of response frequency regarding high PPKI index by age. (I) Probability of response frequency regarding high PPKI by the public sector. (J) Probability of response frequency regarding PPKI by public region. (K) Probability of response frequency regarding PPKI by country. IV Aging. (L) Proportion of responses about aging stigma. (M) Proportion of responses about interest in aging and dementia manual. (N) Proportion of responses about interest in a data-sharing platform. Significance (p values): effects significance (**p ≤ 0.05, ***p ≤ 0.01), model significance (°p ≤ 0.1, °p ≤ 0.05, °p ≤ 0.01). Academic degree: 1: No reported education, 2: Technicians, 3: Tertiaries, 4; Certificates, 5: Undergrads, 6: Hospital Interns, 7: Post-graduate Specialization, 8: Master's Degree, 9: Ph.D. Reproduced with authorization from (127).
A Knowledge-to-Action Framework for a Regional Action Plan
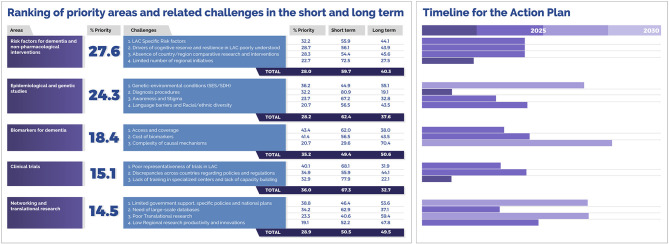
LAC-CD has advanced a Knowledge to Action Framework (KtAF) toward regional action plan for dementia (2). Initially, we identified cross-regional priority areas (Figure 7), namely: (a) risk factors for dementia and non-pharmacological interventions, (b) epidemiological and genetic studies, (c) biomarkers for dementia, (d) clinical trials, and (f) networking and translational research. Evidence-based strategies were proposed to tackle ensuing challenges while considering key sources of complexity (genetic isolates, admixture in populations, environmental factors, and barriers to effective interventions). These strategies were mapped to the above priorities, laying the conceptual groundwork for our further KtAF. These procedures have been endorsed by experts as vehicles to third-generation knowledge (128).
Figure 7.
Priority levels assigned to core areas and challenges via a knowledge inquiry and related actions timelines. LAC-CD regional experts (N = 248) were presented with a survey and were asked to rank the 5 areas and associated challenges in order of priority. We calculated the percentage of respondents who rated these within the top two priorities and used these to rank both areas and challenges. The right inset shows the timeline for the proposed actions. Experts were also asked to deliver their views about a feasible timeline to address these challenges and actions (0–5 or 5–10 years) (% = Mean % of responses). Reproduced with authorization from (2).
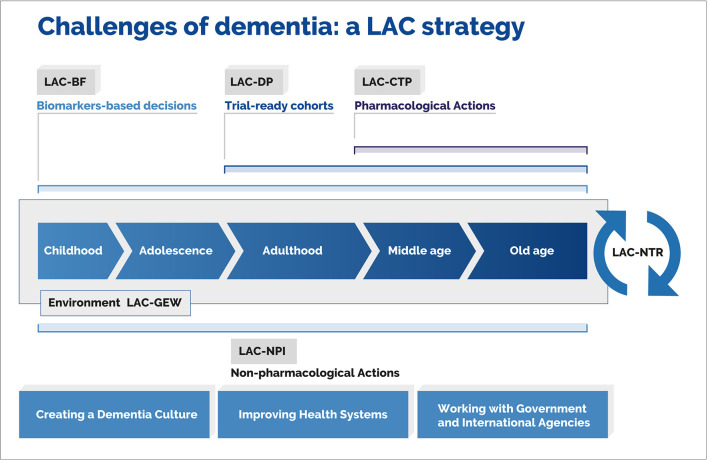
The KtAF comprises five workgroups, each responsible for specific tasks (Figure 8). A Non-pharmacological Interventions Workgroup (LAC-NPI) will address regional risk factors. It will align with international initiatives, reinforce surveillance by incorporating the World Health Organization (WHO) STEPwise approach, foster national dementia plans across the region, develop research on cognitive reserve and resilience, and improve training and educational programs via the GBHI and the Alzheimer's Association. The Genetic and Epidemiology Workgroup (LAC-GEW) will aim to implement epidemiological studies across LACs, identify lifelong factors impacting neurocognitive development, expand family history and genetic protocols, develop a harmonized digital data-sharing platform, boost research on genetic heterogeneity, and support the creation of a regional LAC dementia observatory. The Biomarker Framework (LAC-BF) will strive to validate complementary affordable biomarkers against the A/T/N framework, focusing on cognitive assessment, eye-tracking, non-invasive peripheral markers (i.e., plasma markers.), and multimodal neuroimaging (e.g., EEG, MRI, fMRI, DTI) combined with machine- and deep-learning algorithms (65, 66, 68, 71, 82, 87, 112, 126, 129–134) and novel theoretical approaches (118, 119, 135–139). These unspecific markers can be validated with the measurement and comparison with A/T/N framework's canonical markers. Thus, affordable measures can support the validation of low-cost biomarkers in the region. The Clinical Trial Program (LAC-CTP) will identify main countries and hubs possessing the infrastructure for prevention trials, connect these programs with national regulatory agencies for regional harmonization, develop trial-ready cohorts across countries, and launch a clinical trial training program to empower less experienced centers. Finally, a Network for Translational Research (LAC-NTR) will promote translational research through a network of scientists, clinicians, pharmaceutical leaders, and government representatives. It will also develop digital platforms to maximize collaboration and exchange of resources, while promoting synergy among regional initiatives. Through the joint effort of these workgroups, the KtA will increase awareness, knowledge, and resources leading to global equity in the fight against dementia.
Figure 8.
Knowledge-to-action framework. The diagram captures challenges posed by dementia and the related mapping of key actions. Such actions are linked to specific working groups that have been included in the framework. This approach comprises a biomarker framework (LAC-BF), genetics and epidemiology workgroup (LAC-GEW), dementia platform (LAC-DP), clinical trial program (LAC-CTP), non-pharmacological interventions (LAC-NPI), and an LAC network for translational research (LAC-NTR). Reproduced with authorization from (2).
Conclusions
Our recently launched consortium grasps relevant features for upcoming progress and expansion. Regarding multidisciplinary innovation, ReDLat focuses on the largely ignored convergence of genetic and SDH risks, especially considering multimodal (clinical, cognitive, neuroimaging) effects and innovative machine learning techniques. Regarding translational impact, our project is research-based, but geared to capacity building and implementation science (diagnosis, education, support, evidence-based policy), favoring regional commitment. We also aim to empower local ideas in a global networking landscape, as our initiative merges bottom-up LAC proposals into a single landscape. We promote win-win HIC-LMIC collaborations facing local needs from a local-global perspective. We also focus on barriers, by tackling HIC-LMIC cultural-communicative differences between researchers, assessing underrepresented populations, pushing changes regarding lack of trust among teams based on an equitable platform for collective decision making, and bringing collective support to minimize emergent leaders' disregards. We believe these actions promoting brain health and dementia across LACs and globally will help to truly transform challenges into opportunities.
Author Contributions
AI designed the proposal under supervision of BM. AI wrote the drafts, discussed contributions from all co-authors. All authors searched the literature, participated in discussing the contents of the paper, contributed to editing, and approved the final version of the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1The manual is available here: http://lac-cd.org/en/2020/06/17/manual-for-best-practices-for-the-dementia-diagnosis/
Funding. The consortium was supported by the Multi-partner Consortium to Expand Dementia Research in Latin America [ReDLat, by National Institutes of Health, National Institutes of Aging (R01 AG057234), Alzheimer's Association (SG-20-725707), Rainwater Charitable Fundation- Tau, and Global Brain Health Institute]. AI was partially supported by grants from Takeda CW2680521, Alzheimer's Association GBHI ALZ UK-20-639295, CONICET, ANID/FONDAP/15150012, Sistema General de Regalías (BPIN2018000100059), Universidad del valle (CI 5316), and The Inter-American Development Bank (IDB). ASI was also partially supported by ANID/FONDAP/15150012. CD-A was partially supported by 2018-AARG-591107 and ANID/FONDEF ID20I10152. The contents of this publication are solely the responsibility of the authors and do not represent the official views of these institutions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2021.631722/full#supplementary-material
References
- 1.Ibanez A, Parra MA, Butler C. For Latin America and the Caribbean Consortium on Dementia (LAC-CD). The Latin America and the Caribbean Consortium on Dementia (LAC-CD): From Networking to Research to Implementation Science. J Alzheimers Dis. (2021). 10.3233/JAD-201384. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parra M, Baez S, Sedeño L, Gonzalez Campo C, Santamaría-García H, Aprahamian I, et al. Dementia in Latin America: paving the way towards a regional action plan. Alzheimers Dement. (2021) 17:295–13. 10.1002/alz.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu YT, Beiser AS, Breteler MMB, Fratiglioni L, Helmer C, Hendrie HC, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. (2017) 13:327–39. 10.1038/nrneurol.2017.63 [DOI] [PubMed] [Google Scholar]
- 4.Alladi S, Hachinski V. World dementia: one approach does not fit all. Neurology. (2018) 91:264–70. 10.1212/WNL.0000000000005941 [DOI] [PubMed] [Google Scholar]
- 5.Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Arlington, VA: American Psychiatric Pub; (2013). [Google Scholar]
- 6.Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. (2012) 2:CD005562. 10.1002/14651858.CD005562.pub2 [DOI] [PubMed] [Google Scholar]
- 7.Prince M, Bryce R, Ferr C. World Alzheimer Report 2011. Alzheimer's Disease International; (2011). [Google Scholar]
- 8.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. (2013) 9:63–75 e2. 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 9.Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, et al. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, risk factors. Lancet Neurol. (2008) 7:812–26. 10.1016/S1474-4422(08)70169-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . Dementia: A Public Health Priority. World Health Organization; (2012). [Google Scholar]
- 11.Parra MA, Baez S, Allegri R, Nitrini R, Lopera F, Slachevsky A, et al. Dementia in Latin America: assessing the present and envisioning the future. Neurology. (2018) 90:222–31. 10.1212/WNL.0000000000004897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibáñez A, Sedeño L, García AM, Deacon RMJ, Cogram P. Human and Animal Models for Translational Research on Neurodegeneration: Challenges and Opportunities From South America. Switzerland: Frontiers Media; (2018). 10.3389/978-2-88945-494-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baez S, Ibanez A. Dementia in Latin America: an emergent silent Tsunami. Front Aging Neurosci. (2016) 8:253. 10.3389/fnagi.2016.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reitz C, Mayeux R. Genetics of Alzheimer's disease in Caribbean hispanic and African American populations. Biol Psychiatry. (2014) 75:534–41. 10.1016/j.biopsych.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tosto G, Bird TD, Tsuang D, Bennett DA, Boeve BF, Cruchaga C, et al. Polygenic risk scores in familial Alzheimer disease. Neurology. (2017) 88:1180–6. 10.1212/WNL.0000000000003734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguirre-Acevedo DC, Lopera F, Henao E, Tirado V, Munoz C, Giraldo M, et al. Cognitive decline in a Colombian kindred with autosomal dominant Alzheimer disease: a retrospective cohort study. JAMA Neurol. (2016) 73:431–8. 10.1001/jamaneurol.2015.4851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meeker KL, Wisch JK, Hudson D, Coble D, Xiong C, Babulal GM, et al. Socioeconomic Status mediates racial differences seen using the AT(N) framework. Ann Neurol. (2021) 89:254–65. 10.1002/ana.25948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vega IE, Cabrera LY, Wygant CM, Velez-Ortiz D, Counts SE. Alzheimer's disease in the Latino community: intersection of genetics and social determinants of health. J Alzheimers Dis. (2017) 58:979–92. 10.3233/JAD-161261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardona-Gomez GP, Lopera Dementia F. Preclinical studies in neurodegeneration and its potential for translational medicine in South America. Front Aging Neurosci. (2016) 8:304. 10.3389/fnagi.2016.00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammadi D. Hunting for help in South America. Lancet Neurol. (2016) 15:1120–1. 10.1016/S1474-4422(16)30166-1 [DOI] [PubMed] [Google Scholar]
- 21.Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. World Alzheimer Report 2016: Improving Healthcare for People Living With Dementia: Coverage, Quality and Costs Now and in the Future. London: Alzheimer's Disease International (ADI) (2016). [Google Scholar]
- 22.Tan CH, Fan CC, Mormino EC, Sugrue LP, Broce IJ, Hess CP, et al. Alzheimer's Disease Neuroimaging, Polygenic hazard score: an enrichment marker for Alzheimer's associated amyloid and tau deposition. Acta Neuropathol. (2018) 135:85–93. 10.1007/s00401-017-1789-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desikan RS, Fan CC, Wang Y, Schork AJ, Cabral HJ, Cupples LA, et al. Genetic assessment of age-associated Alzheimer disease risk: development and validation of a polygenic hazard score. PLoS Med. (2017) 14:e1002258. 10.1371/journal.pmed.1002289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacour A, Espinosa A, Louwersheimer E, Heilmann S, Hernandez I, Wolfsgruber S, et al. Genome-wide significant risk factors for Alzheimer's disease: role in progression to dementia due to Alzheimer's disease among subjects with mild cognitive impairment. Mol Psychiatry. (2017) 22:153–60. 10.1038/mp.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan CH, Hyman BT, Tan JJX, Hess CP, Dillon WP, Schellenberg GD, et al. Polygenic hazard scores in preclinical Alzheimer disease. Ann Neurol. (2017) 82:484–8. 10.1002/ana.25029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdes Hernandez MDC, Abu-Hussain J, Qiu X, Priller J, Parra Rodriguez M, Pino M, et al. Structural neuroimaging differentiates vulnerability from disease manifestation in colombian families with Huntington's disease. Brain Behav. (2019) 9:e01343. 10.1002/brb3.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia AM, Sedeno L, Trujillo N, Bocanegra Y, Gomez D, Pineda D, et al. Language deficits as a preclinical window into Parkinson's disease: evidence from asymptomatic Parkin and Dardarin mutation carriers. J Int Neuropsychol Soc. (2017) 23:150–8. 10.1017/S1355617716000710 [DOI] [PubMed] [Google Scholar]
- 28.Garcia AM, Abrevaya S, Kozono G, Cordero IG, Cordoba M, Kauffman MA, et al. The cerebellum and embodied semantics: evidence from a case of genetic ataxia due to STUB1 mutations. J Med Genet. (2017) 54:114–24. 10.1136/jmedgenet-2016-104148 [DOI] [PubMed] [Google Scholar]
- 29.Pietto M, Parra MA, Trujillo N, Flores F, Garcia AM, Bustin J, et al. Behavioral and electrophysiological correlates of memory binding deficits in patients at different risk levels for Alzheimer's disease. J Alzheimers Dis. (2016) 53:1325–40. 10.3233/JAD-160056 [DOI] [PubMed] [Google Scholar]
- 30.Rios-Romenets S, Lopera F, Sink KM, Hu N, Lian Q, Guthrie H, et al. Baseline demographic, clinical, and cognitive characteristics of the Alzheimer's Prevention Initiative (API) Autosomal-Dominant Alzheimer's Disease Colombia Trial. Alzheimers Dement. (2020) 16:1023–30. 10.1002/alz.12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos C, Aguillon D, Cordano C, Lopera F. Genetics of dementia: insights from Latin America. Dement Neuropsychol. (2020) 14:223–36. 10.1590/1980-57642020dn14-030004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quiroz YT, Zetterberg H, Reiman EM, Chen Y, Su Y, Fox-Fuller JT, et al. Plasma neurofilament light chain in the presenilin 1 E280A autosomal dominant Alzheimer's disease kindred: a cross-sectional and longitudinal cohort study. Lancet Neurol. (2020) 19:513–21. 10.1016/S1474-4422(20)30137-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guzman-Velez E, Martinez J, Papp K, Baena A, Vila-Castelar C, Artola A, et al. Associative memory and in vivo brain pathology in asymptomatic presenilin-1 E280A carriers. Neurology. (2020) 95:e1312–21. 10.1212/WNL.0000000000010177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arboleda-Velasquez JF, Lopera F, O'Hare M, Delgado-Tirado S, Marino C, Chmielewska N, et al. Resistance to autosomal dominant Alzheimer's disease in an APOE3 Christchurch homozygote: a case report. Nat Med. (2019) 25:1680–3. 10.1038/s41591-019-0611-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parra MA, Mikulan E, Trujillo N, Sala SD, Lopera F, Manes F, et al. Brain information sharing during visual short-term memory binding yields a memory biomarker for familial Alzheimer's disease. Curr Alzheimer Res. (2017) 14:1335–47. 10.2174/1567205014666170614163316 [DOI] [PubMed] [Google Scholar]
- 36.Giraldo M, Lopera F, Siniard AL, Corneveaux JJ, Schrauwen I, Carvajal J, et al. Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer's disease. Neurobiol Aging. (2013) 34:2077 e11–8. 10.1016/j.neurobiolaging.2013.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takada LT, Bahia VS, Guimaraes HC, Costa TV, Vale TC, Rodriguez RD, et al. GRN and MAPT mutations in 2 frontotemporal dementia research centers in Brazil. Alzheimer Dis Assoc Disord. (2016) 30:310–7. 10.1097/WAD.0000000000000153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahia VS, Kok F, Marie SN, Shinjo SO, Caramelli P, Nitrini R. Polymorphisms of APOE and LRP genes in Brazilian individuals with Alzheimer disease. Alzheimer Dis Assoc Disord. (2008) 22:61–5. 10.1097/WAD.0b013e31815a9da7 [DOI] [PubMed] [Google Scholar]
- 39.Fanganiello RD, Kimonis VE, Corte CC, Nitrini R, Passos-Bueno MR. A Brazilian family with hereditary inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia. Braz J Med Biol Res. (2011) 44:374–80. 10.1590/S0100-879X2011007500028 [DOI] [PubMed] [Google Scholar]
- 40.Huang N, Marie SK, Kok F, Nitrini R. Familial Creutzfeldt-Jakob disease associated with a point mutation at codon 210 of the prion protein gene. Arquivos Neuro Psiquiatria. (2001) 59:932–5. 10.1590/S0004-282X2001000600017 [DOI] [PubMed] [Google Scholar]
- 41.Nishimura AL, Oliveira JR, Matioli SR, Brito-Marques PR, Bahia VS, Nitrini R, et al. Analysis of the disease risk locus DXS1047 polymorphism in Brazilian Alzheimer patients. Mol Psychiatry. (2000) 5:563–6. 10.1038/sj.mp.4000767 [DOI] [PubMed] [Google Scholar]
- 42.Nishimura AL, Oliveira JR, Mitne-Neto M, Guindalini C, Nitrini R, Bahia VS, et al. Lack of association between the brain-derived neurotrophin factor (C-270T) polymorphism and late-onset Alzheimer's disease (LOAD) in Brazilian patients. J Mol Neurosci. (2004) 22:257–60. 10.1385/JMN:22:3:257 [DOI] [PubMed] [Google Scholar]
- 43.Baez S, Couto B, Herrera E, Bocanegra Y, Trujillo-Orrego N, Madrigal-Zapata L, et al. Tracking the cognitive, social, and neuroanatomical profile in early neurodegeneration: type III cockayne syndrome. Front Aging Neurosci. (2013) 5:80. 10.3389/fnagi.2013.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arboleda-Velasquez JF, Lopera F, Lopez E, Frosch MP, Sepulveda-Falla D, Gutierrez JE, et al. C455R notch3 mutation in a Colombian CADASIL kindred with early onset of stroke. Neurology. (2002) 59:277–9. 10.1212/WNL.59.2.277 [DOI] [PubMed] [Google Scholar]
- 45.Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gutierrez Gomez M, Langois CM, et al. Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA Neurol. (2015) 72:316–24. 10.1001/jamaneurol.2014.3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kosik KS, Munoz C, Lopez L, Arcila ML, Garcia G, Madrigal L, et al. Homozygosity of the autosomal dominant Alzheimer disease presenilin 1 E280A mutation. Neurology. (2015) 84:206–8. 10.1212/WNL.0000000000001130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norton DJ, Amariglio R, Protas H, Chen K, Aguirre-Acevedo DC, Pulsifer B, et al. Subjective memory complaints in preclinical autosomal dominant Alzheimer disease. Neurology. (2017) 89:1464–70. 10.1212/WNL.0000000000004533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parra MA, Abrahams S, Logie RH, Mendez LG, Lopera F, Della Sala S. Visual short-term memory binding deficits in familial Alzheimer's disease. Brain. (2010) 133:2702–13. 10.1093/brain/awq148 [DOI] [PubMed] [Google Scholar]
- 49.Parra MA, Saarimaki H, Bastin ME, Londono AC, Pettit L, Lopera F, et al. Memory binding and white matter integrity in familial Alzheimer's disease. Brain. (2015) 138:1355–69. 10.1093/brain/awv048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quiroz YT, Ally BA, Celone K, McKeever J, Ruiz-Rizzo AL, Lopera F, et al. Event-related potential markers of brain changes in preclinical familial Alzheimer disease. Neurology. (2011) 77:469–75. 10.1212/WNL.0b013e318227b1b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quiroz YT, Schultz AP, Chen K, Protas HD, Brickhouse M, Fleisher AS, et al. Brain imaging and blood biomarker abnormalities in children with autosomal dominant Alzheimer disease: a cross-sectional study. JAMA Neurol. (2015) 72:912–9. 10.1001/jamaneurol.2015.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, et al. Dominantly inherited Alzheimer, symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology. (2014) 83:253–60. 10.1212/WNL.0000000000000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sepulveda-Falla D, Barrera-Ocampo A, Hagel C, Korwitz A, Vinueza-Veloz MF, Zhou K, et al. Familial Alzheimer's disease-associated presenilin-1 alters cerebellar activity and calcium homeostasis. J Clin Invest. (2014) 124:1552–67. 10.1172/JCI66407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parra MA. Overcoming barriers in cognitive assessment of Alzheimer's disease. Dement Neuropsychol. (2014) 8:95–98. 10.1590/S1980-57642014DN82000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Custodio N, Wheelock A, Thumala D, Slachevsky A. Dementia in Latin America: epidemiological evidence and implications for public policy. Front Aging Neurosci. (2017) 9:221. 10.3389/fnagi.2017.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibanez A Kosik KS Latin America and the Caribbean Consortium on Dementia (LAC-CD) . COVID-19 in older people with cognitive impairment in Latin America. Lancet Neurol. (2020) 19:719–21. 10.1016/S1474-4422(20)30270-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Resende EPF, Llibre Guerra JJ, Miller BL. Health and socioeconomic inequities as contributors to brain health. JAMA Neurol. (2019) 76:633–4. 10.1001/jamaneurol.2019.0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kavanagh N. Dementia Leaders Gather for Launch of Latin American Research Initiative, News. San Francisco, CA: GBHI; (2020). [Google Scholar]
- 59.MHAS . Parents and Help to Parents. Gateway to Global Aging Data. Mexican Health and Aging Study; (2015). Available online at: https://g2aging.org/?section=module&moduleid=752&display=flowchart (accessed January 15). [Google Scholar]
- 60.Brewster PW, Melrose RJ, Marquine MJ, Johnson JK, Napoles A, MacKay-Brandt A, et al. Life experience and demographic influences on cognitive function in older adults. Neuropsychology. (2014) 28:846–58. 10.1037/neu0000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santoro N, Sutton-Tyrrell K. The SWAN song: Study of Women's Health Across the Nation's recurring themes. Obstet Gynecol Clin North Am. (2011) 38:417–23. 10.1016/j.ogc.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nabe-Nielsen K, Hansen AM, Ishtiak-Ahmed K, Grynderup MB, Gyntelberg F, Islamoska S, et al. Night shift work, long working hours and dementia: a longitudinal study of the Danish Work Environment Cohort Study. BMJ Open. (2019) 9:e027027. 10.1136/bmjopen-2018-027027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.N.C.f.Statistics H . Survey Description. Hyattsville, MD: National Health Interview Survey; (2015). [Google Scholar]
- 64.Santamaria-Garcia H, Baez S, Gomez C, Rodriguez-Villagra O, Huepe D, Portela M, et al. The role of social cognition skills and social determinants of health in predicting symptoms of mental illness. Transl Psychiatry. (2020) 10:165. 10.1038/s41398-020-0852-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bachli MB, Sedeno L, Ochab JK, Piguet O, Kumfor F, Reyes P, et al. Evaluating the reliability of neurocognitive biomarkers of neurodegenerative diseases across countries: a machine learning approach. Neuroimage. (2020) 208:116456. 10.1016/j.neuroimage.2019.116456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.C . Gonzalez Campo, Salamone PC, Rodriguez-Arriagada N, Richter F, Herrera E, Bruno D, et al. Fatigue in multiple sclerosis is associated with multimodal interoceptive abnormalities. Mult Scler. (2020) 26:1845–53. 10.1177/1352458519888881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torres-Prioris MJ, Lopez-Barroso D, Camara E, Fittipaldi S, Sedeno L, Ibanez A, et al. Neurocognitive signatures of phonemic sequencing in expert backward speakers. Sci Rep. (2020) 10:10621. 10.1038/s41598-020-67551-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abrevaya S, Fittipaldi S, Garcia AM, Dottori M, Santamaria-Garcia H, Birba A, et al. At the Heart of neurological dimensionality: cross-nosological and multimodal cardiac interoceptive deficits. Psychosom Med. (2020) 82:850–61. 10.1097/PSY.0000000000000868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ipina IP, Kehoe PD, Kringelbach M, Laufs H, Ibanez A, Deco G, et al. Modeling regional changes in dynamic stability during sleep and wakefulness. Neuroimage. (2020) 215:116833. 10.1016/j.neuroimage.2020.116833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eyigoz E, Courson M, Sedeno L, Rogg K, Orozco-Arroyave JR, Noth E, et al. From discourse to pathology: automatic identification of Parkinson's disease patients via morphological measures across three languages. Cortex. (2020) 132:191–205. 10.1016/j.cortex.2020.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moguilner S, Garcia AM, Perl YS, Tagliazucchi E, Piguet O, Kumfor F, et al. Dynamic brain fluctuations outperform connectivity measures and mirror pathophysiological profiles across dementia subtypes: a multicenter study. Neuroimage. (2021) 225:117522. 10.1016/j.neuroimage.2020.117522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perl YS, Pallavicini C, Pérez Ipiña I, Demertzi A, Bonhomme V, Martial C, et al. Perturbations in dynamical models of whole-brain activity dissociate between the level and stability of consciousness. bioRxiv. (2020) 185157:2020.07.02.185157. 10.1101/2020.07.02.185157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arbabyazd DM, Shen K, Wang Z, Hofmann-Apitius M, McIntosh AR, Battaglia D, et al. Completion and augmentation of connectomic datasets in dementia and Alzheimer's Disease using Virtual Patient Cohorts. bioRxiv. (2020) 911248:2020.01.18.911248. 10.1101/2020.01.18.911248 [DOI] [Google Scholar]
- 74.Perl YS, Pallavicini C, Ipiña IP, Kringelbach M, Deco G, Laufs H, et al. Data augmentation based on dynamical systems for the classification of brain states. Chaos Solitons Fract. (2020) 139:110069. 10.1016/j.chaos.2020.110069 [DOI] [Google Scholar]
- 75.Yoris A, Legaz A, Abrevaya S, Alarco S, Lopez Pelaez J, Sanchez R, et al. Multicentric evidence of emotional impairments in hypertensive heart disease. Sci Rep. (2020) 10:14131. 10.1038/s41598-020-70451-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Quesque F, Coutrot A, Cox S, de Souza Leonardo C, Baez S, Felipe CJ, et al. Culture shapes our understanding of others' thoughts and emotions: an investigation across 12 countries. PsyArXiv [Preprints]. (2020). 10.31234/osf.io/tg2ay [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rossini PM, Di Iorio R, Vecchio F, Anfossi M, Babiloni C, Bozzali M, et al. Early diagnosis of Alzheimer's disease: the role of biomarkers including advanced EEG signal analysis. Report from the IFCN-sponsored panel of experts. Clin Neurophysiol. (2020) 131:1287–310. 10.1016/j.clinph.2020.03.003 [DOI] [PubMed] [Google Scholar]
- 78.McMackin R, Muthuraman M, Groppa S, Babiloni C, Taylor JP, Kiernan MC, et al. Measuring network disruption in neurodegenerative diseases: new approaches using signal analysis. J Neurol Neurosurg Psychiatry. (2019) 90:1011–20. 10.1136/jnnp-2018-319581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hampel H, Toschi N, Babiloni C, Baldacci F, Black KL, Bokde ALW, et al. Revolution of alzheimer precision neurology. Passageway of systems biology and neurophysiology. J Alzheimers Dis. (2018) 64:S47–105. 10.3233/JAD-179932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Josefsson A, Ibanez A, Parra M, Escudero J. Network analysis through the use of joint-distribution entropy on EEG recordings of MCI patients during a visual short-term memory binding task. Healthc Technol Lett. (2019) 6:27–31. 10.1049/htl.2018.5060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoris A, Abrevaya S, Esteves S, Salamone P, Lori N, Martorell M, et al. Multilevel convergence of interoceptive impairments in hypertension: New evidence of disrupted body-brain interactions. Hum Brain Mapp. (2018) 39:1563–81. 10.1002/hbm.23933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salamone PC, Esteves S, Sinay VJ, Garcia-Cordero I, Abrevaya S, Couto B, et al. Altered neural signatures of interoception in multiple sclerosis. Hum Brain Mapp. (2018) 39:4743–54. 10.1002/hbm.24319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mikulan E, Hesse E, Sedeno L, Bekinschtein T, Sigman M, Garcia MDC, et al. Intracranial high-gamma connectivity distinguishes wakefulness from sleep. Neuroimage. (2018) 169:265–77. 10.1016/j.neuroimage.2017.12.015 [DOI] [PubMed] [Google Scholar]
- 84.Garcia-Cordero I, Esteves S, Mikulan EP, Hesse E, Baglivo FH, Silva W, et al. Attention, in and out: scalp-level and intracranial EEG correlates of interoception and exteroception. Front Neurosci. (2017) 11:411. 10.3389/fnins.2017.00411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dottori M, Sedeno L, Martorell Caro M, Alifano F, Hesse E, Mikulan E, et al. Towards affordable biomarkers of frontotemporal dementia: a classification study via network's information sharing. Sci Rep. (2017) 7:3822. 10.1038/s41598-017-04204-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amoruso L, Ibanez A, Fonseca B, Gadea S, Sedeno L, Sigman M, et al. Variability in functional brain networks predicts expertise during action observation. Neuroimage. (2017) 146:690–700. 10.1016/j.neuroimage.2016.09.041 [DOI] [PubMed] [Google Scholar]
- 87.Melloni M, Billeke P, Baez S, Hesse E, de la Fuente L, Forno G, et al. Your perspective and my benefit: multiple lesion models of self-other integration strategies during social bargaining. Brain. (2016) 139:3022–40. 10.1093/brain/aww231 [DOI] [PubMed] [Google Scholar]
- 88.Gonzalez-Gadea ML, Sigman M, Rattazzi A, Lavin C, Rivera-Rei A, Marino J, et al. Neural markers of social and monetary rewards in children with Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder. Sci Rep. (2016) 6:30588. 10.1038/srep30588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melloni M, Sedeno L, Hesse E, Garcia-Cordero I, Mikulan E, Plastino A, et al. Cortical dynamics and subcortical signatures of motor-language coupling in Parkinson's disease. Sci Rep. (2015) 5:11899. 10.1038/srep11899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gonzalez-Gadea ML, Chennu S, Bekinschtein TA, Rattazzi A, Beraudi A, Tripicchio P, et al. Predictive coding in autism spectrum disorder and attention deficit hyperactivity disorder. J Neurophysiol. (2015) 114:2625–36. 10.1152/jn.00543.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Couto B, Adolfi F, Velasquez M, Mesow M, Feinstein J, Canales-Johnson A, et al. Heart evoked potential triggers brain responses to natural affective scenes: a preliminary study. Auton Neurosci. (2015) 193:132–7. 10.1016/j.autneu.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 92.Canales-Johnson A, Silva C, Huepe D, Rivera-Rei A, Noreika V, Garcia Mdel C, et al. Auditory feedback differentially modulates behavioral and neural markers of objective and subjective performance when tapping to your heartbeat. Cereb Cortex. (2015) 25:4490–503. 10.1093/cercor/bhv076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ibanez A, Parra MA. Mapping memory binding onto the connectome's temporal dynamics: toward a combined biomarker for Alzheimer's disease. Front Hum Neurosci. (2014) 8:237. 10.3389/fnhum.2014.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ibanez A, Aguado J, Baez S, Huepe D, Lopez V, Ortega R, et al. From neural signatures of emotional modulation to social cognition: individual differences in healthy volunteers and psychiatric participants. Soc Cogn Affect Neurosci. (2014) 9:939–50. 10.1093/scan/nst067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Escobar MJ, Huepe D, Decety J, Sedeno L, Messow MK, Baez S, et al. Brain signatures of moral sensitivity in adolescents with early social deprivation. Sci Rep. (2014) 4:5354. 10.1038/srep05354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barttfeld P, Petroni A, Baez S, Urquina H, Sigman M, Cetkovich M, et al. Functional connectivity and temporal variability of brain connections in adults with attention deficit/hyperactivity disorder and bipolar disorder. Neuropsychobiology. (2014) 69:65–75. 10.1159/000356964 [DOI] [PubMed] [Google Scholar]
- 97.Amoruso L, Sedeno L, Huepe D, Tomio A, Kamienkowski J, Hurtado E, et al. Time to Tango: expertise and contextual anticipation during action observation. Neuroimage. (2014) 98:366–85. 10.1016/j.neuroimage.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 98.Ibanez A, Cardona JF, Dos Santos YV, Blenkmann A, Aravena P, Roca M, et al. Motor-language coupling: direct evidence from early Parkinson's disease and intracranial cortical recordings. Cortex. (2013) 49:968–84. 10.1016/j.cortex.2012.02.014 [DOI] [PubMed] [Google Scholar]
- 99.Escobar MJ, Rivera-Rei A, Decety J, Huepe D, Cardona JF, Canales-Johnson A, et al. Attachment patterns trigger differential neural signature of emotional processing in adolescents. PLoS ONE. (2013) 8:e70247. 10.1371/journal.pone.0070247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chennu S, Noreika V, Gueorguiev D, Blenkmann A, Kochen S, Ibanez A, et al. Expectation and attention in hierarchical auditory prediction. J Neurosci. (2013) 33:11194–205. 10.1523/JNEUROSCI.0114-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barttfeld P, Amoruso L, Ais J, Cukier S, Bavassi L, Tomio A, et al. Organization of brain networks governed by long-range connections index autistic traits in the general population. J Neurodev Disord. (2013) 5:16. 10.1186/1866-1955-5-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ibanez A, Riveros R, Hurtado E, Gleichgerrcht E, Urquina H, Herrera E, et al. The face and its emotion: right N170 deficits in structural processing and early emotional discrimination in schizophrenic patients and relatives. Psychiatry Res. (2012) 195:18–26. 10.1016/j.psychres.2011.07.027 [DOI] [PubMed] [Google Scholar]
- 103.Petroni A, Canales-Johnson A, Urquina H, Guex R, Hurtado E, Blenkmann A, et al. The cortical processing of facial emotional expression is associated with social cognition skills and executive functioning: a preliminary study. Neurosci Lett. (2011) 505:41–6. 10.1016/j.neulet.2011.09.062 [DOI] [PubMed] [Google Scholar]
- 104.Ibanez A, Toro P, Cornejo C, Urquina H, Manes F, Weisbrod M, et al. High contextual sensitivity of metaphorical expressions and gesture blending: a video event-related potential design. Psychiatry Res. (2011) 191:68–75. 10.1016/j.pscychresns.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 105.Ibanez A, Riveros R, Aravena P, Vergara V, Cardona JF, Garcia L, et al. When context is difficult to integrate: cortical measures of congruency in schizophrenics and healthy relatives from multiplex families. Schizophr Res. (2011) 126:303–5. 10.1016/j.schres.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 106.Ibanez A, Petroni A, Urquina H, Torrente F, Torralva T, Hurtado E, et al. Cortical deficits of emotional face processing in adults with ADHD: its relation to social cognition and executive function. Soc Neurosci. (2011) 6:464–81. 10.1080/17470919.2011.620769 [DOI] [PubMed] [Google Scholar]
- 107.Dufey M, Hurtado E, Fernandez AM, Manes F, Ibanez A. Exploring the relationship between vagal tone and event-related potentials in response to an affective picture task. Soc Neurosci. (2011) 6:48–62. 10.1080/17470911003691402 [DOI] [PubMed] [Google Scholar]
- 108.Aravena P, Hurtado E, Riveros R, Cardona JF, Manes F, Ibanez A. Applauding with closed hands: neural signature of action-sentence compatibility effects. PLoS ONE. (2010) 5:e11751. 10.1371/journal.pone.0011751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hurtado E, Haye A, Gonzalez R, Manes F, Ibanez A. Contextual blending of ingroup/outgroup face stimuli and word valence: LPP modulation and convergence of measures. BMC Neurosci. (2009) 10:69. 10.1186/1471-2202-10-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guerra S, Ibanez A, Martin M, Bobes MA, Reyes A, Mendoza R, et al. N400 deficits from semantic matching of pictures in probands and first-degree relatives from multiplex schizophrenia families. Brain Cogn. (2009) 70:221–30. 10.1016/j.bandc.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 111.Cornejo C, Simonetti F, Ibanez A, Aldunate N, Ceric F, Lopez V, et al. Gesture and metaphor comprehension: electrophysiological evidence of cross-modal coordination by audiovisual stimulation. Brain Cogn. (2009) 70:42–52. 10.1016/j.bandc.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 112.García-Cordero I, Sedeño L, de la Fuente L, Slachevsky A, Forno G, Klein F, et al. Feeling, learning from, and being aware of inner states: Interoceptive dimensions in neurodegeneration and stroke. Philos Trans R Soc Lond B Biol Sci. (2016) 371:20160006. 10.1098/rstb.2016.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garcia-Cordero I, Sedeno L, Fraiman D, Craiem D, de la Fuente LA, Salamone P, et al. Stroke and neurodegeneration induce different connectivity aberrations in the insula. Stroke. (2015) 46:2673–7. 10.1161/STROKEAHA.115.009598 [DOI] [PubMed] [Google Scholar]
- 114.Hesse E, Mikulan E, Decety J, Sigman M, Garcia Mdel C, Silva W, et al. Early detection of intentional harm in the human amygdala. Brain. (2016) 139:54–61. 10.1093/brain/awv336 [DOI] [PubMed] [Google Scholar]
- 115.Hesse E, Mikulan E, Sitt JD, Garcia MDC, Silva W, Ciraolo C, et al. Consistent gradient of performance and decoding of stimulus type and valence from local and network activity. IEEE Trans Neural Syst Rehabil Eng. (2019) 27:619–29. 10.1109/TNSRE.2019.2903921 [DOI] [PubMed] [Google Scholar]
- 116.Garcia AM, Hesse E, Birba A, Adolfi F, Mikulan E, Caro MM, et al. Time to face language: embodied mechanisms underpin the inception of face-related meanings in the human brain. Cereb Cortex. (2020) 30:6051–68. 10.1093/cercor/bhaa178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Birba A, Beltran D, Martorell Caro M, Trevisan P, Kogan B, Sedeno L, et al. Motor-system dynamics during naturalistic reading of action narratives in first and second language. Neuroimage. (2020) 216:116820. 10.1016/j.neuroimage.2020.116820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ibanez A, Billeke P, de la Fuente L, Salamone P, Garcia AM, Melloni M. Reply: Towards a neurocomputational account of social dysfunction in neurodegenerative disease. Brain. (2017) 140:e15. 10.1093/brain/aww316 [DOI] [PubMed] [Google Scholar]
- 119.Ibanez A. Brain oscillations, inhibition and social inappropriateness in frontotemporal degeneration. Brain. (2018) 141:e73. 10.1093/brain/awy233 [DOI] [PubMed] [Google Scholar]
- 120.Fittipaldi S, Abrevaya S, Fuente A, Pascariello GO, Hesse E, Birba A, et al. A multidimensional and multi-feature framework for cardiac interoception. Neuroimage. (2020) 212:116677. 10.1016/j.neuroimage.2020.116677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Musa G, Slachevsky A, Munoz-Neira C, Mendez-Orellana C, Villagra R, Gonzalez-Billault C, et al. Alzheimer's disease or behavioral variant frontotemporal dementia? Review of key points toward an accurate clinical and neuropsychological diagnosis. J Alzheimers Dis. (2020) 73:833–48. 10.3233/JAD-190924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.New manual aims to create common standards for dementia diagnosis across Latin America . Alzheimers Dement. (2020) 16:1099. 10.1002/alz.12147 [DOI] [PubMed] [Google Scholar]
- 123.Dawson W, Bobrow K, Ibanez A, Booi L, Pintado-Caipa M, Yamamoto S, et al. Brain health diplomacy: a dynamic model of international action. Lancet Neurol. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smith E, Ali D, Wilkerson B, Dawson WD, Sobowale K, Reynolds C, et al. A brain capital grand strategy: toward economic reimagination. Mol. Psychiatry. (2021) 26:3–22. 10.1038/s41380-020-00918-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ternes K, Iyengar V, Lavretsky H, Dawson WD, Booi L, Ibanez A, et al. Brain health INnovation Diplomacy: a model binding diverse disciplines to manage the promise and perils of technological innovation. Int Psychogeriatr. (2020) 32:955–79. 10.1017/S1041610219002266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ibanez A, Santamaria-Garcia H, Guerrero Barragan A, Kornhuber A, Ton AMM, Slachevsky A, et al. The impact of SARS-CoV-2 in dementia across Latin America: a call for an urgent regional plan and coordinated response. Alzheimers Dement. (2020) 6:e12092. 10.1002/trc2.12092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ibanez A, Flichtentrei D, Hesse E, Dottori M, Tomio A, Slachevsky A, et al. The power of knowledge about dementia in Latin America across health professionals working on aging. Alzheimers Dement. (2020) 12:e12117. 10.1002/dad2.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Profess. (2006) 26:13–24. 10.1002/chp.47 [DOI] [PubMed] [Google Scholar]
- 129.Donnelly-Kehoe PA, Pascariello GO, Garcia AM, Hodges JR, Miller B, Rosen H, et al. Robust automated computational approach for classifying frontotemporal neurodegeneration: multimodal/multicenter neuroimaging. Alzheimers Dement. (2019) 11:588–98. 10.1016/j.dadm.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moguilner S, Garcia AM, Mikulan E, Hesse E, Garcia-Cordero I, Melloni M, et al. Weighted Symbolic Dependence Metric (wSDM) for fMRI resting-state connectivity: a multicentric validation for frontotemporal dementia. Sci Rep. (2018) 8:11181. 10.1038/s41598-018-29538-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baez S, Pino M, Berrio M, Santamaria-Garcia H, Sedeno L, Garcia AM, et al. Corticostriatal signatures of schadenfreude: evidence from Huntington's disease. J Neurol Neurosurg Psychiatry. (2018) 89:112–6. 10.1136/jnnp-2017-316055 [DOI] [PubMed] [Google Scholar]
- 132.Sedeno L, Piguet O, Abrevaya S, Desmaras H, Garcia-Cordero I, Baez S, et al. Tackling variability: a multicenter study to provide a gold-standard network approach for frontotemporal dementia. Hum Brain Mapp. (2017) 38:3804–22. 10.1002/hbm.23627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Santamaria-Garcia H, Baez S, Reyes P, Santamaria-Garcia JA, Santacruz-Escudero JM, Matallana D, et al. A lesion model of envy and Schadenfreude: legal, deservingness and moral dimensions as revealed by neurodegeneration. Brain. (2017) 140:3357–77. 10.1093/brain/awx269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sedeno L, Couto B, Garcia-Cordero I, Melloni M, Baez S, Morales Sepulveda JP, et al. Brain network organization and social executive performance in frontotemporal dementia. J Int Neuropsychol Soc. (2016) 22:250–62. 10.1017/S1355617715000703 [DOI] [PubMed] [Google Scholar]
- 135.Ibanez A, Manes F. Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology. (2012) 78:1354–62. 10.1212/WNL.0b013e3182518375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baez S, Garcia AM, Ibanez A. The social context network model in psychiatric and neurological diseases. Curr Top Behav Neurosci. (2017) 30:379–96. 10.1007/7854_2016_443 [DOI] [PubMed] [Google Scholar]
- 137.Ibáñez A, García AM. Contextual Cognition: The Sensus Communis of a Situated Mind. 1st ed. Springer International Publishing; (2018). 10.1007/978-3-319-77285-1 [DOI] [Google Scholar]
- 138.Ibáñez A. Insular networks and intercognition in the wild. Cortex. (2019) 115:341–4. 10.1016/j.cortex.2019.01.024 [DOI] [PubMed] [Google Scholar]
- 139.Ibanez A, Schulte M. Situated minds: conceptual and emotional blending in neurodegeneration and beyond. Brain. (2020) 143:3523–5. 10.1093/brain/awaa392 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.