Abstract
Background and Aims:
α2 agonists have been utilised in regional blocks, but very little data is available for their use in transversus abdominis plane (TAP) block in paediatric laparoscopic (LAP) surgeries. This study investigated the analgesic effect of ropivacaine alone versus its combination with dexmedetomidine for TAP block in children undergoing LAP surgery.
METHODS:
A randomised, double-blind trial was conducted in 50 American Society of Anesthesiologists (ASA) 1 and 2 children of 2–8 years undergoing LAP abdominal surgery. Children were randomised to receive a total volume of 0.5 ml/kg of 0.2% ropivacaine (LA group) or 0.2% ropivacaine with 1 μg/kg dexmedetomidine (LAD group) for performing ultrasound-guided bilateral TAP block postoperatively (PO). Patients were monitored PO for vital signs, pain, sedation, time to first rescue analgesic and total analgesic consumption for 24 h. Time to first rescue analgesic was expressed as mean ± standard deviation (SD) and analysed using Kaplan–Meier survival analysis. Pain and sedation scores were expressed as median [interquartile range (IQR)] and analysed using Mann–Whitney U test.
Results:
First rescue analgesic demand was significantly longer (P = 0.001) in LAD (474.8 min) versus LA group (240.9 min) but total analgesics consumption in first 24 h was comparable. Pain scores were significantly lower (P < 0.05) in LAD compared to LA group at all times PO. Each group had comparable but significantly lower sedation scores up to 24 h PO.
Conclusion:
Addition of dexmedetomidine to ropivacaine in TAP block prolongs the time to first analgesic requirement without a difference in the total analgesic consumption.
Keywords: Analgesia, child, dexmedetomidine, laparoscopy, nerve block, pain, postoperative
INTRODUCTION
Peripheral nerve blocks are favoured over central neuraxial blocks as they cause minimal haemodynamic alterations and complications, such as urinary retention, spinal or epidural haematoma as well as short hospital stay. Transversus abdominis plane (TAP) block is the most popular peripheral nerve block providing adequate postoperative (PO) analgesia for a range of abdominal procedures.[1,2] α2 agonists, dexmedetomidine and clonidine have been utilised as adjuncts to a local anaesthetic (LA) to prolong the effect of TAP block.[1] Dexmedetomidine has higher selectivity for α2A receptors as compared to clonidine. In a meta-analysis by El-Boghdadly et al., dexmedetomidine prolonged the duration of motor and sensory block and shortened the onset of block compared to clonidine in supraclavicular nerve block.[3]
Although adult studies have described the successful usage of adjuncts in regional blocks, very little data is available regarding the use of adjuncts in TAP block in paediatric laparoscopic (LAP) surgeries. Thus, the present study aimed to investigate the analgesic effect of a combination of dexmedetomidine and LA compared to LA alone when used for TAP block in children undergoing LAP surgery. The primary objective of the study was to compare the time to requirement of first rescue analgesic dose in patients receiving TAP block and secondary objective was to compare the requirement of total number of analgesic doses in the first 24 h PO.
METHODS
After approval of Institute Ethics Committee (INT/IEC/2016/2654) and written informed consent from parents, this randomised controlled double-blinded trial was conducted in 50 American Society of Anesthesiologists (ASA) physical status 1 and 2 children of 2–8 years undergoing elective LAP abdominal surgery under general anaesthesia from 8.09.16 to 13.10.17. The study was registered (CTRI/2017/09/009749) in Clinical Trial Registry of India. The study followed the principles of the Declaration of Helsinki.
Participants were allocated to receive 0.2% plain ropivacaine (LA group) or 0.2% ropivacaine with dexmedetomidine 1 μg/kg (LAD group) in a total volume of 0.5 ml/kg for performing bilateral TAP block PO. Group allocation was based on a randomised computer-generated sequence in which both assessor and patients were blinded to group allocation by an opaque sealed envelope method. The envelope was opened and the test drugs were prepared based on the assigned group by an anaesthesiologist who was not a part of the study. Children having local infection at injection site, LA allergy and known contraindication to peripheral nerve blockade were excluded.
After the standard nil per oral instructions, the children were premedicated with oral midazolam (0.5 mg/kg) 30 min before the procedure. They underwent either inhalational induction using oxygen, nitrous oxide (FiO2 = 0.5) and graded sevoflurane (1-8%) or intravenous (IV) induction using propofol (2–2.5 mg/kg)/thiopentone sodium (3–4 mg/kg) depending upon the presence of venous access. Nitrous oxide was discontinued after the IV access was secured and patient was given 100% oxygen till intubation. IV morphine 0.1 mg/kg was administered for intraoperative analgesia at induction. Trachea was intubated utilising IV atracurium (0.5 mg/kg) and an appropriate-sized cuffed endotracheal tube (Microcuff endotracheal tube- Kimberly Clark, Halyard). Anaesthesia was maintained with isoflurane [minimum alveolar concentration (MAC) 1.0–1.2], oxygen and air combination (FiO2 = 0.5) with the patient on pressure controlled mechanical ventilation keeping target end-tidal carbon-dioxide (ETCO2) of 32-42 mmHg. Electrocardiogram, pulse oximetry (SPO2), ETCO2, end tidal anaesthetic gas concentration, MAC, non-invasive blood pressure (NIBP) and temperature were monitored for all patients. Patients were administered additional opioid (IV fentanyl 0.5–1 μg/kg) if the surgery duration was prolonged (>3 h) or there was intraoperative tachycardia (rise in HR >20% from baseline).
Ultrasound (US)-guided TAP block was performed bilaterally at the end of surgery under aseptic conditions using a 38 mm, 6–13 MHz linear array US transducer. US probe was placed in the axial plane across the mid-axillary line midway between costal margin and iliac crest. Following the identification of three different layers of the abdominal wall, a 25 mm needle was advanced in plane until its tip lay between the transversus abdominis and the internal oblique muscle. After negative aspiration, confirmation of correct tip location was obtained by hydrodissection of the plane with normal saline. The end point of injection was the spread of LA (0.25 ml/kg on each side) between the transversus abdominis and internal oblique muscle observed as hypoechoic layer on US. Prophylactic IV ondansetron (0.15 mg/kg) and paracetamol (15 mg/kg) were administered at the end of surgery. Neuromuscular blockade was reversed with IV neostigmine (0.05 mg/kg) and glycopyrrolate (0.01 mg/kg) and trachea was extubated.
Patients were administered IV paracetamol 15 mg/kg 8 hourly. They were monitored in the postanaesthesia care unit (PACU) at half hourly interval for 3 h after the TAP block and then in the ward at 4, 8, 12, 18 and 24 h. The parameters recorded were vital signs [pulse rate (PR), NIBP and SPO2], pain [Children's Hospital of Eastern Ontario pain scale (CHEOPS)], sedation [University of Michigan Sedation Score (UMSS)], time to first rescue analgesic (IV tramadol 1 mg/kg when CHEOPS was ≥6), total analgesic consumption in the first 24 h (dose and number) and postoperative vomiting (number of episodes). Intraoperatively block was considered a success if HR and BP were stable and <20% of baseline and a failure when postoperatively CHEOPS was >6 within 30 min after shifting to PACU.
Calculation of the sample size was based on a previous study on paediatric LAP surgeries for undescended testis where the mean time to first rescue analgesia was 67.3 min [standard deviation (SD) 62.3 min].[4] Using a proposed non-inferiority design and assuming a mean difference to first rescue analgesia of 60 min and power of 0.9, a total of 25 patients were allotted to each group. Type 1 error associated with this test is 0.05.
Data were analysed using International Business Machine (IBM) Statistical Package for the Social Sciences (SPSS) version 23.0 computer software. The data were presented as mean ± SD or median [interquartile range (IQR)]. The data were tested for normality using Kolmogorov–Smirnov test. For normally distributed data, t-test was applied. For the analysis of categorical variables with two categories, χ2 test was used. CHEOPS and UMSS were analysed using Mann–Whitney U test. Time to first rescue analgesic was expressed as mean ± SD and was analysed using Kaplan–Meier survival analysis and Log Rank (Mantel–Cox) test. A P value of <0.05 was considered statistically significant.
RESULTS
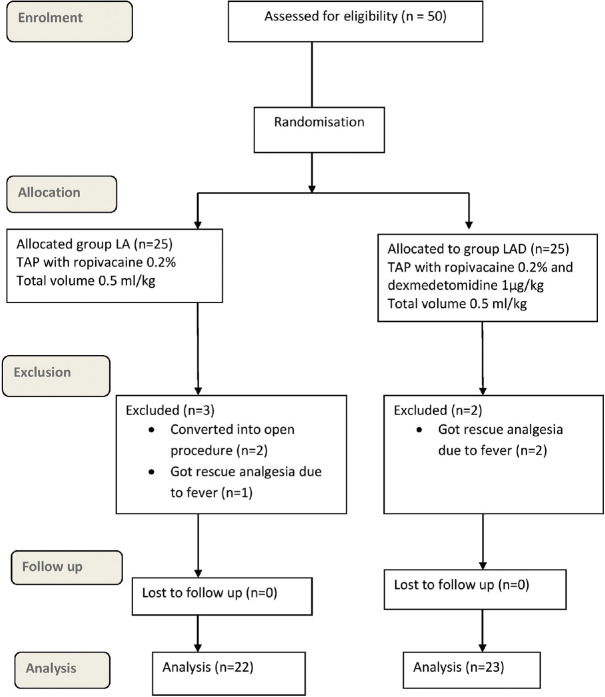
Totally, 50 cases were enroled. Two cases in LA group were converted into open procedure, one patient in LA group and two patients in LAD group received rescue analgesic (paracetamol) due to fever and were thus excluded from the study. So, 22 cases in LA group and 23 cases in LAD group were analysed [Figure 1]. Demographic and operative data for both groups were comparable [Table 1]. Intraoperative fentanyl was given to three patients in LAD group and four patients in LA group.
Figure 1.
CONSORT flow diagram
Table 1.
Demographic characteristics
| LA group (n=22) | LAD group (n=23) | P | |
|---|---|---|---|
| Age (years)* | 5.0 (8-3.25) | 7.0 (8-4.5) | 0.330 |
| Weight (kg)† | 19.2±5.8 | 22.1±6.1 | 0.112 |
| Gender (M: F) number (%)‡ | 16:6 (73:27) | 13:10 (56:44) | 0.256 |
| Duration of anaesthesia (min)† | 167.8±60.4 | 169.4±54.7 | 0.925 |
| Duration of surgery (min)† | 130.7±57.5 | 128.8±52.3 | 0.906 |
| Laparoscopic surgical procedures‡ | |||
| Appendicectomy | 2 (40) | 3 (60) | 0.817 |
| Cholecystectomy | 5 (42) | 7 (58) | |
| Choledochal cyst removal | 5 (45) | 6 (55) | |
| Nephrectomy | 6 (75) | 2 (25) | |
| Pyeloplasty | 1 (50) | 1 (50) | |
| Splenectomy | 1 (33) | 2 (67) | |
| Others | 2 (50) | 2 (50) |
*Values expressed as median (IQR) and analysed using Mann-Whitney U test; †Values expressed as mean±SD and analysed using unpaired t test; ‡Values expressed as number (%) and analysed using Chi-square test. Group LA: Total 0.5 ml/kg of 0.2% ropivacaine, Group LAD: Total 0.5 ml/kg of 0.2% ropivacaine with 1 μg/kg dexmedetomidine
The mean time to first rescue analgesic was 474.8 ± 81.1 min (95% confidence interval (CI):393.75–555.87) in LAD group which was significantly longer (P = 0.001, df = 1) as compared to 240.9 ± 52.7 min (CI:188.17–293.55) in LA group [Figure 2]. However, there was no significant difference (P = 0.298) in total analgesic consumption in first 24 h in the two groups (LA group:3.6 ± 0.7 vs LAD group:3.4 ± 1.1). A total of 19 patients in LA group and 9 patients in LAD group received rescue analgesia.
Figure 2.
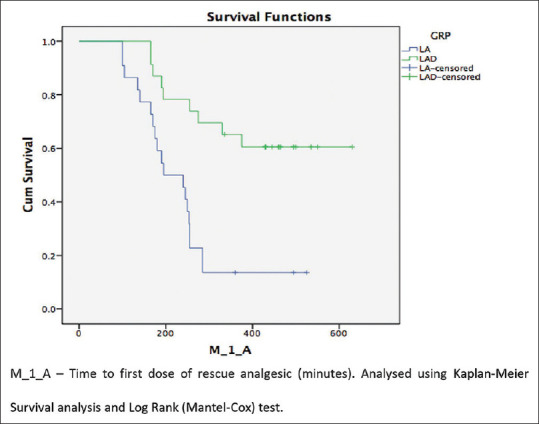
Kaplan–Meier survival curve for time to 1st analgesic
In the LA as well as LAD group, CHEOP score was significantly higher at 3rd (LA, P = 0.016, LAD, P = 0.035) and 4th (LA, P = 0.001, LAD, P = 0.006) PO hour when compared to baseline (first reading after arrival in PACU). CHEOP score was significantly lower in LAD group compared to Group LA at all-time points (P < 0.05) except at 3rd hour PO (P = 0.058) [Table 2]. When compared with baseline, both groups had significantly lower sedation scores at all times of observation (P < 0.001). UMSS scores were comparable between the two groups at all-time intervals up to 24 h PO [Table 3].
Table 2.
CHEOPS in the postoperative period in the two groups
| TIME POINT | LA group (n=22) | LAD group (n=23) | P between groups LA and LAD | 95% CI of the mean difference |
|---|---|---|---|---|
| T0 | 2.0 [2.0-3.0] | 2.0 [1.0-2.0] | 0.022 | 0.63 (0.05-1.2) |
| T0.5 | 2.0 [2.0-3.0] | 2.0 [2.0-2.0] | 0.029 | 0.54 (-0.03-1.1) |
| T1 | 3.0 [2.0-4.0] | 2.0 [2.0-2.0] | 0.001 | 1.1 (0.51-1.6) |
| T1.5 | 3.0 [3.0-5.0] | 2.0 [2.0-3.0] | 0.001 | 1.7 (0.7-2.7) |
| T2 | 4.0 [3.0-5.0] | 2.0 [2.0-3.0] | 0.002 | 1.5 (0.47-2.6) |
| T2.5 | 5.0 [3.0-5.0] | 3.0 [2.0-4.0] | 0.016 | 1.6 (0.28-2.9) |
| T3 | 4.0 [3.5-5.0]* | 3.0 [2.0-4.5]* | 0.058 | 0.76 (-0.28-1.8) |
| T4 | 5.0 [4.0-7.5]* | 4.0 [3.0-4.0]* | 0.004 | 1.8 (0.63-3.0) |
| T8 | 4.0 [3.0-5.0] | 3.0 [3.0-4.0] | 0.012 | 0.82 (0.16-1.4) |
| T12 | 5.0 [3.0-5.0] | 3.0 [2.0-4.0] | 0.022 | 1.0 (-0.13-2.3) |
| T18 | 4.0 [3.75-5.0] | 3.0 [2.0-4.0] | 0.012 | 1.1 (0.30-2.0) |
| T24 | 3.0 [2.75-4.0] | 3.0 [2.0-4.0] | 0.033 | 0.3 (-0.39 to 1.0) |
Values are expressed as median [IQR] and analysed using Mann-Whitney U test. *Intragroup comparison (3rd h LA, P=0.016, LAD, P=0.035 and 4th h LA, P=0.001, LAD, P=0.006). T0: on arrival to PACU, T0.5: at 30 min, T1: at 1 h, T1.5: at 1.5 h, T2: at 2 h, T2.5: at 2.5 h, T3: at 3 h, T4: at 4 h, T8: at 8 h, T12: at 12 h, T18: at 18 h, T24: at 24 h. Group LA: Total 0.5 ml/kg of 0.2% ropivacaine, Group LAD: Total 0.5 ml/kg of 0.2% ropivacaine with 1 μg/kg dexmedetomidine. CHEOPS: Children’s Hospital of Eastern Ontario Pain Scale
Table 3.
Sedation score (UMSS) in the postoperative period in both the groups
| Time point | LA group (n=22) | LAD group (n=23) | P between groups LA and LAD | 95% CI of the mean difference |
|---|---|---|---|---|
| T0 | 2.0 [2.0-3.0] | 2.0 [2.0-3.0] | 0.382 | -0.21 (-0.64-0.21) |
| T0.5 | 2.0 [1.75-2.0]* | 2.0 [2.0-2.0]* | 0.488 | -0.22 (-0.67-0.21) |
| T1 | 1.0 [0.75-2.0]* | 1.0 [1.0-2.0]* | 0.446 | -0.21 (-0.64-0.21) |
| T1.5 | 1.0 [0.0-1.0]* | 1.0 [1.0-1.0]* | 0.486 | -0.14 (-0.56-0.28) |
| T2 | 0.0 [0.0-1.0]* | 1.0 [0.0-1.0]* | 0.109 | -0.28 (-0.65-0.08) |
| T2.5 | 0.0 [0.0-0.0]* | 0.0 [0.0-1.0]* | 0.085 | -0.30 (-0.63-0.03) |
| T3 | 0.0 [0.0-0.0]* | 0.0 [0.0-1.0]* | 0.279 | -0.17 (-0.46-0.11) |
| T4 | 0.0 [0.0-0.0]* | 0.0 [0.0-0.5]* | 0.778 | 0.00 (-0.30-0.30) |
| T8 | 1.0 [0.0-1.0]* | 0.0 [0.0-1.0]* | 0.126 | 0.29 (-0.05-0.63) |
| T12 | 1.0 [0.0-1.0]* | 1.0 [1.0-1.0]* | 0.886 | -0.01 (-0.35-0.33) |
| T18 | 1.0 [0.0-1.0]* | 0.0 [0.0-1.0]* | 0.329 | 0.15 (-0.19-0.51) |
| T24 | 0.0 [0.0-0.0]* | 0.0 [0.0-0.0]* | 0.322 | -0.08 (-0.25-0.08) |
Values are expressed as median [IQR] and analysed using Mann-Whitney U test. *Intragroup comparison P<0.05 as compared with baseline. T0: on arrival to PACU, T0.5: at 30 min, T1: at 1 h, T1.5: at 1.5 h, T2: at 2 h, T2.5: at 2.5 h, T3: at 3 h, T4: at 4 h, T8: at 8 h, T12: at 12 h, T18: at 18 h, T24: at 24 h. Group LA: Total 0.5 ml/kg of 0.2% ropivacaine, Group LAD: Total 0.5 ml/kg of 0.2% ropivacaine with 1 μg/kg dexmedetomidine. UMSS: University of Michigan Sedation Score
In group LA as well as group LAD, there was no significant change in pulse rate at all times of observation up to 24 h when compared to baseline. However, the PR was significantly lower (P < 0.05) in group LAD at all-time intervals up to 24 h [Figure 3].
Figure 3.
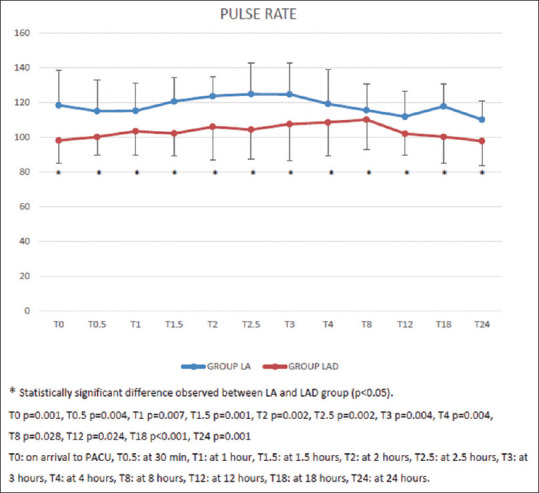
Postoperative pulse rate in both the groups at various time intervals
The two groups had no significant difference in systolic and diastolic blood pressure at all-time intervals up to 24 h when compared to the baseline and also between the two groups.
Out of 45 patients, only three patients had episode of postoperative nausea and vomiting (PONV). Two patients in LAD group had single episode of vomiting for which one received dexamethasone and another received ondansetron. In LA group, one patient had three episodes of vomiting for which metoclopramide and pantoprazole were given.
DISCUSSION
TAP block has been successfully utilised in both adults and children for providing PO analgesia for a variety of surgical procedures showing reduced PO analgesic requirement.[5] Al-Sadek et al. found that children receiving TAP block for LAP correction of undescended testes had lower pain scores, required less intraoperative opioid, longer time to first supplemental analgesia and less analgesic requirement during first 24 h PO.[4]
The duration of analgesia after TAP block is limited to the duration of effect of LA being administered. Therefore, α2 agonists like clonidine and dexmedetomidine have been utilised as adjuncts for prolonging the analgesic effect of LAs in various types of regional blocks.[1,6,7,8] α2 agonists prolong both duration and degree of the LA effect of lidocaine in a dose dependent pattern.[9] Marhofer et al. found the prolongation of duration of ulnar nerve block after the addition of dexmedetomidine to ropivacaine by approximately 60%.[10] Various authors have shown that addition of dexmedetomidine (0.3 μg/kg – 2 μg/kg) to various LA agents in different types of peripheral nerve blocks results in the prolongation of analgesia.[1,6,10,11,12,13,14] However, very little data are available regarding the usage of adjuvants in TAP block for paediatric LAP surgeries.[6,11]
In this study, we preferred to use dexmedetomidine as an adjunct over clonidine because dexmedetomidine is more α2 selective and associated with less haemodynamic fluctuations.[3]. Their mechanism of action is explained by hyperpolarisation of neuronal cation currents resulting in localised inhibitory effect. Dexmedetomidine may have greater inhibitory effect on neuronal action potentials as compared to clonidine. Further, dexmedetomidine provides differential sensory block due to its more pronounced inhibitory effect on Aδ and C fibre action potentials than motor neurons as compared to clonidine. In a meta-analysis by El-Boghdadly et al., it was shown that the onset of action is shortened and duration of sensory and motor blockade is prolonged with dexmedetomidine as compared to clonidine in supraclavicular nerve block.[3]
Dexmedetomidine dose of 1 μg/kg chosen for this study is an intermediate dose in comparison with previous studies where it has been used in a dose range from 0.3-2 μg/kg.[6,11,12,13,14,15] Various studies have utilised a volume of 0.4–2 ml/kg of LA for performing the TAP block.[4,6,16,17] Disma et al. compared the postoperative analgesia of three different concentrations of levobupivacaine 0.375%, 0.25% and 0.125% for ilioinguinal or iliohypogastric block in paediatric inguinal hernia repair and found out that 0.4 mL/kg of 0.25% levobupivacaine provided good pain relief.[17] Sandeman et al. used 0.5 ml/kg ropivacaine 0.2% for US-guided TAP block for paediatric laparoscopic appendicectomy.[16] Mishra et al. utilised ropivacaine 0.2% for TAP block in adult patients undergoing lower abdominal surgery.[15] Based on this, we used 0.2% concentration and 0.5 ml/kg volume of ropivacaine in our study.
The results of our study showed that time to first rescue analgesic was significantly prolonged in LAD group (474.8 ± 81.1 min) as compared to LA group (240.7 ± 52.7 min). Roaf et al. found that time to first rescue analgesic was significantly prolonged in dexmedetomidine group (255 min) compared to the plain bupivacaine group (145 min) in paediatric laparoscopic unilateral herniorrhaphy or hydrocelectomy.[6] Lundblad et al. have also shown that median time to first dose for supplemental analgesics was prolonged by 88% in dexmedetomidine group when compared with plain ropivacaine group (4.05 and 7.61 h respectively, P = 0.071) in paediatric inguinal hernia repair after receiving US-guided ilioinguinal/iliohypogastric nerve block, although this difference was not statistically significant.[11] The authors attributed this to smaller sample size.
No significant difference (P = 0.298) was observed in the total number of analgesic doses required PO in the first 24 h in the two groups. The most probable explanation for this finding maybe that both the groups were receiving supplemental analgesia with paracetamol at 8 hourly intervals with the first dose given when CHEOPS was ≥6. Lundblad et al. also found no significant difference in the number of analgesic doses required postoperatively in the first 24 h.[11] On the contrary, significant reduction in the 24 h postoperative analgesic requirement was observed with dexmedetomidine as compared to the LA in TAP block by many authors.[1,4,5,6,12]
Postoperative pain was assessed using CHEOP score in our study. CHEOPS has been recommended as a valid, reliable and practical tool for assessing pain scores in children.[18,19] Pain score was significantly lower in the dexmedetomidine group at all-time points except 3rd h PO (P = 0.058). In a meta-analysis conducted by Sun et al., addition of dexmedetomidine significantly reduced PO pain scores for 8 h at rest (P = 0.001) and 4 h at movements (P < 0.001).[20] Several other studies have shown lower pain score in the dexmedetomidine group.[1,4,8,11,12,15,16,21] Efficacy of reduction of pain scores has further been validated by significant reduction in the pulse rate PO at all-time points in the dexmedetomidine group.
In our study, both groups showed significantly lower sedation scores at all time-points after 30 min PO when compared with baseline. However, sedation scores were not significantly different between the two groups at all-time intervals up to 24 h. No significant difference in the systolic and diastolic blood pressure as well as PONV was observed between the two groups. The sedation produced by dexmedetomidine is dose related and probably the dose of 1 μg/kg used in our study did not produce sedation. Out of 45 patients, only three patients experienced PONV which can be attributed to increased incidence of PONV in laparoscopic surgery.[22] Therefore, a prophylactic anti-emetic was administered to all patients.
Laparoscopic port site placement was decided by the surgeon. The procedure required three ports: one at right iliac fossa (T12), second at umbilicus (T10) and third at left flank (T8) or suprapubic region (T12-L1). Lateral TAP block performed in our study covers T9-L1 dermatomes making it effective for lower abdominal surgeries. For upper abdominal surgeries, T8 dermatome is missed by the lateral TAP block. However, an additive such as dexmedetomidine may also act by systemic absorption providing more effective analgesia than ropivacaine alone.
The present study was powered to detect a significant difference in time to first rescue analgesic in the two groups. However, it was not powered to detect a difference in the number of rescue analgesics required in 24 h. A study with larger sample size would be required to detect this difference. A single dose of 1 μg/kg dexmedetomidine was used in combination with 0.2% ropivacaine. The effect of varying doses of dexmedetomidine as well as concentration of ropivacaine on pain and requirement of postoperative analgesics cannot be confirmed. Further studies using varying concentrations and doses are warranted. Efficacy of block could only be assessed by lower pain scores in the PO period as it was not possible to observe incision response or to check dermatomes for sensation in paediatric population.
CONCLUSION
Addition of dexmedetomidine to ropivacaine in TAP block prolongs the time to first analgesic requirement without a difference in the total analgesic consumption during first 24 h.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors wish to acknowledge all the parents and their children who gave consent to participate in this study.
REFERENCES
- 1.Almarakbi WA, Kaki AM. Addition of dexmedetomidine to bupivacaine in transversus abdominis plane block potentiates post-operative pain relief among abdominal hysterectomy patients: A prospective randomized controlled trial. Saudi J Anaesth. 2014;8:161–6. doi: 10.4103/1658-354X.130683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karnik PP, Dave NM, Shah HB, Kulkarni K. Comparison of ultrasound-guided transversus abdominis plane (TAP) block versus local infiltration during paediatric laparoscopic surgeries. Indian J Anaesth. 2019;63:356–60. doi: 10.4103/ija.IJA_89_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Boghdadly K, Brull R, Sehmbi H, Abdallah FW. Perineural dexmedetomidine Is more effective than clonidine when added to local anesthetic for supraclavicular brachial plexus block: A systematic review and meta-analysis. Anesth Analg. 2017;124:2008–20. doi: 10.1213/ANE.0000000000002014. [DOI] [PubMed] [Google Scholar]
- 4.Al-Sadek WM, Rizk SN, Selim MA. Ultrasound guided transversus abdominis plane block in pediatric patients undergoing laparoscopic surgery. Egypt J Anaesth. 2014;30:273–8. [Google Scholar]
- 5.Baeriswyl M, Kirkham KR, Kern C, Albrecht E. The analgesic efficacy of ultrasound-guided transversus abdominis plane block in adult patients: A meta-analysis. Anesth Analg. 2015;121:1640–54. doi: 10.1213/ANE.0000000000000967. [DOI] [PubMed] [Google Scholar]
- 6.Raof RA, El Metainy SA, Alia DA, Wahab MA. Dexmedetomidine decreases the required amount of bupivacaine for ultrasound-guided transversus abdominis plane block in pediatrics patients: A randomized study. J Clin Anesth. 2017;37:55–60. doi: 10.1016/j.jclinane.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Ali QE, Manjunatha L, Amir SH, Jamil S, Quadir A. Efficacy of clonidine as an adjuvant to ropivacaine in supraclavicular brachial plexus block: A prospective study. Indian J Anaesth. 2014;58:709–13. doi: 10.4103/0019-5049.147150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand VG, Kannan M, Thavamani A, Bridgit MJ. Effects of dexmedetomidine added to caudal ropivacaine in paediatric lower abdominal surgeries. Indian J Anaesth. 2011;55:340–6. doi: 10.4103/0019-5049.84835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshitomi T, Kohjitani A, Maeda S, Higuchi H, Shimada M, Miyawaki T. Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor. Anesth Analg. 2008;107:96–101. doi: 10.1213/ane.0b013e318176be73. [DOI] [PubMed] [Google Scholar]
- 10.Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2013;110:438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 11.Lundblad M, Marhofer D, Eksborg S, Lönnqvist PA. Dexmedetomidine as adjunct to ilioinguinal/iliohypogastric nerve blocks for pediatric inguinal hernia repair: An exploratory randomized controlled trial. Paediatr Anaesth. 2015;25:897–905. doi: 10.1111/pan.12704. [DOI] [PubMed] [Google Scholar]
- 12.Rai P, Negi DS, Singh SK, Malviya D, Bagwan MC. Effect of addition of dexmedetomidine to ropivacaine in Transversus Abdominis Plane Block on post-operative pain in lower segment caesarean section: A randomized controlled trial. IOSR JDMS. 2016;15:122–5. [Google Scholar]
- 13.Keplinger M, Marhofer P, Kettner SC, Marhofer D, Kimberger O, Zeitlinger M. A pharmacodynamic evaluation of dexmedetomidine as an additive drug to ropivacaine for peripheral nerve blockade: A randomised, triple-blind, controlled study in volunteers. Eur J Anaesthesiol. 2015;32:790–6. doi: 10.1097/EJA.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 14.Meenakshi Karuppiah NP, Shetty SR, Patla KP. Comparison between two doses of dexmedetomidine added to bupivacaine for caudal analgesia in paediatric infraumbilical surgeries. Indian J Anaesth. 2016;60:409–14. doi: 10.4103/0019-5049.183394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra M, Mishra SP, Singh SP. Ultrasound-guided transversus abdominis plane block: What are the benefits of adding dexmedetomidine to ropivacaine. Saudi J Anaesth. 2017;11:58–61. doi: 10.4103/1658-354X.197348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandeman DJ, Bennett M, Dilley AV, Perczuk A, Lim S, Kelly KJ. Ultrasound-guided transverses abdominis plane blocks for laparoscopic appendicectomy in children: A prospective randomized trial. Br J Anaesth. 2011;106:882–6. doi: 10.1093/bja/aer069. [DOI] [PubMed] [Google Scholar]
- 17.Disma N, Tuo P, Pellegrino S, Astuto M. Three concentrations of levobupivacaine for ilioinguinal/iliohypogastric nerve block in ambulatory pediatric surgery. J ClinAnesth. 2009;21:389–93. doi: 10.1016/j.jclinane.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Suraseranivongse S, Santawat U, Kraiprasit K, Petcharatana S, Prakkamodom S, Muntraporn N. Cross-validation of a composite pain scale for preschool children within 24 hours of surgery. Br J Anaesth. 2001;87:400–5. doi: 10.1093/bja/87.3.400. [DOI] [PubMed] [Google Scholar]
- 19.Hesselgard K, Larsson S, Romner B, Strömblad LG, Reinstrup P. Validity and measurement in children 1-7 years of age. Pediatr Crit Care Med. 2007;8:102–8. doi: 10.1097/01.PCC.0000257098.32268.AA. [DOI] [PubMed] [Google Scholar]
- 20.Sun Q, Liu S, Wu H, Ma H, Liu W, Fang M, et al. Dexmedetomidine as an adjuvant to local anesthetics in transversus abdominis plane block. Clin J Pain. 2019;35:375–84. doi: 10.1097/AJP.0000000000000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kendigelen P, Tutuncu AC, Erbabacan E, Ekici B, Köksal G, Altındas F, et al. Ultrasound-assisted transversus abdominis plane block vs wound infiltration in pediatric patient with inguinal hernia: Randomized controlled trial. J Clin Anesth. 2016;30:9–14. doi: 10.1016/j.jclinane.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Mehrotra S. Postoperative anaesthetic concerns in children: Postoperative pain, emergence delirium and postoperative nausea and vomiting. Indian J Anaesth. 2019;63:763–70. doi: 10.4103/ija.IJA_391_19. [DOI] [PMC free article] [PubMed] [Google Scholar]