Abstract
To report a case of linezolid-induced toxic optic neuropathy. Clinical examination and imaging are presented over a 4-month interval from initial presentation to subsequent follow-up of 4 months after discontinuation of linezolid. The patient was found to have optic neuropathy as demonstrated by clinical presentation and examination. Upon discontinuation of linezolid, the patient's visual acuity, visual fields, and color vision significantly improved. Linezolid has previously been reported to cause toxic optic neuropathy and retinopathy. We hereby describe a tuberculosis patient with linezolid-associated toxic optic neuropathy. Our report aims to describe the ocular side effects of linezolid use to enhance awareness.
Keywords: Linezolid, optic neuropathy, toxic neuropathy
Introduction
Toxic optic neuropathy is a complex and multifactorial medical condition characterized by bilateral painless progressive visual impairment preceded by a change in color vision. It occurs secondary to exposure to a toxin that damages the optic nerve. Common etiologies known to cause toxicity are drugs (Ethambutol, Amiodarone, and Tamoxifen) and nutritional deficiencies (Vitamin B12, folate).[1] We report a case of bilateral toxic optic neuropathy in a 25-year-old male known to have extensive drug-resistant tuberculosis (XDR-TB) presented with bilateral sudden visual impairment after 5 months of linezolid use along with other anti-TB medications.
Case Report
A 25-year-old male was referred to ophthalmology from the infectious diseases department with a complaint of painless blurred vision in both eyes for a few days. He denied headache, proximal muscle ache, or recent trauma. Medical history showed that the patient was diagnosed with XDR-TB on treatment with INH (300 mg/day) for 1 month, ethionamide (500 mg/twice per day) for 3 months, clofazimine (100 mg/day), moxifloxacin (400 mg/day), linezolid (600 mg/twice per day), cyscloserin (750 mg/day), and bedaquiline and pyridoxine (80 mg/day) for the past 5 months. Ocular history was unremarkable. The dosage of linezolid was increased to 1200 mg 3 weeks before the presentation.
On examination, visual acuity was 20/200 in both eyes. The intraocular pressure was 17 mmHg in the right eye and 16 mmHg in the left eye. Ishihara color plate test revealed a decrease in color vision, mainly in the left eye (3 of 15 plates) while the right eye was (7 of 15 plates). Pupils were regular, round, and reactive without relative afferent pupillary defect. The anterior segment examination was unremarkable. Fundi showed blurred disk margins in both eyes with mild nasal elevation in the left eye. His visual field revealed ceco-central scotomas in both eyes [Figure 1]. Magnetic resonance imaging of the brain and orbit was unremarkable. Leber's hereditary optic neuropathy genetic testing was negative.
Figure 1.
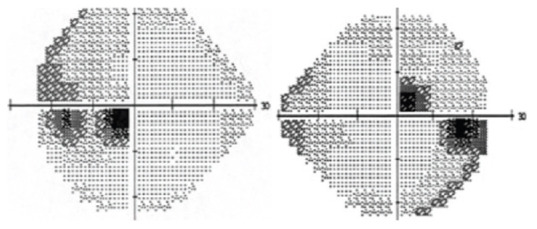
Humphry visual field testing at the presentation which showed cecocentral scotomas in both eyes
Out of the medications he is using, we concluded that the patient had a drug-induced toxic optic neuropathy secondary to a high dose of linezolid. Therefore, after informing the primary team, we suggested substituting linezolid with another alternative and they decided to stop linezolid and continue the rest of the medications. Subsequently, the patient reported improvement of vision 1-week post discontinuation of linezolid. Visual acuity improved to 20/60 in both eyes. However, color vision did not improve within the same period and inversely worsened in the right eye (5 of 15 plates). Three months after the presentation, visual acuity improved remarkably to 20/25 in the right eye and 20/28 in the left eye. Furthermore, color vision was fully restored in both eyes (15 of 15 plates). Furthermore, examination of fundi showed mild temporal disk pallor more prominent in the left eye. Visual field testing was unremarkable [Figure 2].
Figure 2.
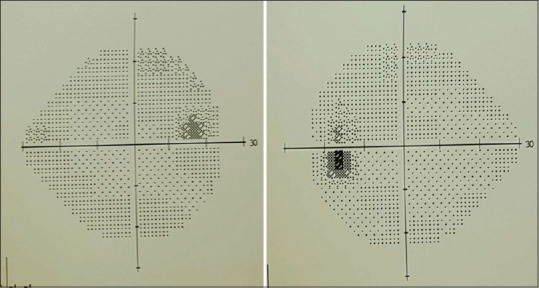
Humphry visual field testing 3 months after linezolid cessation
Discussion
Linezolid is a synthetic antibiotic that is used in the treatment of Gram-positive bacteria.[2] It belongs to the oxazolidinones group of antibiotics, which exert their action by blocking protein synthesis in bacterial cells. Linezolid has been used effectively in the treatment of multidrug-resistant TB as a second line.[2] While being effectively used in treating drug-resistant bacteria and such as numerous antibiotics, linezolid has been linked to side effects. These side effects can vary from mild headaches, nausea, diarrhea, and vomiting to potentially serious ones such as myelosuppression, serotonin syndrome, lactic acidosis, and peripheral neuropathy.
The possibility of experiencing side effects was linked to the dosage and duration of treatment, mainly when used for more than 2 weeks. Therefore, it requires weekly complete blood count monitoring and discontinuation when myelosuppression or neuropathy is suspected.[1] The main ocular side effect linked to linezolid use was optic neuropathy when used for more than 28 days that mandates frequent visual function monitoring. There were previous reports in the literature of changes in visual acuity or color vision, blurred vision, and visual field defects attributed to linezolid use.[3,4,5,6,7,8,9,10,11,12,13,14]
Toxic optic neuropathy is a bilaterally symmetrical optic nerve disorder characterized by central or cecocentral scotomas and color vision impairment. The papillomacular bundle is frequently damaged due to its high demand for energy and the inability of mitochondria to generate enough adenosine triphosphate.[15] Frequent substances were linked to toxic optic neuropathy including chemotherapeutic agents, alcohols, antiarrhythmics, PDE inhibitors, heavy metals, antimalarials, and other antibiotics such as chloramphenicol, sulfonamides, isoniazid, ethambutol, and streptomycin.
Nutritional deficiencies can also result in toxic optic neuropathies, with Vitamin B and folic acid deficiencies being the most significant and related to alcohol use or bariatric surgery. In addition to optic neuropathy, linezolid has been associated with photoreceptor dysfunction.[16,17] The photoreceptor dysfunction mechanism is attributed to mitochondrial dysfunction, causing an imbalance of free radical homeostasis resembling Leber's hereditary optic neuropathy.[16] A novel screening to prevent optic neuropathy has been proposed by Sean et al., based on the previously reported cases in which they recommend screening adult patients “within 1 month after initiating linezolid, followed by a subsequent evaluation every 30–60 days beginning 3 months from initiation.”[18] The primary treatment is the immediate cessation of linezolid with visual acuity improving first before color vision.[19]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kesler A, Pianka P. Toxic optic neuropathy. Curr Neurol Neurosci Rep. 2003;3:410–4. doi: 10.1007/s11910-003-0024-y. [DOI] [PubMed] [Google Scholar]
- 2.Who Expert Committee. The Selection and Use of Essential Medicines. WHO Technical Report Series. 2015. [Last accessed on 2020 Feb 24]. p. 31. Available from: https://apps.who.int/iris/bitstream/handle/10665/189763/9789241209946_eng.pdf; jsessionid=1A8F9E6BBB9BC6DB622EF371E6C6F4DC?sequence=1 .
- 3.Rana P, Roy V, Ahmad J. Drug-induced optic neuropathy in a case of extensively drug-resistant pulmonary tuberculosis. J Basic Clin Physiol Pharmacol. 2018;30:139–40. doi: 10.1515/jbcpp-2018-0007. [DOI] [PubMed] [Google Scholar]
- 4.Mehta S, Das M, Laxmeshwar C, Jonckheere S, Thi SS, Isaakidis P. Linezolid-associated optic neuropathy in drug-resistant tuberculosis patients in Mumbai, India. PLoS One. 2016;11:e0162138. doi: 10.1371/journal.pone.0162138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishii N, Kinouchi R, Inoue M, Yoshida A. Linezolid-induced optic neuropathy with a rare pathological change in the inner retina. Int Ophthalmol. 2016;36:761–6. doi: 10.1007/s10792-016-0196-5. [DOI] [PubMed] [Google Scholar]
- 6.Libershteyn Y. Ethambutol/linezolid toxic optic neuropathy. Optom Vis Sci. 2016;93:211–7. doi: 10.1097/OPX.0000000000000783. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal R, Addison P, Saihan Z, Pefkianaki M, Pavesio C. Optic neuropathy secondary to Linezolid for multidrug-resistant mycobacterial spinal tuberculosis. Ocul Immunol Inflamm. 2015;23:90–2. doi: 10.3109/09273948.2013.874447. [DOI] [PubMed] [Google Scholar]
- 8.Xerri O, Lemaire B, Nasser G, Rousseau-Huvey B, Labetoulle M, Rousseau A. Severe linezolid-induced toxic optic neuropathy. J Fr Ophtalmol. 2015;38:e55–8. doi: 10.1016/j.jfo.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Karuppannasamy D, Raghuram A, Sundar D. Linezolid-induced optic neuropathy. Indian J Ophthalmol. 2014;62:497–500. doi: 10.4103/0301-4738.118451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaitali P, Suresh R. Comment on linezolid induced optic neuropathy. Indian J Ophthalmol. 2015;63:75–6. doi: 10.4103/0301-4738.151484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han J, Lee K, Rhiu S, Lee JB, Han SH. Linezolid-associated optic neuropathy in a patient with drug-resistant tuberculosis. J Neuroophthalmol. 2013;33:316–8. doi: 10.1097/WNO.0b013e31829b4265. [DOI] [PubMed] [Google Scholar]
- 12.Khadilkar SV, Yadav RS, Rajan S. Linezolid optic neuropathy: Be careful and quick. J Assoc Physicians India. 2013;61:866–7. [PubMed] [Google Scholar]
- 13.Eisenack J, Landau K, Wildberger H, Knecht P. Linezolid-associated optic neuropathy? Klin Monbl Augenheilkd. 2012;229:433–4. doi: 10.1055/s-0031-1299168. [DOI] [PubMed] [Google Scholar]
- 14.Schecter GF, Scott C, True L, Raftery A, Flood J, Mase S. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2010;50:49–55. doi: 10.1086/648675. [DOI] [PubMed] [Google Scholar]
- 15.De Vriese AS, Coster RV, Smet J, Seneca S, Lovering A, Van Haute LL, et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis. 2006;42:1111–7. doi: 10.1086/501356. [DOI] [PubMed] [Google Scholar]
- 16.Grohmann SM, Berman A, Grassi MA. Linezolid-induced photoreceptor dysfunction masquerading as autoimmune retinopathy. Doc Ophthalmol. 2020;140:77–82. doi: 10.1007/s10633-019-09725-3. [DOI] [PubMed] [Google Scholar]
- 17.Park DH, Park TK, Ohn YH, Park JS, Chang JH. Linezolid induced retinopathy. Doc Ophthalmol. 2015;131:237–44. doi: 10.1007/s10633-015-9518-6. [DOI] [PubMed] [Google Scholar]
- 18.Dempsey SP, Sickman A, Slagle WS. Case report: Linezolid optic neuropathy and proposed evidenced-based screening recommendation. Optom Vis Sci. 2018;95:468–74. doi: 10.1097/OPX.0000000000001216. [DOI] [PubMed] [Google Scholar]
- 19.Sharma P, Sharma R. Toxic optic neuropathy. Indian J Ophthalmol. 2011;59:137–41. doi: 10.4103/0301-4738.77035. [DOI] [PMC free article] [PubMed] [Google Scholar]


