Key Points
The recombinant zoster vaccine is safe and tolerable in allogeneic HCT recipients.
The vaccine does not increase the rate of GVHD, relapse, or death and results in infrequent breakthrough VZV reactivation.
Abstract
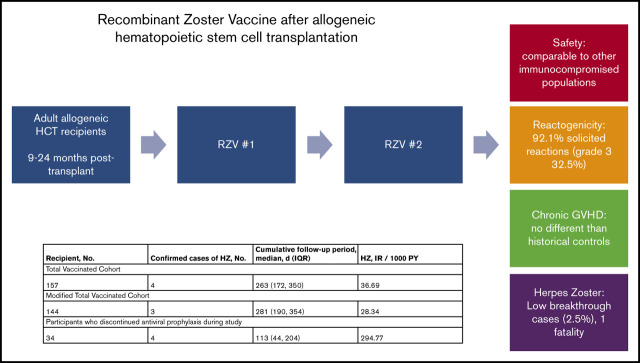
Allogeneic hematopoietic cell transplantation (HCT) recipients are at increased risk for varicella zoster virus (VZV) reactivation and associated complications. A nonlive adjuvanted recombinant zoster vaccine (RZV) has been developed to prevent herpes zoster (HZ), but there are no recommendations for use in this population. In this single-center prospective observational cohort study, we assessed the safety and reactogenicity of RZV, as well as incidence of graft-versus-host disease (GVHD) and confirmed cases of HZ after vaccination. Between December of 2018 and June of 2020, patients aged ≥18 years received 2 doses of RZV between 9 and 24 months after HCT, with the doses separated by ≥8 weeks. One hundred and fifty-eight patients (mean age, 55 years; 42% women) received ≥1 dose (total vaccinated cohort), and 150 patients (95%) received 2 doses (modified total vaccinated cohort). Solicited reactions occurred in 92.1% of patients (grade 3, 32.5%), owing mostly to injection site pain, which occurred in 86% (grade 3, 16%). The cumulative incidence of GVHD in the peri-vaccination period was no different than in historical controls (adjusted incidence rate ratio, 1.05; 95% confidence interval, 0.8-1.38). There were 4 cases of HZ in the total vaccinated cohort (2.5%) and 3 cases in the modified total vaccinated cohort (28.3/1000 person-years). Among recipients of allogeneic HCT, RZV was safe, tolerable, and did not increase rates of GVHD. Future clinical trials are needed to determine the immunogenicity and efficacy of RZV in this population.
Visual Abstract
Introduction
Varicella zoster virus VZV reactivation in allogeneic hematopoietic cell transplantation (HCT) recipients is common, with 20% to 53% of patients affected without the use of prophylactic strategies.1-4 Prolonged antiviral prophylaxis with acyclovir (ACV) or valacyclovir is the cornerstone of VZV prevention in this population and has been shown to be effective in 2 randomized controlled trials (RCTs)5,6 and multiple observational studies.7-11
ACV prophylaxis is recommended for ≥12 months following HCT; however, the duration of prophylaxis varies among transplant centers, and rates of herpes zoster (HZ) remain higher in this population after 1 year.5,7,12 Furthermore, severe HZ still occurs with high morbidity and mortality despite antiviral prophylaxis strategies.13
To better protect against VZV reactivation after allogeneic HCT, a vaccination approach has been recommended by the American Society for Blood and Marrow Transplantation, the European Group of Blood and Marrow Transplantation, and the Infectious Disease Society of America.12,14,15 The varicella vaccine (VARIVAX; Merck & Co., Whitehouse Station, NJ) can be given to recipients >24 months from transplant who do not have ongoing graft-versus-host disease (GVHD) and are not receiving immunosuppression. The zoster vaccine live (ZVL) (ZOSTAVAX; Merck and Co.) has been administered in single-center trials and demonstrates overall safety; however, it is not widely recommended.16,17
The recombinant zoster vaccine (RZV) (SHINGRIX, GlaxoSmithKline, Research Triangle Park, NC) contains VZV glycoprotein E in combination with an adjuvant (AS01B).18 RZV was approved by the US Food and Drug Administration in 2017 for HZ prevention in immunocompetent adults older than 50 years of age and is now recommended by the Advisory Committee on Immunization Practices.19 Studies in specific groups, including HIV, renal transplant, hematologic malignancy, solid organ malignancy, and, importantly, autologous HCT have shown overall safety and immunogenicity20-24; however, there are no Advisory Committee on Immunization Practices recommendations in this population.
In this study, we evaluate the safety and reactogenicity of RZV in adult allogeneic HCT recipients, including any effects on GVHD occurrence or exacerbation given the presence of an adjuvant.
Methods
Study design and participants
This is a single-center prospective observational cohort study that was conducted at the Dana-Farber Cancer Institute between December of 2018 and June of 2020. As part of updated Dana-Farber/Brigham and Women’s Hospital (DF/BWH) vaccination guidelines, allogeneic HCT recipients were eligible for vaccination with RZV if they were >9 months from the time of transplant. The recommendation to use RZV was made by a multidisciplinary team given the continued risk for VZV reactivation after ACV discontinuation, associated morbidity and mortality, improved safety profile of RZV compared to zoster vaccine live (ZVL), and recent data documenting safety in autologous HCT recipients.24
Adult allogeneic HCT recipients who were ≥18 years old at the time of transplant, who were between 9 and 24 months from the time of transplant, and who were deemed appropriate for RZV by the treating clinician were eligible for the study. Patients who had disease relapse prior to administration of the first dose of RZV vaccination (V1) were excluded.
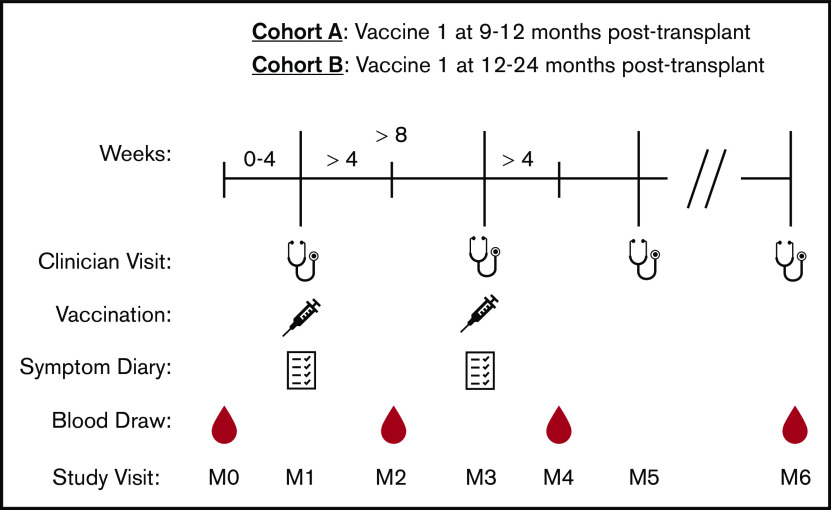
Patients were given 2 intramuscular doses (0.5 mL each) of RZV separated by ≥8 weeks. Cohort A consisted of patients who received V1 at 9 to 12 months after transplant in accordance with DF/BWH vaccination guidelines. Cohort B consisted of patients who received V1 at 12 to 24 months after transplant as part of a “catch-up” vaccination effort (Figure 1). RZV was given alongside other vaccines in the vaccine schedule (supplemental Data). Patients were followed until June of 2020 and for ≥30 days after final vaccination. Written informed consent was obtained from all patients. This study was approved by the Office for Human Research Studies at the Dana-Farber Cancer Institute and was conducted per the Declaration of Helsinki.
Figure 1.
Study design for participants receiving RZV. Stethoscopes represent clinician visits that occurred on the day of V1 (M1), the day of vaccine 2 (M3), 6 months after V1 (M5), and the most recent follow-up (M6). Syringes represent RZV administration with ≥8 weeks between V1 and vaccine 2. Notebooks represent symptom diary collection that occurred at clinician visits M1 and M3 and collected by study staff via telephone or e-mail. Drops represent blood samples that were collected 0 to 4 weeks prior to V1 (M0), >4 weeks after V1 (M2), >4 weeks after vaccine 2 (M4), and at 10 to 14 months after V1 (M6), if follow-up allowed.
Study end points
The primary end points were safety and reactogenicity in the total vaccinated cohort (TVC), which included all participants who received ≥1 vaccine dose. The secondary end points included incidence and severity of GVHD in the TVC compared with historical controls and the incidence rates of HZ in the TVC and modified TVC (mTVC), which included only patients who received both doses of the vaccine.
Assessment of reactogenicity and safety
Reactogenicity and safety data were collected in the TVC using a standardized toxicity assessment.18,25 Reactogenicity was measured with solicited injection-site reactions (pain, redness, and swelling) and systemic reactions (fever, headache, fatigue, gastrointestinal symptoms, myalgia, and shivering) on diary cards for 7 days after each vaccination. Solicited reactions were graded on a scale from 0 (absent) to 3 (prevention of normal everyday activities or injection-site reactions >100 mm). Unsolicited adverse events (AEs) were collected for 30 days after each dose, and serious AEs (SAEs) related to the study intervention were collected until the end of the study follow-up period.
Assessment of GVHD, relapse, and death
Given concerns about potential immune-mediated disorders (pIMDs) related to RZV, a subgroup observational cohort analysis of cohort A was performed to determine differences in chronic GVHD (cGVHD), relapse, and death compared with historical controls. Controls were Dana-Farber Cancer Institute patients who underwent a single allogeneic HCT between January of 2015 and December of 2016 prior to RZV approval and who had no relapse or death at 9 months after transplant. The concern in the field is that the adjuvant, ASO1B, is most likely to cause an aberrant immune response shortly after vaccination associated with immune stimulation; thus, study patients were followed for incidence and severity of cGVHD at 9, 12, and 15 months from the time of transplant, as well as for death and relapse during this period. Transplant demographics, cGVHD, relapse, and survival data from historical control patients were retrieved from the bone marrow transplant data repository of the Dana-Farber Cancer Institute. Analysis of incidence rate ratios (IRRs) were adjusted for factors known to influence GVHD, including age, sex, HLA matching, GVHD prophylaxis medications, and source of stem cells.
HZ case definition and incidence rate assessments
HZ cases were defined as (1) a new rash that was characteristic (unilateral, dermatomal, painful, vesicular) and that was documented as HZ by a treating clinician or (2) an atypical rash or suspected disseminated VZV infection that was confirmed by VZV culture, direct fluorescent antibody detection, polymerase chain reaction, or immunohistochemical staining on tissue specimen.
HZ cases were ascertained by systematic chart review at the end of the study period using prescription drug history for valacyclovir, famciclovir, and IV formulation of ACV, as well as the search terms “VZV,” “varicella,” “zoster,” and “shingles.” Additionally, treating clinicians were instructed to report all HZ cases to study staff throughout the study period. Patients who met the above criteria underwent manual chart review by 2 investigators to confirm HZ cases.
Incidence ratios (IRs) were calculated for the TVC, mTVC, and the subgroup of patients who discontinued antiviral prophylaxis during the study period.
Statistical analysis
Descriptive statistics were reported as mean with standard deviation, median with interquartile range (IQR), or frequency with percentage. For the safety data analysis, 95% confidence intervals (CI) for the estimated proportions of individuals experiencing AEs were calculated using the exact Clopper-Pearson binomial proportion approach as the result of some proportions being close to 0 or 1.
The cumulative incidence (with 95% CI) of cGVHD, cGVHD severity, relapse, and death were calculated for cases and controls at predefined follow-up time points. Individuals were right censored at their final visit or the end of the study; death was treated as a competing risk and accounted for in the cumulative incidence calculations. The IRR for cGVHD, relapse, and death for cases and controls was calculated using Poisson regression with a log link and robust standard errors; follow-up was up to 24 months after transplant in cases and controls, and a log offset was used to account for individual follow-up. A 95% CI was constructed for the IRR to explore the hypothesis that the IRR will be near 1.0. An adjusted analysis was performed for cGVHD by including age (continuous), sex (dichotomous), acute GVHD prophylaxis regimen (7 levels), source of stem cells (dichotomous), and HLA matching (dichotomous). Age was assessed for having a nonlinear effect on the response using Akaike information criterion. Because there were few relapse and death events, a propensity score analysis was used for the relapse and death outcomes. Specifically, a binary logistic regression model including the aforementioned covariates was fit predicting case and control status; propensity score trimming was not necessary. Stabilized inverse probability of treatment weighting was used to account for propensity scores, and robust standard errors were used to account for correlation introduced through weighting. Adequacy of the propensity score balancing was determined by calculating the standardized mean differences of covariates between study groups; a difference <0.2 was considered appropriate.
The IR of HZ following initial vaccination was calculated in the TVC and mTVC in which follow-up was right censored at relapse, death, or end of follow-up. Patients who had HZ within 30 days of vaccine 2 (V2) were excluded from the mTVC analysis. A sensitivity analysis was performed looking at follow-up only in individuals who discontinued antiviral prophylaxis. A cumulative incidence curve was constructed for the mTVC, treating relapse and death as competing risks. All testing was 2 tailed, and P values < .05 were considered statistically significant. Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Study participants
Of the 178 volunteers who consented to participate, 158 received V1 (TVC group), and 150 (95%) received V2 (Figure 2). One hundred seventeen of 158 (74%) volunteers received V1 9 to 12 months after HCT (cohort A), whereas 51 of 158 (26%) volunteers received V1 13 to 24 months after HCT (cohort B), with a median time from HCT to V1 of 280 days (IQR, 267-407) and a median time between V1 and V2 of 91 days (IQR, 70-105) (Table 1). Cumulative follow-up time was 109 person-years (PYs) in the TVC and 13.6 PYs after discontinuation of ACV prophylaxis.
Figure 2.
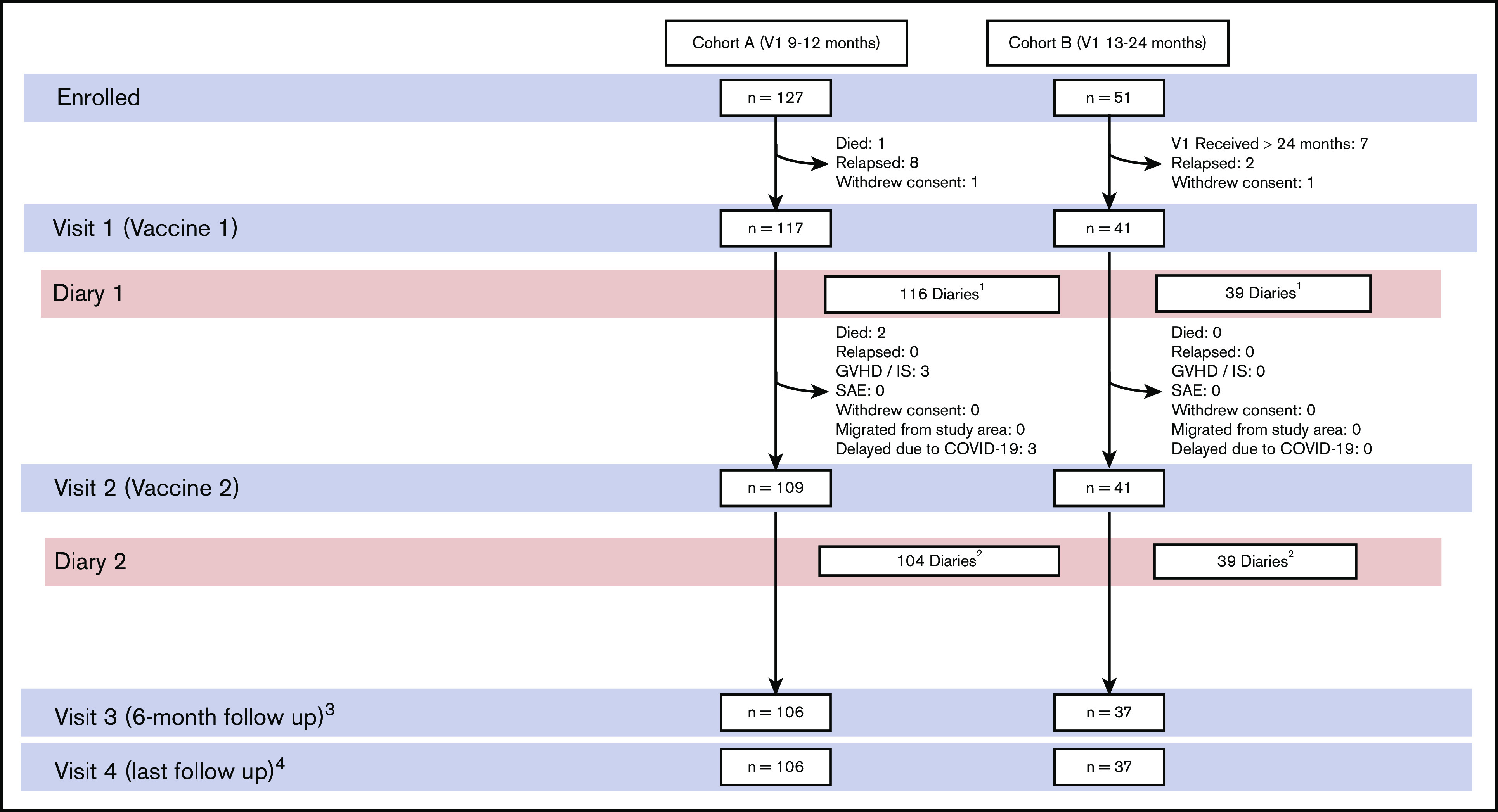
Participant flow. Cohort A received V1 between 9 and 12 months after HCT. Cohort B received V1 between 13 and 24 months after HCT. Participants who did not return symptom diaries were monitored at the subsequent clinician visit for unsolicited AEs and SAEs. 1Three of 3 participants with missing diaries had follow-up visits with no SAE. One patient died secondary to infection not related to trial intervention. 2Six of 7 participants with missing diaries had follow-up visits with no SAE or death. 3Six of 7 participants who did not have follow-up after V2 returned diaries and were monitored for SAEs at 30 days. Lack of follow-up was attributed primarily to COVID-related delays. 4Sixteen participants had ≤6-month follow-up after V1. IS, immunosuppression.
Table 1.
HZ characteristics of participants
| HZ and vaccine characteristics | Data |
|---|---|
| VZV Immunoglobulin G | |
| Positive | 146 (92) |
| Negative | 9 (6) |
| Indeterminate | 3 (2) |
| HZ prior to transplant | |
| Yes | 22 (14) |
| No | 135 (85) |
| Unknown | 1 (1) |
| ZVL prior to transplant | |
| Yes | 29 (18) |
| No | 113 (72) |
| Unknown | 16 (10) |
| Time from transplantation to V1, median (IQR), d | 280.50 (267.0-407.0) |
| Time between V1 and V2, median (IQR), d | 91.0 (70.0-105.0) |
| Active chronic GVHD | |
| At V1 | 60 (38) |
| At V2* | 61 (41) |
| Systemic immunosuppression | |
| At V1 | 112 (71) |
| At V2 | 90 (60) |
| Coadministered vaccines | |
| At V1 | 148 (94) |
| At V2 | 126 (84) |
| Antiviral prophylaxis† | |
| At V1 | 157 (99) |
| At V2 | 147 (98) |
Unless otherwise indicated, data are n (%).
Missing data (n = 1).
Antiviral treatment with activity against VZV used as prophylaxis against HZ, herpes simplex virus, or cytomegalovirus.
Participants had a mean age of 55 years (range, 19-76) and were predominantly male (58%) and white (87%); acute myeloid leukemia (34%) and myelodysplastic syndrome (18%) were the most common indications for HCT (Table 2). The majority (92%) had positive VZV immunoglobulin G serologies, and the minority reported a history of HZ (14%) or administration of ZVL (18%) prior to HCT. At the time of V1, 38% of participants had active cGVHD; 71% and 60% were on systemic immunosuppression at the time of V1 and V2, respectively. Ninety-eight percent of participants remained on antiviral prophylaxis from the time of HCT through administration of V2.
Table 2.
Participant demographics
| Participant characteristics | Data |
|---|---|
| Age, mean ± SD (range), y | 55.05 ± 13.83 (19-76) |
| Sex | |
| Male | 91 (58) |
| Female | 67 (42) |
| Race | |
| White | 137 (87) |
| Asian | 7 (4) |
| Black or African-American | 4 (3) |
| Other | 10 (6) |
| Primary indication for transplant | |
| AML | 53 (34) |
| MDS | 29 (18) |
| ALL | 24 (15) |
| MPD | 12 (8) |
| Anemia, red cell disorder | 9 (6) |
| NHL | 10 (6) |
| Other* | 21 (13) |
| Regimen | |
| Myeloablative | 55 (35) |
| Nonmyeloablative | 103 (65) |
| Donor | |
| Matched related | 24 (15) |
| Mismatched related | 23 (15) |
| Matched unrelated | 87 (55) |
| Mismatched unrelated | 24 (15) |
| Source of stem cells | |
| Peripheral blood | 118 (75) |
| Bone marrow | 37 (23) |
| Cord blood | 3 (2) |
| GVHD prophylaxis medications | |
| Tacrolimus | 155 (98) |
| Sirolimus | 54 (34) |
| Methotrexate | 98 (62) |
| Mycophenolate mofetil | 39 (25) |
| CD34 selection | 1 (1) |
| Other | 38 (24) |
| Acute GVHD | 48 (30) |
| Skin | 41 (85) |
| Gastrointestinal | 13 (27) |
| Liver | 4 (8) |
Unless otherwise indicated, data are n (%).
ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorder; NHL, non-Hodgkin lymphoma; SD, standard deviation.
CLL, chronic lymphocytic leukemia; CML, chronic myelogenous; HL, Hodgkin lymphoma; other indication.
Reactogenicity and safety
Solicited AEs were collected in 155 of 158 (98%) participants for V1 and 143 of 150 (95%) participants for V2 (Figure 2). The majority of participants had coadministered vaccines at V1 (94%) and V2 (84%) (Table 1). Overall, solicited reactions within 7 days of vaccination occurred in 92.1% of participants (Table 3). Solicited injection site reactions occurred in 87.3% (grade 3, 18.7%), owing mostly to injection site pain, which occurred in 86% (grade 3 16%). Injection site reactogenicity was similar after V1 (78.1%) and V2 (75.5%). Solicited general reactions occurred in 82.8% (grade 3 26.5%); fatigue (70.2%) and myalgia (50.7%) were the most common general symptoms reported. General symptoms were also similar after V1 (67.7%) and V2 (66.4%).
Table 3.
Safety and reactogenicity in TVC
| Data | |
|---|---|
| AEs | |
| Any grade | 139/151 (92.1); 86.5-95.8 |
| Grade 3 | 49/151 (32.5); 25.1-40.5 |
| Injection site AEs | |
| All types | |
| Any grade | 131/150 (87.3); 80.9-92.2 |
| Grade 3 | 28/150 (18.7); 12.8-25.8 |
| Pain | |
| Any grade | 129/150 (86.0); 79.4-91.1 |
| Grade 3 | 24/150 (16.0); 10.5-22.9 |
| Redness | |
| Any grade | 34/149 (22.8); 16.3-30.4 |
| Grade 3 | 7/149 (4.7); 1.9-9.4 |
| Swelling | |
| Any grade | 34/149 (22.8); 16.3-30.4 |
| Grade 3 | 3/149 (2.0); 0.4-5.8 |
| General AEs | |
| Any AE | |
| Any grade | 125/151 (82.8); 75.8-88.4 |
| Grade 3 | 40/151 (26.5); 19.6-34.3 |
| Fever | |
| Any grade | 34/149 (22.8); 16.3-30.4 |
| Grade 3 | 4/149 (2.7); 0.7-6.7 |
| Headache | |
| Any grade | 69/150 (46.0); 37.8-54.3 |
| Grade 3 | 10/150 (6.7); 3.2-11.9 |
| Fatigue | |
| Any grade | 106/151 (70.2); 62.2-77.4 |
| Grade 3 | 27/151 (17.9); 12.1-24.9 |
| Gastrointestinal | |
| Any grade | 58/150 (38.7); 30.8-47.0 |
| Grade 3 | 8/150 (5.3); 2.3-10.2 |
| Myalgia | |
| Any grade | 88/149 (59.1); 50.7-67.0 |
| Grade 3 | 17/149 (11.4); 6.8-17.6 |
| Shivering | |
| Any grade | 54/150 (36.0); 28.3-44.2 |
| Grade 3 | 11/150 (7.3); 3.7-12.7 |
| Unsolicited AEs | |
| Within 30 d of vaccination | 11/150 (7.3); 3.7-12.7 |
| Related to trial intervention* | 6/150 (4.0); 1.5-8.5 |
| SAEs† | |
| Within 30 d of vaccination‡ | 2/150 (1.3); 0.2-4.7 |
| Within total follow-up period | 2/150 (1.3); 0.2-4.7 |
| Relapse of malignancy | 13/158 (8.2); 4.5-13.7 |
| Death | 5/158 (3.2); 1.0-7.2 |
Participants with missing symptom diaries at V1 or V2 were removed for missing data unless grade 3 was reported. Data are n/N (%); 95% CI.
The 6 unsolicited AEs related to the trial intervention were injection site pain (n = 1), bone pain (n = 1), pruritus (n = 2), rash (n = 1), and dizziness (n = 1).
SAEs were defined as events that were life-threatening, required hospitalization or prolonged hospitalization, or resulted in disability or incapacity or birth defect in offspring.
The 2 SAEs that may be related to vaccination were hospitalization for metabolic acidosis and weakness 3 days after V1 and hospitalization for fever and weakness 3 days after V1.
During the 30-day postvaccination period, unsolicited AEs were reported in 7.3% of participants; 4% were deemed related to the trial intervention. Two SAEs (1.3%) were identified and considered possibly related to vaccination: hospitalization for metabolic acidosis and weakness 3 days after V1 and hospitalization for fever and weakness 3 days after V1. During the study period, there were 5 (3.2%) deaths; they were secondary to disease relapse and infection and were not considered related to vaccination.
GVHD, relapse, and mortality
Disease-related outcomes were assessed prevaccination at 9 months after HCT (116 cases, 282 controls) and postvaccination at 12 months (111 cases, 259 controls) and at 15 months (82 cases, 233 controls). Follow-up time was variable, with 50 cases reaching 18 months of follow-up and 5 cases reaching 24 months of follow-up. The cumulative incidence of cGVHD in cohort A was 0.45 (95% CI, 0.36-0.54), 0.57 (95% CI, 0.47-0.65), and 0.60 (95% CI, 0.5-0.68) at 9, 12, and 15 months after HCT, respectively, compared with 0.42 (95% CI, 0.36-0.48), 0.56 (95% CI, 0.5-0.62), and 0.6 (95% CI, 0.54-0.66) in historical controls (unadjusted IRR, 1.1; 95% CI, 0.84-1.44 and adjusted IRR, 1.05; 95% CI, 0.8-1.38). Controls had a higher cumulative incidence of severe GVHD at all 3 time points, although this did not reach statistical significance. Patients on immunosuppression at the time of vaccination (n = 94) had a higher prevaccination rate for chronic GVHD compared with those not on immunosuppression (n = 22) (0.52 vs 0.13), and they also experienced a higher incidence of chronic GVHD in the peri-vaccination period between 9 and 15 months (IRR, 4.3; 95% CI, 1.04-17.72).
There was no evidence of a difference in the rates of relapse (unadjusted IRR, 1.15; 95% CI, 0.57-2.35 and adjusted IRR, 0.97; 95% CI, 0.46-2.04) or mortality (unadjusted IRR, 0.61; 95% CI, 0.21-1.74 and adjusted IRR, 0.41; 95% CI, 0.14-1.25) between participants and historical controls in the peri-vaccination period (9-15 months post-HCT).
Efficacy
During the study period, 4 of 157 (2.5%) patients experienced an episode of HZ at a median of 705 days (range, 383-911) after HCT, a median of 76.5 days (range, 10-115) after V2, and a median of 25.5 days (range, 9-206) after discontinuation of antiviral prophylaxis (Table 4). The time between V1 and V2 varied (range, 49-140 days) in comparison with the study median of 91 days (IQR, 70-105) (Table 4). No patient was taking immunosuppressive medications at the time of HZ. One case (0.6%, 1/109 PYs) was fatal, presenting as disseminated vesicular rash, pneumonitis, and hepatitis in a cord blood recipient.
Table 4.
HZ case characteristics
| Age at HCT, y | VZV IgG | Prior HZ | Stem cell source | HLA matching | Time between V1 and V2, d | Time from HCT to HZ, d | Time from V2 to HZ, d | Time from antiviral discontinuation to HZ, d | Immunosuppression at HZ | Clinical presentation |
|---|---|---|---|---|---|---|---|---|---|---|
| 22* | + | No | Bone marrow | Matched unrelated | 49 | 383 | 10 | 10 | None | Dermatomal |
| 47 | + | Yes | Cord blood | Mismatched unrelated | 63 | 668 | 102 | 41 | None | Disseminated cutaneous, pneumonia, hepatitis, death |
| 68 | + | No | Peripheral blood | Matched related | 91 | 743 | 115 | 206 | None | Dermatomal |
| 25 | + | No | Peripheral blood | Matched related | 140 | 911 | 51 | 9 | None | Dermatomal |
IgG, immunoglobulin G; +, present.
Excluded from modified TVC as the result of an HZ episode <30 days after V2.
The incidence of HZ was 36.7/1000 PYs with a median follow-up of 263 days (IQR, 172-350) in the TVC and 28.3/1000 PYs with a median follow-up of 281 days (IQR, 190-354) in the mTVC (Figure 3). Thirty-four (21.5%) participants discontinued antiviral prophylaxis after receiving both doses of RZV at a median of 544 days (IQR, 442-718) post-HCT. HZ occurred in 4 of 34 patients (11.8%) with a median follow-up of 113 days (IQR, 44-204) after discontinuation.
Figure 3.
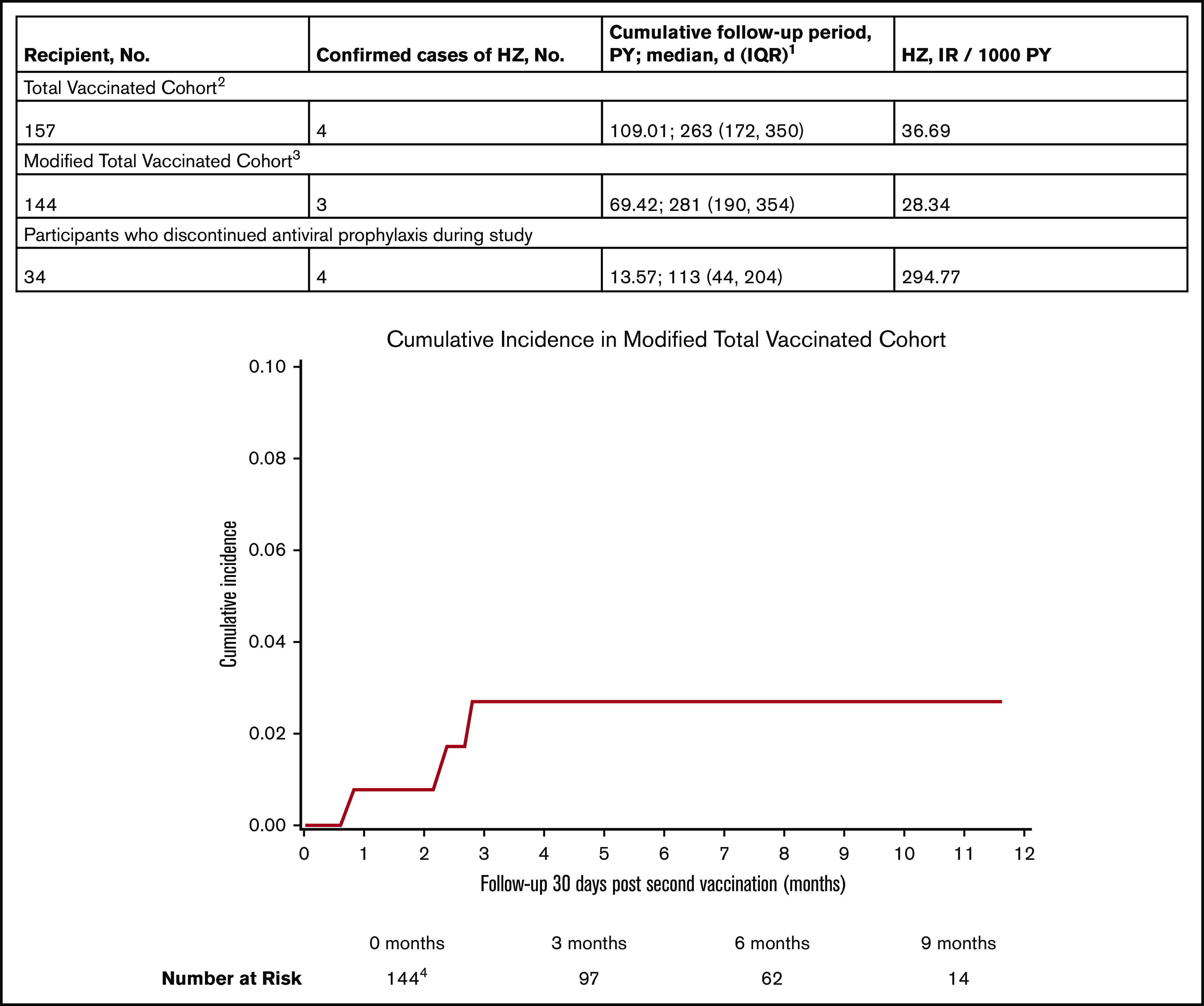
Cumulative incidence and incidence rates of HZ during the study. 1Cumulative follow-up period is the sum of follow-up periods censored at the occurrence of HZ, relapse, or death. Patients were followed from V1 to study end (June of 2020). 2One participant was excluded from the TVC because an episode of HZ occurred before V1. 3Participants who developed HZ <30 days from V2. Participants excluded from the mTVC include 8 patients who did not receive V2, 4 patients who relapsed before V2, and 1 patient who had HZ prior to 30 days after V2. 4The median follow-up time at risk was 157 days (IQR, 84-237) in the mTVC. Eleven patients did not reach 30 days at risk, including 7 patients without follow-up after V2, 2 patients with <30 days follow-up after V2, and 2 patients with relapse at <30 days follow-up after V2.
Discussion
Two doses of RZV demonstrated safety and tolerability in allogeneic HCT without increased rates of cGVHD, relapse, or death, as well as overall low rates of breakthrough HZ compared with historical controls. As reported previously, RZV is more reactogenic than placebo.18,25 Solicited reactions were reported in the majority of participants; they occurred more frequently than in healthy adults18,25 but at rates similar to autologous HCT recipients.24 Grade 3 general symptoms occurred at a higher rate than did those reported in other immunocompromised populations.20-24
Vaccines were coadministered with RZV in the majority of participants which may have led to a higher incidence of solicited AEs than if RZV was administered alone. Furthermore, many participants reported baseline disease and treatment-related symptoms that overlapped with solicited general symptoms. This may have contributed to the higher rates of grade 3 reactions observed. Despite the reactogenicity, there was high acceptability of the vaccine, with 95% of participants completing V2; the reasons why the remaining 5% did not receive V2 were attributed to clinical or logistical barriers (Figure 2). Unsolicited AEs related to the vaccine and SAEs were also infrequent and similar to other cohorts.20-24
Although there have been concerns related to pIMDs with the use of the RZV novel adjuvant AS01B system, there was no increased incidence or severity of cGVHD observed in the subgroup that was vaccinated between 9 and 12 months compared with historical controls in the peri-vaccination 9 to 15–month period. This finding is in keeping with renal transplant recipients who did not experience higher rates of solid organ rejection21 and healthy adults who did not have increased pIMDs after RZV.18,25
Many participants received RZV with active cGVHD and/or ongoing immunosuppression (60-71%) and had similar disease-related outcomes compared with historical controls. Patients on immunosuppression during vaccination had a higher IRR of cGVHD in the peri-vaccination period compared with those not on immunosuppression; however, they also likely represent a cohort with a higher baseline risk for alloreactivity. VARIVAX and ZVL are strictly contraindicated in this subgroup of transplant recipients15,26; therefore, RZV can be administered more generally in this highly immunocompromised and heterogenous population.
Through prospective case collection, we found an IR of 28.34/1000 PYs for HZ in the mTVC, which is higher than that seen after RZV in healthy older adults (IR, 0.8/1000 PYs)25 but similar to autologous HCT recipients (IR, 30.0/1000 PYs).24 RZV immune response may be lower after allogeneic HCT than after autologous HCT; however, this finding has not been validated using vaccine-specific B- and T-cell responses, and the relationship with efficacy has not been determined.27 Allogeneic HCT recipients currently experience a 4% to 9% incidence of VZV reactivation at 2 years posttransplant, despite extended ACV prophylaxis.5,7,28-30 Our observed incidence (2.5%) was lower with longer median follow-up. Although RZV likely offers additional protection, larger prospective RCTs are needed to determine efficacy.
There was 1 fatal case of disseminated HZ during the study period in a patient with T-cell acute lymphocytic leukemia after cord blood transplantation; it occurred +668 days post-HCT off ACV prophylaxis. Although this patient was on hemodialysis, he was off immunosuppression, and there was no notable immune deficiency (absolute lymphocyte count, immunoglobulin levels) in the months prior to the event. We previously described that severe HZ continues to occur after allogeneic HCT with an IR of 1/228 PYs, despite extended antiviral prophylaxis, with a peak in the 12 to 24–month posttransplant period.13
The timing of RZV was determined by expert consensus and in concordance with administration of other nonlive vaccines. Patients may benefit from alternative dosing schedules, including delay of series in those on immunosuppression or consideration of pretransplant dosing of donor or recipient to generate immune memory, such as has been evaluated with pneumococcal vaccination.31 Discontinuation of antiviral prophylaxis was left to the discretion of the treating clinician. Notably, there was a case of HZ 10 days after V2, suggesting that ACV should be continued for some time after series completion. Immunogenicity data are forthcoming and will help to guide decision making about vaccination timing and the duration of antiviral prophylaxis.
As a study of the DF/BWH vaccination protocol, it was limited to a single center, and there was no prospective control group. Comparison of cGVHD, relapse, and mortality were limited to historical controls, and small event rates for relapse and mortality must be interpreted with caution. Study participants received vaccination based on clinician discretion and, therefore, may represent a healthier subgroup of allogeneic HCT recipients. Furthermore, the population was predominantly white (87%), limiting generalizability. Lastly, the short follow-up limits the assessment of durability of RZV response, and findings might not reflect long-term efficacy.
In summary, among recipients of allogeneic HCT, RZV was safe, tolerable, and did not increase rates of GVHD. There were few breakthrough cases of HZ in this cohort; however, future RCTs are needed to determine vaccine efficacy.
Supplementary Material
The full-text version of this article contains a data supplement.
Footnotes
Presented in abstract form at IDWeek, Virtual Conference, 21-25 October 2020.
Data sharing requests should be sent to Emily Baumrin (emily.baumrin@pennmedicine.upenn.edu).
Authorship
Contribution: E.B. designed and performed research and wrote the manuscript; N.E.I., B.B., and M.M.F., performed research; C.P.B. analyzed data; Q.Y. performed research and contributed data for participants and historical controls; and V.T.H., L.R.B., and N.C.I. designed research and revised the manuscript.
Conflict-of-interest disclosure: N.C.I. has received research grants from GlaxoSmithKline, Merck, Astellas, and AiCuris. The remaining authors declare no competing financial interests.
Correspondence: Emily Baumrin, University of Pennsylvania, 3400 Civic Center Blvd, South Tower, 7th Floor, Suite 776, Philadelphia, PA 19104; e-mail: emily.baumrin@pennmedicine.upenn.edu.
References
- 1.Koc Y, Miller KB, Schenkein DP, et al. Varicella zoster virus infections following allogeneic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Marrow Transplant. 2000;6(1):44-49. [DOI] [PubMed] [Google Scholar]
- 2.Su SH, Martel-Laferriere V, Labbe AC, et al. High incidence of herpes zoster in nonmyeloablative hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(7):1012-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locksley RM, Flournoy N, Sullivan KM, Meyers JD. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152(6):1172-1181. [DOI] [PubMed] [Google Scholar]
- 4.Ho DY, Arvin AM. Varicella zoster virus infections. In: Forman SJ, Negrin RS, Antin JH, Appelbaum FR, eds. Thomas’ Hematopoietic Cell Transplantation, 5th ed.. West Sussex, UK: John Wiley & Sons, Ltd; 2016:1085-1110. [Google Scholar]
- 5.Boeckh M, Kim HW, Flowers ME, Meyers JD, Bowden RA. Long-term acyclovir for prevention of varicella zoster virus disease after allogeneic hematopoietic cell transplantation–a randomized double-blind placebo-controlled study. Blood. 2006;107(5):1800-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selby PJ, Powles RL, Easton D, et al. The prophylactic role of intravenous and long-term oral acyclovir after allogeneic bone marrow transplantation. Br J Cancer. 1989;59(3):434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood. 2007;110(8):3071-3077. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Kumar D, Messner HA, et al. Clinical efficacy of prophylactic strategy of long-term low-dose acyclovir for varicella-zoster virus infection after allogeneic peripheral blood stem cell transplantation. Clin Transplant. 2008;22(6):770-779. [DOI] [PubMed] [Google Scholar]
- 9.Asano-Mori Y, Kanda Y, Oshima K, et al. Long-term ultra-low-dose acyclovir against varicella-zoster virus reactivation after allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2008;83(6):472-476. [DOI] [PubMed] [Google Scholar]
- 10.Thomson KJ, Hart DP, Banerjee L, Ward KN, Peggs KS, Mackinnon S. The effect of low-dose aciclovir on reactivation of varicella zoster virus after allogeneic haemopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35(11):1065-1069. [DOI] [PubMed] [Google Scholar]
- 11.Kanda Y, Mineishi S, Saito T, et al. Long-term low-dose acyclovir against varicella-zoster virus reactivation after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;28(7):689-692. [DOI] [PubMed] [Google Scholar]
- 12.Lee CJ, Savani BN, Ljungman P. Varicella zoster virus reactivation in adult survivors of hematopoietic cell transplantation: how do we best protect our patients? Biol Blood Marrow Transplant. 2018;24(9):1783-1787. [DOI] [PubMed] [Google Scholar]
- 13.Baumrin E, Cheng MP, Kanjilal S, Ho VT, Issa NC, Baden LR. Severe herpes zoster requiring intravenous antiviral treatment in allogeneic hematopoietic cell transplantation recipients on standard acyclovir prophylaxis. Biol Blood Marrow Transplant. 2019;25(8):1642-1647. [DOI] [PubMed] [Google Scholar]
- 14.Rubin LG, Levin MJ, Ljungman P, et al. ; Infectious Diseases Society of America . 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):309-318. [DOI] [PubMed] [Google Scholar]
- 15.Ljungman P, Cordonnier C, Einsele H, et al. ; Centers for Disease Control and Prevention . Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2009;44(8):521-526. [DOI] [PubMed] [Google Scholar]
- 16.Issa NC, Marty FM, Leblebjian H, et al. Live attenuated varicella-zoster vaccine in hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2014;20(2):285-287. . [DOI] [PubMed] [Google Scholar]
- 17.Naidus E, Damon L, Schwartz BS, Breed C, Liu C. Experience with use of Zostavax(®) in patients with hematologic malignancy and hematopoietic cell transplant recipients. Am J Hematol. 2012;87(1):123-125. [DOI] [PubMed] [Google Scholar]
- 18.Lal H, Cunningham AL, Godeaux O, et al. ; ZOE-50 Study Group . Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087-2096. [DOI] [PubMed] [Google Scholar]
- 19.Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berkowitz EM, Moyle G, Stellbrink HJ, et al. ; Zoster-015 HZ/su Study Group . Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis. 2015;211(8):1279-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vink P, Ramon Torrell JM, Sanchez Fructuoso A, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in chronically immunosuppressed adults following renal transplant: a phase III, randomized clinical trial. Clin Infect Dis. 2020;70(2):181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dagnew AF, Ilhan O, Lee WS, et al. ; Zoster-039 study group . Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988-1000. [DOI] [PubMed] [Google Scholar]
- 23.Vink P, Delgado Mingorance I, Maximiano Alonso C, et al. ; Zoster-028 Study Group . Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial [published correction appears in Cancer. 2020;126(12):2941]. Cancer. 2019;125(8):1301-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastidas A, de la Serna J, El Idrissi M, et al. ; ZOE-HSCT Study Group Collaborators . Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322(2):123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham AL, Lal H, Kovac M, et al. ; ZOE-70 Study Group . Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019-1032. [DOI] [PubMed] [Google Scholar]
- 26.Bhalla P, Forrest GN, Gershon M, et al. Disseminated, persistent, and fatal infection due to the vaccine strain of varicella-zoster virus in an adult following stem cell transplantation. Clin Infect Dis. 2015;60(7):1068-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camargo JF, Lin RY, Natori Y, et al. Reduced immunogenicity of the adjuvanted recombinant zoster vaccine after hematopoietic cell transplant: a pilot study. Blood Adv. 2020;4(19):4618-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamani K, MacDonald J, Lavoie M, et al. Zoster prophylaxis after allogeneic hematopoietic cell transplantation using acyclovir/valacyclovir followed by vaccination. Blood Adv. 2016;1(2):152-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo HM, Kim YS, Bang CH, Lee JH, Lee JY, Lee DG, et al. Antiviral prophylaxis for preventing herpes zoster in hematopoietic stem cell transplant recipients: a systematic review and meta-analysis. Antiviral Res. 2017;140:106-115. [DOI] [PubMed] [Google Scholar]
- 30.Mascarenhas K, Teh JB, Peng K, et al. Efficacy of low-dose zoster prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2020;55(8):1662-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locke FL, Menges M, Nishihori T, Nwoga C, Alsina M, Anasetti C. Boosting humoral and cellular immunity to pneumococcus by vaccination before and just after autologous transplant for myeloma. Bone Marrow Transplant. 2016;51(2):291-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.