Key Points
CAR T-cell therapy is associated with 2 potentially costly and resource-intensive AEs: CRS and NEs.
Reducing the incidence/severity of these AEs improves safety outcomes and may lower management costs associated with CAR T-cell therapy.
Abstract
Chimeric antigen receptor (CAR) T-cell therapies have demonstrated high response rates in patients with relapsed/refractory large B-cell lymphoma (LBCL); however, these therapies are associated with 2 CAR T cell–specific potentially severe adverse events (AEs): cytokine release syndrome (CRS) and neurological events (NEs). This study estimated the management costs associated with CRS/NEs among patients with relapsed/refractory LBCL using data from the pivotal TRANSCEND NHL 001 trial of lisocabtagene maraleucel, an investigational CD19-directed defined composition CAR T-cell product with a 4-1BB costimulation domain administered at equal target doses of CD8+ and CD4+ CAR+ T cells. This retrospective analysis of patients from TRANSCEND with prospectively identified CRS and/or NE episodes examined relevant trial-observed health care resource utilization (HCRU) associated with toxicity management based on the severity of the event from the health care system perspective. Cost estimates for this analysis were taken from publicly available databases and published literature. Of 268 treated patients as of April 2019, 127 (47.4%) experienced all-grade CRS and/or NEs, which were predominantly grade ≤2 (77.2%). Median total AE management costs ranged from $1930 (grade 1 NE) to $177 343 (concurrent grade ≥3 CRS and NE). Key drivers of cost were facility expenses, including intensive care unit and other inpatient hospitalization lengths of stay. HCRU and costs were significantly greater among patients with grade ≥3 AEs (22.8%). Therefore, CAR T-cell therapies with a low incidence of severe CRS/NEs will likely reduce HCRU and costs associated with managing patients receiving CAR T-cell therapy. This clinical trial was registered at www.clinicaltrials.gov as #NCT02631044.
Visual Abstract
Introduction
Non-Hodgkin lymphoma (NHL) is the most common blood cancer in the United States and includes numerous subtypes with distinct biological and clinical behaviors.1,2 Diffuse large B-cell lymphoma (DLBCL) is an aggressive and common NHL subtype, accounting for 30% to 40% of all newly diagnosed cases.2,3 First-line treatment of patients with DLBCL usually comprises chemotherapy and rituximab with or without radiotherapy, with the most established accepted therapy being a combination of rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone (R-CHOP).4 Although most patients respond to first-line treatment, 20% to 50% of patients with DLBCL experience relapse or are unable to achieve remission after first-line therapy5,6; prognosis is poor in these cases. Patients with progressive disease after receipt of R-CHOP are typically treated with combination salvage chemotherapy, and those who respond to treatment may subsequently undergo autologous hematopoietic stem cell transplantation (HSCT) if they are sufficiently fit.5,7 Historically, there have been limited effective treatment options for patients with relapsed/refractory DLBCL who are ineligible for or relapse after autologous HSCT.5,8 However, recent developments in chimeric antigen receptor (CAR) T-cell therapy now provide an option with the possibility of cure.
CAR T-cell therapies have demonstrated high response rates in patients with relapsed/refractory LBCL, including DLBCL (de novo or transformed from any indolent lymphoma), high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements, primary mediastinal B-cell lymphoma, and grade 3B follicular lymphoma.9-11 With the introduction of CAR T-cell therapies, 2 key adverse events (AEs) have emerged: cytokine release syndrome (CRS) and neurological events (NEs). CRS, an inflammatory reaction that can be mild (eg, fever alone) or severe (resulting in multiorgan failure), results from immune activation and the release of inflammatory cytokines.12 In addition to CRS, NEs, ranging from confusion and aphasia to encephalopathy, may occur among patients who receive CAR T-cell therapy. The incidence and severity of CRS and NEs vary among CAR T-cell therapies, with all-grade and grade ≥3 CRS events ranging from 42% to 93% and 2% to 22%, respectively, and NEs ranging from 21% to 64% and 10% to 28%, respectively.9-11 Both high- and low-grade CRS and NEs are associated with various clinical and economic consequences, including potentially expensive medications and extended hospitalization stays.13,14 Currently, there is limited research to estimate the health care resource utilization (HCRU) and economic burden of CRS and NEs among patients who received lisocabtagene maraleucel (liso-cel), an investigational CD19-directed defined composition 4-1BB–costimulated CAR T-cell product administered at equal target doses of CD8+ and CD4+ CAR+ T cells.13-15
Previous studies have modeled the costs associated with CRS and NE management after CAR T-cell therapy.15,16 This study used data from the pivotal TRANSCEND NHL 001 clinical trial (hereafter referred to as TRANSCEND) of liso-cel to estimate the management costs associated with CRS and NEs in the LBCL cohort comprising patients with relapsed/refractory DLBCL not otherwise specified (either de novo or transformed from any indolent lymphoma), high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements, primary mediastinal B-cell lymphoma, or grade 3B follicular lymphoma.11 TRANSCEND is a multicenter seamless-design phase 1 study, with the first patient enrolled in January 2016.11 Patients with LBCL who subsequently relapsed after receiving prior anti-CD20–containing chemoimmunotherapy and/or undergoing prior autologous or allogeneic HSCT were treated with liso-cel at 1 of 3 target dose levels (50 × 106, 100 × 106, or 150 × 106 CAR+ T cells).
Methods
The study protocol and protocol amendments were approved by the following institutional review boards (IRBs) at participating sites: Memorial Sloan Kettering Cancer Center IRB/Privacy Board-B, University of Texas MD Anderson Cancer Center IRB, Advarra IRB (formerly Chesapeake IRB), Dana-Farber/Harvard Cancer Center IRB, University of Alabama IRB for Human Use, Beckman Research Institute of the City of Hope National Medical Center IRB, University of Nebraska Medical Center IRB, University of California San Francisco IRB, Fred Hutchinson Cancer Consortium IRB, Northside Hospital IRB, Western IRB, and University of Pittsburgh IRB. The study was conducted in accordance with the Declaration of Helsinki.
This study is a retrospective microcosting analysis of data from TRANSCEND. The clinical trial database (data cutoff date, 12 April 2019) contained data on patient demographics, clinical and treatment information, AEs (including CRS and NEs, grade of AEs, and treatments for CRS and NEs), and HCRU. The analysis included adult patients with relapsed/refractory LBCL in the TRANSCEND trial who were treated with liso-cel11 and who experienced a treatment-emergent (defined as starting any time from initiation of liso-cel administration through and including 90 days afterward) CRS or NE episode after receiving liso-cel. CRS was prospectively identified by investigators and defined as an AE using the Medical Dictionary for Regulatory Activities preferred term “cytokine release syndrome.” An NE was identified by investigators as a central nervous system AE that was reported as related to liso-cel. CRS was graded according to the Lee et al12 criteria. NEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).17 For patients who experienced multiple occurrences of the same AE, subsequent events starting within 7 days of resolution of the previous event were considered a single episode.11 The data were deidentified and compliant with Section 164.514(a) of the Health Insurance Portability and Accountability Act Privacy Rule; thus, no institutional board review was required, because this study was exempt under Exemption 45 CFR 46.101(b)(4). This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology checklist for cohort studies.18
Patient classification
Patients were included if they experienced any treatment-emergent CRS and/or NE episode.17 Patients were stratified by the treatment-emergent AE they experienced: (1) CRS only, (2) NE only, (3) nonconcurrent CRS and NE (defined as experiencing both with a gap between AE episodes), or (4) concurrent CRS and NE (defined as experiencing both with any overlap in AE episodes). Stratifications were further refined by AE severity. Patients who experienced an episode of CRS or NE only were classified according to individual grade, whereas patients who experienced episodes of both events were classified based on the highest grade of either the CRS and/or NE episode (grade 1-2 or ≥3).
HCRU and unit cost estimation
Relevant HCRU associated with the treatment and management of CRS and NEs was identified in the trial based on CRS and NE management guidelines. HCRU was defined as any office visits, standard inpatient (non–intensive care unit [ICU]) hospitalizations, ICU stays, diagnostic laboratory work or imaging, procedures, or medications consistent with management protocols and expert opinion on the specific AE (either CRS or NE) and severity that occurred on or between the dates of CRS and NE onset and resolution. A unit cost was applied to each incidence of HCRU, accounting for frequency and duration of each resource (Figure 1). Patient follow-up for this HCRU analysis was defined as the time from CRS or NE onset after liso-cel treatment to resolution. Any AEs that occurred after anticancer treatment subsequent to liso-cel or after liso-cel retreatment were not considered in this analysis.
Figure 1.
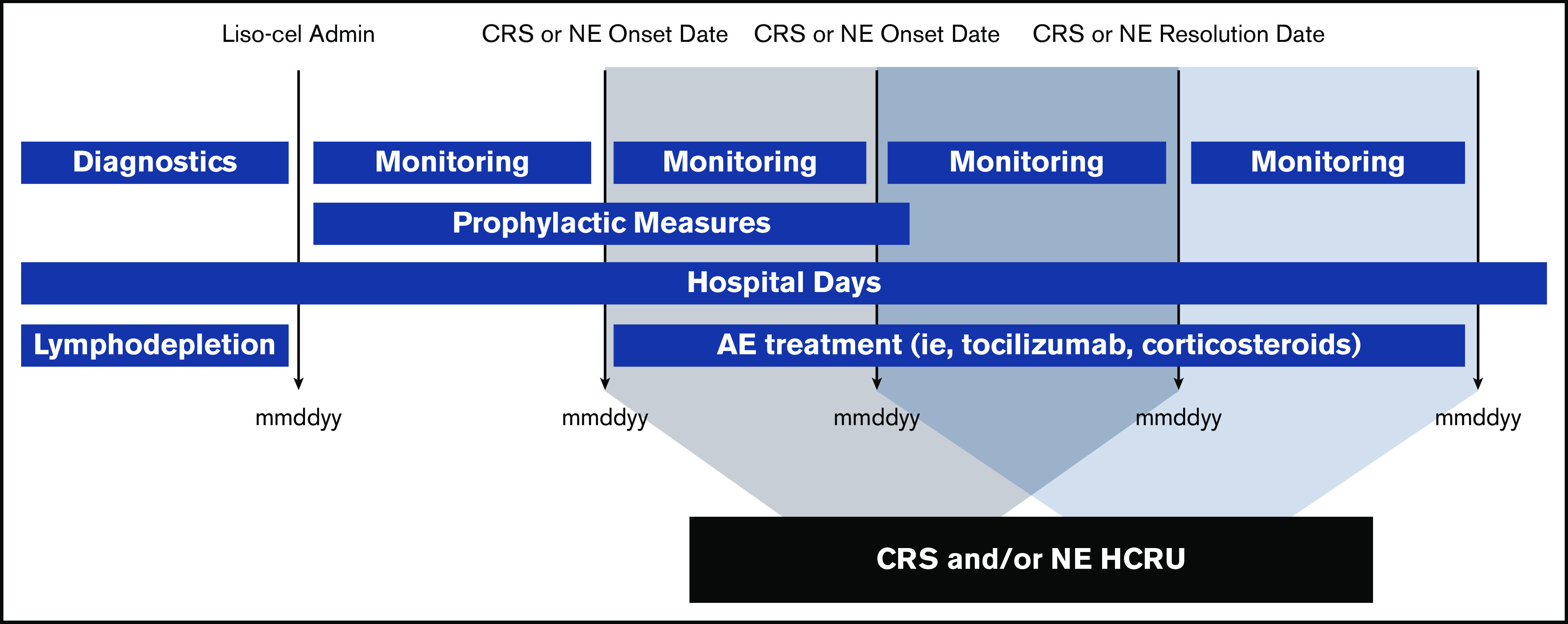
Study methodology. Dates were recorded to accurately account for the frequency and duration of any events experienced by individual patients to inform the cost calculations for this study.
The cost analysis was performed from a health care system perspective. Unit costs were obtained from 2019 public databases, including the National Inpatient Sample, Centers for Medicare and Medicaid Services (CMS) Physician Fee Schedule, CMS Outpatient Prospective Payment System, CMS Durable Medical Equipment Fee Schedule, and CMS Laboratory Fee Schedule.19-23 Costs were limited to those observed within the clinical trial setting and thus did not capture all potential costs that might be incurred in a real-world setting (eg, professional fees). The national payment amounts were used from these sources. Wholesale acquisition costs were obtained from the IBM Micromedex RED BOOK for unit costs of medications and evaluated at the dispensed amounts to incorporate drug wastage.24 When necessary, unit costs were obtained from peer-reviewed literature and inflated to 2019 US dollars using the US Bureau of Labor Statistics consumer price index for medical care.25,26
Given that reimbursement rates do not reflect actual costs, a payment/cost ratio was applied to Medicare payment rates to estimate the true cost of HCRU incurred by the health care system.27 Furthermore, we used cost ratios from the literature for cost adjustments to reflect the site of care where the health services occurred or were administered.28,29 Values of key unit costs are provided in Table 1.
Table 1.
Key unit costs and probabilistic sensitivity analysis parameters
| Parameter | Base case input | Reference | Detail | Low | High | Assumed distribution* |
|---|---|---|---|---|---|---|
| Reimbursement/cost ratio, % | 87.0 | 27 | — | 43.5 | 1.31 | β |
| Clinic/HOPD cost ratio, % | 38.0 | 28 | — | 19 | 57 | β |
| HOPD/IP cost ratio, % | 35.0 | 29 | — | 17.5 | 52.5 | β |
| HOPD office visit, $ | 115.85 | 21 | APC 5012 | 57.93 | 173.78 | γ |
| Standard inpatient bed day, $ | 2420.77 | 19 | Adjusted to 2019 US$ | 1210.38 | 3631.15 | γ |
| ICU bed day, $ | 7181.84 | 25 | Adjusted to 2019 US$ | 3590.92 | 10 772.75 | γ |
| CT head scan with contrast, $ | ||||||
| Nonfacility TC | 107.76 | 20 | HCPCS 70460 | 53.88 | 161.64 | γ |
| 26; phys. comp. | 58.38 | 20 | HCPCS 70460 | 29.19 | 87.57 | γ |
| TC; APC | 480.77 | 21 | APC 8006 | 240.39 | 721.16 | γ |
| MRI brain scan with contrast, $ | ||||||
| Nonfacility TC | 227.77 | 20 | HCPCS 70552 | 113.89 | 341.66 | γ |
| 26; phys. comp. | 91.54 | 20 | HCPCS 70552 | 45.77 | 137.31 | γ |
| TC; APC | 855.60 | 21 | APC 8008 | 427.80 | 1283.40 | γ |
| PET scan with contrast, $ | ||||||
| Nonfacility TC | 1375.61 | 20 | HCPCS 78608 | 687.81 | 2063.42 | γ |
| 26; phys. comp. | 73.52 | 20 | HCPCS 78608 | 36.76 | 110.28 | γ |
| EEG, $ | ||||||
| Nonfacility TC | 375.89 | 20 | HCPCS 95819 | 187.95 | 563.84 | γ |
| 26; phys. comp. | 59.46 | 20 | HCPCS 95819 | 29.73 | 89.19 | γ |
| TC; APC | 252.31 | 21 | APC 85722 | 126.16 | 378.47 | γ |
| Lumbar puncture, $ | ||||||
| Nonfacility | 152.09 | 20 | HCPCS 62270 | 76.05 | 228.14 | γ |
| Facility | 80.37 | 20 | HCPCS 62270 | 40.19 | 120.56 | γ |
| Mechanical ventilation, $ per d | 2644.91 | 25 | Adjusted to 2019 US$ | 1322.46 | 3967.37 | γ |
| Dialysis, $ per d | 611.73 | 20 | APC 5401 | 305.87 | 917.60 | γ |
| Vasopressin, $ | 301.25 | 24 | NDC: 63323-0302-09 | 150.63 | 451.88 | γ |
| Norepinephrine, $ | 77.67 | 24 | NDC: 55390-0002-10; 25 μg/min | 38.84 | 116.51 | γ |
| Siltuximab, $ | 8571.74 | 24 | NDC: 50242-0135-01 | 4285.87 | 12 857.61 | γ |
| Anakinra, $ | 155.01 | 24 | NDC: 66658-0234-07; 100 mg | 77.51 | 232.52 | γ |
| Tocilizumab, $ | 4477.30 | 24 | NDC: 50242-0135-01; 800 mg | 2238.65 | 6715.95 | γ |
26; phys. comp., physician component; APC, Ambulatory Payment Classification; CT, computed tomography; EEG, electroencephalogram; HCPCS, Healthcare Common Procedure Coding System; HOPD, hospital outpatient department; IP, inpatient hospital; MRI, magnetic resonance imaging; NDC, National Drug Code; PET, positron emission tomography; TC, technical component.
The β distribution is applied to model the behavior of random variables limited to finite length. Given that estimates cannot be <0, the β distribution was selected. The β distribution is a suitable model for the random behavior of percentages and proportions. γ distribution is assumed for cost data, specifically for using small sample sizes that often have nonnormal distribution (mean and median are not similar); thus, cost studies often use a γ distribution to address the skewness of cost data that are subject to wide variance and likely a wide range, with a few outliers that affect the mean cost.
Study outcomes
The primary study outcome was the cost of CRS and NE management, which was aggregated to estimate the total cost for an individual patient. Costs were grouped into 4 HCRU categories: (1) diagnostics, (2) procedures, (3) medications, and (4) facility costs. The total cost was calculated per patient; a median total cost of CRS and NEs was evaluated and reported. All cost outcomes are presented in 2019 US dollars.
Secondary outcomes included incidence and cost of CRS and/or NEs by AE severity grade. We also evaluated the rates of key HCRU of interest, such as tocilizumab, corticosteroid, and vasopressor use, mechanical ventilation, and ICU admission. Lastly, the hospital length of stay (LOS) was calculated during the time in which a patient experienced a CRS or NE episode, categorized by standard inpatient and ICU days.
Statistical analysis
Descriptive statistics were employed to summarize the costs of CRS and NE management; because of the small sample size, median rather than mean costs were reported. Median costs were aggregated by HCRU category, specifically medication, diagnostic, procedure, and facility costs. Medication costs included any medications that were given to manage CRS or NEs. Diagnostics included laboratory work and imaging costs. The procedures category included dialysis and mechanical ventilation costs; the costs for outpatient visits, standard inpatient hospitalizations, and ICU stays were represented in the facilities category. Counts and rates were calculated for key HCRU of interest. Times to CRS/NE onset and resolution were evaluated relative to the date of liso-cel administration (ie, defined as day 1). Descriptive values were generated for times to CRS/NE onset and resolution, LOS, and cost outcomes. All statistical analyses were performed in Microsoft Excel for Office 365 (version 2002).
Sensitivity analysis
We conducted a probabilistic sensitivity analysis using a Monte Carlo simulation modeling approach on key cost parameters with 1000 iterations to address median cost parameter uncertainty and obtain 95% confidence intervals. Base case inputs were randomly varied using an assumed distribution (β distribution was assumed for key cost ratios, and γ distribution was assumed for unit cost inputs) to generate cost estimates.
Results
Patient demographics and characteristics
The LBCL cohort of TRANSCEND comprised 268 patients who received liso-cel. Overall, 127 (47.4%) of 268 patients experienced a CRS and/or NE episode; of these 127 patients, 47 (37.0%) had CRS only (17.5% of the total population), 14 (11.0%) had NEs only (5.2% of the total population), and 66 (52.0%) had both CRS and NEs (24.6% of the total population). Most patients with CRS and/or NEs (n = 98 [77.2%] of 127) experienced a grade 1 or 2 event (Table 2).
Table 2.
Patient demographics and characteristics of liso-cel administration by AE (N = 127)
| AE stratification | CRS only (n = 47)* | NE only (n = 14)* | Nonconcurrent CRS and NE (n = 21)* | Concurrent CRS and NE (n = 45)* | Any CRS and/or NE (n = 127) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade ≥3 | Grade 1 | Grade 2 | Grade ≥3 | Grade ≤2 CRS and NE | Grade ≥3 CRS or NE | Grade ≤2 CRS and NE | Grade ≥3 CRS or NE | Grade ≥3 CRS and NE | ||
| Total, n (%)† | 33 (26.0) | 13 (10.2) | 1 (0.8) | 4 (3.1) | 3 (2.4) | 7 (5.5) | 16 (12.6) | 5 (3.9) | 29 (22.8) | 12 (9.4) | 4 (3.1) | 127 (100.0) |
| Age, y | ||||||||||||
| Mean (SD) | 56.3 (14.2) | 58.8 (13.6) | 47 (NA) | 60.8 (10.2) | 63.3 (8.1) | 62.3 (21.0) | 57.4 (11.2) | 57.6 (15.3) | 62.7 (14.3) | 60.4 (16.5) | 43.0 (23) | 58.8 (14.6) |
| Median | 58 | 63 | 47 | 64 | 62 | 66 | 58 | 58 | 64 | 64.5 | 45.5 | 62 |
| Range | 27-81 | 26-74 | NA | 46-69 | 56-72 | 19-82 | 33-74 | 33-73 | 29-86 | 37-79 | 18-63 | 18-86 |
| Male sex, n (%)‡ | 19 (57.6) | 10 (76.9) | 1 (100.0) | 3 (75.0) | 3 (100.0) | 5 (71.4) | 9 (56.3) | 4 (80.0) | 18 (62.1) | 7 (58.3) | 4 (100.0) | 83 (65.4) |
| White race, n (%)‡ | 27 (81.8) | 11 (84.6) | 1 (100.0) | 4 (100.0) | 3 (100.0) | 4 (57.1) | 15 (93.8) | 5 (100.0) | 28 (96.6) | 9 (75.0) | 4 (100.0) | 111 (87.4) |
| Non-Hispanic/non-Latino ethnicity, n (%)‡ | 29 (87.9) | 11 (84.6) | 1 (100.0) | 3 (75.0) | 3 (100.0) | 7 (100.0) | 14 (87.5) | 5 (100.0) | 25 (86.2) | 11 (91.7) | 2 (50.0) | 111 (87.4) |
| Site of administration, n (%)†‡ | ||||||||||||
| Inpatient | 30 (90.9) | 12 (92.3) | 1 (100.0) | 2 (50.0) | 3 (100.0) | 6 (85.7) | 13 (81.3) | 5 (100.0) | 25 (86.2) | 12 (100.0) | 3 (75.0) | 112 (88.2) |
| Outpatient | 3 (9.1) | 1 (7.7) | 0 | 2 (50.0) | 0 | 1 (14.3) | 3 (18.8) | 0 | 4 (13.8) | 0 | 1 (25.0) | 15 (11.8) |
Table includes all patients who experienced ≥1 CRS or NE episode (n = 127).
NA, not applicable; SD, standard deviation.
Lee et al12 criteria were used to determine CRS toxicity grade, and National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03)17 were used to determine NE toxicity grade.
Percentages based on patients who experienced a CRS and/or NE episode.
Percentages calculated within each column as percentage of AE type and severity.
The median age of patients with a CRS and/or NE episode was 62 years, ranging from age 18 to 86 years. These patients were predominantly male (65.4%) and White (87.4%). Patient demographics are grouped by AE category (CRS and NEs) and severity (grade) in Table 1. Demographic characteristics were similar across AE cohorts. Most patients who experienced a CRS and/or NE episode (n = 112 [88.2%] of 127) were administered liso-cel in the inpatient setting, whereas 15 patients (11.8%) were administered liso-cel in the outpatient setting (Table 2).
Onset and duration of CRS and NE episodes
From liso-cel infusion, the median (range) time to onset and duration of the first CRS episode were 5.0 (1.0-14.0) and 5.0 (1.0-17.0) days, respectively. From liso-cel infusion, the median (range) time to onset and duration of the first NE episode were 9.0 (1.0–66.0) and 11.0 (1.0–86.0) days, respectively. Median times to onset and resolution from the first CRS onset were similar across AE grade stratifications. The duration of CRS and/or NEs was longest among patients with concurrent grade ≥3 CRS and NEs (Table 3). Among patients who developed NEs after CRS onset, the median gap between CRS onset and NE onset was 5 days.
Table 3.
CRS and NE characteristics (N = 127)
| Grade* | n (%)† | Median (range) | n (%)‡ | |||||
|---|---|---|---|---|---|---|---|---|
| CRS onset, d | CRS resolution, d | NE onset, d | NE resolution, d | CRS onset before NE | NE onset before CRS | Same onset | ||
| CRS only | ||||||||
| Any | 47 (37.0) | 5.0 (1.0-14.0) | 9.0 (2.0-17.0) | — | — | — | — | — |
| 1 | 33 (26.0) | 5.0 (2.0-14.0) | 8.0 (2.0-17.0) | — | — | — | — | — |
| 2 | 13 (10.2) | 5.0 (1.0-9.0) | 9.0 (6.0-15.0) | — | — | — | — | — |
| ≥3 | 1 (0.8) | 3.0 (3.0-3.0) | 8.0 (8.0-8.0) | — | — | — | — | — |
| NE only | ||||||||
| Any | 14 (11.0) | — | — | 10.0 (2.0-34.0) | 20.0 (7.0-94.0) | — | — | — |
| 1 | 4 (3.1) | — | — | 12.5 (8.0-20.0) | 23.5 (18.0-32.0) | — | — | — |
| 2 | 3 (2.4) | — | — | 17.0 (14.0-20.0) | 20.0 (20.0-20.0) | — | — | — |
| ≥3 | 7 (5.5) | — | — | 8.0 (2.0-34.0) | 19.0 (7.0-94.0) | — | — | — |
| Nonconcurrent CRS and NE | ||||||||
| Any | 21 (16.5) | 4.0 (1.0-11.0) | 9.0 (2.0-19.0) | 13.0 (6.0-66.0) | 25.0 (6.0-92.0) | 21 (100.0) | 0 | 0 |
| CRS and NE ≤2 | 16 (12.6) | 3.0 (1.0-7.0) | 9.0 (2.0-19.0) | 14.5 (6.0-66.0) | 27.5 (6.0-92.0) | 16 (100.0) | 0 | 0 |
| CRS or NE ≥3 | 5 (3.9) | 6.0 (3.0-11.0) | 12.0 (8.0-13.0) | 13.0 (9.0-15.0) | 23.0 (22.0-55.0) | 5 (100.0) | 0 | 0 |
| Concurrent CRS and NE | ||||||||
| Any | 45 (35.4) | 4.0 (1.0-12.0) | 10.0 (3.0-24.0) | 7.0 (1.0-18.0) | 16.0 (3.0-90.0) | 31 (68.9) | 8 (17.8) | 6 (13.3) |
| CRS and NE ≤2 | 29 (22.8) | 5.0 (2.0-8.0) | 10.0 (3.0-24.0) | 7.0 (1.0-16.0) | 14.0 (3.0-42.0) | 20 (69.0) | 6 (20.7) | 3 (10.3) |
| CRS or NE ≥3 | 12 (9.4) | 4.0 (1.0-12.0) | 11.5 (6.0-23.0) | 8.0 (1.0-18.0) | 18.0 (12.0-90.0) | 8 (66.7) | 2 (16.7) | 2 (16.7) |
| CRS and NE ≥3 | 4 (3.1) | 4.0 (3.0-8.0) | 12.5 (9.0-16.0) | 5.5 (4.0-12.0) | 12.5 (25.0-30.0) | 3 (75.0) | 0 | 1 (25.0) |
| Any CRS | 113 | 5.0 (1.0-14.0) | 9.0 (2.0-24.0) | — | — | — | — | — |
| Any NE | 80 | — | 9.0 (1.0-66.0) | 19.0 (3.0-94.0) | — | — | — | |
Lee et al12 criteria were used to determine CRS toxicity grade, and National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03)17 were used to determine NE toxicity grade.
Percentages based on patients who experienced a CRS and/or NE episode.
Percentages calculated within each row.
Among the 66 patients with both CRS and NEs (21 [31.8%] nonconcurrent and 45 [68.1%] concurrent), CRS onset occurred before NE onset in 52 (78.8%). Specifically, of the 45 patients within the concurrent CRS and NE group, CRS preceded NE onset in 31 (68.9%), NE onset occurred first in 8 (17.8%), and 6 (13.3%) experienced CRS onset and NE onset on the same day. For all 21 patients with nonconcurrent CRS and NEs, CRS onset occurred before NE onset.
HCRU
Most patients were either admitted to the hospital (if treated as an outpatient) or remained in the hospital (if treated as an inpatient) for CAR T-cell infusion and monitoring. Among the 127 patients who experienced a CRS and/or NE episode, only 17 (13.4%) required any ICU stay for CRS and/or NE management. For management of CRS with or without NEs, 18 patients (14.2%) received tocilizumab only, 23 (18.1%) received corticosteroids only, and 33 (26.0%) received tocilizumab plus corticosteroids. In addition, 1 patient with concurrent grade ≥3 CRS and NE episodes remained in the ICU for the entire duration of CRS and NE management. Corticosteroid use increased as the severity of CRS and NEs increased (Table 4).
Table 4.
HCRU by AE type and severity (N = 127)
| Key HCRU | CRS only* (n = 47) | NE only* (n = 14) | Nonconcurrent CRS and NE (n = 21)*† | Concurrent CRS and NE (n = 45)* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade ≥3 | Grade 1 | Grade 2 | Grade ≥3 | Grade ≤2 CRS and NE | Grade ≥3 CRS or NE | Grade ≤2 CRS and NE | Grade ≥3 CRS or NE | Grade ≥3 CRS and NE | |
| n (%)‡ | 33 (26.0) | 13 (10.2) | 1 (0.8) | 4 (3.1) | 3 (2.4) | 7 (5.5) | 16 (12.6) | 5 (3.9) | 29 (22.8) | 12 (9.4) | 4 (3.1) |
| Facility, n (%)§ | |||||||||||
| Standard inpatient | 33 (100.0) | 13 (100.0) | 1 (100.0) | 0 | 3 (100.0) | 7 (100.0) | 16 (100.0) | 5 (100.0) | 29 (100.0) | 11 (91.7) | 3 (75.0) |
| ICU | 0 | 2 (15.4) | 1 (100.0) | 0 | 1 (33.3) | 1 (14.3) | 1 (6.3) | 1 (20.0) | 2 (6.9) | 4 (33.3) | 4 (100.0) |
| Medications, n (%)§ | |||||||||||
| Tocilizumab only | 6 (18.2) | 5 (38.5) | 0 | 0 | 0 | 0|| | 2 (12.5) | 0 | 4 (13.8) | 1 (8.3) | 0 |
| Corticosteroids only | 0 | 2 (15.4) | 0 | 0 | 3 (100.0) | 4 (57.1) | 1 (6.3) | 2 (40.0) | 6 (20.7) | 5 (41.7) | 0 |
| Tocilizumab + corticosteroids | 0 | 2 (15.4) | 1 (100.0) | 0 | 0 | 0 | 6 (37.5) | 3 (60.0) | 11 (37.9) | 6 (50.0) | 4 (100.0) |
| Vasopressor | 0 | 1 (7.7) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.4) | 1 (8.3) | 4 (100.0) |
| Mean (SD) tocilizumab doses | 0.21 (0.5) | 0.77 (0.9) | 2 | 0 | 0 | 0 | 0.63 (0.7) | 0.8 (0.8) | 0.72 (0.8) | 0.83 (0.9) | 1.25 (0.5) |
| Mean (SD) corticosteroids, d | 0 | 0.8 (1.7) | 8 | 0 | 14.7 (22.8) | 1.4 (1.4) | 1.6 (2.3) | 11.6 (11.0) | 3.9 (7.0) | 9.5 (7.5) | 24 (28.9) |
| Procedures, n (%)§ | |||||||||||
| Dialysis | 0 | 0 | 1 (100.0) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (8.3) | 2 (50.0) |
| Mean (SD), d | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0.08 (0.3) | 5.75 (10.2) |
| Median (range) | 0 (0-0) | 0 (0-0) | 3 | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-1) | 0 (0-21) |
| Mechanical ventilation | 0 | 1 (7.7) | 1 (100.0) | 0 | 0 | 1 (14.3) | 1 (6.3) | 0 | 1 (3.4) | 2 (16.7) | 2 (50.0) |
| Mean (SD), d | 0 | 0.08 (0.3) | 4 | 0 | 0 | 0.14 (0.4) | 0.06 (0.25) | 0 | 0.72 (0.2) | 0.75 (2.3) | 6.5 (13) |
| Median (range) | 0 (0-0) | 0 (0-1) | 4 | 0 (0-0) | 0 (0-0) | 0 (0-1) | 0 (0-1) | 0 (0-0) | 0 (0-21) | 0 (0-8) | 4 (0-26) |
| Standard inpatient LOS, d | |||||||||||
| Median (range) | 3 (1-13) | 6 (1-11) | 1 | 0 (0-0) | 6 (1-17) | 6 (2-12) | 7 (2-14) | 21 (12-35) | 11 (2-42) | 14.5 (0-43) | 5 (0-30) |
| Mean (SD) | 4 (2.7) | 6 (2.6) | 1 (NA) | 0 (0) | 8 (8.2) | 6.1 (3.7) | 7.2 (3.4) | 20.2 (9.4) | 12.6 (8.0) | 16.8 (12.0) | 10 (13.6) |
| ICU LOS, d | |||||||||||
| Median (range) | 0 (0-0) | 0 (0-2) | 5 | 0 (0-0) | 0 (0-2) | 0 (0-3) | 0 (0-6) | 0 (0-4) | 0 (0-39) | 0 (0-57) | 15.5 (3-30) |
| Mean (SD) | 0 (0) | 0.2 (0.6) | 5 (NA) | 0 (0) | 0.7 (1.2) | 0.4 (1.1) | 0.4 (1.5) | 0.8 (1.8) | 1.4 (7.2) | 6.9 (16.3) | 16 (14.0) |
| Total LOS, d | |||||||||||
| Median (range) | 3 (1-13) | 6 (1-11) | 6 | 0 (0-0) | 6 (1-19) | 6 (2-12) | 7 (2-14) | 21 (12-35) | 11 (2-81) | 16 (9-57) | 21 (6-56) |
| Mean (SD) | 4 (2.7) | 6.2 (2.6) | 6 (NA) | 0 (0) | 8.7 (9.3) | 6.6 (4.2) | 7.6 (3.4) | 21 (9.7) | 14.1 (14.2) | 23.7 (14.6) | 26.0 (22.4) |
Lee et al12 criteria were used to determine CRS toxicity grade, and National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03)17 were used to determine NE toxicity grade.
There were no patients with nonconcurrent CRS and NEs where both events were grade ≥3.
Percentages based on patients who experienced a CRS and/or NE episode.
Percentages calculated within each column as percentage of AE type and severity.
One patient received tocilizumab as prophylaxis for CRS. However, this patient did not have graded CRS and did not receive tocilizumab for CRS management; therefore, this instance was excluded.
An additional analysis of LOS was performed. Total LOS ranged from 0 to 81 days (median, 7 days), with most time spent in a standard (non-ICU) inpatient bed (median ICU LOS, 0 days). Patients with both CRS and NEs had generally longer total LOSs than those with only a CRS or NE episode. The longest median LOS was experienced by those with nonconcurrent grade ≥3 CRS and NEs and with any-grade concurrent CRS and NEs. The median ICU stay was longest for patients with concurrent grade ≥3 CRS and NEs (Table 4).
Total cost of CRS and NE management
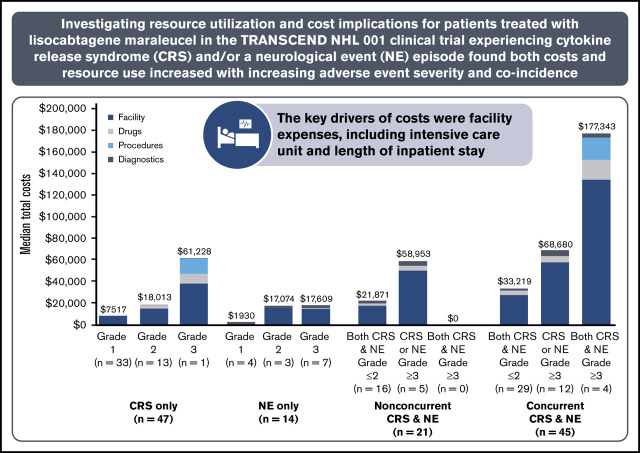
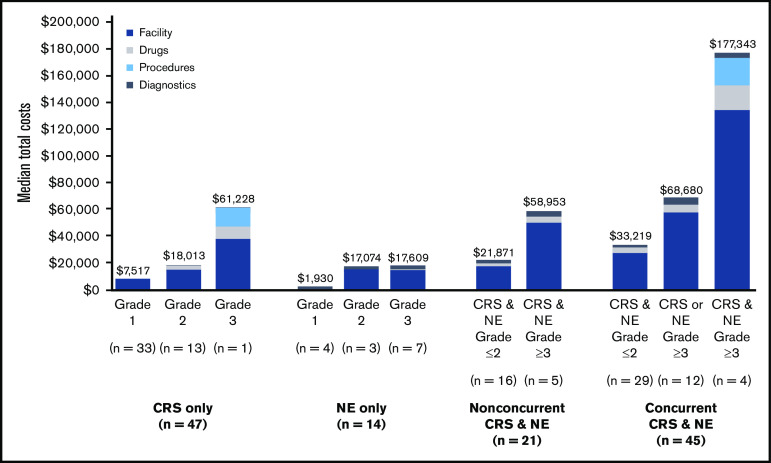
Median component and total costs to manage CRS and NEs are presented by AE type and severity with 95% confidence intervals from the probabilistic sensitivity analysis (Table 5; Figure 2). Median total costs for AE management ranged from $1930 to $177 343. More severe AEs were associated with higher median total costs. Median costs for CRS or NEs only, respectively, were $7517 (range, $2421-$37 616) and $1930 ($0-$3992) for grade 1, $18 013 ($5630-$46 972) and $17 074 ($4947-$64 214) for grade 2, and $61 228 (no range; n = 1) and $17 609 ($6584-$48 485) for grade 3. As expected, costs were highest among patients who experienced concurrent CRS and NEs, especially when both AEs were grade ≥3. Additional summary statistics were calculated for AE management costs across all patients and ranged from $0 to $454 093 (Table 5; Figure 2).
Table 5.
Total CRS and NE management costs by AE type and severity (N = 127)
| Stratification | CRS only (n = 47)* | NE only (n = 14)* | Nonconcurrent CRS and NE (n = 21)* | Concurrent CRS and NE (n = 45)* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade ≥3 | Grade 1 | Grade 2 | Grade ≥3 | Grade ≤2 CRS and NE | Grade ≥3 CRS or NE | Grade ≤2 CRS and NE | Grade ≥3 CRS or NE | Grade ≥3 CRS and NE | |
| n (%)† | 33 (26.0) | 13 (10.2) | 1 (0.8) | 4 (3.1) | 3 (2.4) | 7 (5.5) | 16 (12.6) | 5 (3.9) | 29 (22.8) | 12 (9.4) | 4 (3.1) |
| Median otal cost, $ | |||||||||||
| Diagnostics | 121 | 279 | 363 | 1 530 | 2 395 | 2 732 | 2 698 | 4 410 | 2 053 | 5 247 | 3 647 |
| Medications | 0 | 3 209 | 8 793 | 0 | 155 | 219 | 1 895 | 3 706 | 4 405 | 5 616 | 18 613 |
| Procedures | 0 | 0 | 13 741 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 861 |
| Facility | 7 395 | 14 525 | 38 330 | 399 | 14 525 | 14 658 | 17 278 | 50 836 | 26 762 | 57 817 | 134 221 |
| Total | 7 517 | 18 013 | 61 228 | 1 930 | 17 074 | 17 609 | 21 871 | 58 953 | 33 219 | 68 680 | 177 343 |
| Summary cost statistics, $ | |||||||||||
| Mean | 11 042 | 21 750 | 61 228 | 1 963 | 28 508 | 22 670 | 26 296 | 64 481 | 50 903 | 106 714 | 187 058 |
| SD | 7 498 | 10 807 | NA | 2 136 | 31 445 | 16 783 | 13 555 | 33 240 | 80 375 | 109 590 | 159 971 |
| Minimum | 2 421 | 5 630 | 61 228 | 0 | 4 947 | 6 584 | 10 497 | 34 386 | 5 935 | 27 007 | 38 858 |
| 25th percentile | 5 257 | 16 486 | NA | 179 | 10 654 | 9 893 | 16 752 | 34 672 | 25 911 | 45 833 | 54 869 |
| 75th percentile | 14 768 | 28 050 | NA | 3 713 | 40 288 | 34 051 | 32 467 | 96 849 | 44 442 | 121 474 | 314 031 |
| Maximum | 37 616 | 46 972 | 61 228 | 3 992 | 64 214 | 48 485 | 61 925 | 102 728 | 454 093 | 432 675 | 345 689 |
| 95% CI per probabilistic sensitivity analysis‡ | |||||||||||
| Lower | 7 399 | 17 769 | 60 597 | 1 762 | 16 786 | 17 303 | 21 509 | 58 040 | 32 749 | 67 834 | 174 995 |
| Upper | 7 635 | 18 257 | 61 979 | 2 098 | 17 362 | 17 915 | 22 233 | 59 866 | 33 689 | 69 526 | 179 691 |
CI, confidence interval.
Lee et al12 criteria were used to determine CRS toxicity grade, and National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03)17 were used to determine NE toxicity grade.
Percentages based on patients who experienced a CRS and/or NE episode.
95% CIs estimated from probabilistic sensitivity analysis applied to base case median costs to impute 95% CIs for base case.
Figure 2.
Median total costs of CRS and NE management by HCRU category.
Exploratory analysis
An exploratory analysis was conducted for patients treated with liso-cel in the outpatient setting. Among the 15 patients with CRS and NEs who were treated in the outpatient setting, 13 required hospital admission for toxicity management, with a median LOS of 5 days. Furthermore, only 1 of 15 patients who received liso-cel in the outpatient setting required ICU monitoring. The median total cost of all-grade CRS and/or NE management for patients who received liso-cel in the outpatient setting was $14 566 compared with $26 186 for patients who received liso-cel in the inpatient setting (n = 112).
Discussion
CRS or NE episodes occurred in 127 patients (47.4%) who were administered liso-cel in the TRANSCEND clinical trial. Among these patients, HCRU and CRS/NE management costs varied extensively between AE stratifications. Median costs ranged from $7517 to $61 228 for those with CRS only and from $1930 to $17 609 for those with NEs only. Median costs ranged from $21 871 to $58 953 for nonconcurrent CRS and NEs and from $33 219 to $177 343 for concurrent CRS and NEs. Costs increased significantly by grade for both CRS and NEs.
This analysis found that inpatient hospitalization and ICU LOS were the key drivers of CRS and/or NE management costs. Facility costs comprised an average of 79.7% of total management costs across all patients. Medications, including tocilizumab for the management of CRS, were not a major component of costs, most likely because of the low rate of use in TRANSCEND. Drug expenditures comprised an average of 9.4% of total costs, although drug costs for liso-cel and lymphodepletion were not part of this calculation, because they were provided to patients who enrolled in TRANSCEND and were not used in the treatment of CRS or NEs. A recent site-of-care analysis by Lyman et al15 indicated that CAR T-cell therapy acquisition costs can be substantial, but administration in an outpatient setting does have posttreatment cost-saving implications. Additional consideration should be given to reimbursement rates for inpatient vs outpatient administration of CAR T-cell therapy, which vary by site and insurer.
Moreover, HCRU and costs differed substantially between patients who experienced a grade ≥3 CRS or NE episode compared with those who did not. Eleven (37.9%) of the 29 patients with grade ≥3 events were admitted to the ICU for AE management compared with 6 (6.1%) of the 98 patients with grade ≤2 events. Given that 77.2% of patients treated with liso-cel who experienced CRS and/or NEs had grade ≤2 events, this further limited the need for ICU management, which was required in only 17 patients (13.4%). Management of grade ≥3 events resulted in a 193.3% increase in aggregated median costs vs grade ≤2 events ($50 586 vs $17 246, respectively). These HCRU and cost differences are significant, because most patients who experienced CRS and/or NE episodes after liso-cel administration did not have grade ≥3 events.
The low incidence of severe CRS and NEs and time to onset of these toxicities among those treated with liso-cel support the ongoing investigation of outpatient administration for some patients, which would also significantly limit health care expenditures.11 Of the 25 patients who received liso-cel in the outpatient setting of TRANSCEND, 10 (40.0%) did not experience any CRS or NE episodes. The 15 outpatients with CRS and/or NEs had decreased rates of HCRU and lower median costs for AE management compared with inpatients, which is consistent with the site-of-care analysis by Lyman et al.15
Although CAR T-cell therapies have shown promising response rates and durable clinical efficacy, the financial considerations of such treatments continue to be a topic of concern for key stakeholders.30,31 CAR T-cell products with better safety profiles may provide more consistent cost estimates.15 Furthermore, there are growing concerns about the overall impact of treatments on the health care system, particularly with regard to inpatient and ICU capacities. A CAR T-cell therapy that is associated with a low incidence of grade ≥3 events, a low rate of transfer to the ICU, and a safety profile that supports the option of outpatient administration in some patients would further reduce the strain on the health care system. This study provides further context for resource allocation and the cost implications for the management of CRS and NEs associated with liso-cel.
Our results are generally in accordance with other CAR T cell–associated AE management cost analyses. Hernandez et al16 found nondrug costs for treating CRS were $30 000 to $36 000 for the average patient and as high as $56 000 for those with severe CRS. We observed a relatively similar cost range in this study, with escalating costs associated with CRS severity. Lyman et al15 also performed a cost analysis with similar findings for inpatients ($81 611 total for drugs, procedures, and hospitalization), although the main focus of this study was to compare the costs of care between inpatient and nonacademic specialty oncology network outpatient settings. In the latter setting, medical procedure costs were universally lower, suggesting that treating patients with CAR T-cell therapy as outpatients could mitigate some of the associated costs (40.4% cost reduction). Additionally, cost-effectiveness analyses tend to suggest that CAR T-cell therapy may be more cost effective than salvage chemotherapy and stem cell transplantation, although all 3 studies caution their findings are preliminary.32-34
Various assumptions were required for the clinical considerations and economic evaluation. It was assumed that the database captured all clinical and resource utilization; no methods to address missing data were employed. Because of the dynamic nature of CRS and NEs, patients may experience a range of AE severity. However, patients were stratified based on the maximum AE grade that occurred. In addition, the management of care may transition between sites of care over the course of an AE episode. On days where AE management took place in multiple facilities, the higher-level unit was selected. Costs were limited to those observed within the clinical trial and therefore did not include additional costs that may be incurred in the real-world setting (eg, professional fees and cost of the liso-cel product once commercially available).
This analysis was performed from the health care system perspective; however, data on the true costs for each site of care in the health care system were not available. Estimates by site of care relied on ratios found in the literature. Reimbursement rates were used as source values for cost estimation, and a reimbursement/ cost ratio was applied. Drug costs were assumed to be equivalent for all sites of care. True drug acquisition costs may differ across hospitals as a result of 340B program eligibility, contracting power, and other factors. However, despite the limited availability of hospital-specific cost data, this analysis provides a relative relationship on HCRU and costs between AE grade severity levels. Although unit cost estimates were based on the most recently available public databases or peer-reviewed literature, they may not reflect the current cost burden of resources. However, these sources are consistent with recent CAR T-cell therapy economic modeling efforts.15,16,34,35 Because of the uncertainty surrounding cost inputs, the validity of the results were tested in a sensitivity analysis. Even with the introduction of uncertainty, the association of increasing costs with AE severity was consistent. Moreover, the estimations represent national averages and may not be generalizable to specific institutions or geographic areas.
This study focused exclusively on the management of CRS and NEs. Other prevalent AEs such as cytopenias and hypogammaglobulinemia were not included. In a conservative approach, this resource and cost analysis was restricted to those HCRUs listed in the clinical trial management protocol by AE and severity or those that were informed by expert opinion.
This analysis was restricted to patients who received liso-cel in the TRANSCEND trial; thus, the findings for HCRU and cost estimations may not represent outcomes for other CAR T-cell therapies or in settings outside of a clinical trial given different AE profiles or management strategies.10,36-39 Furthermore, given the small sample size for many of the AE stratifications, particularly regarding average or median cost estimates for grade ≥3 AEs, generalizability is limited.
We believe this study is among the first to estimate costs for CRS and NEs by AE type and severity. In addition, it explores the resource and cost implications when a patient experiences both CRS and NE episodes. The findings contribute to the existing literature estimating the economic impact of treatment with a CAR T-cell therapy, while highlighting the significant difference in costs to manage CRS and NEs depending on the severity of the event. Liso-cel is associated with low rates of grade ≥3 AEs, which may reduce the HCRU and economic costs of managing patients who are treated with CAR T-cell therapy.
Acknowledgments
This study was funded by Bristol-Myers Squibb. Medical writing and editorial support were provided by Meredith Rogers and Jeremy Henriques of The Lockwood Group (Stamford, CT), funded by Bristol-Myers Squibb.
Footnotes
Data requests may be submitted to Celgene, a Bristol-Myers Squibb company, at https://vivli.org/ourmember/celgene/ and must include a description of the research proposal.
Authorship
Contribution: A.N., J.G., J.S.A., M.G., N.M., and T.S. were responsible for the conception and design of the study; A.N., J.S.A., M.G., N.M., and T.S. wrote the manuscript; A.N., C.P., J.G., J.S.A., M.G., M.P.J., N.M., T.S., and Y.K. critically revised the manuscript for important intellectual content; A.N. and Y.K. conducted the statistical analysis; M.P.J., C.D., C.P., and S.S. provided administrative, technical, or material support; M.P.J. was responsible for supervision; and all authors had full access to all of the data, carefully reviewed the manuscript, and approved the final version.
Conflict-of-interest disclosure: J.S.A. declares consulting for Allogene, Bristol-Myers Squibb, Celgene, Gilead, Kite Pharma, and Novartis. T.S. declares consulting for AstraZeneca, BeiGene, Celgene, Juno Therapeutics, Kite Pharma, and Pharmacyclics/AbbVie; institutional research funding from AstraZeneca, BeiGene, Celgene, Juno Therapeutics, Kite Pharma, Oncternal, Pharmacyclics, and TG Therapeutics; speakers bureau for AstraZeneca, Janssen, Pharmacyclics, and Seattle Genetics; and travel, accommodations, and expenses from AstraZeneca. J.G., C.D., C.P., M.P.J., and Y.K. are employees of Bristol-Myers Squibb and may own stock in Bristol-Myers Squibb. S.S., A.N., N.M., and M.G. are employees of BluePath Solutions, which was contracted by Bristol-Myers Squibb to perform the analyses.
Correspondence: Jeremy S. Abramson, Massachusetts General Hospital, Zero Emerson Pl, Suite 118, Boston, MA 02114; e-mail: jabramson@mgh.harvard.edu.
References
- 1.US Cancer Statistics Working Group. US Cancer Statistics data visualizations tool, based on November 2018 submission data (1999-2016). Available at: https://gis.cdc.gov/Cancer/USCS/DataViz.html. Accessed 5 March 2019.
- 2.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16(8):2780-2795. [DOI] [PubMed] [Google Scholar]
- 3.The Non-Hodgkin’s Lymphoma Classification Project . A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89(11):3909-3918. [PubMed] [Google Scholar]
- 4.Roschewski M, Staudt LM, Wilson WH. Diffuse large B-cell lymphoma-treatment approaches in the molecular era. Nat Rev Clin Oncol. 2014;11(1):12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study [published correction appears in Blood. 2018;131(5):587-588]. Blood. 2017;130(16):1800-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedele R, Salooja N, Martino M. Recommended screening and preventive evaluation practices of adult candidates for hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2016;16(11):1361-1372. [DOI] [PubMed] [Google Scholar]
- 8.Skrabek P, Assouline S, Christofides A, et al. Emerging therapies for the treatment of relapsed or refractory diffuse large B cell lymphoma. Curr Oncol. 2019;26(4):253-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 11.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. [DOI] [PubMed] [Google Scholar]
- 12.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome [published corrections appear in Blood. 2015;126(8):1048 and Blood. 2016;128(11):1533]. Blood. 2014;124(2):188-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqi T, Garcia J, Dehner C, et al. Estimation of the resource utilization and costs of cytokine release syndrome observed in the transcend-NHL clinical trial: a micro-costing study [abstract]. Blood. 2018;132(suppl 1). Abstract 319.
- 14.Abramson JS, Siddiqi T, Garcia G, et al. Burden of cytokine release syndrome (CRS) and neurologic events (NE) in patients (pts) with relapsed/refractory non-Hodgkin lymphoma (NHL) receiving lisocabtagene maraleucel (liso-cel; JCAR017) in TRANSCEND NHL 001 [abstract]. J Clin Oncol. 2019;37(suppl 15). Abstract 6637.
- 15.Lyman GH, Nguyen A, Snyder S, Gitlin M, Chung KC. Economic evaluation of chimeric antigen receptor T-cell therapy by site of care among patients with relapsed or refractory large B-cell lymphoma. JAMA Netw Open. 2020;3(4):e202072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez I, Prasad V, Gellad WF. Total costs of chimeric antigen receptor T-cell immunotherapy. JAMA Oncol. 2018;4(7):994-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. Available at: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed 23 October 2019.
- 18.University of Bern. Strengthening the Reporting of Observational studies in Epidemiology (STROBE). STROBE statement: checklist of items that should be included in reports of cohort studies. Available at: https://www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_cohort.pdf. Accessed 20 April 2020.
- 19.Agency for Healthcare Research and Quality. National Inpatient Sample. Healthcare Cost and Utilization Project. Available at: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed 5 March 2019.
- 20.Centers for Medicare and Medicaid Services. Medicare physician fee schedule. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched. Accessed 5 March 2019.
- 21.Centers for Medicare and Medicaid Services. Hospital Outpatient Prospective Payment System. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS. Accessed 5 March 2019.
- 22.Centers for Medicare and Medicaid Services. Durable medical equipment (DME) center. Available at: https://www.cms.gov/Center/Provider-Type/Durable-Medical-Equipment-DME-Center. Accessed 20 April 2020.
- 23.Centers for Medicare and Medicaid Services. Clinical laboratory fee schedule. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched. Accessed 20 April 2020.
- 24.IBM Watson Health Product Education. IBM Micromedex RED BOOK. Available at: https://truvenhealth.com/Training/Product/IBM-Micromedex-Clinical-Knowledge/IBM-Micromedex-RED-BOOK. Accessed 5 March 2019.
- 25.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266-1271. [DOI] [PubMed] [Google Scholar]
- 26.US Bureau of Labor Statistics. Consumer price index for all urban consumers: medical care in the U.S. city average [CPIMEDNS], retrieved from FRED, Federal Researve Band of St. Louis. Available at: https://fred.stlouisfed.org/series/CPIMEDNS. Accessed 7 April 2020.
- 27.American Hospital Association. Fact sheet: underpayment by Medicare and Medicaid. Available at: https://www.aha.org/system/files/media/file/2020/01/2020-Medicare-Medicaid-Underpayment-Fact-Sheet.pdf. Accessed 20 April 2020.
- 28.Leavitt Partners. Cancer treatment costs are consistently lower in the community setting versus the hospital outpatient department. Available at: https://leavittpartners.com/whitepaper/cancer-treatment-costs-are-consistently-lower-in-the-community-setting-versus-the-hospital-outpatient-department/. Accessed 30 March 2020.
- 29.Meisenberg BR, Ferran K, Hollenbach K, Brehm T, Jollon J, Piro LD. Reduced charges and costs associated with outpatient autologous stem cell transplantation. Bone Marrow Transplant. 1998;21(9):927-932. [DOI] [PubMed] [Google Scholar]
- 30.Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book. 2019;39:433-444. [DOI] [PubMed] [Google Scholar]
- 31.Hay AE, Cheung MC. CAR T-cells: costs, comparisons, and commentary. J Med Econ. 2019;22(7):613-615. [DOI] [PubMed] [Google Scholar]
- 32.Lin JK, Muffly LS, Spinner MA, Barnes JI, Owens DK, Goldhaber-Fiebert JD. Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult large B-cell lymphoma. J Clin Oncol. 2019;37(24):2105-2119. [DOI] [PubMed] [Google Scholar]
- 33.Roth JA, Sullivan SD, Lin VW, et al. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States [published correction appears in J Med Econ. 2018;21(12):1255]. J Med Econ. 2018;21(12):1238-1245. [DOI] [PubMed] [Google Scholar]
- 34.Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term survival and cost-effectiveness associated with axicabtagene ciloleucel vs chemotherapy for treatment of B-cell lymphoma. JAMA Netw Open. 2019;2(2):e190035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Institute for Clinical and Economic Review . Chimeric antigen receptor T-cell therapy for B-cell cancers: effectiveness and value. Available at: https://collections.nlm.nih.gov/catalog/nlm:nlmuid-101744954-pdf. Accessed 20 April 2020.
- 36.Kite Pharma. YESCARTA (axicabtagene ciloleucel) prescribing information. Available at: https://www.gilead.com/-/media/files/pdfs/medicines/oncology/yescarta/yescarta-pi.pdf. Accessed 9 March 2019.
- 37.Novartis Pharmaceuticals Corporation. KYMRIAH (tisagenlecleucel) prescribing information. Available at: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kymriah.pdf. Accessed 9 March 2019.
- 38.Teachey DT, Bishop MR, Maloney DG, Grupp SA. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit “ALL”. Nat Rev Clin Oncol. 2018;15(4):218. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen A, Garcia G, Gitlin M, Jun MP. An economic model to estimate the cost of cytokine release syndrome (CRS) and neurologic events (NE) observed in chimeric antigen receptor (CAR) T-cell therapies administered to patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL). Presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Annual Meeting; 18-20 May 2020.