ABSTRACT
Bacterial infection is one of the most common and serious diseases. Extracellular vesicles (EVs) expressed by bacterial cells during infection and their biological functions have been a growing field in recent years. The study of the immune interaction mechanism between EVs and bacteria has become more significant. EVs are released into the extracellular microenvironment during bacterial infection. EVs carry various lipids, proteins, nucleic acids, and other substances of host bacteria and participate in various physiological and pathological processes. EV-based vaccines against bacterial infection are also being evaluated. This review focuses on the biological characteristics of EVs, the interaction between EVs and the host immune system, and the potential of EVs as new vaccines. A deeper understanding of the interaction between EVs and the immune system informs on the biological function and heterogeneity of EVs. This knowledge also can facilitate the development and application of EVs and their potential as vaccines.
KEYWORDS: EVs vaccine active immunity
Introduction
EVs are involved in a range of physiological and pathological processes, such as angiogenesis, tumor generation, and tumor immune response,1 which not only maintain the body’s homeostasis but also participate in the development of various diseases. In pathogen infection, EVs could transmit infectious substances such as proteins and RNAs, which had strong immunogenicity to causes immune responses, further changed the function of uninfected cells and affected the interaction between pathogens and host cells.2In brief, EVs acted as a spherical lipid bilayer to deliver the immune antigen of bacterium. Nowadays, EVs are attracting more and more attention as a new vaccine.
This review briefly introduces the relationship between bacterial EVs and the immune system, more importantly, the potential of EVS as an emerging vaccine is highlighted.
1. Biogenesis of bacterial extracellular vesicles
EVs are important intercellular communication agents in the intercellular space and which reach target cells through blood circulation. EVs were first thought to be useless particles (“garbage bags” and “pure cell products”) or waste products made during bacterial cell growth, metabolism, and death.3 However, EVs are now considered the third intercellular communication channel besides intercellular connections and soluble molecules,4 targeting the host’s cells with bioactive substances to regulate the expression of genes and affecting cell proliferation, invasion, and death.5 EVs represent the total vesicles secreted by bacterial cells and are divided into three categories according to size: 1) microvesicles from budding cell membranes, diameter of 100–1000 nm; 2) apoptotic bodies secreted during apoptosis, diameter of 1000–5000 nm; and 3) exosomes formed and germinated inwards by early endosome cavitation, originating from the polyvesicular body, produced by membrane fusion, and released from cells, diameter of 40–100 nm.6
EVs were secreted without affecting the integrity of bacteria, which were spherical structure wrapped by a lipid bilayer. This structure was mainly composed of the outer membrane and periplasmic substances, which were a mixture of bioactive molecules, including proteins, nucleic acids, phospholipids, and lipopolysaccharides (LPS), as well as other substances such as ions, metabolites, and signaling molecules7 (Figure 1). Nucleic acids mainly included sRNA, miRNAs, mRNAs, and non-coding RNAs.8 Based on EVs, sRNA was transmitted to interacting microorganisms to inhibit infection, which provided an innovative method for the control of pathogens, especially fungi, in agriculture and biomedicine.9 These transcriptional materials were completely encapsulated in extracellular vesicles and translated, thus affecting the expression of host cell genes, EVs after Akt gene modification promoted the expression of platelet-derived growth factor-D.10 When infected by various pathogens, such as bacteria, fungi, and viruses, they would release EVs to play an important role, what is more, Gram-positive bacteria EVs could be secreted into the extracellular environment through thick walls.11,12 When EVs were released, they were not immediately engulfed by the surrounding cells, which was an important safeguard for long-distance signal communication.13,14Although this interesting phenomenon has been well known for many years, EVs still lacked systematic description, which were still in the process of in-depth characterization15,16.
Figure 1.
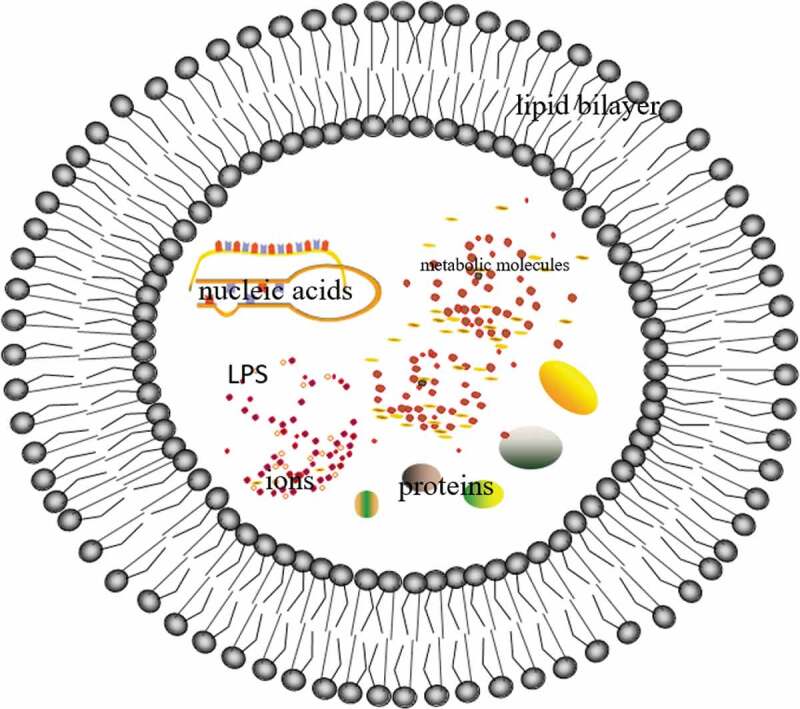
EVs is a spherical structure enclosed by a lipid bilayer, which contains proteins, nucleic acids, phospholipids, and lipopolysaccharides (LPS), as well as other substances such as ions, metabolic molecules, and signaling molecules
The current data suggested that EVs had complex and highly dynamic structures that had not received enough attention in the past.17 Due to different cells, tissues, and physiological conditions, EVs varied greatly. The plasticity of EVs allowed them to adapt to changes in external environment, which suggested that they had important biological functions, and intercellular communication within the host is a research hotspot.18,19 Studying and understanding EVs would challenge conventional notions of cellular communication and open up new opportunities in areas of translational medicine.
2. The interaction of bacteria EVs with the immune system
As a heterogeneous, protein-rich, and lipid-rich molecular carrier, EVs were capable to release substances at the extracellular, activating the host’s innate and acquired immune response pathways.20 EVs carried pathogen-associated molecular patterns (PAMP) that bound to pattern recognition receptors (PRR) to mediate the production of inflammatory factors. LPS and lipoprotein in EVs were ligands of TLR4 and TLR2 receptors,21 respectively. ClyA in EVs of Escherichia coli activated TLR4 pathway.22 Peptidoglycan in the EVs of Helicobacter pylori activated immune responses through the cell peptidoglycan sensor NOD1.23 EVs of Pseudomonas aeruginosa activated atypical inflammation in human monocytes through caspase-5 and induced activation of inflammasomes in mouse macrophages, manifested by “spot” formation, as well as secretion of IL-1β and cell death.24
EVs were the “hub” and “scaffold” of bacterial extracellular activities which could transport various virulence factors at the time of latent infection, leading to changes in the function of host cells and helping the bacteria escape from the killing of the monocyte-macrophage system. EVs of Chlamydia muridarum contained a large amount of serine protease HtrA, which lysed cell calcarein and facilitated invasion of epithelial cells by Chlamydia muridarum.25 ClyA in EVs of Escherichia coli had much higher hydrolysis activity than ClyA monomer.26 The interaction between EVs and immune cells facilitated the uptake of EVs, the maturation of macrophages and dendritic cells, inducing the expression and releasing of inflammatory factors. LPS and outer membrane proteins in EVs stimulated antigen-presenting cells and enhanced immune response.27 The EVs of Klebsiella pneumoniae stimulated the secretion of IL-1 and IL-8 by epithelial cells.28 Escherichia coli transmitted active heat-resistant enterotoxin (LT) to epithelial cells through EVs, accompanied by the up-regulation of IL-6.29 EVs in the air was a key cause of chronic pneumonia. Long-term exposure to EVs induced chronic immune responses, leading to the development of a variety of respiratory diseases.30 Respiratory exposure to indoor dust with EVs of Escherichia coli increased the production of pro-inflammatory cytokines. Continuous inhalation of EVs for 4 weeks would lead to neutrophils inflammation and emphysema, which was shown as an increase in elastase activity, as well as the pro-inflammatory factors IFN-γ, IL-17A, and TNF-α.31
Bacteria-host interactions mediated by EVs led to multiple responses, from non-immunogenic to proinflammatory or cytotoxic. Intranasal injection of EVs derived from Pseudomonas aeruginosa mediated pulmonary inflammation through TLR 2 and TLR 4.32 EVs of Lactobacillus gasseri BC12 and Lactobacillus crispatus BC5 could protect human T cells, cervix and tonsil tissue from HIV-1 infection, and this protective effect was caused by inhibiting viral attachment and entrance of envelope proteins after EVs treatment,33 so EVs altered cell’s susceptibility by influencing the condition of cell membrane proteins.
These studies suggested that EVs had potential to develop novel vaccines because they could active the host immune response, including facilitating the T cell response, promoting B cells to secrete antibodies and mediating inflammatory responses. In addition, EVs as vaccines were easy to be recognized in the form of nano-level, with high efficiency and low toxicity.34 Bacterial EVs might be a crucial factor in the pathogenesis of specific infectious diseases, and their latent use as a vaccine to prevent infection has begun.35
3. Bacterial EVs as target of immune intervention
Vaccination is the administration of antigenic substances to produce protective immunity, which is considered the most cost-effective way to prevent infectious diseases. Vaccines should resemble the pathogen without causing the associated disease,36 it meant that they should have the right size and contain pathogen-specific antigens. EVs were very complex super-molecular structures. The appropriate size of EVs was easily processed by antigen-presenting cells. They naturally contained ingredients that stimulated the immune responses, which were similar to the bacterial antigen surfaces of pathogens and delivered to immune receptor cells to trigger maturation and activate signals. Until now EVs have been studied in animal models, but none have progressed to clinical trials.37–39
3.1. EVs promote active immunity by promoting T lymphocytes response in vivo
In vivo, Th1 cells mainly mediate cellular immunity, which secrete proinflammatory cytokines such as IFN-γ, TNF-α, and IL-2, promote the cytotoxic activity of immune cells and facilitate the proliferation of Tc cells, while Th2 cells mainly mediate humoral immunity, which secrete anti-inflammatory factors like IL-4, IL-5, IL-6, IL-10, and IL-13, promote anti-inflammatory and antibody-dependent immune response;40 Th17 cells play an important role in autoimmunity by releasing pro-inflammatory cytokines such as IL-17, IL-6, IL-22, and TNF-α41
Klebsiella pneumoniae is the most general cause of respiratory infections, and it is also the second most common cause of gram-negative bacteremia. In recent years, Klebsiella pneumoniae has become an important pathogen of nosocomial infection.42 A study had shown that34 EVs of Klebsiella pneumoniae effectively activated the innate immune response to induce specific IgG antibodies, along with the enhanced expression of IFN-γ, IL-17, and IL-4 in CD4 + T cells and a strong Th1, IFN-γ + T cell response. EVs of Klebsiella pneumoniae provided protection, and the survival rate of mice was up to 100% after EVs treatment, so preventive measures could be formulated to reduce Klebsiella pneumoniae infection based on the EVs. Helicobacter pylori is a gram-negative microaerobic, which could induce chronic gastritis, peptic ulcer, and gastric cancer.43 Oral administration of EVs derived from Helicobacter pylori caused strong humoral and mucosal immune responses, mainly manifested as the Th2 immune response and a significant reduction in bacterial load.44Lacking of whooping cough bacillus adhesion was the main reason why traditional whooping cough bacillus vaccines could not provide lasting immunity.45 EVs as new vaccines protected mice from infection by the adhesion-deficient strain of bacillus pertussis through the activation of INF-γ+/IL-17 + T and CD4 + T memory cells in respiratory tract tissues effectively and persistently.46 Staphylococcus aureus is one of the major pathogens of nosocomial infections.47 EVs of staphylococcus aureus contained peptidoglycan, lipoic acid, and many pathogenic molecules,48 which promoted the production of antibodies. In addition, staphylococcus aureus-derived EVs prevented pneumonia infection through the Th1 cell response mostly.49 After co-culture with EVs derived from Shigella freund, the levels of MHC-II and co-stimulating molecule CD40 of macrophages were increased, above all, EVs enhanced adaptive immunity by acting on antigen-presenting cells, which was particularly important in the vaccination process, further toxicity studies in rats also demonstrated the safety of this kind of vaccine.50 Therefore, EVs had the ability to induce protective immunity, promote the up-regulation of co-stimulus molecules, and the expression of cytokines.51,52
In summary, EVs affected the expression levels of inflammatory and anti-inflammatory factors by affecting the reaction of Th1/Th2/Th17, thus improving the active immunity of the body. The EV-based vaccines amplified the ability to fight pathogens through active immunity, which played an important role in the prevention and treatment of bacterial infections.
3.2. EVs not only immunize the same-origin strains but also cross-protect the heterogeneous strains
A kind of bacteria might cause a wide range of diseases, and different diseases had respective mechanisms and protective antigens. Traditional vaccines usually targeted a sort of disease, while EVs could be used as new vaccines to prevent multiple diseases, providing all-round protection.
EVs of Acinetobacter baumannii showed strong responses to a variety of outer membrane proteins, and bacterial load was significantly reduced after EVs treatment.53,54 Shigella freund, particularly serotype 2a, caused the highest prevalence of bacterial dysentery in developing countries.55 EVs derived from Shigella fregii type 2a induced protective immune responses against the lethal attack of homologous strains.56 Neisseria gonorrhoeae is a highly adaptable human pathogen that can be transmitted sexually.57 Female mice were immunized intravaginally with EVs, then attacked by a model of Neisseria gonorrhoeae genital infection. EVs could clear the homologous gonorrhoeae strains within 6–9 d, depending on the production of antibodies and IFN-γ. A significant protective effect also existed after the attack with different strains, and this immunologic memory lasted at least 6 months.58 EVs isolated from nonpathogenic Streptococcus pneumoniae not only protected it from attack by homologous strains but also provided cross-protection against pathogenic heterologous strains.59 Brucella used stealth strategies to evade the host’s innate and adaptive immune responses, then establishing persistent and chronic infections.60 EVs induced specific anti-brucella-mediated immune response, which prevented infection in mouse models and had a protective effect against wild-type infection.61
Therefore, the potential of EVs as new vaccines were not only limited to the protection of homologous strains but also offered the cross-protection for heterologous strains. In the case of effective induction of protective immunity, EVs could reduce the bacterial load and improve the efficiency of active immunity, so as to realize “one kind of vaccine against multiple strains.”
3.3. EVs as an important part of vaccine enhances immunity and promotes safety
Vaccines are immune substances that induce to produce positive protective effects, which are specific to infectious pathogens and toxins. The effectiveness determines the success or failure of the product.62 Immunization is the most effective measure to prevent and control of communicable diseases. Vaccination population is mostly healthy population, especially with children and infants mainly, so the safety problem is particularly important. The safety and effectiveness of are two key factors that determine the success of vaccines.
Haemophilus influenzae is an important pathogen of upper respiratory tract infection.63 The virulence factor D protein in Haemophilus influenzae was highly immunogenic and genetically conserved, so it was commonly used to design vaccines.64 The IgG response after treatment of the combination of D-protein and EVs was significantly stronger than that of D-protein alone, in other words, EVs, as an adjuvant, enhanced the immunogenicity of D-protein.65 Adjuvants injected in advance could enhance immunogenicity or alter the type of immune response.66 Byoppertussis bordetella can cause respiratory problems, and its symptoms are similar to pertussis.67 The O-antigen associated with the EVs was an important part of the prevention of Byoppertussis bordetella infection.68
Shiga toxin-producing strains of Escherichia coli (STEC) might cause severe hemolytic uremic syndrome (HUS), leading to chronic renal failure.69 EVs had protective effect in mouse model and immunogenicity in calf, which were immune neutralization of toxins.70 EVs isolated from nonpathogenic Streptococcus pneumoniae protected mice from infection by pathogenic Streptococcus pneumoniae strains. Even when co-cultured with cells at high concentrations of EVs, the cell viability and apoptosis rate remained unchanged, so the safety of the vaccine was guaranteed.59 Moreover, Acinetobacter baumannii strains which lacked LPS could also produce EVs, which provided protective immunity against Acinetobacter baumannii, further ensuring the safety of EVs.71
Above, through continuous research on EVs in the field of vaccine, its safety and immune efficacy would be gradually improved. EVs as vaccines have great potential in bacterial infections. Therefore, screening and identifying safe and effective EVs have great significance in the search for more effective bacterial vaccines.
4. The superiority of Bacterial EVs as a new type of vaccine
The classic live attenuated and inactivated vaccines were the basis for the development of bacterial vaccines. However, these two kinds of vaccines had complex components, and there were substances that caused immune side effects. In other words, the safety and efficacy needed to be further improved. With the deepening of the understanding of specific pathogenic components of bacteria, the development of component vaccines had emerged and rapidly became a research hotspot in the field of bacterial vaccines. The main pathogenic components were polysaccharides and proteins. Because vesicle proteins required the internalization of macrophages, the pro-inflammatory immune response induced by EVs was stronger than that induced by LPS.72 Therefore, EVs had a stronger ability to induce active immunity than pathogenic agents such as LPS. A kind of bacterium always caused a wide range of diseases, but it usually infected multiple organs and its pathogenesis was different. Component vaccines usually targeted only one organ or tissue or disease. The pertussis vaccine was a classic vaccine as whole-cell inactivation. However, in the 1990s, it was largely replaced by acellular pertussis vaccine in developed countries, but this acellular pertussis vaccine was less reactive and they did not effectively reduce bacterial infection.73 DNA vaccines could clone multiple target genes on the same plasmid carrier to achieve to prevent multiple diseases. DNA vaccines had attracted a lot of attention from scientists, but they were still being explored. With the development of electron microscopy, EVs have been proved to be secreted into the extracellular environment through thick walls, which were used as a carrier to enrichment cavity material degradation and protect them from extracellular.11
Compared with traditional vaccines, EV-based vaccines have the following advantages: the appropriate size allows them to be easily processed by antigen-presenting cells;74 EVs naturally contain components that stimulate the body’s immune response,29 all in one go, overcoming the defect of single-antigen target; the use of EVs eliminates the possibility of live cell vaccine activation, which guarantees the safety to some extent; with the development of proteins and transcriptomics, the chemical components of EVs have become increasingly clear, so their application prospect is very attractive, which will provide a more effective weapon for the control of infectious diseases.
EVs have great potential as new vaccines in bacterial infection (Figure 2), which also are proved to be more diverse than originally thought. How to find the EVs with good immunogenicity, how to choose the immune pathway, how to evaluate the safety must be considered among them. As a result, we face significant challenges in developing new vaccines.
Figure 2.

EVs vaccine can affect the immune response of T lymphocytes to change the levels of various cytokines in the body for active immunity; EVs vaccine can not only protect homologous strains, but also cross-protect heterologous strains; EVs vaccine can also be used as an adjuvant or an important part of the vaccine to enhance the effect of active immunity and further enhance its safety
5. Summary and Prospect
As a new vaccine inoculation reagent, EVs had their unique advantages over traditional vaccines. The lipid seal structure of EVs maintained the stability of protein in the circulation of the blood. Moreover, the specific lipid components (such as sphingomyelin and cholesterol) helped the fusion of EVs and host’s cell membranes. Their nanoscale size and composition reduced the probability of being recognized by mononuclear phagocytic system, further avoiding ineffective vaccines. EVs were widely found in biological fluids and remained stable in the blood circulation, which persisted during infection. These characteristics made EVs become an excellent biomarker and potential vaccine inoculation reagent. Due to the existence of immunogenicity proteins and pathogenic molecules in EVs, therefore, EVs have immunogenicity and helper effects, causing they become a new target for vaccine research. Further researches about the potential of EVs as vaccines might advance the field of “extracellular vesicles and immunology.” Studies of the properties in EVs promoted research on related applications, including disease diagnosis, treatment, vaccines, and so on. However, the existing research methods and means had become the bottleneck of clinical translational application, which needed to be further improved.
Deep researches of the potential of EVs in vaccines, on the one hand, it helps us understand the biological function and heterogeneity of EVs preferably; on the other hand, it is beneficial to the modification, clinical application, and engineering and standardization of EVs. With the deepening of research and the innovation of methodology, these problems will be gradually solved in the future.
Funding Statement
This study was supported by Clinical Medical Technology Innovation Guidance Project of Hunan Provincial Finance Department (grant no. 2017SK50122).
References
- 1.Kegelman TP, Das SK, Emdad L, Hu B, Menezes ME, Bhoopathi P, Wang X-Y, Pellecchia M, Sarkar D, Fisher PB.. Targeting tumor invasion: the roles of MDA-9/Syntenin. Expert Opin Ther Targets PMID:25219541. 2015;19(1):97–112. doi: 10.1517/14728222.2014.959495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev PMID:22722893. 2012;64:676–705. doi: 10.1124/pr.112.005983.. [DOI] [PubMed] [Google Scholar]
- 3.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol PMID:19144520. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol PMID:23420871. 2013;200:373–83. doi: 10.1083/jcb.201211138.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic MM, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. Tumour exosome integrins determine organotropic metastasis. Nature PMID:26524530. 2015;527:329–35. doi: 10.1038/nature15756.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol PMID:19692638. 2009;183:3720–30. doi: 10.94049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Wu XH, Wang D, Luo CL, Chen LX. Bladder cancer cell-derived exosomes inhibit tumor cell apoptosis and induce cell proliferation in vitro. Mol Med Rep PMID:23969721. 2013;8:1272–78. doi: 10.3892/mmr.2013.1634. [DOI] [PubMed] [Google Scholar]
- 8.Lécrivain AL, Beckmann BM. Bacterial RNA in extracellular vesicles: A new regulator of host-pathogen interactions? Biochim Biophys Acta Gene Regul Mech PMID:32142907. 2020;1863:194–519. doi: 10.1016/j.bbagrm.2020.194519. [DOI] [PubMed] [Google Scholar]
- 9.Cai Q, He B, Weiberg A, Buck AH, Jin H. Small RNAs and extracellular vesicles: new mechanisms of cross-species communication and innovative tools for disease control. PLoS Pathog PMID:31887135. 2019;15:e1008090. doi: 10.1371/journal.ppat.1008090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, Qian H, Xu W, Zhu W. Exosomes Derived from Akt -Modified Human Umbilical Cord Mesenchymal Stem Cells Improve Cardiac Regeneration and Promote Angiogenesis via Activating Platelet-Derived Growth Factor D. Stem Cells Transl Med PMID:28170176. 2017;6(1):51–59. doi: 10.5966/sctm.2016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol PMID:26324094. 2015;13:620–30. doi: 10.1038/nrmicro3480.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH, Lee J, Park J, Gho YS. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol PMID:25704309. 2015;40:97–104. doi: 10.1016/j.semcdb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol PMID:19442504. 2009;21:575–81. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Tkach M, Théry C. Communication by Extracellular Vesicles: where We Are and Where We Need to Go. Cell PMID:26967288. 2016;164:1226–32. doi: 10.1016/j.cell.2016.01.043.. [DOI] [PubMed] [Google Scholar]
- 15.Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int J Mol Sci. 2017. 10.3390/ijms18061153. PMID:28555055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piotrowska M, Ciura K, Zalewska M, Dawid M, Correia B, Sawicka P, Lewczuk B, Kasprzyk J, Sola L, Piekoszewski W, et al. Capillary zone electrophoresis of bacterial extracellular vesicles: A proof of concept. J Chromatogr A. 2020. PMID:32197757. 10.1016/j.chroma.2020.461047 [DOI] [PubMed] [Google Scholar]
- 17.Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013. 10.3402/jev.v2i0.20389. PMID:24009890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macia L, Nanan R, Hosseini-Beheshti E, Grau GE. Host- and Microbiota-Derived Extracellular Vesicles, Immune Function, and Disease Development. Int J Mol Sci. 2019. 10.3390/ijms21010107. PMID:31877909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagnelie MA, Corvec S, Khammari A, Dréno B. Bacterial extracellular vesicles: A new way to decipher host-microbiota communications in inflammatory dermatoses. Exp Dermatol PMID:31633842. 2020;29:22–28. doi: 10.1111/exd.14050.. [DOI] [PubMed] [Google Scholar]
- 20.Roier S, Zingl FG, Cakar F, Schild S. Bacterial outer membrane vesicle biogenesis: a new mechanism and its implications. Microb Cell PMID:28357362. 2016;3:257–59. doi: 10.15698/mic2016.06.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol PMID:12524386. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni HM, Nagaraj R, Jagannadham MV. Protective role of E. coli outer membrane vesicles against antibiotics. Microbiol Res PMID:26640046. 2015;181:1–7. doi: 10.1016/j.micres.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Thay B, Damm A, Kufer TA, Wai SN, Oscarsson J. Aggregatibacter actinomycetemcomitans outer membrane vesicles are internalized in human host cells and trigger NOD1- and NOD2-dependent NF-kappaB activation. Infect Immun PMID:25024364. 2014;82:4034–46. doi: 10.1128/IAI.01980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bitto NJ, Baker PJ, Dowling JK, Wray-McCann G, De Paoli A, Tran LS, Leung PL, Stacey KJ, Mansell A, Masters SL, et al. Membrane vesicles from Pseudomonas aeruginosa activate the noncanonical inflammasome through caspase-5 in human monocytes. Immunology and Cell Biology PMID:30003588. 2018;96:1120–30. doi: 10.1111/imcb.12190. [DOI] [PubMed] [Google Scholar]
- 25.Bartolini E, Ianni E, Frigimelica E, Petracca R, Galli G, Berlanda SF, Norais N, Laera D, Giusti F, Pierleoni A, et al. Recombinant outer membrane vesicles carrying Chlamydia muridarum HtrA induce antibodies that neutralize chlamydial infection in vitro. J Extracell Vesicles. 2013. PMID:24009891. 10.3402/jev.v2i0.20181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gujrati V, Kim S, Kim S-H, Min JJ, Choy HE, Kim SC, Jon S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano PMID:24410085. 2014;8:1525–37. doi: 10.1021/nn405724x. [DOI] [PubMed] [Google Scholar]
- 27.Bobat S, Flores-Langarica A, Hitchcock J, Marshall JL, Kingsley RA, Goodall M, Gil-Cruz C, Serre K, Leyton DL, Letran SE, et al. Soluble flagellin, FliC, induces an Ag-specific Th2 response, yet promotes T-bet-regulated Th1 clearance of Salmonella typhimurium infection. Eur J Immunol PMID:21469112. 2011;41:1606–18. doi: 10.1002/eji.201041089. [DOI] [PubMed] [Google Scholar]
- 28.Turner KL, Cahill BK, Dilello SK, Gutel D, Brunson DN, Alberti S, Ellis TN. Porin Loss Impacts the Host Inflammatory Response to Outer Membrane Vesicles of Klebsiella pneumoniae. Antimicrob Agents Chemother PMID:26666932. 2015;60:1360–69. doi: 10.1128/AAC.01627-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev PMID:20197500. 2010;74:81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Kim EK, Park HJ, McDowell A, Kim YK. The impact of bacteria-derived ultrafine dust particles on pulmonary diseases. Exp Mol Med PMID:32203101. 2020;52:338–47. doi: 10.1038/s12276-019-0367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YS, Lee WH, Choi EJ, Choi JP, Heo YJ, Gho YS, Jee YK, Oh YM, Kim YK. Extracellular vesicles derived from Gram-negative bacteria, such as Escherichia coli, induce emphysema mainly via IL-17A-mediated neutrophilic inflammation. J Immunol PMID:25716999. 2015;194:3361–68. doi: 10.4049/jimmunol.1402268. [DOI] [PubMed] [Google Scholar]
- 32.Park K-S, Lee J, Jang SC, Kim SR, Jang MH, Lötvall J, Kim Y-K, Gho YS. Pulmonary inflammation induced by bacteria-free outer membrane vesicles from Pseudomonas aeruginosa. American Journal of Respiratory Cell and Molecular Biology PMID:23713467. 2013;49:637–45. doi: 10.1165/rcmb.2012-0370OC. [DOI] [PubMed] [Google Scholar]
- 33.Nahui PRA, Vanpouille C, Laghi L, Parolin C, Melikov K, Backlund P, Vitali B, Margolis L. Extracellular vesicles from symbiotic vaginal lactobacilli inhibit HIV-1 infection of human tissues. Nat Commun. 2019. 10.1038/s41467-019-13468-9. PMID:31827089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee WH, Choi HI, Hong SW, Kim KS, Gho YS, Jeon SG. Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp Mol Med PMID:26358222. 2015;47:e183. doi: 10.1038/emm.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unal CM, Schaar V, Riesbeck K. Bacterial outer membrane vesicles in disease and preventive medicine. Seminars in Immunopathology PMID:21153593. 2011;33:395–408. doi: 10.1007/s00281-010-0231-y. [DOI] [PubMed] [Google Scholar]
- 36.Di Pasquale A, Bonanni P, Garçon N, Stanberry LR, El-Hodhod M, Tavares Da Silva F. Vaccine safety evaluation: practical aspects in assessing benefits and risks. Vaccine PMID:27836435. 2016;34:6672–80. doi: 10.1016/j.vaccine.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 37.McConnell MJ, Rumbo C, Bou G, Pachón J. Outer membrane vesicles as an acellular vaccine against Acinetobacter baumannii. Vaccine PMID:21679737. 2011;29:5705–10. doi: 10.1016/j.vaccine.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Nieves W, Petersen H, Judy BM, Blumentritt CA, Russell-Lodrigue K, Roy CJ, Torres AG, Morici LA. A Burkholderia pseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin Vaccine Immunol PMID:24671550. 2014;21:747–54. doi: 10.1128/cvi.00119-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitra S, Chakrabarti MK, Koley H. Multi-serotype outer membrane vesicles of Shigellae confer passive protection to the neonatal mice against shigellosis. Vaccine PMID:23684822. 2013;31:3163–73. doi: 10.1016/j.vaccine.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Hirahara K, Nakayama T. CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol PMID:26874355. 2016;28:163–71. doi: 10.1093/intimm/dxw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol PMID:30891627. 2019;41:283–97. doi: 10.1007/s00281-019-00733-8. [DOI] [PubMed] [Google Scholar]
- 42.Holt KE, Wertheim H, Zadoks RN, Baker S, Whitehouse CA, Dance D, Jenney A, Connor TR, Hsu LY, Severin J, et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci U S A PMID:26100894. 2015;112:E3574–3581. doi: 10.1073/pnas.1501049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camilo V, Sugiyama T, Touati E. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2017;22(Suppl). 10.1111/hel.12405. PMID:28891130. [DOI] [PubMed] [Google Scholar]
- 44.Liu Q, Li X, Zhang Y, Song Z, Li R, Ruan H, Huang X. Orally-administered outer-membrane vesicles from Helicobacter pylori reduce H. pylori infection via Th2-biased immune responses in mice. Pathogens and Disease. 2019;77:PMID:31504509. doi: 10.1093/femspd/ftz050. [DOI] [PubMed] [Google Scholar]
- 45.Hegerle N, Guiso N. Bordetella pertussis and pertactin-deficient clinical isolates: lessons for pertussis vaccines. Expert Rev Vaccines PMID:24953157. 2014;13:1135–46. doi: 10.1586/14760584.2014.932254. [DOI] [PubMed] [Google Scholar]
- 46.Zurita ME, Wilk MM, Carriquiriborde F, Bartel E, Moreno G, Misiak A, Mills KHG, Hozbor D. A Pertussis Outer Membrane Vesicle-Based Vaccine Induces Lung-Resident Memory CD4 T Cells and Protection Against, Including Pertactin Deficient Strains. Frontiers in Cellular and Infection Microbiology PMID:31106160. 2019;9:125. doi: 10.3389/fcimb.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerver SM, Mihalkova M, Bion JF, Wilson APR, Chudasama D, Johnson AP, Hope R. Surveillance of Bloodstream Infections in Intensive Care Units in England, May 2016-April 2017: epidemiology and Ecology. J Hosp Infect. 2020. doi: 10.1016/j.jhin.2020.05.010. PMID:32422311. [DOI] [PubMed] [Google Scholar]
- 48.Lee E-Y, Choi D-Y, Kim D-K, Kim J-W, Park JO, Kim S, Kim S-H, Desiderio DM, Kim Y-K, Kim K-P, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics PMID:19834908. 2009;9:5425–36. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 49.Choi SJ, Kim M-H, Jeon J, Kim OY, Choi Y, Seo J, Hong S-W, Lee W-H, Jeon SG, Gho YS, et al. Active Immunization with Extracellular Vesicles Derived from Staphylococcus aureus Effectively Protects against Staphylococcal Lung Infections, Mainly via Th1 Cell-Mediated Immunity. PloS One PMID:26333035. 2015;10:e0136021. doi: 10.1371/journal.pone.0136021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pastor Y, Camacho A, Gil AG, Ramos R, ALd C, Peñuelas I, Irache JM, Gamazo C. Effective protection of mice against Shigella flexneri with a new self-adjuvant multicomponent vaccine. Journal of Medical Microbiology PMID:28721849. 2017;66:946–58. doi: 10.1099/jmm.0.000527. [DOI] [PubMed] [Google Scholar]
- 51.Cho KS, Kang SA, Kim SD, Mun SJ, Yu HS, Roh HJ. Dendritic cells and M2 macrophage play an important role in suppression of Th2-mediated inflammation by adipose stem cells-derived extracellular vesicles. Stem Cell Res. 2019. 10.1016/j.scr.2019.101500. PMID:31344653. [DOI] [PubMed] [Google Scholar]
- 52.Kim HY, Lim Y, An SJ, Choi BK. Characterization and immunostimulatory activity of extracellular vesicles from Filifactor alocis. Mol Oral Microbiol PMID:31675472. 2020;35:1–9. doi: 10.1111/omi.12272. [DOI] [PubMed] [Google Scholar]
- 53.Badmasti F, Ajdary S, Bouzari S, Fooladi AAI, Shahcheraghi F, Siadat SD. Immunological evaluation of OMV(PagL)+Bap(1-487aa) and AbOmpA(8-346aa)+Bap(1-487aa) as vaccine candidates against Acinetobacter baumannii sepsis infection. Molecular Immunology PMID:26277277. 2015;67:552–58. doi: 10.1016/j.molimm.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 54.Huang W, Yao Y, Long Q, Yang X, Sun W, Liu C, Jin X, Li Y, Chu X, Chen B, et al. Immunization against multidrug-resistant Acinetobacter baumannii effectively protects mice in both pneumonia and sepsis models. PloS One. 2014;9. PMID:24956279. 10.1371/journal.pone.0100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacLennan CA, Riddle MS, Chen WH, Talaat KR, Jain V, Bourgeois AL, Frenck R, Kotloff K, Porter CK. Consensus Report on Shigella Controlled Human Infection Model: clinical Endpoints. Clin Infect Dis PMID:31816065. 2019;69:S591–s595. doi: 10.1093/cid/ciz891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camacho AI, de Souza J, Sánchez-Gómez S, Pardo-Ros M, Irache JM, Gamazo C. Mucosal immunization with Shigella flexneri outer membrane vesicles induced protection in mice. Vaccine PMID:21911022. 2011;29:8222–29. doi: 10.1016/j.vaccine.2011.08.121. [DOI] [PubMed] [Google Scholar]
- 57.Chitneni P, Beksinska M, Dietrich JJ, Jaggernath M, Closson K, Smith P, Lewis DA, Matthews LT, Smit J, Ndung’u T, et al. Partner notification and treatment outcomes among South African adolescents and young adults diagnosed with a sexually transmitted infection via laboratory-based screening. Int J STD AIDS. 2020;956462420915395. PMID:32403988. doi: 10.1177/0956462420915395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Hammer LA, Liu W, Hobbs MM, Zielke RA, Sikora AE, Jerse AE, Egilmez NK, Russell MW. Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunology PMID:28272393. 2017;10:1594–608. doi: 10.1038/mi.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi C-W, Park EC, Yun SH, Lee S-Y, Kim SI, Kim G-H. Potential Usefulness of Extracellular Membrane Vesicles as Antibacterial Vaccines. Journal of Immunology Research PMID:28210633. 2017;2017:7931982. doi: 10.1155/2017/7931982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG. Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. The American Journal of Pathology PMID:25892682. 2015;185:1505–17. doi: 10.1016/j.ajpath.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bagheri Nejad R, Yahyaraeyat R, Es-Haghi A, Nayeri Fasaei B, Zahraei Salehi T. Induction of specific cell-mediated immune responses and protection in BALB/c mice by vaccination with outer membrane vesicles from a Brucella melitensis human isolate. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica PMID:31514254. 2019;127:797–804. doi: 10.1111/apm.12997. [DOI] [PubMed] [Google Scholar]
- 62.Vetter V, Denizer G, Friedland LR, Krishnan J, Shapiro M. Understanding modern-day vaccines: what you need to know. Ann Med PMID:29172780. 2018;50:110–20. doi: 10.1080/07853890.2017.1407035. [DOI] [PubMed] [Google Scholar]
- 63.Langereis JD, de Jonge MI. Unraveling Haemophilus influenzae virulence mechanisms enable discovery of new targets for antimicrobials and vaccines. Curr Opin Infect Dis PMID:32304471. 2020;33:231–37. doi: 10.1097/qco.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 64.Michel LV, Kaur R, Zavorin M, Pryharski K, Khan MN, LaClair C, O’Neil M, Xu Q, Pichichero ME. Intranasal coinfection model allows for assessment of protein vaccines against nontypeable Haemophilus influenzae in mice. J Med Microbiol PMID:30136923. 2018;67:1527–32. doi: 10.1099/jmm.0.000827. [DOI] [PubMed] [Google Scholar]
- 65.Behrouzi A, Bouzari S, Siadat SD, Oloomi M, Davari M, Mazaheri H. Evaluation of the immunogenic property of NT H. influenzae protein D with Neisseria meningitidis OMV in BALB/c. Journal of Infection in Developing Countries PMID:28036315. 2016;10:1345–51. doi: 10.3855/jidc.7513. [DOI] [PubMed] [Google Scholar]
- 66.Batista-Duharte A, Martínez DT, Carlos IZ. Efficacy and safety of immunological adjuvants. Where is the cut-off? Biomed Pharmacother PMID:29894962. 2018;105:616–24. doi: 10.1016/j.biopha.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 67.Trainor EA, Nicholson TL, Merkel TJ. Bordetella pertussis transmission. Pathog Dis PMID:26374235. 2015;73:ftv068. doi: 10.1093/femspd/ftv068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bottero D, Zurita ME, Gaillard ME, Carriquiriborde F, Martin Aispuro P, Elizagaray M, Bartel E, Castuma C, Hozbor D. Outer-Membrane-Vesicle-Associated O Antigen, a Crucial Component for Protecting Against Infection. Frontiers in Immunology PMID:30459769. 2018;9:2501. doi: 10.3389/fimmu.2018.02501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ylinen E, Salmenlinna S, Halkilahti J, Jahnukainen T, Korhonen L, Virkkala T, Rimhanen-Finne R, Nuutinen M, Kataja J, Arikoski P, et al. Hemolytic uremic syndrome caused by Shiga toxin-producing Escherichia coli in children: incidence, risk factors, and clinical outcome. Pediatr Nephrol. 2020. PMID:32323005. doi: 10.1007/s00467-020-04560-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fingermann M, Avila L, De Marco MB, Vázquez L, Di Biase DN, Müller AV, Lescano M, Dokmetjian JC, Fernández Castillo S, Pérez Quiñoy JL. OMV-based vaccine formulations against Shiga toxin producing Escherichia coli strains are both protective in mice and immunogenic in calves. Human Vaccines & Immunotherapeutics PMID:29923791. 2018;14:2208–13. doi: 10.1080/21645515.2018.1490381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pulido MR, García-Quintanilla M, Pachón J, McConnell MJ. A lipopolysaccharide-free outer membrane vesicle vaccine protects against Acinetobacter baumannii infection. Vaccine PMID:31843268. 2020;38:719–24. doi: 10.1016/j.vaccine.2019.11.043. [DOI] [PubMed] [Google Scholar]
- 72.Ellis TN, Leiman SA, Kuehn MJ. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect Immun PMID:20605984. 2010;78:3822–31. doi: 10.1128/iai.00433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Pol L, Stork M. van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol J PMID:26912077. 2015;10:1689–706. doi: 10.1002/biot.201400395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol PMID:20948547. 2010;10:787–96. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]


