ABSTRACT
People Living with HIV (PLWH) remain disproportionately susceptible to vaccine-preventable illnesses due to increased morbidity and mortality from common pathogens, increased transmission related to epidemiologic factors, and decreased vaccination rates. We aimed to describe patient-specific predictive factors that may impact adherence to the CDC’s recommended vaccination schedules in PLWH. We retrospectively evaluated adult PLWH in care at the University of Nebraska Medical Center’s HIV clinic and collected information related to demographics, clinic visits, vaccination status, and measures of HIV disease control. Patients were categorized as “Adherent” if they had received all vaccinations for which they were eligible and were categorized as “Non-Adherent” if they were deficient or delayed in receiving one or more vaccinations. Participant characteristics were compared between groups by multivariable logistic regression to identify predictors associated with vaccine schedule non-adherence. We evaluated 502 PLWH who met our inclusion criteria; 206 of these (41%) had received all eligible vaccinations, while 296 (59%) were missing one or more vaccinations. The mean age of participants was 48 years old, 76% were male, and 53% were white. Our participants had a median of 2.83 clinic visits per year and missed 8.3% of scheduled clinic visits. Factors associated with non-adherence to vaccination schedules included a high frequency of missed clinic appointments (>10%), men who have sex with men, and a CD4 count <200 cells/mm3. Knowledge of variables associated with vaccination rates may be beneficial in identifying patients at-risk for under-vaccination and designing targeted education programs for providers and patients.
KEYWORDS: HIV, PLWH, vaccination, vaccines, adherence
Introduction
Human Immunodeficiency Virus (HIV) disease progression is characterized by a gradual decline in the number of CD4+ T lymphocytes, eventually leading to a decline in adaptive immune function if left untreated. As a result, people living with HIV (PLWH) are at increased risk for many viral, bacterial, and fungal infections. Accordingly, the Centers for Disease Control and Prevention (CDC) has released a vaccination schedule specific for PLWH in order to better protect this vulnerable population against vaccine-preventable illness.1
PLWH suffer more severe infections from vaccine-preventable diseases, leading to increased morbidity and mortality. Several studies have shown an increased prevalence of Hepatitis B Virus (HBV) co-infection in PLWH, potentially due to a common mode of transmission.2–4 Other studies have shown an increase in all-cause and liver-related mortality in patients with HIV and HBV co-infection compared to HBV infection alone.5 Additionally, a recent study investigated the prevalence and virulence of Human Papillomavirus (HPV) genotypes in men who have sex with men (MSM), comparing PLWH to those without HIV.6 The study revealed that MSM PLWH experienced significantly higher rates and severity of oral, anal, and penile HPV infection, with approximately 80% of infections caused by genotypes protected against by the HPV vaccination. Furthermore, a study conducted in Malawi investigated the severity and mortality of influenza infection in PLWH compared to individuals without HIV.7 HIV infection itself was found to be a risk factor for acquiring influenza, with PLWH experiencing increased severity of influenza infection, requiring higher rates of hospitalization and health-care utilization.
Several barriers exist in vaccinating PLWH. Surveys of primary care providers (PCP) reveal that many do not feel that they possess adequate knowledge or experience to effectively manage individuals at risk for HIV infection or patients already living with HIV.8–10 Consequently, most PLWH are co-managed by an infectious disease or HIV specialist. With multiple health-care providers, complex medication regimens, and possibilities for multiple co-morbidities, lack of coordination in patient care may be one of many contributing factors to lower rates of vaccination in this patient population. Secondly, provider-specific barriers to vaccine schedule adherence in PLWH include safety concerns, concerns about vaccine efficacy, and reimbursement issues.11 Competing provider priorities during a health-care visit may be another contributing factor.12 Lastly, patient hesitancy and lack of knowledge regarding vaccinations remains an additional barrier to vaccination. Common reasons cited for vaccine declination include fear of side effects, lack of concern about vaccine-preventable disease, worry about the vaccine worsening the course of HIV infection, and a belief that vaccination would fail due to a compromised immune system.13,14
Despite the increased risk for infections and the widespread availability of vaccines, vaccination rates in PLWH remain lower than the general population.15–19 While vaccination rates for PLWH are well-documented, factors contributing to low vaccination are not. The aim of our study is to better understand predictor variables and disparities in vaccination rates amongst PLWH. Knowledge of these patient-specific variables may allow for identification of patients at-risk for under-vaccination and more targeted education programs for both patients and providers.
Patients and methods
This single-center retrospective cohort study utilized the electronic health-care records (EHR) from the Nebraska Medicine/University of Nebraska Medical Center (UNMC) HIV clinic to explore patient-specific factors that influenced vaccination rates in PLWH. Information from clinic records was cross-compared with the Nebraska State Immunization Information System (NESIIS) registry for completeness and consistency. Inclusion criteria for the study were HIV-positive serostatus (as documented via ICD-10 codes), greater than 19 years of age (the age of majority in Nebraska), HIV diagnosis greater than 1 year prior to medical record search, first visit to the UNMC HIV clinic greater than 1 year prior to medical record search (to allow time for receipt of all eligible vaccinations), a current patient of the UNMC HIV clinic (defined as most recent clinic visit occurring within 1 year of medical record search), and medically eligible to receive vaccinations. Medical eligibility for vaccination was defined as the absence of a documented contraindication to receiving the individual vaccination and absence of past severe reaction upon vaccine administration documented in the medical record. This study was approved by the UNMC Institutional Review Board (336–18-EP).
Vaccine adherence
The primary outcome was overall vaccination regimen adherence. Adherence was coded in a dichotomous manner, with patients categorized as either “Adherent” or “Non-Adherent” according to the CDC’s 2018 recommended vaccination guidelines for PLWH. Patients were categorized as “Adherent” if they had received all vaccinations for which they were eligible at the time of medical record review. Patients were categorized as “Non-Adherent” if they were deficient or delayed in receiving one or more vaccinations for which they were eligible at the time of medical record review. A deficiency in vaccination was defined as non-receipt of a vaccination for which the patient was eligible and delay in vaccination was defined as the presence of at least one clinic visit where a patient was eligible to receive a second or third dose of a vaccine series yet did not receive the vaccine dose for any reason.
Criteria utilized to assess vaccine eligibility
The CDC’s 2018 guideline was used to assess patient eligibility to receive each vaccine listed in Table 1. Prior to data collection, Recombinant Zoster Vaccine (RZV) and Meningococcal A, C, W, and Y (MenACWY) were excluded from the overall adherence analysis as these were new recommendations to the 2018 CDC guidelines and adherence rates were expected to be lower than other vaccinations. Traditional childhood vaccinations, such as Measles, Mumps, Rubella (MMR), Haemophilus influenzae type b (Hib), Varicella (Var), Inactivated Poliovirus (IPV), and Rotavirus (RV) were not considered as this information was not consistently documented in our databases.
Table 1.
Vaccinations analyzed per the CDC’s 2018 immunization guidelines for PLWH
| 1. HepA (Hepatitis A) |
| 2. HepB (Hepatitis B) |
| 3. HPV (Human Papillomavirus) |
| 4. Influenza |
| 5. PCV13 (Pneumococcal Conjugate) |
| 6. PPSV23 (Pneumococcal Polysaccharide) |
| 7. Tdap/Td (Tetanus and Diptheria, with or without acellular pertussis) |
| 8. ZVL (Zoster Vaccine Live) |
CDC = Centers for Disease Control and Prevention, PLWH = People Living with HIV
Patients were designated as “Non-Adherent” for Hepatitis B vaccine (HepB) if they did not have documented immunity via HBV surface antibody titers (nonimmune) and did not have documentation of receipt of a Hepatitis B-containing vaccine series. MSM status was used to delineate eligibility for vaccination with Hepatitis A vaccine (HepA), with MSM participants considered eligible for HepA vaccination as defined in the 2018 CDC Immunization Guidelines. MSM patients were designated as “Non-Adherent” if they did not have documented immunity via HAV surface antibody titers (nonimmune) and did not have documentation of receipt of a Hepatitis A-containing vaccine series. Nebraska Medicine administers Havrix (GlaxoSmithKline) as a 2-dose series with doses administered 6–12 months apart. If Twinrix (GlaxoSmithKline) was administered (combined HepA and HepB vaccination), the 3-dose HepB schedule (with doses at 0, 1–2 months, and 6 months) was used to assess adherence status.
Patients could receive vaccinations from an outside provider, but proper documentation was required in the EHR or NESIIS to receive classification as “received.” Eligibility for the remaining vaccines was determined by age and CDC-specified criteria; the maximum number of vaccinations an individual patient was eligible to receive was eight (Table 1). Patients were deemed ineligible for any vaccine if they had a documented allergy to any specific vaccination.
Patient characteristics
Patient-specific characteristics that were collected as potential predictors for vaccine adherence included age, sex, race/ethnicity, MSM status, number of clinic appointments within the past year, percentage of missed clinic appointments (defined as a “no show” for a scheduled appointment with no prior notice to the clinic), insurance/assistance programs, distance of residence from clinic, employment status, primary care provider hospital affiliation, most recent CD4 cell count, and most recent HIV RNA level. Patients were categorized as MSM if they were assigned male sex at birth, including transgender women, and if they had documented sexual exposures with a male listed as a risk factor in the EHR. Non-MSM (females and males without documented male-to-male sexual exposures) were compared to MSM to investigate potential disparities faced by the MSM population.
Age, number of clinic visits per year, and percentage of missed clinic appointments were converted to dichotomous variables based on the sample population mean/median. Percentage of missed clinic appointments was calculated using the number of documented “no shows” to clinic appointments divided by the total number of clinic visits scheduled since May 2012, the date our institution implemented the EHR, to time of medical record review; patient or provider-canceled appointments or rescheduled clinic visits were not counted as missed appointments. Distance of residence from clinic was based on surrounding area geography. Patients within 15 miles of the clinic were considered to live in the local metropolitan area. Patients who lived 15–70 miles from clinic were typically from a nearby metropolitan area or surrounding communities in Eastern Nebraska and Western Iowa. Patients who lived greater than 70 miles from clinic generally lived in rural communities and were considered outside of either of these two geographic areas.
The HIV RNA level of 50 copies/mL was the chosen breakpoint as this was the least sensitive lower limit of detection for any HIV RNA test documented in the EHR. Measures above and below 50 copies/mL corresponded to detectable and undetectable HIV RNA levels, respectively. For CD4 cell count, 200 cells/mm3 was chosen to compare immunocompetent to immunocompromised patients.
Statistical analysis
Patient characteristics were compared between the Adherent and Non-Adherent groups using chi-square and independent sample t-test. A multivariable logistic regression model was used to identify characteristics associated with adherence. Variables were analyzed dichotomously in order to perform binary logistic regression. White race was compared to Nonwhite races, with Nonwhite races grouped together given their relatively low prevalence in the sampled population. Multicollinearity was tested using Variance Inflation Factor (VIF). All predictor variables were linearly independent and the empiric model included all collected predictors; there was not stepwise or backwards stepwise inclusion of variables. Analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC). P values <.05 were considered significant.
Results
Between May 14 and June 1, 2018, electronic medical records from 503 individuals were reviewed. Five hundred and two patients met inclusion criteria; one patient was excluded due to a history of Guillain–Barre upon past vaccination. Of the 502 patients, 206 (41%) were completely adherent to the 2018 CDC-recommended vaccine regimen and 296 (59%) had some delay or deficiency. Of the studied population, the mean (Standard Deviation [SD]) age was 49 (12) years old, approximately 76% were male, 2% identified as transgender, and 53% were white (Table 2). Patients were eligible to receive a mean (SD) of 5 (1) of the eight studied vaccinations. Our participants had a median (Interquartile Range [IQR]) of 2.83 (2.40–3.88) clinic visits per year and missed a median (IQR) of 8.3% (0.0–25.0%) of scheduled appointments (calculated using number of documented “no shows” to clinic appointments divided by the total number of clinic visits scheduled since EHR implementation to time of medical record review). Statistically significant differences were noted between adherent and non-adherent patients in terms of race/ethnicity, MSM status, percentage of clinic visits missed, HIV RNA level, and CD4 cell count.
Table 2.
Study population demographics
| Variable | Value | Total Population n = 502; n (%) |
Adherent n = 206; n (%) |
Non-Adherent n = 296; n (%) |
P Value |
|---|---|---|---|---|---|
| Age, years | < 49 | 245 (48.8) | 100 (48.5) | 145 (49.0) | .59 |
| ≥ 49 | 257 (51.2) | 106 (51.5) | 151 (51.0) | ||
| Sex/gender | Female | 112 (22.3) | 50 (24.3) | 62 (20.9) | .37 |
| Male | 381 (75.9) | 154 (74.8) | 227 (76.7) | ||
| Transgender MTF | 9 (1.8) | 2 (1.0) | 7 (2.4) | ||
| Race/ethnicity | White | 268 (53.4) | 105 (51.0) | 163 (55.1) | <.01a |
| Hispanic/Latino | 70 (13.9) | 32 (15.5) | 38 (12.8) | ||
| Black/African American | 143 (28.5) | 52 (25.2) | 91 (30.7) | ||
| American Indian/Alaskan Native | 4 (0.8) | 2 (1.0) | 2 (0.7) | ||
| Asian | 17 (3.4) | 15 (7.3) | 2 (0.7) | ||
| MSM statusb | Non-MSM | 231 (46.0) | 110 (53.4) | 121 (40.9) | .01 |
| MSM | 271 (54.0) | 96 (46.4) | 175 (59.1) | ||
| Distance from clinic, miles | < 15 | 402 (80.1) | 171 (83.0) | 231 (78.0) | .30 |
| 15–70 | 62 (12.4) | 20 (9.7) | 42 (14.2) | ||
| > 70 | 38 (7.6) | 15 (7.3) | 23 (7.8) | ||
| Number of clinic visits/year | < 3 | 255 (50.8) | 105 (51.0) | 150 (50.7) | .95 |
| ≥ 3 | 247 (49.2) | 101 (49.0) | 146 (49.3) | ||
| Percentage of missed clinic appointments | ≤ 10% | 264 (52.6) | 131 (63.6) | 133 (44.9) | <.01 |
| > 10% | 238 (47.4) | 75 (36.4) | 163 (55.1) | ||
| Employment status | Unemployed | 174 (34.7) | 63 (30.6) | 111 (37.5) | .11 |
| Employed/Retired | 328 (65.3) | 143 (69.4) | 185 (62.5) | ||
| Insurance status and assistance programs | Uninsured | 37 (7.4) | 17 (8.3) | 20 (6.8) | .25 |
| Ever received Medicare/Medicaid | 173 (34.5) | 61 (29.6) | 112 (37.8) | ||
| Ryan White/ADAP | 204 (40.6) | 87 (42.2) | 117 (39.5) | ||
| Private Insurance Only | 88 (17.5) | 41 (19.9) | 47 (15.9) | ||
| Primary care clinic | UNMC HIV Clinic | 152 (30.3) | 63 (30.6) | 89 (30.1) | .61 |
| Other UNMC Clinic | 112 (22.3) | 50 (24.3) | 62 (20.9) | ||
| Non-UNMC Clinic | 238 (47.4) | 93 (45.1) | 145 (49.0) | ||
| Most recent CD4 count, cells/mm3 | > 200 | 471 (93.8) | 204 (99.0) | 267 (90.2) | <.01 |
| ≤ 200 | 31 (6.2) | 2 (1.0) | 29 (9.8) | ||
| Most recent HIV RNA level, copies/mL | ≤ 50 | 437 (87.1) | 187 (90.8) | 250 (84.5) | .04 |
| > 50 | 65 (12.9) | 19 (9.2) | 46 (15.5) |
Data are presented as n (%). P-values are based on univariate analysis.
Abbreviations: Transgender MTF = Transgender Male to Female; MSM = Men who have sex with men
aP-value indicates a higher percentage of Asian population in the Adherent cohort.
bMSM was used to delineate eligibility for vaccination with HepA as defined in the 2018 CDC Immunization Guidelines.
In multivariable analysis, patients who identified as MSM and who had >10% missed clinic visits were associated with twofold lower adherence to the vaccination schedule. Additionally, those with most recent CD4 cell count <200 cells/mm3 were approximately ninefold less likely to be up to date on all vaccinations (Table 3). Data for adherence to individual vaccinations are visualized in Figure 1.
Table 3.
Adjusted odds ratios of non-adherence by predictor variable
| Variable | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|
| Age ≥ 49 years | 1.28 (0.86–1.92) | .23 |
| Male sex at birth | 0.78 (0.45–1.36) | .38 |
| Nonwhite race | 0.87 (0.58–1.31) | .50 |
| MSM | 2.03 (1.26–3.27) | <.01 |
| Distance ≥ 15 miles | 1.57 (0.95–2.58) | .08 |
| Office visits ≥ 3 per year | 1.12 (0.76–1.65) | .56 |
| Missed appointments > 10% | 2.21 (1.48–3.29) | <.01 |
| Currently employed | 0.78 (0.51–1.19) | .25 |
| Currently insured/receiving assistancea | 1.18 (0.55–2.53) | .67 |
| PCP outside of HIV clinic | 1.13 (0.90–1.41) | .29 |
| CD4 ≤ 200 cells/mm3* | 9.44 (2.14–41.57) | <.01 |
| HIV RNA > 50 copies/mL | 1.30 (0.70–2.41) | .40 |
P-values are based on multivariable logistic regression. Abbreviations: MSM = men who have sex with men; PCP = primary care provider
aDefined as private health insurance, Medicare or Medicaid, Ryan White or ADAP assistance, or ADAP-facilitated health insurance.
Figure 1.
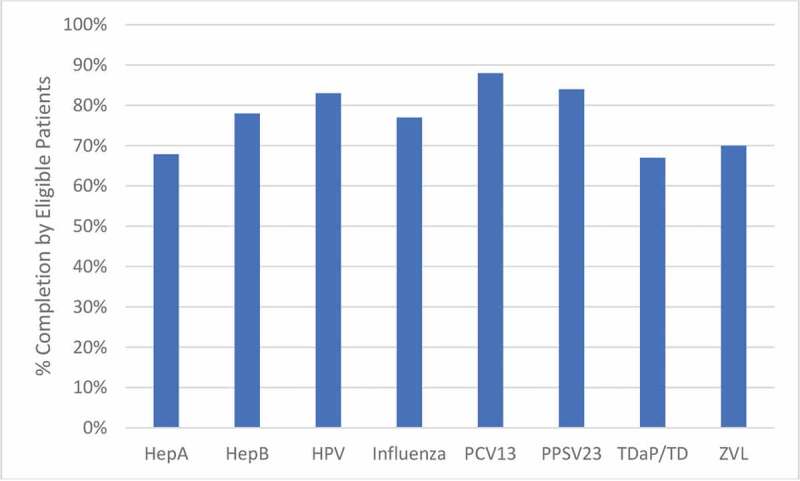
Percentage of eligible patients that received each vaccine. All patients (n = 502) were eligible for vaccination with Hepatitis B (HepB), Influenza, Pneumococcal Conjugate (PCV13), and Tetanus, Diptheria, with/without acellular pertussis (TDaP/TD) vaccines. The number of eligible patients for other vaccines is as follows: Hepatitis A (HepA): 271; Human Papillomavirus (HPV): 48; Pneumococcal Polysaccharide (PPSV23): 452; Zoster Vaccine Live (ZVL): 70
Discussion
We found nearly 60% of PLWH who had been attending our clinic for at least 1 year to have a delay or deficiency in the 2018 CDC-recommended vaccination regimen, even after excluding two recent vaccine recommendations. Vaccination rates were comparable to previously reported rates for HAV, HBV, and influenza vaccination in PLWH.14–18 A previous study has reported an association of lack of insurance coverage, low education level, and financial stressors with decreased vaccination rates.20 Here, we identify three previously unreported patient factors associated with vaccination non-adherence: a high frequency of missed clinic appointments (>10%), MSM status, and a CD4 cell count ≤200 cells/mm.3
Patients with a high frequency of missed clinic appointments (>10%) were less likely to receive vaccinations for which they were eligible compared to patients who kept their clinic appointments (missed ≤10% of appointments). Patients with missed clinic appointments had a lower number of visits to a health-care provider and fewer opportunities for vaccine administration. An additional barrier in this subpopulation is that the health-care provider may prioritize higher acuity medical issues over vaccine administration when these patients are present for scheduled visits. With competing priorities and limited visit time, vaccines are often deferred to subsequent visits. Our study highlights the importance of provider vigilance in assessing vaccine eligibility and ensuring their administration at the earliest available opportunity.
The MSM cohort displayed greater non-adherence to the CDC’s recommended vaccination schedule when compared to the non-MSM cohort, representing another health disparity faced by MSM living with HIV. This represents an important finding as MSM living with HIV are at even greater risk for certain vaccine-preventable disease than other individuals with HIV. HAV, HBV, HPV, and meningococcal disease are all more prevalent in MSM living with HIV due to transmission risks, further highlighting the importance of vaccinations in this patient population. Adoption of strategies to limit health disparities in the MSM population could prove useful in improving vaccination rates.
Our subset of patients with CD4 cell count less than 200 cells/mm3 was significantly less likely to receive all vaccines for which they were eligible compared to patients with CD4 cell counts greater than 200 cells/mm,3 even when controlling for other studied variables. Prior to our study, Nebraska Medicine’s clinical protocol was to delay non-live virus vaccinations, pneumococcal and hepatitis B vaccines specifically, in PLWH with CD4 count ≤200 cells/mm3 until the patients achieved immune reconstitution (as defined by CD4 > 200 cells/mm3) to increase the likelihood of an effective vaccine response. This delay in administration likely contributed to lower vaccination rates in this cohort. A recent, practice-changing study from Malawi tested the PCV7 vaccine in adults living with HIV in a randomized, double-blind, placebo-controlled fashion.21 The study demonstrated 74% clinical efficacy at preventing vaccine-type invasive pneumococcal disease, with similar efficacy even among those with CD4 counts <200 cells/mm.3 A 2014 review article on HIV vaccination guidelines concluded patients living with HIV should be vaccinated with PCV regardless of the CD4 count.22 In contrast to the 2018 European AIDS Clinical Society (EACS) guidelines,23 CDC recommendations for other non-live virus vaccinations also recommend earlier vaccination in PLWH, regardless of CD4 count. Earlier vaccination also provides protection to patients at-risk for missed clinic appointments and those that are lost to follow-up. These factors support the utility of earlier vaccination in PLWH with low CD4 counts, despite historic provider concerns that these patients may not mount as effective of an immune response to vaccination. We suggest earlier vaccination, when clinically appropriate, and periodically reassessing local clinical protocols to ensure they reflect the most up to date recommendations in the vaccination guidelines and most recent literature.
Our single-center, retrospective study has limitations which must be considered. Patient deaths or transfers of HIV-specific care to outside facilities during the study period may have contributed to lower vaccination rates if this information was not recorded in the EHR. Information on provider-offered and patient-declined vaccinations, vaccine coverage by individual insurance plans, and availability of specific vaccines within the clinic at each time-point was not consistently available. Furthermore, not all insurances covered all recommended vaccines for clinic administration and may account for some degree of nonadherence. These patients may have received these vaccines at outside facilities (community pharmacies) and this documentation would have been captured in the Nebraska State Immunization Information System (NESIIS) if the outside facility was registered with NESIIS. Additionally, a large proportion of our patient population received support from the Ryan White HIV/AIDS Program, which covers all recommended vaccines. Nevertheless, true vaccination rates may be higher than depicted due to the retrospective nature of the study and limitations of the electronic health record.
PLWH remain disproportionately susceptible to vaccine-preventable illnesses compared to the general population, however, factors influencing vaccination rates in PLWH have been infrequently reported. Our study reveals that a high percentage of missed clinic appointments (>10%), MSM status, and CD4 cell counts ≤200 cells/mm3 were associated with decreased vaccination rates in PLWH. Awareness of these patient-specific variables, combined with knowledge of local clinical vaccination protocols, may be important in identifying patients at risk for under-vaccination and provide for targeted education programs for both providers and patients.
Acknowledgments
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure of potential conflicts of interest
The authors declare no relevant conflicts of interest or financial relationships.
References
- 1.Kim DK, Riley LE, Hunter P.. Recommended immunization schedule for adults aged 19 years or older. Ann Intern Med. 2018;168(3):210–20. doi: 10.7326/M17-3439. [DOI] [PubMed] [Google Scholar]
- 2.Umutesi J, Simmons B, Makuza JD, Dushimiyimana D, Mbituyumuremyi A, Uwimana JM, Ford N, Mills EJ, Nsanzimana S. Prevalence of hepatitis B and C infection in persons living with HIV enrolled in care in Rwanda. BMC Infect Dis. 2017;17(1):315. doi: 10.1186/s12879-017-2422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tengan FM, Abdala E, Nascimento M, Bernardo WM, Barone AA. Prevalence of hepatitis B in people living with HIV/AIDS in Latin America and the Caribbean: a systematic review and meta-analysis. BMC Infect Dis. 2017;17(1):587. doi: 10.1186/s12879-017-2695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Truong HM, Fatch R, Do TD, McFarland W. Hepatitis B vaccination and infection prevalence among men who have sex with men who travel internationally. Sex Transm Dis. 2018;45(5):e25–e28. doi: 10.1097/OLQ.0000000000000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornton AC, Jose S, Bhagani S, Chadwick D, Dunn D, Gilson R, Main J, Nelson M, Rodger A, Taylor C, et al. Hepatitis B, Hepatitis C, and mortality among HIV-positive individuals. AIDS. 2017;31(18):2525–32. doi: 10.1097/QAD.0000000000001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ucciferri C, Tamburro M, Falasca K, Sammarco ML, Ripabelli G, Vecchiet J. Prevalence of anal, oral, penile and urethral human Papillomavirus in HIV infected and HIV uninfected men who have sex with men. J Med Virol. 2018;90(2):358–66. doi: 10.1002/jmv.24943. [DOI] [PubMed] [Google Scholar]
- 7.Ho A, Aston SJ, Jary H, Mitchell T, Alaerts M, Menyere M, Mallewa J, Nyirenda M, Everett D, Heyderman RS, et al. Impact of HIV on the burden and severity of influenza illness in Malawian adults: a prospective cohort and parallel case-control study. Clin Infect Dis. 2018;66(6):865–76. doi: 10.1093/cid/cix903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez KM, Khalili J, Trevillyan J, Currier J. What is the best model for HIV primary care? Assessing the influence of provider type on outcomes of chronic comorbidities in HIV. J Infect Dis. 2018;218(2):337–39. doi: 10.1093/infdis/jiy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maude R, Volpe G, Stone D. Knowledge, Attitude, and Practice of Pre-exposure prophylaxis (PrEP) against HIV infection of medical providers at an academic center. Open Forum Infect Dis. 2017;4(1):S437. doi: 10.1093/ofid/ofx163.1106. [DOI] [Google Scholar]
- 10.Wood BR, McMahan VM, Naismith K, Stockton JB, Delaney LA, Stekler JD. Knowledge, practices, and barriers to HIV pre-exposure prophylaxis (PrEP) prescribing among Washington state medical providers. Sex Transm Dis. 2018;45(7):452–58. doi: 10.1097/OLQ.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 11.Aziz M, Kessler H, Huhn G. Providers’ lack of knowledge about herpes zoster in HIV-infected patients is among barriers to herpes zoster vaccination. Int J STD AIDS. 2013;24(6):433–39. doi: 10.1177/0956462412472461. [DOI] [PubMed] [Google Scholar]
- 12.Hurley LP, Bridges CB, Harpaz R, Allison MA, O’Leary ST, Crane LA, Brtnikova M, Stokley S, Beaty BL, Jimenez-Zambrano A, et al. Physician attitudes toward adult vaccines and other preventive practices. Public Health Rep. 2016;131(2):320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moyroud L, Hustache S, Goirand L, Hauzanneau M, Epaulard O. Negative perceptions of hepatitis B vaccination among attendees of an urban free testing center for sexually transmitted infections in France. Hum Vaccin Immunother. 2017;13(5):998–1004. doi: 10.1080/21645515.2016.1264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison N, Poeppl W, Herkner H, Tillhof KD, Grabmeier-Pfistershammer K, Rieger A, Forstner C, Burgmann H, Lagler H. Predictors for and coverage of influenza vaccination among HIV-positive patients: a cross-sectional survey. HIV Med. 2017;18(7):500–06. doi: 10.1111/hiv.12483. [DOI] [PubMed] [Google Scholar]
- 15.Weiser J, Perez A, Bradley H, King H, Shouse RL. Low prevalence of hepatitis b vaccination among patients receiving medical care for HIV infection in the United States, 2009 to 2012. Ann Intern Med. 2018;168(4):245–54. doi: 10.7326/M17-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tedaldi EM, Baker RK, Moorman AC, Wood KC, Fuhrer J, McCabe RE, Holmberg SD. Hepatitis A and B vaccination practices for ambulatory patients infected with HIV. Clin Infect Dis. 2004;38:1478–84. doi: 10.1086/420740. [DOI] [PubMed] [Google Scholar]
- 17.Bailey CL, Smith V, Sands M. Hepatitis B vaccine: A seven-year study of adherence to the immunization guidelines and efficacy in HIV-1-positive adults. Int J Infect Dis. 2008;12(6):e77–e83. doi: 10.1016/j.ijid.2008.05.1226. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher KM, Juhasz M, Harris NS, Teshale EH. Predictors of influenza vaccination in HIV-infected patients in the United States, 1990–2002. J Infect Dis. 2007;196:339–46. doi: 10.1086/519165. [DOI] [PubMed] [Google Scholar]
- 19.Sticchi L, Bruzzone B, Caligiuri P, Rappazzo E, Lo Casto M, De Hoffer L, Gustinetti G, Viscoli C, Di Biagio A. Seroprevalence and vaccination coverage of vaccine-preventable diseases in perinatally HIV-1-infected patients. Hum Vaccin Immunother. 2015;11(1):263–69. doi: 10.4161/hv.36162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsachouridou O, Georgiou A, Naoum VD, Papagianni M, Kotoreni G, Forozidou E, Tsoukra P, Gogou C, Chatzidimitriou D, Skoura L, et al. Factors associated with poor adherence to vaccination against hepatitis viruses, streptococcus pneumoniae and seasonal influenza in HIV-infected adults. Hum Vaccin Immunother. 2019;15(2):295–304. doi: 10.1080/21645515.2018.1509644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, Mwaiponya M, Zijlstra EE, Molyneux ME, Gilks CF. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. New Eng J Med. 2010;362(9):812–22. doi: 10.1056/NEJMoa0903029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crum-Cianflone NF, Wallace MR. Vaccination in HIV-Infected Adults. AIDS Patient Care STDS. 2014;28(8):397–410. doi: 10.1089/apc.2014.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behrens G, Pozniak A, Puoti M, Miro JM. European AIDS clinical society guidelines version 9.1. 2018.


