Abstract
Vascular wilt, caused by the pathogen Fusarium oxysporum f. sp. physali (Foph), is a major disease of cape gooseberry (Physalis peruviana L.) in Andean countries. Despite the economic losses caused by this disease, there are few studies related to molecular mechanisms in the P. peruviana—Foph pathosystem as a useful tool for crop improvement. This study evaluates eight candidate genes associated with this pathosystem, using real-time quantitative PCR (RT-qPCR). The genes were identified and selected from 1,653 differentially expressed genes (DEGs) derived from RNA-Seq analysis and from a previous genome-wide association study (GWAS) of this plant-pathogen interaction. Based on the RT-qPCR analysis, the tubuline (TUB) reference gene was selected for its highly stable expression in cape gooseberry. The RT-qPCR validation of the candidate genes revealed the biological variation in their expression according to their known biological function. Three genes related to the first line of resistance/defense responses were highly expressed earlier during infection in a susceptible genotype, while three others were overexpressed later, mostly in the tolerant genotype. These genes are mainly involved in signaling pathways after pathogen recognition, mediated by hormones such as ethylene and salicylic acid. This study provided the first insight to uncover the molecular mechanism from the P. peruviana—Foph pathosystem. The genes validated here have important implications in the disease progress and allow a better understanding of the defense response in cape gooseberry at the molecular level. Derived molecular markers from these genes could facilitate the identification of tolerant/susceptible genotypes for use in breeding schemes.
Keywords: Fusarium, Cape gooseberry, RNAseq, GWAS, qPCR
Introduction
Plant diseases are the cause of significant crop losses in agriculture worldwide, and when combined with weeds, pathogens and pests, account for 20–30% reduced yield (Savary et al., 2019). Therefore, developing genetically improved varieties with increased yields and resistance to biotic stresses are promising alternatives for disease control (Vyska, Cunniffe & Gilligan, 2016). Members of the genus Fusarium represent the most widespread pathogens that cause various diseases on economically major crops (Husaini, Sakina & Cambay, 2018). The vascular wilt disease caused by Fusarium oxysporum has been studied in detail in some species of the Solanaceae family. For instance, in tomato, a specific Fusarium strain (F. oxysporum f. sp. lycopersici) is able to trigger a monogenic-type of resistance (Takken & Rep, 2010; Prihatna, Barbetti & Barker, 2018). However, considering that Fusarium pathogenicity is host specific, understanding the mechanisms of interaction in other pathosystems are crucial to provide alternative strategies for disease control.
Cape gooseberry (Physalis peruviana L.) is a valuable fruit-bearing species, recognized as a source of pharmacological compounds with anti-inflammatory, antibacterial and antitumor activities (Fang, Liu & Li, 2012; Abou Baker & Rady, 2020), as well as a functional food with vitamins A, C and B complex, minerals and antioxidant components (Puente, Nocetti & Espinosa, 2019; Ramadan, 2020). In Colombia, cape gooseberry is the second most important export fruit, mainly to European markets (Fischer et al., 2005). However, its production has decreased from 17.13 ton/ha in 2007 to 15.01 ton/ha in 2017 (MinAgricultura, 2017), mostly due to the lack of breeding materials with desirable traits such as disease resistance. Although, in Colombia, two cape gooseberry varieties (Andina and Dorada) with increased yield and reduced fruit-cracking incidence had been released, both varieties are susceptible to different pathogens (Núñez et al., 2016a, 2016b). F. oxysporum f. sp physali (Foph) has been identified as one of the major constraints in cape gooseberry (Simbaqueba et al., 2018). Nevertheless, the genetic basis underlying Foph recognition and cape gooseberry defense responses remain unclear.
Plants have different strategies to distinguish pathogens, involving different recognition layers. The first level relies on the recognition of conserved microbial elicitors known as pathogen-associated molecular patterns (PAMPs). This recognition triggers a resistance response in the plant known as PAMPs-triggered immunity (PTI) (Jones & Dangl, 2006; Choi & Klessig, 2016). When pathogens evade PTI, translocating effector proteins into the host, a second level of recognition called effector-triggered immunity (ETI) is activated. This defense level involves the detection of these effector proteins through intracellular receptors, called R proteins, which have a canonical plant nucleotide-binding, leucine-rich repeat (NLR) domain (Jones & Dangl, 2006; Van der Burgh & Joosten, 2019). Pathogen recognition through PTI or ETI triggers a whole-genome transcriptional reprograming including the synthesis of pathogenesis-related (PR) proteins. Some of the PR proteins later act by targeting pathogen cell walls or membranes, strengthening plant cell walls, and initiating hypersensitive responses that block the pathogen attack (Saboki, Usha & Singh, 2011).
Approaches such as RNA sequencing (RNA-Seq) and genome wide association studies (GWAS) have allowed the profiling of global gene expression patterns and the mapping of simple or complex traits, respectively, in both model and non-model plants. These approaches have been useful resources for the understanding of how plants respond to biotic or abiotic stimuli through the discovery of candidate genes involved in different biological processes (Iquebal et al., 2019; Sicilia et al., 2019; Safavi-Rizi, Herde & Stöhr, 2020), including those involved in plant-microbe interactions (Bartoli & Roux, 2017).
In cape gooseberry, the assembly and annotation of its leaf transcriptome was the first attempt to provide valuable resources for developing molecular tools in this species (Garzón-Martínez et al., 2012). This leaf transcriptome was used to search for proteins that encode conserved domains related to plant immunity (Enciso-Rodríguez et al., 2013). Enciso-Rodríguez et al. (2013) identified 74 immunity-related candidate genes in P. peruviana. These genes comprised 17 receptor-like kinase (RLKs) and 57 NLRs related to ETI, including eight and nine NLRs associated with toll/interleukin-1 receptor (TIR) and coiled-coil (CC) domains, respectively. However, this study was limited to constitutively expressed genes in leaves, which may not represent the actual mechanisms underlying the Foph response since it is a soil-borne pathogen. Later on, Osorio-Guarín et al. (2016) identified 17 additional candidate genes related to defense/resistance responses against Foph through a GWAS using 100 accessions of P. peruviana. More recently, Vera Alvarez et al. (2017) generated a workflow for transcriptome annotation from stem and root RNA-Seq data of the P. peruviana—Foph interaction. Nevertheless, no further analyses on candidate genes have been conducted to date. Therefore, further investigation is required to understand the P. peruviana—Foph pathosystem.
Understanding the expression patterns of critical regulatory genes contribute to elucidating the mechanisms involved in disease development and defense responses. The use of contrasting genotypes, according to their response against biotic stresses, and molecular techniques such as real-time quantitative PCR (RT-qPCR), are suitable approaches to validate the relationship between candidate genes and resistance/defense responses. RT-qPCR is a robust and cost-effective technique for quantifying messenger RNA (mRNA) (Schmittgen & Livak, 2008; Abdallah & Bauer, 2016). To date, this is the most frequently used tool to validate the molecular regulation of genes related to defense on several plant species (Użarowska et al., 2009; Casassola et al., 2015; Cregeen et al., 2015; Pombo et al., 2019).
This study aims to validate key genes identified by an RNA-Seq analysis and a previous GWAS study (Osorio-Guarín et al., 2016) of the P. peruviana—Foph pathosystem, by means of RT-qPCR. This will contribute to widening the knowledge for this orphan crop, and to help understand the resistance/defense responses in cape gooseberry. The results presented in this study will aid in the development of tolerant/resistant cape gooseberry varieties.
Materials and Methods
Plant material and growth conditions
One P. peruviana tolerant genotype (09U279-1) was used for the RNA-seq analysis based on previous studies of the differential resistance responses against F. oxysporum f.sp. physali pathogenic strain MAP5 (Enciso-Rodríguez et al., 2013; Liberato et al., 2014). Plantlets were in vitro propagated using MS medium (Murashige & Skoog, 1962), supplemented with 0.1 mg/L gibberellic acid (GA3) and 0.1 mg/L indole-3-butyric acid (IBA). In vitro plantlets were acclimatized in peat moss-based substrate under greenhouse conditions at 25 ± 2 °C with a 12/12h photoperiod. Three month-old plants with 10 cm height and three true leaves were transplanted to plastic bags filled with 500 g of sterilized substrate (3:1 ratio of soil:rice:husk) for subsequent inoculation experiments.
For RT-qPCR experiments, cape gooseberry genotypes, 09U128-5 and 09U140-5, previously reported as susceptible and tolerant to Foph (Osorio-Guarín et al., 2016), respectively, were used to validate the selected candidate genes. Plant material was in vitro propagated and grown according to the conditions mentioned above.
Inoculation assays
The highly virulent monosporic Foph strain (Map5) was used for inoculum production (Enciso-Rodríguez et al., 2013). The strain was reactivated in liquid potato dextrose agar (PDA) for 8 days at 27 °C in constant agitation (120 rpm). Inoculum concentration was adjusted to 1 × 106 CFU/ml according to Namiki et al. (1994). Plants were inoculated by directly applying the inoculum into the soil, while mock-inoculated plants were watered with sterile water.
Transcriptome sequencing and in silico analysis of RNA-Seq data
Root and stem tissue from two biological replicates of an inoculated and mock-inoculated tolerant genotype (09U279-1) were collected at 0, 24, 48, 72, and 96 days post-inoculation (dpi), and flash frozen in liquid nitrogen. Samples from each time point were combined into one pool for RNA extraction, cDNA synthesis (Bio S&T Inc. Montreal, QC, Canada) and Illumina sequencing (Emory Genomics Center. Atlanta, GA, USA). RNA-Seq data from all libraries were downloaded from the Sequence Read Archive (SRA) of the National Center for Biotechnology Information (NCBI) database under BioProject ID 67621 (Table S1) (Vera Alvarez et al., 2017).
Reads were assessed for quality using FastQC version 0.11.1 (Andrews et al., 2012). Adapters trimming and quality filtering were done using Fastq-mcf from Ea-utils (Aronesty, 2011). Low quality reads with short sequence length (<50 bp) and low base sequence quality (<30) were removed. NCBI’s UniVec database (build 9.0) was used for filtering any possible contamination sequences. An in silico normalization of the filtered high quality reads was done in order to reduce the use of computational resources. Reads were normalized with a maximum coverage of reads set to 30.
Normalized reads were used for de novo assembly of the cape gooseberry transcriptome using Trinity version 2.0.2 (Grabherr et al., 2011) with default parameters. After assembly, the length distribution of assembled transcripts was compared with the well-known tomato transcriptome assembly (ITAG2.3). Furthermore, Fusarium-related sequences were filtered using a BLAST-Like Alignment Tool (BLAT) against a database containing the super contigs from different F. oxysporum strains retrieved from the Fusarium Comparative Database of the Broad Institute (Ma et al., 2010). Fusarium transcripts greater than 500 bp were removed from de novo cape gooseberry transcriptome assembly.
Two biological replicates from an inoculated tolerant genotype (SRA: SRX972116, SRX971469) and mock-inoculated read libraries (SRA: SRX980678, SRX978916) were mapped to the de novo cape gooseberry transcriptome assembly for transcript abundance estimation using the Trinity tool kit (Garber et al., 2011) with the RSEM method (Li & Dewey, 2011). Differentially expressed genes (DEGs) across both inoculated and mock-inoculated plants were identified using the edgeR package version 3.0 (Robinson, McCarthy & Smyth, 2009). Transcripts with a fold change >2 and a false discovery rate (FDR) < 0.01 were considered as significant DEGs. Functional annotation and gene ontology (GO) analyses were carried out by a local BLASTX search against the UniprotKB/Swiss-Prot and the tomato database (ITAG2.3), using an expected value threshold of 1e−3. BLAST files were filtered and parsed for the best high score match using the perl script BlastAddDescriptor (Bombarely, 2012).
Candidate genes selection and primer design
From the DEGs identified, candidate genes were selected for further RT-qPCR validation assays based on their functional annotation. The closest tomato gene (Build SL3.0) to the DEGs was used for designing primers after identifying the exon-intron boundaries from the tomato genes using the IDT tool (Integrated DNA Technologies, 2018). Genes were named using the corresponding tomato ID used for the primer pairs design.
Furthermore, seven candidate genes, flanking genomic regions associated with resistance/defense to Foph, were selected from a previous GWAS study of the P. peruviana—Foph interaction (Osorio-Guarín et al., 2016). The primer design strategy consisted of the alignment of genotyping-by-sequencing (GBS) reads from the candidate genes, of about 64 bp, against the de novo cape gooseberry transcriptome. Primers were designed from selected Physalis transcript sequences using Primer3 (Rozen & Skaletsky, 2000). Amplicon sizes were confirmed using 2% (w/v) agarose gel stained with SYBR Safe (1X) (Invitrogen, Waltham, MA, USA). Genes were named according to the corresponding Physalis transcript ID used for the primer pairs design.
The pyrophosphatase (PPA2), β-tubulin (TUB), actin (ACT) and elongation factor (EF-1α) were evaluated as reference genes as previously reported in different RT-qPCR analyses (Czechowski et al., 2005; Hong et al., 2010). The selection of the best reference gene for cape gooseberry was based on analyses of melting curves with four-fold serial dilutions (1:1, 1:4, 1:16 and 1:64) and by calculating their variation among samples.
RT-qPCR assay
Forty plants from the susceptible (09U128-5) and tolerant (09U140-5) genotypes selected were inoculated with the Foph strain MAP5. Ten plants were mock-inoculated using sterile water. Plants were arranged in a completely randomized block design with two replicates (10 plants per replicate) per treatment and genotype. Inoculation assays were done at the Tibaitatá Research Center of the Corporación Colombiana de Investigacion Agropecuaria (Agrosavia).
Plant symptoms were scored according to the disease severity scale described by Enciso-Rodríguez et al. (2013) with some modifications (Table S2). The severity scale of the disease consisted of five degrees. These levels were: 0: plant without symptoms, 1–4: plant with increasing degrees of wilting and 5: dead plant. Root tissue was collected at 1, 3 and 5 degrees of the scale. To avoid conflating the disease with nutrient deficiency symptoms, plants were fertilized with 2 ml/L of foliar fertilizer and a 10-20-10 edaphic fertilizer. All samples were immediately frozen in liquid nitrogen and stored at −80 °C for RNA extraction.
RNA isolation and cDNA synthesis
Total RNA was isolated from root tissue using the Plant RNA Purification Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. RNA samples (1 μg) were treated with DNase TURBO (Invitrogen, Waltham, MA, USA) to discard contaminating DNA. The purified RNA was quantified using a NanoDrop 2000 UV-Vis spectrophotometer and visualized in 1.5% (w/v) agarose gel. Complementary DNA (cDNA) synthesis was performed using 500 ng of total RNA with the iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions.
RT-qPCR analysis
Real-time quantitative PCR reactions were performed on an iCycler IQ5 real-time PCR System using the iQ SYBR Green supermix System (Bio-Rad, Hercules, CA, USA), with the following thermocycler conditions: one cycle of initial denaturation at 95 °C for 3 min, followed by 40 cycles of 95 °C for 15 s and a final extension at 56 °C for 60 s in a 10 µl final volume. Each reaction was performed in triplicate. Melting curves were generated at 65–95 °C after 40 cycles to check for primer pairs specificity.
Finally, the 2−ΔCT method was used to compare the differential expression from each candidate gene at the three degrees of the disease scale from the tissue collected. One-way ANOVA followed by Tukey HDS for multiple pairwise comparisons were applied. The gene expression profiling was done using the following equation (Schmittgen & Livak, 2008):
2−ΔCT where ΔCT = (CT gene of interest – CT reference gene)
Results
P. peruviana transcriptome assembly and DEGs profiling
A reference transcriptome was assembled for cape gooseberry, as is common for non-model organisms. Here, we used 98 million reads (Table S3), with an average length of 100 bp, to generate a de novo transcriptome assembly from previous data (Vera Alvarez et al., 2017) and subsequent identification of DEGs from the P. peruviana—Foph pathosystem. After trimming adapters, primers, contaminants and low-quality sequences, a total of 87,488,110 reads were used for an in silico normalization process, which resulted in 17,664,763 sequences used for a de novo transcriptome assembly (Table S3). Based on the assembly’s information provided by the assembly, a total of 60,934 transcripts with a read length greater than 500 bp and an N50 of 1,511 bp, were generated (Table S4). After extracting F. oxysporum sequences, 59,476 transcripts were used as reference for mapping reads for downstream differential expression and functional analysis.
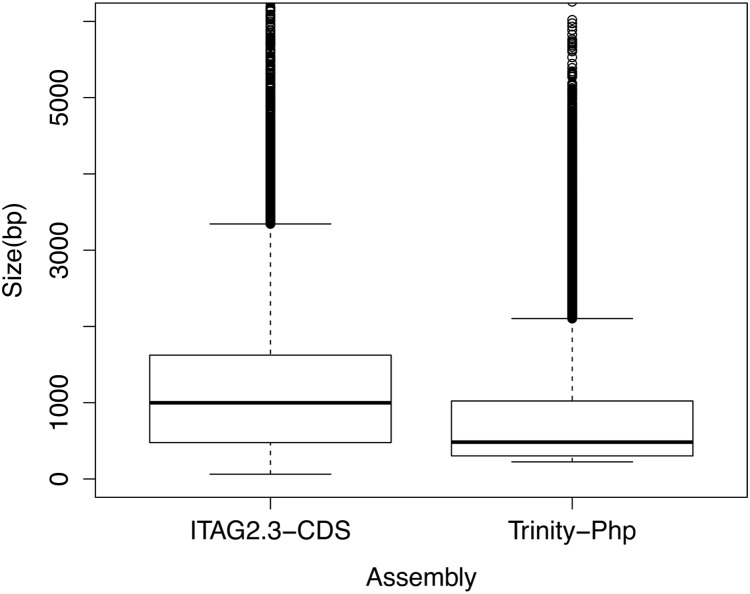
To assess the quality of the transcriptome assembly, the length distribution of assembled transcripts was compared to gene models of a well-annotated closely related species (Solanum lycopersicum), as done by Villarino et al. (2014) (Fig. 1). Even though, it was observed a significant difference (p < 0.01) between the mean size distribution of all P. peruviana trinity transcripts (125,590 sequences, mean size transcripts 779 bp) and the tomato full annotated transcriptome (34,727 sequences, mean size transcripts 1,208 bp) (Fig. 1), the N50 value of P. peruviana assembly (N50 25,226 sequences with 1,207 bp average length) was close to the tomato N50 annotated transcriptome (N50 7000 sequences with 1,400 bp average length).
Figure 1. Boxplot comparison of the de novo assembled transcripts length distribution using the Trinity software.
First box indicates tomato cDNA gene models (ITAG2.3 CDS) and second box represents Trinity de novo assembly from P. peruviana (Trinity-Php). Statistical significance between both transcripts length means was analyzed by a t-test, p < 0.01.
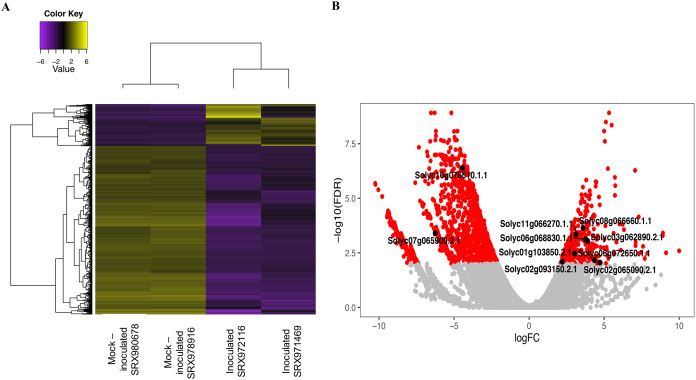
Next, four transcriptome libraries were analyzed to find DEGs related to the P. peruviana—Foph pathosystem. When comparing inoculated and mock-inoculated libraries, 329 up-regulated transcripts and 1,323 down-regulated transcripts were found (Fig. 2A; Data S1). A gene ontology analysis was done to determine the functions of the DEGs (Fig. S1; Table S5). GO classification showed that the 1,652 DEGs were classified in at least one GO term at level 2 (molecular function, biological process or cellular component). In the molecular function category, binding (GO:0005488) and catalytic activity (GO:0003824), were the most abundant GO terms. The biological process category revealed different ontologies, including cellular process (GO:0009987), response to stimulus (GO:0050896) and metabolic process (GO:0008152). Finally, for cellular component, transcripts involved in cell (GO:0005623) and cell part (GO:0044464) were the most highly represented (Fig. S1).
Figure 2. Heat map and volcano plot of differential expressed transcripts from two biological replicates of a tolerant genotype inoculated and mock-inoculated read libraries.
(A) The yellow color represents upregulated transcripts and the purple color indicates down-regulated transcripts from both inoculated and mock-inoculated libraries. (B) The red dots indicates differentially expressed genes, while the gray dots represents genes that are not differentially expressed between the inoculated and mock-inoculated libraries. Black dots indicate the 10 candidate genes selected for further study.
Candidate gene selection and primer design
Based on the DEGs, 10 candidate genes associated with plant defense responses were selected (Fig. 2B; Table 1). These genes included transcription factors like Apetala2/Ethylene Response Factors (AP2/ERFs), patatin, Xyloglucan Endotransglucosylase/Hydrolase (XTHs), among other genes which are known to be involved in responsive pathways related to stress regulation (Olsen, Popper & Krause, 2016; Najafi, Sorkheh & Nasernakhaei, 2018; Cheng, Song & Wu, 2019). On the other hand, seven candidate genes from a previous GWAS study (Osorio-Guarín et al., 2016) were selected based on (1) mapping to the tomato genome genes with a complete functional annotation and (2) a functional annotation related to defense responses (Table 1). Among these genes, the WD40 repeats, legume lectines, G proteins, and others were included (Smith et al., 1999; Roopashree et al., 2006; Trusov et al., 2009).
Table 1. Selected candidate genes identified in RNA-Seq and a preliminary GWAS study and primers sequences for RT-qPCR analysis.
ID correspond to the tomato gene ID and Physalis transcript ID used for the primer pairs design.
| ID | Functional annotation | Primer sequences | Tm (°C) | Size (bp) | Source |
|---|---|---|---|---|---|
| Solyc01g103850.2.1 | Ubiquitin-like domain-containing CTD phosphatase | F-TCGACTGCAAGCCTTTAGG | 55.5 | 148 | DEGs |
| R-GCGTGTGCCTTTCTGAATG | |||||
| Solyc02g065090.2.1 | Patatin-like protein 3 | F-TTGATGGAGGTGTAGCTGC | 55.3 | 142 | |
| R-CCTGTGCCTAGTGATAGAACC | |||||
| Solyc02g093150.2.1 | AP2-like ethylene-responsive transcription factor | F-ATTCACTGGCATGTATCCTGG | 54.8 | 149 | |
| R-AGTACTGGACACGAACTTGC | |||||
| Solyc03g062890.2.1 | Superoxide dismutase [Cu-Zn] 2 | F-GGAACGATAGTGTGCAAGGATC | 55.6 | 136 | |
| R-CCGTTAGTGGTATCACCCAAG | |||||
| Solyc06g068830.1.1 | Ethylene-responsive transcription factor ERF115 | F-GCAAAACCAATTACCTCAGCTC | 54.4 | 150 | |
| R-AAAGTACCCAACCATACACGAG | |||||
| Solyc06g072650.1.1 | Auxin responsive SAUR protein | F-GCCAAATATGCAAACCATCC | 53.0 | 217 | |
| R-CTCCATGTCCCAAGACCCTA | |||||
| Solyc07g065900.2.1 | Fructose-bisphosphate aldolase | F-CTTAGTGGAATTATCTTGTTCGAGG | 52.7 | 141 | |
| R-GGGTAGTTGTCTCACCATTGG | |||||
| Solyc08g066660.1.1* | Ethylene-responsive transcription factor ERF096 | F-CACGTATTTGGCTTGGGACT | 55.3 | 157 | |
| R-TGAATGTCACGCGGACTAAG | |||||
| Solyc10g076510.1.1 | Pyruvate decarboxylase 1 | F-CACAACGGGCAAGGAAAATG | 55.6 | 139 | |
| R-TGCTGGTGTCATCCTTGTG | |||||
| Solyc11g066270.1.1 | Xyloglucan endotransglucosylase/hydrolase 9 | F-CCTTCTAGCTCTCCTTCGGG | 55.5 | 76 | |
| R-AGTTCCTATGCACCCACAAC | |||||
| R-GGGTAGTTGTCTCACCATTGG | |||||
| Php_TR3359* | Proteasome subunit beta type, proteinaceous elicitor of plant defense reactions | F-GATCCCACAGCATCATAAGTGA | 55.8 | 119 | Osorio-Guarín et al. (2016) |
| R-CAGCTGCTTTCGAACACACTAT | |||||
| Php_TR3653* | Legume lectin beta domain, signaling molecule in defense response | F-ATGCAAATGTTGGAACAATCAG | 51.4 | 92 | |
| R-TTCTCCTTTTTCGGTTCTTCTG | |||||
| Php_TR7902* | WD-40 repeats, signal transduction and hypersensitive response | F-TTGCTGACTTCCATGAGCTTTA | 52.6 | 103 | |
| R-CCAGGCAATAGATGTTTGATGA | |||||
| Php_TR8981* | Lipase, class 3, related to PAD4, EDS1 y SAG101 (Accumulation of salicylic acid) | F-TTTCATCCTCACCACACTATGC | 55.0 | 103 | |
| R-GAGGACAGAAATCCACCATCTC | |||||
| Php_TR46393* | G-protein-coupled receptor, immunity in plants | F-AAACAGGACACACAGGAAAGGT | 53.2 | 115 | |
| R-GCATTGTTTAGCATCCGTATCA | |||||
| Php_TR64396* | Thioredoxin domain, oxidative stress tolerance | F-TTACCCTTACTGGCGATACGTT | 51.4 | 101 | |
| R-CAGGTTCTTGCAGTGCTTACAC | |||||
| Php_TR69681* | Major facilitator superfamily transporter, exporting toxins | F-GCTGTTGTTGTTCAGATGGAAA | 53.6 | 89 | |
| R-CCTCTATCGCACATATCCACAA | |||||
| Elongation factor (EF) | – | F-TGGTTTTGAAGCTGGTATCTCC | 54.6 | 140 | Reference Genes |
| R-CATACCTAGCCTTGGAGTACTTG | |||||
| Pyrophosphatase (PPA2) | – | F-GATGAGTTTCCTGATGTTCGTTTG | 54.0 | 136 | |
| R-GAACCCTCCAGTGTCTATCTTC | |||||
| Tubulin (TUB) | – | F-GTCTGGTGCTGGAAACAATTG | 54.1 | 133 | |
| R-TGAATGGCACACTTGAAAACC | |||||
| Actin (ACT) | – | F-GTACAGTGTCTGGATTGGAGG | 54.7 | 73 | |
| R-GCCCTTTGAAATCCACATCTG |
Notes:
Set of genes evaluated by RT-qPCR.
Tm, melting temperature; bp, base pair.
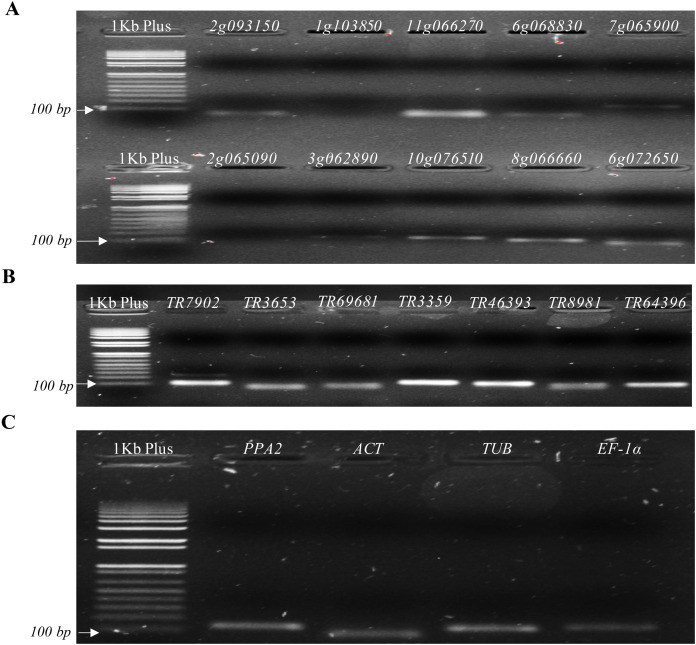
A BLAST comparing the 10 DEGs primer pairs sequences to the de novo P. peruviana transcriptome showed a partial alignment (∼10 bp from 20 pb), mismatches between primer pairs and transcripts (∼3 bp), as well as secondary annealing of the primer pairs with different cape gooseberry transcripts (Table S6). In addition, electrophoresis in 2% (w/v) agarose gels from the 10 DEGs primer pairs showed that some primer pairs amplified a single PCR product with the expected target size (Solyc11g066270.1.1, Solyc10g076510.1.1, Solyc08g066660.1.1, Solyc06g072650.1.1), while others did not amplify (Solyc01g103850.2.1, Solyc02g065090.2.1, Solyc03g062890.2.1) or showed a weak amplification (Solyc02g093150.2.1, Solyc06g068830.1.1, Solyc07g065900.2.1) (Fig. 3A). When evaluating a melting curve of three out of 10 of the genes with the best BLAST results, we found a low specificity of two primer pairs (Solyc06g068830.1.1, Solyc11g066270.1.1) and a strong specificity for one (Solyc08g066660.1.1) (Figs. S2A, S2B and S2C). Considering these results, we decided to continue with the candidate gene Solyc08g066660.1.1, which showed good results in the BLAST alignment and melting curve.
Figure 3. Agarose gels from candidate and reference genes selected.
(A) Genes selected from the differentially expressed genes (DEGs) analysis. (B) Genes selected from Osorio-Guarín et al. (2016). (C) Reference genes.
In contrast, agarose gels from the seven primer pairs selected from a GWAS study (Osorio-Guarín et al., 2016) showed high specificity and the expected target size (Fig. 3B), which was corroborated with their melting curves (Figs. S2D, S2E and S2F). No amplification was observed in control samples without reverse transcriptase, cDNA template or primers in the RT-qPCR reaction. Based on the results of both primer design strategies, eight candidate genes, one from DEGs and seven from the previous GWAS study (Osorio-Guarín et al., 2016) were used for further RT-qPCR analysis.
Electrophoresis in 2% (w/v) agarose gels revealed that all the reference genes (PPA2, TUB, ACT and EF-1α) amplified a single product with the expected size (Table 1; Fig. 3C). Expression patterns of these four genes in a sample set of inoculated plants were quantified using RT-qPCR. Standard curves revealed a high primer specificity for all selected genes, presenting a good fluorescence quality and primer dimer absence (Fig. S3C). The genes TUB and PPA2 with a similar melting curve temperature of 80 °C were selected to determine which of the two primer pairs showed the least variation between different cDNA samples from the assay. It was found that the reference gene TUB had greater stability and less variation in CT values with a range of −0.767 to −0.131 and −0.004 to −0.893 for both biological samples, while PPA2 showed a greater variation ranging from −1.9 to −10.8 and −0.360 to −1.312. Therefore, the TUB reference gene was selected for subsequent analyses.
Phenotypic differences between selected genotypes in response to Foph
Cape gooseberry genotypes 09U128-5 and 09U140-5 were inoculated with the highly virulent Foph strain Map5. The first phenotypic symptoms were observed at 17 days post-inoculation (dpi) for the susceptible genotype 09U128-5, presenting small yellow spots (chlorosis) on the leaves. At 25 and 30 dpi, this genotype exhibited symptoms associated to scale degrees 3 and 4, respectively, according to the disease severity scale, to finally collapse at 35 dpi. On the other hand, the tolerant genotype 09U140-5 started to present the first symptoms on leaves at 24 dpi, later than the susceptible genotype. At 30 and 40 dpi, this genotype exhibited symptoms related to scale degrees 3 and 4, respectively. This genotype died at the end of the experiment (60 dpi). The tolerant genotype took 25 days longer to die compared to the susceptible genotype (Fig. S4). No symptoms were observed in the mock-inoculated plants.
Validation of candidate genes using RT-qPCR
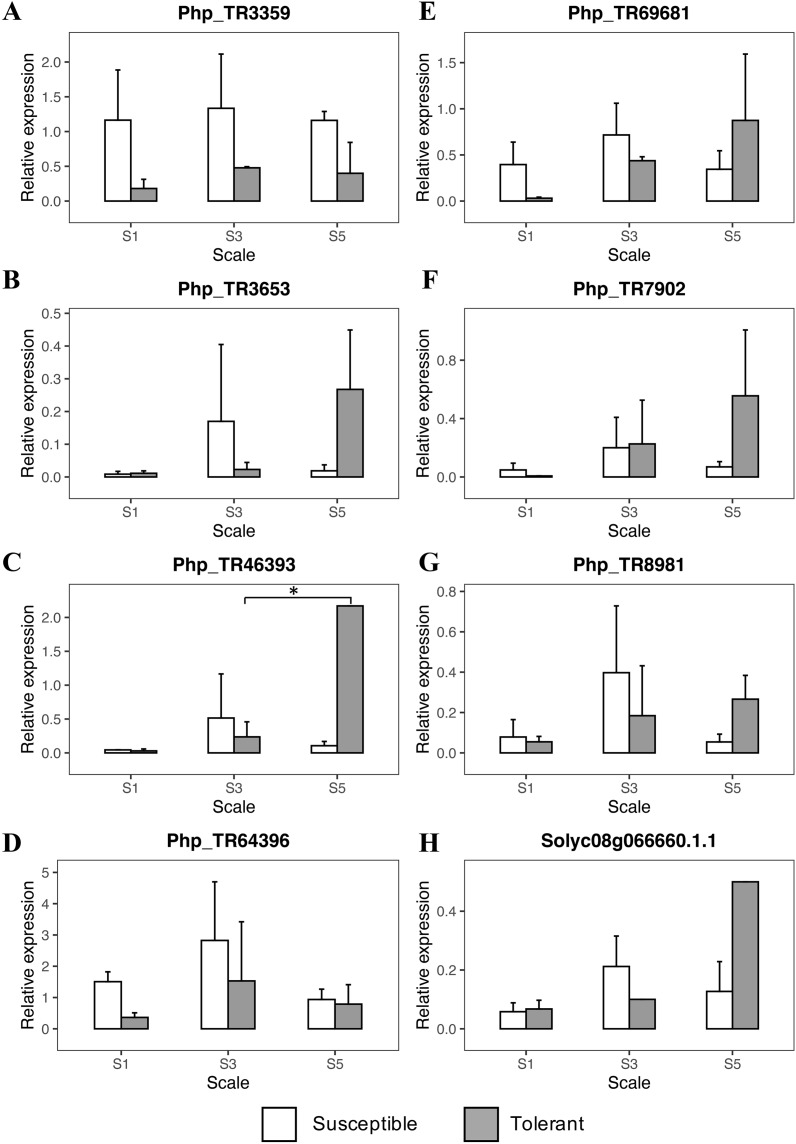
The eight candidate genes selected were evaluated using the aforementioned cape gooseberry genotypes exposed to Foph. Plant material was collected at three different scale degrees of the disease to ensure a broad spectrum of the scale in the expression profile. The TUB gene was used as the reference gene to normalize the data and determine the relative expression level of the candidate genes.
The RT-qPCR analysis showed an initial trend in the scale degrees 1 and 3 from the genes Php_TR3359, Php_TR64396, and Php_TR69681 with an increased response to the initial infection stages of Foph in the susceptible genotype (Fig. 4; Table S7). Once the disease progressed to scale degree 5, the transcript level of genes, mainly related to signal transduction and hormone-regulated induced defense responses (ethylene and salicylic acid), increased in the tolerant genotype (Php_TR8981, Php_TR46393, Solyc08g066660.1.1) (Fig. 4). On the other hand, the expression profile of Php_TR3653 and Php_TR7902 increased at the scale degree 5, in the tolerant genotype. Overall, as the disease progresses, the plant triggered a stronger response against Foph, which extends the lifespan of the tolerant plant.
Figure 4. Quantitative real-time PCR (qRT-PCR) analyses of eight candidate genes related to Foph resistance response in cape gooseberry root tissue.
Genes: (A) PhpTR3359, (B) PhpTR3653, (C) PhpTR46393, (D) PhpTR64396, (E) PhpTR69681, (F) PhpTR7902, (G) PhpTR8981, (H) Solyc08g066660.1.1 Asterisk indicates the significant diference of the tolerant genotype among the disease severity scale (One-way ANOVA, Tukey HSD test, p < 0.05).
Discussion
Candidate defense-related genes in the P. peruviana—Foph pathosystem were selected through DEGs and GWAS strategies
Understanding the molecular mechanisms by which plants respond to their environment has been key to tackling biotic and abiotic stresses in plants. The development of next-generation sequencing (NGS) technologies has accelerated the identification of genes associated with agronomic-related traits for important crops (Nguyen et al., 2019), enabling the earlier selection of progenies with desirable attributes through marker-assisted selection (MAS). In this study, we used the results from two different NGS approaches, RNA-Seq and GWAS, to identify and select candidate genes associated with the P. peruviana—Foph pathosystem.
RNA-Sequencing has been widely used for analyzing gene expression patterns in different tissues and developmental stages under various conditions (Tsukagoshi et al., 2015; Brito et al., 2017; Howlader et al., 2020). Here, we analyzed the data of previously reported transcriptome libraries (Vera Alvarez et al., 2017) from cape gooseberry’s root and stem tissues derived from the interaction with Foph. A comparison between a de novo cape gooseberry transcriptome assembly with the tomato transcriptome, as the most closely related species from the Solanaceae family with full annotated transcriptome, showed a similar distribution. According to Villarino et al. (2014), this means an accurate assembly approach. After the assembly, the DEGs were screened using a comparative analysis of two libraries, corresponding to the Foph inoculated tolerant genotype (SRA: SRX972116, SRX971469) and its mock-inoculated (SRA: SRX980678, SRX978916) plants. Previous studies have used the cape gooseberry’s leaf transcriptome (Garzón-Martínez et al., 2012) to identify immunity-related genes and derived markers (Enciso-Rodríguez et al., 2013). This is the first root/stem specific study that aims to identify candidate genes related to the response of cape gooseberry to Foph.
In this study, 10 DEGs and seven candidate genes, reported from a previous GWAS study, have the characteristic gene architectures from genes involved in downstream recognition pathways related to biotic stresses. Some DEGs were associated with gene ontologies such as response to stress with the typical conserved domains related to plant immunity such as kinase domains. These domains are well known for being involved in downstream signaling (i.e., mitogen-activated protein kinases) after pathogen recognition through either PTI or ETI (Jones & Dangl, 2006) We also found candidate genes with associated domains such as AP2, ubiquitin and patatine-like domains, as well as: plant auxin responsive proteins, glycosyl hydrolases proteins, superoxide dismutase, and others (Table 1). In general, these candidate genes are involved with the expression or synthesis of defense-related compounds, triggered by a possible Foph recognition through either PTI or ETI in P. peruviana as reported in other species (Dreher & Callis, 2007; Minic, 2008; Bielach, Hrtyan & Tognetti, 2017; Subki, Abidin & Yusof, 2018; Najafi, Sorkheh & Nasernakhaei, 2018; Cheng, Song & Wu, 2019).
RT-qPCR standardization allowed for reliable verification of gene expression in the P. peruviana—Foph pathosystem
Real-time quantitative PCR has been a valuable tool for a cost-efficient validation of candidate gene expression. However, quantification of gene expression is affected by various experimental sources of variation that need to be carefully controlled, such as primer performance, the quantity of the initial material, sample-sample variation, the quality of the RNA, the reference genes used, the experimental design and the statistical methods employed for the analysis (Chen et al., 2011; Bustin & Huggett, 2017).
For designing primers, two approaches were used: (1) homologous sequences of the tomato transcriptome for the DEGs genes and (2) de novo cape gooseberry transcripts for genes or genomic regions derived from GWAS. As expected, the use of the de novo cape gooseberry transcriptome resulted in primer pairs with a higher specificity than the ones designed from tomato gene models. Despite the fact of the high degree of synteny in terms of gene order, orientation and exon/intron structure in the Solanaceae family (Frary, Doganlar & Frary, 2016), chromosomal rearrangements/mutations must be present between the two species, resulting in sequence variation within exon/intron boundaries used for the design of primers from DEGs. Thus, the primer pairs may not match as specifically as when using short sequences (tags) and transcripts from the same species. In addition, various genomic elements and gene features can impact primer specificity. Primers may inadvertently cross-match to non-target transcripts or other members of a gene family (Yuen, Brooks & Li, 2001; Ruiz-Villalba et al., 2017) or to similar but untargeted paralogous sequences such as intron-less pseudogenes (Dapprich et al., 2016). This is particularly uncertain in P. peruviana which currently lacks a reference genome sequence. Thus, plants present a large variety of mRNAs which can be very related and induced to the wrong amplification (Tian et al., 2020). In this study, from the 17 primer pairs designed, eight showed a high efficiency and were selected for further validation. A re-design from the primers for the remaining DEGs using the de novo P. peruviana transcriptome and further RT-qPCR analyses need to be considered.
In order to select appropriate reference genes, which are essential for the normalization of the expression of a target gene (Kozera & Rapacz, 2013), four genes were evaluated. Reference genes are those associated with basic cellular functions and are routinely used due to their expression at relatively constant levels in different organs, developmental stages and different stress conditions. However, a range of variability is expected depending on their stability and expression level under certain biological conditions (Hong et al., 2010; Kozera & Rapacz, 2013; Curis et al., 2019). This study is the first that evaluated different reference genes for Foph response in cape gooseberry. Even though most studies use traditional reference genes (Actin, EF), they are not always stably expressed in different species or experimental conditions (Rebouças et al., 2013; Zhu et al., 2013). Here, from the four genes evaluated, the TUB gene presented the lowest variation among samples. Osorio-Guarín, García-Arias & Yockteng (2019), also recommended TUB as an endogenous gene to evaluate the phytoene desaturase (PDS) gene expression in cape gooseberry leaf tissue, confirming the suitability of this gene for RT-qPCR analyses. This gene is also known to show a highly stable expression in carrot (Tian et al., 2015), sisal (Sarwar et al., 2020) and soybean (Hu et al., 2009).
Contrasting phenotypic responses between selected genotypes in response to Foph allowed proper sampling for RT-qPCR analysis
For the experimental design, it was important to follow the disease progress to ensure proper sampling of genotypes’ responses to Foph. The use of a modified severity scale from Enciso-Rodríguez et al. (2013), helped us to clearly separate the plant’s reponse to Foph, which it is suppported by Manzo et al. (2016), who recommended longer evaluation times, considering that the response to F. oxyxporum disease in a closely related species like tomato is slow.
The greenhouse assay showed similar responses to Foph as in a previous study (Osorio-Guarín et al., 2016). In the tolerant genotype, the vascular wilt disease was gradually increased with time, exhibiting a delay in the disease progress, dying at the end of the experiment. This delay in the infection suggests that the tolerance response against Foph was mediated by minor genes involved in horizontal or polygenic-related resistance, which also implies a reduction in the severity of the symptoms when compared with the susceptible genotype (Pagán & García-Arenal, 2018).
Verification of candidate gene expression in the P. peruviana—Foph pathosystem by RT-qPCR shed light on the interaction for crop improvement
During the first stages of the infection, Foph elicited the expression of the Php_TR3359, Php_TR69681 and Php_TR64396 candidate genes in the cape gooseberry susceptible genotype. Specifically, Php_TR69681 (Tomato gene ID: Solyc05g051900.2) is related to the major facilitator superfamily transporter proteins (MFS). MFS play an important role in controlling the exchange of toxins governing plant-pathogen interactions by exporting the plant pathogen toxins outside the cell (Peng et al., 2011; Menke, Dong & Kistler, 2012). The Zm-mfs1 defense-inducible gene that encodes a protein related to MFS transporters in maize, appears to be part of the initial defense response to fungal pathogen infection. It subsequently displays an expression decline when symptoms are developed in either susceptible or resistant genotypes (Simmons et al., 2003). In the present study, this gene showed a lightly increased expression at the earlier stages of the infection, with a subsequent decrease when the plant was approaching death in the susceptible genotype, which was opposite to the gene response in the tolerant genotype. On the other hand, the gene Php_TR3359 (Tomato gene ID: Solyc02g084920.2) is related to the alpha/beta subunit of the proteasome system, which according to Suty et al. (2003), it translates signals that trigger the systemic acquired response (SAR) against pathogens’ attack. Here, an earlier induction of this gene was observed in the susceptible genotype, lasting until the plant collapse. Similarly, Üstün et al. (2016) showed that proteasome activity is strongly induced during basal defense in Arabidopsis thaliana, being an essential component of molecular pattern-triggered immunity and SAR induction. The third gene, Php_TR64396 encodes thioredoxins proteins that act as antioxidants, playing an essential role in tolerance to oxidative stress and are involved in defense mechanisms to the mosaic virus in tobacco (Tomato gene ID: Solyc11g069690.1) (Vieira Dos Santos & Rey, 2006; Sun et al., 2010). This gene was also induced in the earlier stages of the disease with a high expression in the degree scale 3. All the previous genes were expressed in the susceptible genotype, and although they had been previously associated with defense response in plants, they could also be associated to susceptibility as observed in potato and Arabidopsis. For instance, the defense-associated gene DMR6 is expressed during the downy mildew infection in Arabidopsis, favoring pathogen attack and had also been associated with susceptibility responses in potato (Van Damme et al., 2008; Sun et al., 2016). Since the expression of Php_TR64396 and Php_TR3359 are highly induced in the susceptible cape gooseberry genotype during Foph infection, it is possible that these genes could be associated to susceptibility-related responses (Fig. 4). However, caution must be taken when analyzing the role of these genes in the Foph susceptible genotype. These genes are derived from previously associated genomic regions detected by GWAS (Osorio-Guarín et al., 2016), and likely co-segregate with the actual causal gene located in the same linkage disequilibrium block.
Interestingly, a second group of candidate genes (Php_TR46393, Php_TR8981, Solyc08g066660.1.1), involved in hormone synthesis pathways, presented an induction in later stages of the infection, mainly in the tolerant genotype. The Php_TR46393 gene (Tomato Gene ID: Solyc08g61260.2) is related to a large family of transmembrane receptor proteins called G-protein-coupled receptors (GPCRs) in fungi and metazoans. These genes are believed to be involved in plant defense signaling by mediating responses initiated by multiple RLKs (Liu et al., 2013), and are associated with resistance to necrotrophic pathogens such as F. oxysporum, Alternaria brassicicola and Botrytis cinerea (Trusov et al., 2006, 2009). Trusov et al. (2009) showed in later phases of the disease response that heterometric G proteins enhanced the expression of jasmonic acid (JA)/ethylene (ET)-dependent genes conferring resistance to F. oxysporum in Arabidopsis. These observations suggest that G proteins might play a key role in the tolerance response to Foph in cape gooseberry. The gene Php_TR8981 (Tomato Gene ID: Solyc09g098450.2), also associated with stress response in plants, encodes a class 3 lipase protein and has a relationship with the proteins PAD4, EDS1 and SAG101 that form a systemic signal that functions as the main barrier against pathogens, involving SA accumulation (Wiermer, Feys & Parker, 2005). Finally, the Solyc08g066660.1 gene encodes ethylene response transcription factor (ERF), which act either as an activator or repressor of plant defense responses (Thirugnanasambantham et al., 2015). Overexpression of ERFs has been shown to enhance plant resistance to F. oxysporum (Berrocal-Lobo & Molina, 2004). In general, the overexpression of these genes in the tolerant P. peruviana genotype clearly shows the late response to Foph infection. This response involved the pathogen recognition and posterior signaling cascade that induces local and systemic responses mediated by plant hormones such as ET and SA.
Other important genes that were expressed in the tolerant genotype, at later stages, were Php_TR7902 and Php_TR3653. The first one (Tomato Gene ID: Solyc08g081990.2) encodes a WD-40 repeat involved in different functions, including the hypersensitive response (Smith et al., 1999). The second gene Php_TR3653 (Tomato Gene ID: Solyc12g049500.1) has a beta domain of legume lectin, that according to Roopashree et al. (2006) might be involved in the protection against pathogenic agents by producing lipoxygenases. Specifically, legume-like lectin receptor kinases are involved in sensing cell wall integrity and defense response to Phytophthora infestans (Bouwmeester et al., 2011).
Overall, harnessing genomic regions associated with Foph resistance/susceptibility responses in cape gooseberry will contribute to the establishment of appropriate breeding schemes for early selection of resistant/tolerant genotypes. The validation of candidate genes identified from RNA-Seq and GWAS will contribute to this goal, accelerating the generation of new cape gooseberry varieties with desirable attributes and the understanding of P. peruviana responses against Foph.
Conclusions
This is the first attempt to validate a set of resistance/defense response candidate genes during P. peruviana—Foph interaction using RT-qPCR. This study corroborates the differential expression of eight candidate genes at different stages of the Foph disease severity scale. These genes could be used for developing specific markers to identify susceptible and tolerant cape gooseberry cultivars against Foph. Thus, these genes are great candidates for breeding purposes and marker-assisted or genomic selection programs. Further, analysis of more additional DEG candidate genes would provide a broader understanding of this pathosystem.
Supplemental Information
(A) Molecular Function. (B) Biological process. (C) Cellular component.
(A) DEG: Solyc06g068830.1.1. (B) DEG: Solyc11g066270.1.1. (C) DEG: Solyc08g066660.1.1. (D) GWAS: Php_TR7902. (E) GWAS: Php_TR3653. (F) GWAS: Php_TR69681.
(A) Actina (ACT). (B) Elongation factor (EF-1α). (C) Tubulin (TUB). (D) Pyrophosphatase (PPA2).
Susceptible genotype: 09U128-5, Tolerant genotype: 09U140-5.
Reads were downloaded from the Sequence Read Archive (SRA) under BioProject ID 67621.
Yellow color represents the 10 candidate DGEs genes selected.
Yellow color represents the original alignment with the Physalis transcript ID from the DEGs candidate genes (see Supplemental Table S5)
Acknowledgments
We thank Edgar Jimenez for suppling in vitro plant material for the greenhouse assays. The authors gratefully acknowledge Tatiana Rodríguez and Edwin Rodriguez for their help in the greenhouse assay and assisting with plant care and Johan David Barboza for adapting the disease severity scale. We also thank Dr. Lukas Mueller and Dr. Suzy Strickler from the Boyce Thomson Institute for their guidance in RNA-Seq analysis. Finally, we thank Roxana Yockteng for her support and advice in data analysis and comments on the paper.
Funding Statement
Support for this research was provided by a grant of the Colombian Ministry of Agriculture agreement No. 1726 and TV-18. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Luz Stella Barrero, Email: lbarrero@agrosavia.co.
Jaime A. Osorio Guarín, Email: josoriog@agrosavia.co.
Additional Information and Declarations
Competing Interests
Gina A. Garzón-Martínez, Francy L. García-Arias, Felix E. Enciso-Rodríguez, Mauricio Soto-Suárez, Carolina González, Luz Stella Barrero, and Jaime A. Osorio-Guarín are employed by Corporacion Colombiana de Investigación Agropecuaria Agrosavia.
Author Contributions
Gina A. Garzón-Martínez conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Francy L. García-Arias performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Felix E. Enciso-Rodríguez conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Mauricio Soto-Suárez conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Carolina González conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Aureliano Bombarely analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Luz Stella Barrero conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Jaime A. Osorio Guarín conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Raw data are available in the Supplemental Files.
References
- Abdallah & Bauer (2016).Abdallah HB, Bauer P. Quantitative reverse transcription-qPCR-based gene expression analysis in plants BT—plant signal transduction: methods and protocols. Methods in Molecular Biology. 2016;1363:9–24. doi: 10.1007/978-1-4939-3115-6_2. [DOI] [PubMed] [Google Scholar]
- Abou Baker & Rady (2020).Abou Baker D, Rady H. Bioassay-guided approach employed to isolate and identify anticancer compounds from Physalis peruviana calyces. Plant Archives. 2020;20:3285–3291. [Google Scholar]
- Andrews et al. (2012).Andrews S, Krueger F, Segonds-Pichon A, Biggins L, Krueger C, Wingett S. Babraham: Babraham Institute; 2012. [23 August 2018]. FastQC. FastQC: a quality control tool for high throughput sequence data. [Google Scholar]
- Aronesty (2011).Aronesty E. ea-utils : “Command-line tools for processing biological sequencing data”. GitHub. 2011. https://github.com/ExpressionAnalysis/ea-utils. [28 August 2018]. https://github.com/ExpressionAnalysis/ea-utils
- Bartoli & Roux (2017).Bartoli C, Roux F. Genome-wide association studies in plant pathosystems: toward an ecological genomics approach. Frontiers in Plant Science. 2017;8:1165. doi: 10.3389/fpls.2017.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocal-Lobo & Molina (2004).Berrocal-Lobo M, Molina A. Ethylene response factor 1 mediates arabidopsis resistance to the Soilborne Fungus Fusarium oxysporum. Molecular Plant-Microbe Interactions. 2004;17(7):763–770. doi: 10.1094/MPMI.2004.17.7.763. [DOI] [PubMed] [Google Scholar]
- Bielach, Hrtyan & Tognetti (2017).Bielach A, Hrtyan M, Tognetti VB. Plants under stress: involvement of auxin and cytokinin. International Journal of Molecular Sciences. 2017;18(7):1427. doi: 10.3390/ijms18071427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombarely (2012).Bombarely A. GenoToolBox. 2012. https://github.com/aubombarely/GenoToolBox. [30 August 2019]. https://github.com/aubombarely/GenoToolBox
- Bouwmeester et al. (2011).Bouwmeester K, De Sain M, Weide R, Gouget A, Klamer S, Canut H, Govers F. The lectin receptor kinase LecRK-I.9 is a novel Phytophthora resistance component and a potential host target for a RXLR effector. PLOS Pathogens. 2011;7(3):e1001327. doi: 10.1371/journal.ppat.1001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito et al. (2017).Brito LF, Irla M, Kalinowski J, Wendisch VF. Detailed transcriptome analysis of the plant growth promoting Paenibacillus riograndensis SBR5 by using RNA-seq technology. BMC Genomics. 2017;18(1):846. doi: 10.1186/s12864-017-4235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin & Huggett (2017).Bustin S, Huggett J. qPCR primer design revisited. Biomolecular Detection and Quantification. 2017;14:19–28. doi: 10.1016/j.bdq.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casassola et al. (2015).Casassola A, Brammer SP, Chaves MS, Martinelli JA, Stefanato F, Boyd LA. Changes in gene expression profiles as they relate to the adult plant leaf rust resistance in the wheat cv Toropi. Physiological and Molecular Plant Pathology. 2015;89(4):49–54. doi: 10.1016/j.pmpp.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2011).Chen L, Zhong H-Y, Kuang J-F, Li J-G, Lu W-J, Chen J-Y. Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta. 2011;234(2):377–390. doi: 10.1007/s00425-011-1410-3. [DOI] [PubMed] [Google Scholar]
- Cheng, Song & Wu (2019).Cheng J, Song N, Wu J. A patatin-like protein synergistically regulated by jasmonate and ethylene signaling pathways plays a negative role in Nicotiana attenuata resistance to Alternaria alternata. Plant Diversity. 2019;41(1):7–12. doi: 10.1016/j.pld.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi & Klessig (2016).Choi HW, Klessig DF. DAMPs, MAMPs, and NAMPs in plant innate immunity. BMC Plant Biology. 2016;16(1):379. doi: 10.1186/s12870-016-0921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregeen et al. (2015).Cregeen S, Radisek S, Mandelc S, Turk B, Stajner N, Jakse J, Javornik B. Different gene expressions of resistant and susceptible hop cultivars in response to infection with a highly aggressive strain of verticillium albo-atrum. Plant Molecular Biology Reporter. 2015;33(3):689–704. doi: 10.1007/s11105-014-0767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curis et al. (2019).Curis E, Nepost C, Grillault Laroche D, Courtin C, Laplanche J-L, Etain B, Marie-Claire C. Selecting reference genes in RT-qPCR based on equivalence tests: a network based approach. Scientific Reports. 2019;9(1):16231. doi: 10.1038/s41598-019-52217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski et al. (2005).Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible W-R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology. 2005;139(1):5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapprich et al. (2016).Dapprich J, Ferriola D, Mackiewicz K, Clark PM, Rappaport E, D’Arcy M, Sasson A, Gai X, Schug J, Kaestner KH, Monos D. The next generation of target capture technologies—large DNA fragment enrichment and sequencing determines regional genomic variation of high complexity. BMC Genomics. 2016;17(1):486. doi: 10.1186/s12864-016-2836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher & Callis (2007).Dreher K, Callis J. Ubiquitin, hormones and biotic stress in plants. Annals of Botany. 2007;99(5):787–822. doi: 10.1093/aob/mcl255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enciso-Rodríguez et al. (2013).Enciso-Rodríguez FE, González C, Rodríguez EA, López CE, Landsman D, Barrero LS, Mariño-Ramírez L. Identification of immunity related genes to study the Physalis peruviana—Fusarium oxysporum pathosystem. PLOS ONE. 2013;8(7):e68500. doi: 10.1371/journal.pone.0068500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, Liu & Li (2012).Fang S-T, Liu J-K, Li B. Ten new withanolides from Physalis peruviana. Steroids. 2012;77(1–2):36–44. doi: 10.1016/j.steroids.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Fischer et al. (2005).Fischer G, Miranda D, Piedrahita W, Romero J. Avances en cultivo, poscosecha y exportación de la uchuva (Physalis peruviana L.) en Colombia. Bogotá: Universidad Nacional de Colombia, Facultad de Agronomía; 2005. [Google Scholar]
- Frary, Doganlar & Frary (2016).Frary A, Doganlar S, Frary A. Synteny among solanaceae genomes. In: Causse M, Giovannoni J, Bouzayen M, Zouine M, editors. The Tomato Genome. Berlin: Springer; 2016. pp. 217–243. [Google Scholar]
- Garber et al. (2011).Garber M, Grabherr MG, Guttman M, Trapnell C. Computational methods for transcriptome annotation and quantification using RNA-seq. Nature Methods. 2011;8(6):469–477. doi: 10.1038/nmeth.1613. [DOI] [PubMed] [Google Scholar]
- Garzón-Martínez et al. (2012).Garzón-Martínez GA, Zhu Z, Landsman D, Barrero LS, Mariño-Ramírez L. The Physalis peruviana leaf transcriptome: assembly, annotation and gene model prediction. BMC Genomics. 2012;13(1):151. doi: 10.1186/1471-2164-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr et al. (2011).Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, Di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology. 2011;29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong et al. (2010).Hong SM, Bahn SC, Lyu A, Jung HS, Ahn JH. Identification and testing of superior reference genes for a starting pool of transcript normalization in Arabidopsis. Plant & Cell Physiology. 2010;51(10):1694–1706. doi: 10.1093/pcp/pcq128. [DOI] [PubMed] [Google Scholar]
- Howlader et al. (2020).Howlader J, Robin AHK, Natarajan S, Biswas MK, Sumi KR, Song CY, Park J, Nou I. Transcriptome analysis by RNA-seq reveals genes related to plant height in two sets of parent-hybrid combinations in easter lily (Lilium longiflorum) Scientific Reports. 2020;10(1):9082. doi: 10.1038/s41598-020-65909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2009).Hu R, Fan C, Li H, Zhang Q, Fu Y-F. Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT-PCR. BMC Molecular Biology. 2009;10(1):93. doi: 10.1186/1471-2199-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husaini, Sakina & Cambay (2018).Husaini AM, Sakina A, Cambay SR. Host-pathogen interaction in Fusarium oxysporum infections: where do we stand? Molecular Plant-Microbe Interactions®. 2018;31(9):889–898. doi: 10.1094/MPMI-12-17-0302-CR. [DOI] [PubMed] [Google Scholar]
- Integrated DNA Technologies (2018).Integrated DNA Technologies PrimerQuest® program. 2018. https://www.idtdna.com/pages/tools/primerquest. [3 July 2019]. https://www.idtdna.com/pages/tools/primerquest
- Iquebal et al. (2019).Iquebal MA, Sharma P, Jasrotia RS, Jaiswal S, Kaur A, Saroha M, Angadi UB, Sheoran S, Singh R, Singh GP, Rai A, Tiwari R, Kumar D. RNAseq analysis reveals drought-responsive molecular pathways with candidate genes and putative molecular markers in root tissue of wheat. Scientific Reports. 2019;9(1):13917. doi: 10.1038/s41598-019-49915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones & Dangl (2006).Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444(7117):323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kozera & Rapacz (2013).Kozera B, Rapacz M. Reference genes in real-time PCR. Journal of Applied Genetics. 2013;54(4):391–406. doi: 10.1007/s13353-013-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li & Dewey (2011).Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12(1):323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberato et al. (2014).Liberato S, Sánchez-Betancourt E, Arguelles J, González C, Núñez V, Barrero L. Cytogenetics of Physalis peruviana L. and Physalis floridana Rydb. genotypes with differential response to Fusarium oxysporum. Corpoica Ciencia y Tecnología Agropecuaria. 2014;15:49–59. [Google Scholar]
- Liu et al. (2013).Liu J, Ding P, Sun T, Nitta Y, Dong O, Huang X, Yang W, Li X, Botella JR, Zhang Y. Heterotrimeric G proteins serve as a converging point in plant defense signaling activated by multiple receptor-like kinases. Plant Physiology. 2013;161(4):2146–2158. doi: 10.1104/pp.112.212431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2010).Ma LJ, Van Der Does HC, Borkovich KA, Coleman JJ, Daboussi MJ, Di Pietro A, Dufresne M, Freitag M, Grabherr M, Henrissat B, Houterman PM, Kang S, Shim WB, Woloshuk C, Xie X, Xu JR, Antoniw J, Baker SE, Bluhm BH, Breakspear A, Brown DW, Butchko RAE, Chapman S, Coulson R, Coutinho PM, Danchin EGJ, Diener A, Gale LR, Gardiner DM, Goff S, Hammond-Kosack KE, Hilburn K, Hua-Van A, Jonkers W, Kazan K, Kodira CD, Koehrsen M, Kumar L, Lee YH, Li L, Manners JM, Miranda-Saavedra D, Mukherjee M, Park G, Park J, Park SY, Proctor RH, Regev A, Ruiz-Roldan MC, Sain D, Sakthikumar S, Sykes S, Schwartz DC, Turgeon BG, Wapinski I, Yoder O, Young S, Zeng Q, Zhou S, Galagan J, Cuomo CA, Kistler HC, Rep M. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature. 2010;464(7287):367–373. doi: 10.1038/nature08850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo et al. (2016).Manzo D, Ferriello F, Puopolo G, Zoina A, D’Esposito D, Tardella L, Ferrarini A, Ercolano MR. Fusarium oxysporum f.sp. radicis-lycopersici induces distinct transcriptome reprogramming in resistant and susceptible isogenic tomato lines. BMC Plant Biology. 2016;16(1):53. doi: 10.1186/s12870-016-0740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke, Dong & Kistler (2012).Menke J, Dong YN, Kistler HC. Fusarium graminearum Tri12p influences virulence to wheat and trichothecene accumulation. Molecular Plant-Microbe Interactions. 2012;25(11):1408–1418. doi: 10.1094/MPMI-04-12-0081-R. [DOI] [PubMed] [Google Scholar]
- MinAgricultura (2017).MinAgricultura Área Sembrada, Cosechada, Producción Y Rendimiento Del Cultivo De Uchuva Según Departamento. https://www.agronet.gov.co/Documents/39-UCHUVA_2017.pdf. [5 December 2017];Evaluaciones agropecuarias municipales. 2017 [Google Scholar]
- Minic (2008).Minic Z. Physiological roles of plant glycoside hydrolases. Planta. 2008;227(4):723–740. doi: 10.1007/s00425-007-0668-y. [DOI] [PubMed] [Google Scholar]
- Murashige & Skoog (1962).Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 1962;15(3):473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Najafi, Sorkheh & Nasernakhaei (2018).Najafi S, Sorkheh K, Nasernakhaei F. Characterization of the APETALA2/Ethylene-responsive factor (AP2/ERF) transcription factor family in sunflower. Scientific Reports. 2018;8(1):1–16. doi: 10.1038/s41598-018-29526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki et al. (1994).Namiki F, Shiomi T, Kayamura T, Tsuge T. Characterization of the formae speciales of Fusarium oxysporum causing wilts of cucurbits by DNA fingerprinting with nuclear repetitive DNA sequences. Applied and Environmental Microbiology. 1994;60(8):2684–2691. doi: 10.1128/AEM.60.8.2684-2691.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen et al. (2019).Nguyen KL, Grondin A, Courtois B, Gantet P. Next-generation sequencing accelerates crop gene discovery. Trends in Plant Science. 2019;24(3):263–274. doi: 10.1016/j.tplants.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Núñez et al. (2016a).Núñez V, Sánchez-Betancourt E, Mayorga F, Navas A, Gómez L. Corpoica Andina. Variedad de uchuva para Boyacá, Cundinamarca, Antioquia y Nariño. Bogotá: Corporación Colombiana de Investigación Agropecuaria; 2016a. [Google Scholar]
- Núñez et al. (2016b).Núñez V, Sánchez-Betancourt E, Mayorga F, Navas A, Gómez L. Corpoica Dorada: variedad de uchuva para Boyacá, Cundinamarca y Antioquia. Bogotá: Corporación Colombiana de Investigación Agropecuaria; 2016b. [Google Scholar]
- Olsen, Popper & Krause (2016).Olsen S, Popper ZA, Krause K. Two sides of the same coin: xyloglucan endotransglucosylases/hydrolases in host infection by the parasitic plant Cuscuta. Plant Signaling & Behavior. 2016;11(3):e1145336. doi: 10.1080/15592324.2016.1145336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio-Guarín et al. (2016).Osorio-Guarín JA, Enciso-Rodríguez FE, González C, Fernández-Pozo N, Mueller LA, Barrero LS. Association analysis for disease resistance to Fusarium oxysporum in cape gooseberry (Physalis peruviana L.) BMC Genomics. 2016;17(1):248. doi: 10.1186/s12864-016-2568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio-Guarín, García-Arias & Yockteng (2019).Osorio-Guarín J, García-Arias F, Yockteng R. Virus-induced gene silencing (VIGS) in cape gooseberry (Physalis peruviana L., Solanaceae) Universitas Scientiarum. 2019;24(1):111–133. doi: 10.11144/Javeriana.SC24-1.vigs. [DOI] [Google Scholar]
- Pagán & García-Arenal (2018).Pagán I, García-Arenal F. Tolerance to plant pathogens: theory and experimental evidence. International Journal of Molecular Sciences. 2018;19(3):810. doi: 10.3390/ijms19030810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng et al. (2011).Peng H, Han S, Luo M, Gao J, Liu X, Zhao M. Roles of multidrug transporters of MFS in plant stress responses. International Journal of Bioscience, Biochemistry and Bioinformatics. 2011;1:109–113. doi: 10.7763/IJBBB.2011.V1.20. [DOI] [Google Scholar]
- Pombo et al. (2019).Pombo MA, Ramos RN, Zheng Y, Fei Z, Martin GB, Rosli HG. Transcriptome-based identification and validation of reference genes for plant-bacteria interaction studies using Nicotiana benthamiana. Scientific Reports. 2019;9(1):1–10. doi: 10.1038/s41598-018-38247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prihatna, Barbetti & Barker (2018).Prihatna C, Barbetti MJ, Barker SJ. A novel tomato Fusarium wilt tolerance gene. Frontiers in Microbiology. 2018;9:1226. doi: 10.3389/fmicb.2018.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente, Nocetti & Espinosa (2019).Puente L, Nocetti D, Espinosa A. Physalis peruviana Linnaeus, an update on its functional properties and beneficial effects in human health. In: Mariod AA, editor. Wild Fruits: Composition, Nutritional Value and Products. Cham: Springer International Publishing; 2019. pp. 447–463. [Google Scholar]
- Ramadan (2020).Ramadan MF. Bioactive phytochemicals of cape gooseberry (Physalis peruviana L.) In: Murthy HN, Bapat VA, editors. Bioactive Compounds in Underutilized Fruits and Nuts. Cham: Springer International Publishing; 2020. pp. 75–90. [Google Scholar]
- Rebouças et al. (2013).Rebouças EDL, Costa JJDN, Passos MJ, Passos JRDS, Hurk RVD, Silva JRV. Real time PCR and importance of housekeepings genes for normalization and quantification of mRNA expression in different tissues. Brazilian Archives of Biology and Technology. 2013;56(1):143–154. doi: 10.1590/S1516-89132013000100019. [DOI] [Google Scholar]
- Robinson, McCarthy & Smyth (2009).Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopashree et al. (2006).Roopashree S, Singh SA, Gowda LR, Rao AGA. Dual-function protein in plant defence: seed lectin from Dolichos biflorus (horse gram) exhibits lipoxygenase activity. Biochemical Journal. 2006;395(3):629–639. doi: 10.1042/BJ20051889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen & Skaletsky (2000).Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods in Molecular Biology. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Ruiz-Villalba et al. (2017).Ruiz-Villalba A, Van Pelt-Verkuil E, Gunst QD, Ruijter JM, Van den Hoff MJB. Amplification of nonspecific products in quantitative polymerase chain reactions (qPCR) Biomolecular Detection and Quantification. 2017;14(11):7–18. doi: 10.1016/j.bdq.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboki, Usha & Singh (2011).Saboki E, Usha K, Singh B. Pathogenesis related (PR) proteins in plant defense mechanism age-related pathogen resistance. Current Research and Technology Advances. 2011;2:1043–1054. [Google Scholar]
- Safavi-Rizi, Herde & Stöhr (2020).Safavi-Rizi V, Herde M, Stöhr C. RNA-Seq reveals novel genes and pathways associated with hypoxia duration and tolerance in tomato root. Scientific Reports. 2020;10(1):1692. doi: 10.1038/s41598-020-57884-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar et al. (2020).Sarwar MB, Ahmad Z, Anicet BA, Sajid M, Rashid B, Hassan S, Ahmed M, Husnain T. Identification and validation of superior housekeeping gene(s) for qRT-PCR data normalization in Agave sisalana (a CAM-plant) under abiotic stresses. Physiology and Molecular Biology of Plants. 2020;26(3):567–584. doi: 10.1007/s12298-020-00760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savary et al. (2019).Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nature Ecology & Evolution. 2019;3(3):430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- Schmittgen & Livak (2008).Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sicilia et al. (2019).Sicilia A, Testa G, Santoro DF, Cosentino SL, Lo Piero AR. RNASeq analysis of giant cane reveals the leaf transcriptome dynamics under long-term salt stress. BMC Plant Biology. 2019;19(1):355. doi: 10.1186/s12870-019-1964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simbaqueba et al. (2018).Simbaqueba J, Catanzariti A-MA, González C, Jones DA. Evidence for horizontal gene transfer and separation of effector recognition from effector function revealed by analysis of effector genes shared between cape-gooseberry- and tomato-infecting formae speciales of Fusarium oxysporum. Molecular Plant Pathology. 2018;19(10):2302–2318. doi: 10.1111/mpp.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons et al. (2003).Simmons CR, Fridlender M, Navarro PA, Yalpani N. A maize defense-inducible gene is a major facilitator superfamily member related to bacterial multidrug resistance efflux antiporters. Plant Molecular Biology. 2003;52(2):433–446. doi: 10.1023/A:1023982704901. [DOI] [PubMed] [Google Scholar]
- Smith et al. (1999).Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: a common architecture for diverse functions. Trends in Biochemical Sciences. 1999;24(5):181–185. doi: 10.1016/S0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- Subki, Abidin & Yusof (2018).Subki A, Abidin AAZ, Yusof ZNB. The role of thiamine in plants and current perspectives in crop improvement. B Group Vitamins—Current Uses and Perspectives. InTech.2018. [Google Scholar]
- Sun et al. (2010).Sun L, Ren H, Liu R, Li B, Wu T, Sun F, Liu H, Wang X, Dong H. An h-type thioredoxin functions in tobacco defense responses to two species of viruses and an abiotic oxidative stress. Molecular Plant-Microbe Interactions. 2010;23(11):1470–1485. doi: 10.1094/MPMI-01-10-0029. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2016).Sun K, Wolters AMA, Vossen JH, Rouwet ME, Loonen AEHM, Jacobsen E, Visser RGF, Bai Y. Silencing of six susceptibility genes results in potato late blight resistance. Transgenic Research. 2016;25(5):731–742. doi: 10.1007/s11248-016-9964-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suty et al. (2003).Suty L, Lequeu J, Lançon A, Etienne P, Petitot AS, Blein JP. Preferential induction of 20S proteasome subunits during elicitation of plant defense reactions: towards the characterization of plant defense proteasomes. International Journal of Biochemistry and Cell Biology. 2003;35(5):637–650. doi: 10.1016/S1357-2725(02)00386-2. [DOI] [PubMed] [Google Scholar]
- Takken & Rep (2010).Takken F, Rep M. The arms race between tomato and Fusarium oxysporum. Molecular Plant Pathology. 2010;11:309–314. doi: 10.1111/J.1364-3703.2009.00605.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirugnanasambantham et al. (2015).Thirugnanasambantham K, Durairaj S, Saravanan S, Karikalan K, Muralidaran S, Islam VIH. Role of ethylene response transcription factor (ERF) and its regulation in response to stress encountered by plants. Plant Molecular Biology Reporter. 2015;33(3):347–357. doi: 10.1007/s11105-014-0799-9. [DOI] [Google Scholar]
- Tian et al. (2020).Tian L, Chou H-L, Fukuda M, Kumamaru T, Okita TW. mRNA localization in plant cells. Plant Physiology. 2020;182(1):97–109. doi: 10.1104/pp.19.00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian et al. (2015).Tian C, Jiang Q, Wang F, Wang G-L, Xu Z-S, Xiong A-S. Selection of suitable reference genes for qPCR normalization under abiotic stresses and hormone stimuli in carrot leaves. PLOS ONE. 2015;10(2):e0117569. doi: 10.1371/journal.pone.0117569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusov et al. (2006).Trusov Y, Rookes JE, Chakravorty D, Armour D, Schenk PM, Botella JR. Heterotrimeric G proteins facilitate arabidopsis resistance to necrotrophic pathogens and are involved in jasmonate signaling. Plant Physiology. 2006;140(1):210–220. doi: 10.1104/pp.105.069625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusov et al. (2009).Trusov Y, Sewelam N, Rookes JE, Kunkel M, Nowak E, Schenk PM, Botella JR. Heterotrimeric G proteins-mediated resistance to necrotrophic pathogens includes mechanisms independent of salicylic acid-, jasmonic acid/ethylene- and abscisic acid-mediated defense signaling. Plant Journal. 2009;58(1):69–81. doi: 10.1111/j.1365-313X.2008.03755.x. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi et al. (2015).Tsukagoshi H, Suzuki T, Nishikawa K, Agarie S, Ishiguro S, Higashiyama T. RNA-Seq analysis of the response of the halophyte, Mesembryanthemum crystallinum (Ice Plant) to high salinity. PLOS ONE. 2015;10(2):1–16. doi: 10.1371/journal.pone.0118339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üstün et al. (2016).Üstün S, Sheikh A, Gimenez-Ibanez S, Jones A, Ntoukakis V, Börnke F. The proteasome acts as a hub for plant immunity and is targeted by pseudomonas type III effectors. Plant Physiology. 2016;172(3):1941–1958. doi: 10.1104/pp.16.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Użarowska et al. (2009).Użarowska A, Dionisio G, Sarholz B, Piepho H-P, Xu M, Ingvardsen CR, Wenzel G, Lübberstedt T. Validation of candidate genes putatively associated with resistance to SCMV and MDMV in maize (Zea mays L.) by expression profiling. BMC Plant Biology. 2009;9(1):15. doi: 10.1186/1471-2229-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme et al. (2008).Van Damme M, Huibers RP, Elberse J, Van Den Ackerveken G. Arabidopsis DMR6 encodes a putative 2OG-Fe(II) oxygenase that is defense-associated but required for susceptibility to downy mildew. Plant Journal. 2008;54(5):785–793. doi: 10.1111/j.1365-313X.2008.03427.x. [DOI] [PubMed] [Google Scholar]
- Van der Burgh & Joosten (2019).Van der Burgh AM, Joosten MHAJ. Plant immunity: thinking outside and inside the box. Trends in Plant Science. 2019;24(7):587–601. doi: 10.1016/j.tplants.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Vera Alvarez et al. (2017).Vera Alvarez R, Medeiros Vidal N, Garzón-Martínez GA, Barrero LS, Landsman D, Mariño-Ramírez L. Workflow and web application for annotating NCBI BioProject transcriptome data. Database: The Journal of Biological Databases and Curation. 2017;2017:1135. doi: 10.1093/database/bax008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira Dos Santos & Rey (2006).Vieira Dos Santos C, Rey P. Plant thioredoxins are key actors in the oxidative stress response. Trends in Plant Science. 2006;11(7):329–334. doi: 10.1016/j.tplants.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Villarino et al. (2014).Villarino GH, Bombarely A, Giovannoni JJ, Scanlon MJ, Mattson NS. Transcriptomic analysis of Petunia hybrida in response to salt stress using high throughput RNA sequencing. PLOS ONE. 2014;9(4):e94651. doi: 10.1371/journal.pone.0094651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyska, Cunniffe & Gilligan (2016).Vyska M, Cunniffe N, Gilligan C. Trade-off between disease resistance and crop yield: a landscape-scale mathematical modelling perspective. Journal of the Royal Society Interface. 2016;13(123):20160451. doi: 10.1098/rsif.2016.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiermer, Feys & Parker (2005).Wiermer M, Feys BJ, Parker JE. Plant immunity: the EDS1 regulatory node. Current Opinion in Plant Biology. 2005;8(4):383–389. doi: 10.1016/j.pbi.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Yuen, Brooks & Li (2001).Yuen PS, Brooks KM, Li Y. RNA: a method to specifically inhibit PCR amplification of known members of a multigene family by degenerate primers. Nucleic Acids Research. 2001;29(6):E31. doi: 10.1093/nar/29.6.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2013).Zhu J, Zhang L, Li W, Han S, Yang W, Qi L. Reference gene selection for quantitative real-time PCR normalization in Caragana intermedia under different abiotic stress conditions. PLOS ONE. 2013;8(1):53196. doi: 10.1371/journal.pone.0053196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Molecular Function. (B) Biological process. (C) Cellular component.
(A) DEG: Solyc06g068830.1.1. (B) DEG: Solyc11g066270.1.1. (C) DEG: Solyc08g066660.1.1. (D) GWAS: Php_TR7902. (E) GWAS: Php_TR3653. (F) GWAS: Php_TR69681.
(A) Actina (ACT). (B) Elongation factor (EF-1α). (C) Tubulin (TUB). (D) Pyrophosphatase (PPA2).
Susceptible genotype: 09U128-5, Tolerant genotype: 09U140-5.
Reads were downloaded from the Sequence Read Archive (SRA) under BioProject ID 67621.
Yellow color represents the 10 candidate DGEs genes selected.
Yellow color represents the original alignment with the Physalis transcript ID from the DEGs candidate genes (see Supplemental Table S5)
Data Availability Statement
The following information was supplied regarding data availability:
Raw data are available in the Supplemental Files.