ABSTRACT
The aim of the study was to investigate changes in the incidences of Varicella and Herpes Zoster (HZ) following introduction of single dose Varicella vaccine (VV) in Turkey. Changes in the incidences of varicella and HZ per 100,000 population were compared with pre (2011–2012) and post-VV period (2018–2019) throughout years between years 2011 and 2019 both for children and adults. In children ≤5 years of age, the annual incidences of varicella significantly decreased from 290 per 100000 children in 2011 to 24 per 100000 children in 2019 [p = .0001]. Also, for children ≤5 years the mean annual incidence of varicella decreased significantly [326/100000 ±51/100000 vs 23/100000 ± 1/100000; p = .014] between pre- and post-VV period. Moreover, the annual incidences of varicella significantly decreased from 43 per 100000 children in 2011 to 26 per 100000 children in 2019 in children age between 6 and 17 years. On the other hand, incidence of varicella in adult population (age >17 years) did not change significantly. Besides, the annual incidences of Herpes Zoster did not change significantly in children age stratas but significant increment observed in adult population. This increment was significant in adult age strata of 18–44 years, but non-significant in age strata of 45–64 years and >64 years. Thus, our study showed a significant reduction in the incidences of Varicella in children age stratas whereas significant increment in the incidence of HZ in adult population after the implementation of VV into the NIP of Turkey.
KEYWORDS: Varicella vaccine, varicella zoster, Herpes Zoster, children, adult
Introduction
Varicella-Zoster Virus (VZV) is responsible for two diseases: Varicella (Chicken Pox), which occurs mainly in childhood, and Herpes Zoster (HZ, Shingles), which occurs mainly in older adults. After causing varicella, VZV remains dormant in the sensory ganglia and reactivates later in life, leading to HZ. Although the cause of reactivation remains unclear, diminished cellular immunity might contribute.1 The introduction of universal varicella vaccination in national childhood immunization programs worldwide has resulted in a significant decrease in varicella infection rates.2 On the other hand, decreased circulation of wild-type viruses – brought about by the universal varicella vaccination program – might increase HZ incidence among adults. This is because these adults will have fewer immunity-boosting exogenous wild-type varicella exposures.3 Such as association has not been shown in children.4 Therefore, the monitoring of the long-term impacts of varicella vaccination on HZ incidence is important.
Varicella vaccine (VV) was introduced into the national immunization program (NIP) of Turkey in 2013 with a single dose at 12 months old and a concomitant separate administration of the measles-mumps-rubella vaccine and 13-valent pneumococcal conjugate vaccine (PCV13) vaccine.5 No catch-up program was run for those children unvaccinated with VV aged older than 12 months. The estimated national VV coverage rates for Turkish children at least 12 months old were 98% in 2013, 94% in 2014, 97% in 2015, 98% in 2016, 96% in 2017, and 96% in 2018.6 The Turkish NIP does not recommend routinely HZ vaccination in those older adult population.
Currently, there is no study investigating the impact of single-dose varicella vaccination on the incidences of varicella infection and HZ in Turkey. The aim of this study was, therefore, to evaluate the effects of universal single-dose varicella vaccination on incidences of varicella infection and HZ in Turkish children and adults with a time trend analysis of 9 years.
Materials and methods
We conducted a retrospective record review, including all 1-month to 18-year-old children and adult patients presenting to the study hospitals with suspected varicella or HZ between 2011 and 2019. This study was performed at Ataşehir Memorial Hospital and Şişli Memorial Hospital located within two different districts of Istanbul (estimated population of the two districts: 690,000, nearly 4–5% of the whole city population), Turkey. Both hospitals are large primary and tertiary care hospitals that work mainly with the private health insurance system. The cases were children and adults with a diagnosis of varicella or HZ in our study hospitals’ pediatric and adult outpatient, emergency room (ER), and dermatology units from January 1, 2011, to December 31, 2019. Cases were identified according to the International Statistical Classification of Diseases and Related Health Problems-10th (ICD-10 codes). We included both primary and secondary diagnoses and used ICD codes B01 for varicella and B02 for HZ. Cases with a primary diagnosis of postherpetic trigeminal neuralgia or postherpetic polyneuropathy were not included because of their increased potential to represent long-term follow-up for prior HZ episodes. Also, we could not detect subclinical varicella infection since we did not enroll immunocompromised patients. The number of admitted patients was detected by protocol numbers that also included the identification number of each patient via hospital databases. Repeated-admissions for the same diagnosis were excluded using the patient identification number. Incidences were calculated as follows: incidence of varicella per 100,000 population = number of varicella cases in a year/number of population in a year × 100,000 and incidence of HZ per were 100,000 population = number of HZ cases in a year/number of population in a year × 100,000. Annual varicella vaccination rate was accepted as same with measles vaccination rate since concomitant administration in Turkey.6 The study was approved by the Şişli Memorial Hospital Ethical Committee for inclusion of both study hospitals.
Statistical analysis
Data were entered into a Microsoft Office Excel (Microsoft, Redmond, WA, USA) spreadsheet and analyzed using Stata 10.0 Statistics/Data Analysis (StataCorp, Lakeway, Drive, TX, USA). The year 2011–2012 was considered a pre-vaccination period of varicella immunization (2013 is the first year of VV in the Turkey NIP), and the year 2018–2019 was considered as the post-vaccination period since full vaccination coverage achieved for children ≤5 years old. The years between 2013 and 2017 were considered a transitional period. To measure the linear correlation between the study period (years) and the incidences of varicella and HZ, we used the Pearson correlation coefficient (r). A t-test was performed to determine if there was a difference in the mean incidences of varicella and HZ between the pre-vaccination period and the post-vaccination period. A p-value <0.05 was considered significant.
Results
During the study period (2011–2019), a total of 1,090,803 patients (mean, 121,200 patient/year; range, 91,965–138,254 patient/year) were admitted to our study hospitals. Table 1 shows the age distribution of hospital admissions for the study years. The yearly admission number gradually increased with time, ranging from 91,965 in 2011 to 138,254 in 2019 (p < .05). Among admissions, there were 135,957 (12%) children ≤5 years old age (mean, 15,106 children/year; range, 10,323–18140 children year) (Table 1). The varicella vaccination status of the study population is shown in Table 2.
Table 1.
Incidence of varicella with respect to age stratas and years
| YEAR | No of total patient population | No. of children age ≤ 5 years admitted | No. (%) children diagnosis with varicella age ≤ 5 years | Incidence of varicella per 100000 children age ≤ 5 years | No. of children age 6–17 years admitted | No. (%) children diagnosis with varicella age 6–17 years | Incidence of varicella per 100000 children age 6–17 years | No. of patient age > 17 years admitted | No. (%) patient diagnosis with varicella age > 17 years | Incidence of varicella per 100000 patient age > 17 years |
|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | 91965 | 10323 | 30 | 290 | 5038 | 43 | 853 | 76604 | 4 | 52 |
| 2012 | 110205 | 11844 | 43 | 363 | 5646 | 41 | 726 | 92715 | 8 | 8 |
| 2013 | 112785 | 14489 | 52 | 358 | 6897 | 67 | 971 | 91403 | 21 | 22 |
| 2014 | 118248 | 15491 | 30 | 193 | 8906 | 35 | 392 | 93851 | 5 | 5 |
| 2015 | 122183 | 15613 | 28 | 179 | 8205 | 48 | 585 | 98365 | 13 | 13 |
| 2016 | 128305 | 16122 | 21 | 130 | 9571 | 52 | 543 | 102612 | 7 | 6 |
| 2017 | 136317 | 17906 | 12 | 67 | 10479 | 36 | 343 | 107932 | 15 | 13 |
| 2018 | 132831 | 18140 | 4 | 22 | 11558 | 25 | 216 | 103133 | 9 | 8 |
| 2019 | 138254 | 16029 | 4 | 24 | 15280 | 26 | 170 | 106975 | 5 | 4 |
Table 2.
Varicella vaccine immunization status of study population with respect to age stratas
| Year | Children age ≤ 5 years | Children age 6–17 years | Patient age ≥ 18 years |
|---|---|---|---|
| 2011 | None | None | None |
| 2012 | None | None | None |
| 2013* | Yes for children age ≤ 12 months | None | None |
| 2014 | Yes for children age ≤ 24 months | None | None |
| 2015 | Yes for children age ≤ 36 months | None | None |
| 2016 | Yes for children age ≤ 48 months | None | None |
| 2017 | Yes for children age ≤ 60 months | None | None |
| 2018 | All | Yes for children age ≤ 12 months | None |
| 2019 | All | Yes for children age ≤ 24 months | None |
*Varicella vaccination introduced Turkish national immunization program in year 2013. World Health Organization (WHO) reported a national coverage for the Varicella vaccine 97% in 2013, 96% in 2014, 97% in 2015, 98% in 2016, 96% in 2017 and in 2018.
Incidences of varicella
During the study period (2011–2019), 684 patients were diagnosed as varicella (mean, 76 patient/year; range, 9–140 patient/year) (Table 1). In children ≤5 years of age, the annual incidences of varicella significantly decreased from 290 per 100,000 children in 2011 to 24 per 100,000 children in 2019 (p = .0001, r = −0.94) [Figure 1]. Also, in children age between 6 and 17 years, the annual incidences of varicella significantly decreased from 43 per 100,000 children in 2011 to 26 per 100,000 children in 2019 (Figure 1). The incidence of varicella in the adult population (age >17 years) did not change significantly from 2011 to 2019 (p = .68, r = −063) [Figure 1]. For children, ≤5 years, when the mean annual incidences of varicella between the pre-vaccination period of VV (2011/2012) were compared with a post-vaccination period (2018/2019), the mean annual incidence of varicella decreased significantly (326/100000 ± 51/100000 vs. 23/100000 ± 1/100000; 95% CI: 146–460; p = .014). Moreover, the mean annual incidences of varicella in the post-vaccination period also significantly decreased when compared with the mean annual incidences of the pre-vaccination period (789/100,000 ± 89/100,000 vs. 193/100,000 ± 32/100,000; 95% CI: 305–887; p = .012) in children age 6–17 years. On the other hand, the mean annual incidences of varicella did not differ between the pre-vaccination period and post-post vaccination period in the adult (>17 years) population (30/100,000 ± 31/100,000 vs. 6/100,000 ± 2/100,000; 95% CI: −71–119; p = .39).
Figure 1.
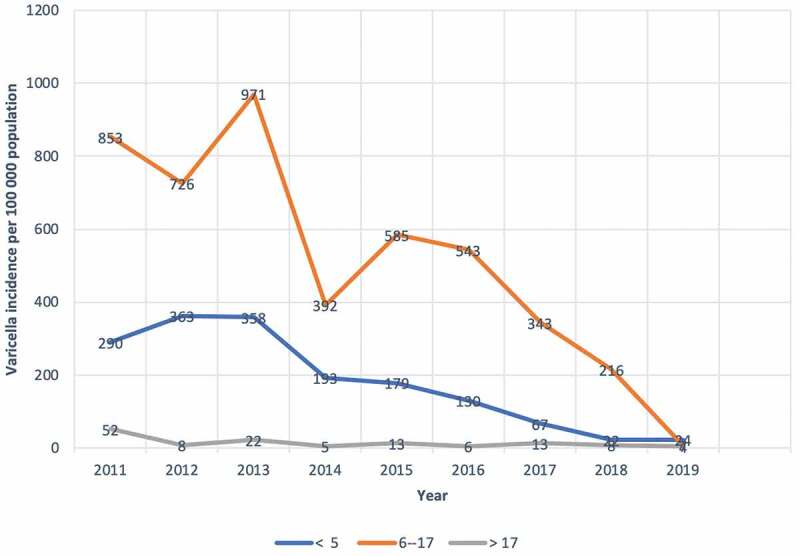
The incidences of varicella with respect to age stratas (year) by calendar year 2011–2019
Incidences of Herpes Zoster
During the 2011 to 2019 study period, 2049 patients were diagnosed as HZ (mean, 227 patient/year; range, 184–300 patient/year) [Table 3]. In children ≤5 years old, the annual incidences of HZ did not change significantly between 2011 and 2019 (p = .22, r = −0.45), and this was also true for 6- to 17-year-old children (p = −0.23, r = −0.55) [Table 3], [Figure 2].
Table 3.
Incidence of Herpes Zoster with respect to age stratas and years
| YEAR | No. of children age ≤ 5 years admitted | No. (%) children diagnosis with zoster age ≤ 5 years | Incidence of zoster per 100000 children age ≤ 5 years | No. of children age 6–17 years admitted | No. (%) children diagnosis with zoster age 6–17 years | Incidence of zoster per 100000 children age 6–17 years | No. of patient age > 17 years admitted | No. (%) patient diagnosis with zoster age > 17 years | Incidence of zoster per 100000 patient age > 17 years |
|---|---|---|---|---|---|---|---|---|---|
| 2011 | 10323 | 1 | 9 | 5038 | 1 | 19 | 76604 | 140 | 182 |
| 2012 | 11844 | 4 | 33 | 5646 | 8 | 141 | 92715 | 184 | 198 |
| 2013 | 14489 | 0 | 0 | 6897 | 4 | 57 | 91403 | 186 | 203 |
| 2014 | 15491 | 1 | 6 | 8906 | 3 | 33 | 93851 | 184 | 196 |
| 2015 | 15613 | 0 | 0 | 8205 | 3 | 36 | 98365 | 223 | 226 |
| 2016 | 16122 | 3 | 18 | 9571 | 4 | 41 | 102612 | 235 | 229 |
| 2017 | 17906 | 3 | 16 | 10479 | 3 | 28 | 107932 | 244 | 226 |
| 2018 | 18140 | 5 | 27 | 11558 | 5 | 43 | 103133 | 249 | 241 |
| 2019 | 16029 | 6 | 37 | 15280 | 9 | 58 | 106975 | 305 | 285 |
Figure 2.
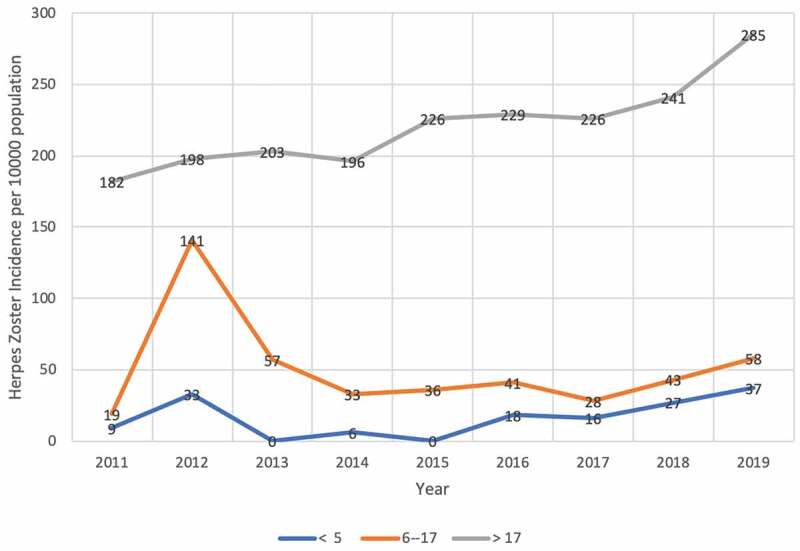
The incidences of Herpes Zoster with respect to age stratas by calendar year 2011–2019
On the other hand, the incidence of HZ in the adult population (age >17 years) significantly increased when comparing 2011 to 2019 (the annual incidences of HZ significantly increased from 182 per 100,000 adults in 2011 to 285 per 100,000 adults in 2019; p = .0006, r = 0.91) [Table 3], [Figure 2]. When we evaluated HZ incidences for adult age strata, the annual incidences of HZ significantly increased in adults age 18–44 years between 2011 and 2019 (the annual incidences of HZ significantly increased from 208 per 100,000 adults in 2011 to 303 per 100,000 adults in 2019; r = 0.80, p = .009) [Figure 2], [Table 4]. On the other hand, the incidences of HZ did not change significantly in either adult age strata 45–64 years (the annual incidences of HZ non-significantly increased from 141 per 100,000 adults in 2011 to 314 per 100,000 adults in 2019; r = 0.54, p = .13) or >64 years (the annual incidences of HZ non-significantly increased from 181 per 100,000 adults in 2011 to 203 per 100,000 adults in 2019; r = 0.49, p = .18) [Figure 2], [Table 4]. When the mean annual incidences of HZ between the pre-vaccination period of VV (2011/2012) were compared with a post-vaccination period (2018/2019) for children ≤5 years, the incidence of HZ did not change significantly (21/100,000 ± 16/100,000 vs. 32/100,000 ± 37/100,000; 95% CI: −66–44; p = .48). Moreover, the mean annual incidences of HZ in the post-vaccination period did not change significantly when compared with the mean annual incidences of the pre-vaccination period (80/100,000 ± 86/100,000 vs. 50/100,000 ± 10/100,000; 95% CI: −234–293; p = .67) for 6–17-year-old children. On the other hand, the mean annual incidences of HZ significantly increased from the pre-vaccination period to post-post vaccination period in adults (190/100,000 ± 8/100,000 vs. 263/100,000 ± 22/100,000; 95% CI: 2–144; p = .047).
Table 4.
Incidences of Herpes Zoster with respect to adulthood age stratas by calendar year
| YEAR | No. of patients age 18–44 years admitted | No. (%) patients diagnosis with zoster age 18–44 years | Incidence of zoster per 100000 patients age 18–44 years | No. of patients age 45–64 years admitted | No. (%) patients diagnosis with zoster age 45–64 years | Incidence of zoster per 100000 patients age 45–64 years | No. of patient age > 65 years admitted | No. (%) patient diagnosis with zoster age ≥ 65 years | Incidence of zoster per 100000 patient age ≥ 65 years |
|---|---|---|---|---|---|---|---|---|---|
| 2011 | 39756 | 83 | 208 | 24758 | 35 | 141 | 12090 | 22 | 181 |
| 2012 | 52849 | 119 | 225 | 26922 | 41 | 152 | 12944 | 22 | 169 |
| 2013 | 62403 | 120 | 192 | 15757 | 52 | 330 | 13243 | 16 | 120 |
| 2014 | 51795 | 118 | 227 | 28921 | 40 | 138 | 13135 | 28 | 213 |
| 2015 | 54544 | 128 | 234 | 30233 | 62 | 205 | 13588 | 33 | 242 |
| 2016 | 61785 | 151 | 244 | 26393 | 60 | 227 | 14434 | 28 | 193 |
| 2017 | 64431 | 149 | 231 | 28340 | 57 | 201 | 15161 | 38 | 250 |
| 2018 | 63361 | 158 | 249 | 23720 | 62 | 261 | 16052 | 32 | 199 |
| 2019 | 62012 | 188 | 303 | 28281 | 89 | 314 | 16682 | 34 | 203 |
Discussion
To our knowledge, this was the first study in a large, middle-income country to assess the impact of universal single-dose VV on incidences of varicella and HZ in both children and adults following VV introduction into the NIP. In this study, we showed a significant impact of varicella vaccination on the incidence of varicella in children ≤5 years of age, and 6–17 years of age after varicella vaccination introduction in Turkey. Our trend analysis revealed that after the implementation of VV into the NIP of our country in 2013, with each passing year, the incidence of varicella decreased significantly with a linear slope in children ≤5 years old who received the varicella vaccination. We also observed a reduction in the incidence of varicella in unvaccinated children (age 6–17 years). Vaccine effectiveness after single dose of VV ranged from 55% to 87%, while vaccine effectiveness ranging from 84% to 98% after two doses.7–10 A single-dose vaccination schedule is effective at controlling the severe form of infection, but varicella breakthrough still occurs.7–10 For this reason, to control varicella cases in the population, the World Health Organization recommends that vaccine coverage should be maintained above 80%.11 The impact of vaccination is expressed as the proportionate reduction in disease burden, comparing incidences and mortality rates in the same population between the pre-vaccine era and after vaccine implementation.12 After one-dose programs, reductions up to 74% have been recorded,13 whereas reductions exceeding 90% have been recorded after two-dose schedules.14–16 In our country, during the study period, VV coverage was found to be at least 96%, and we observed a dramatic decrease (93%) in the incidence of varicella in the vaccinated population (children age ≤5 years). Even in our country, second dose of varicella vaccine is not recommended by Turkish Ministry of Health. In private doctor’s office and in some private hospitals’ children at 4–6 years of age may get second dose of varicella vaccination if their care-givers demanded and accepted to pay cost of vaccination. We did not know how many of children ≤5 years of age had a second dose of varicella vaccine but we think that amount is negligible since only children who was age over 4 years of age had a chance to get second dose of vaccination as international recommendations. We also observed a decrease in incidences of varicella in unvaccinated older children (6–17 years old), supporting the herd immunity effect of VV because of diminished wild virus circulation in the population. But in our study we could not examined subclinical varicella infection since we used just only ICD codes for diagnosis, and also our study population composed of immunocompetent patient population, we think that number of subclinical varicella infection in our study population is negligible and unpredictable since subclinical varicella infection mostly seen in immunocompromised population and its frequency even lower in immunocompromised children. Also, detection of subclinical infection is problematic and mostly diagnosis is done by detection of viral DNA by PCR assay. Moreover, subclinical infection mostly detected in elder population, and viral PCR positivity corelates with age.17,18
On the other hand, we did not observe any increase in the incidence of varicella in the adult population. It has been postulated that vaccination of children might shift in the age of infection to older age groups, where the more severe disease might be more common, due to decreased exposure to circulating VZV.19 Although this might be possible with vaccination coverage below 80%,20 high vaccine coverage, and a two-dose program are likely to be effective at stopping varicella transmission in the population.21 In our country, Turkey with higher single-dose varicella vaccination coverage (96%) without second dose implementation into the national immunization program, we observed reported high rate of reduction in the incidence of varicella infection without any shift to unvaccinated population. On the other hand, many previous studies have shown that the incidence of varicella in non-vaccinated population also decreases, perhaps due to herd immunity acquired by the high coverage obtained in the vaccination programs,14,16,22-24 which is consistent with our study findings, especially for unvaccinated older children (6–17 years old) and unchanged varicella incidence in the adult population.
A second most important finding of this study was that the incidence of HZ in the adult population (age >17 years) significantly increased from the year 2011 to 2019, and this increase was significant in 18–44-year-old adults but non-significant in 45–64-year-old and >64-year-old adults. Several population-based studies of the time trends in the HZ incidence before and after the introduction of varicella vaccination have been conducted to investigate changes in HZ incidence in many countries. Studies in the United States evaluating the pre- and post-VV data and HZ incidence rates detected increases for all adults age strata analyzed over study periods spanning 1945–2013.25,26 Moreover, by using the MarketScan Commercial database for 1993–2006, and Medicare for 1996–2006, the incidences of HZ had increased steadily throughout this time period in all adult age strata, with a discontinuity seen in 2002 in the 55–64 and ≥65 years age strata.27 Hales et al. also reported that the incidence of HZ increased during that period, but after 2006, plateauing in the HZ incidence rate was evident.25 Furthermore, Harpaz et al. extended analyses of the MarketScan Commercial and Medicare databases up to the year 2016.28 The plateauing in the HZ incidence rate in the ≥65-year-old age groups after the year 2006 continued, while the rate continued to increase in the 18–64 age groups and declined in the 0–17 age group.28 Recently, Walfson et al. investigated the incidences of HZ using MarketScan Commercial and Medicare databases between 1991 and 2016. These authors reported that the annual incidences of HZ increased throughout the period of 1991–2012 in all adult age categories, with a plateau in 2013–2016 that was most evident in the ≥65 age group.29 Among the non-US studies that included pre- and post-VV data, studies reported increasing numbers of HZ cases or HZ incidence rates in Australia,30,31 Canada,32,33 Germany,34 Japan,35 Korea,36 Spain,37 and Taiwan38 over study periods. The increase in the incidence of HZ after universal VV introduction can be explained by the exogenous boosting hypothesis: in the absence of varicella vaccination, immunity is boosted after early childhood infection by reexposure to the circulating virus.39 By reducing the circulating virus, universal childhood varicella vaccination might have the unintended consequence of allowing cell-mediated immunity in adults to wane in later decades, hence increasing HZ incidence. In the long term, however, a decreasing incidence of HZ is expected to occur, assuming that vaccinated individuals are less likely to develop HZ when compared to naturally infected individuals.40 Many models have calculated that a temporary increase in HZ incidence, as a result of varicella vaccination, could be anticipated over the next 50–70 years.21,41
This study had several limitations. First, the study population did not include all of the country’s children. However, Istanbul (nearly 20% of the country’s population) is the largest city in Turkey. Istanbul has more than 100 hospitals, including state and private hospitals. Ataşehir Memorial Hospital is located in the Asian region of Istanbul, and Şişli Memorial Hospital is located in the European region of Istanbul. Both hospitals are large hospitals that mainly work with the private health insurance system and have well-documented hospital data systems. We believe that our study hospitals are representative of the Istanbul population, as well as fairly representative of Turkey as a whole, and thus, are likely to yield good data about the impact of VV on varicella and HZ. Second, we could not calculate the incidence of varicella-related hospitalization and mortality since we did not detect any mortality and few hospitalized patients during the study period.
This study supports the powerful impact of single-dose varicella vaccination on the incidence of varicella in vaccinated and unvaccinated children in Turkey. Nine years after the implementation of varicella vaccination into the NIP of Turkey, the incidence of HZ increased in the adult population, a finding that underlines the importance of HZ vaccination in the adult population.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9(3):361–81. doi: 10.1128/CMR.9.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papaloukas O, Giannouli G, Papaevangelou V. Successes and challenges in varicella vaccine. Ther Adv Vaccines. 2014;2(2):39–55. doi: 10.1177/2051013613515621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin MJ, Smith JG, Kaufhold RM, Barber D, Hayward AR, Chan CY, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003;188(9):1336‐1344. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 4.Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: a population-based study. Pediatrics. 2015;135(3):e565–71. doi: 10.1542/peds.2013-4037. [DOI] [PubMed] [Google Scholar]
- 5.WHO World Health Organization . [accessd 2020 May1]. https://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria[country][]=TUR.
- 6.WHO World Health Organization . Immunization summary. [accessed 2020 May1]. http://apps.who.int/gho/data/node.main.PCV3n?lang=en.
- 7.Spackova M, Wiese-Posselt M, Dehnert M, Matysiak-Klose D, Heininger U, Siedler A. Comparative varicella vaccine effectiveness during outbreaks in day-care centres [published correction appears in Vaccine. 2010 May 7;28(21):3754. Vaccine. 2010;28(3):686–91. doi: 10.1016/j.vaccine.2009.10.086. [DOI] [PubMed] [Google Scholar]
- 8.Vazquez M, LaRussa PS, Gershon AA, Niccolai LM, Muehlenbein CE, Steinberg SP, et al. Effectiveness over time of varicella vaccine. Jama. 2004;291(7):851–55. doi: 10.1001/jama.291.7.851. [DOI] [PubMed] [Google Scholar]
- 9.Mahamud A, Wiseman R, Grytdal S, Basham C, Ashgar J, Dang T, et al. Challenges in confirming a varicella outbreak in the two-dose vaccine era. Vaccine. 2012;30(48):6935–39. doi: 10.1016/j.vaccine.2012.07.076. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro ED, Vazquez M, Esposito D, Holabird N, Steinberg SP, Dziura J, LaRussa PS, Gershon AA. Effectiveness of 2 doses of varicella vaccine in children. J Infect Dis. 2011;203(3):312–15. doi: 10.1093/infdis/jiq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varicella and herpes zoster vaccines: WHO position paper, June. Recommendations . WHO positions paper on vaccination against varicella and herpes. Vaccine. 2014;2016(34):198–99. [DOI] [PubMed] [Google Scholar]
- 12.Hanquet G, Valenciano M, Simondon F, Moren A. Vaccine effects and impact of vaccination programmes in post-licensure studies. Vaccine. 2013;31(48):5634–42. doi: 10.1016/j.vaccine.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Avila-Aguero ML, Ulloa-Gutierrez R, Camacho-Badilla K, Soriano-Fallas A, Arroba-Tijerino R, Morice-trejos A. Varicella prevention in Costa Rica: impact of a one-dose schedule universal vaccination. Expert Rev Vaccines. 2016;16(3):229–34. doi: 10.1080/14760584.2017.1247700. [DOI] [PubMed] [Google Scholar]
- 14.Garcia Cenoz M, Castilla J, Chamorro J, Martinez-Artola F, Irisarri F, Arriazu M, et al. Impact of universal two-dose vaccination on varicella epidemiology in Navarre, Spain, 2006 to 2012. Euro Surveill. 2013;18(32):20552. doi: 10.2807/1560-7917.ES2013.18.32.20552. [DOI] [PubMed] [Google Scholar]
- 15.Boccalini S, Bonanni P, Bechini A. Preparing to introduce the varicella vaccine into the Italian immunisation programme: varicella-related hospitalisations in Tuscany, 2004–2012. Euro Surveill. 2016;21(24). doi: 10.2807/15607917.ES.2016.21.24.30257. [DOI] [PubMed] [Google Scholar]
- 16.Baxter R, Tran TN, Ray P, Lewis E, Fireman B, Black S, Shinefield HR, Coplan PM, Saddier P. Impact of vaccination on the epidemiology of varicella: 1995–2009. Pediatrics. 2014;134(1):24–30. doi: 10.1542/peds.2013-4251. [DOI] [PubMed] [Google Scholar]
- 17.Schünemann S, Mainka C, Wolff MH. Subclinical reactivation of varicella-zoster virus in immunocompromised and immunocompetent individuals. Intervirology. 1998;41(2–3):98‐102. doi: 10.1159/000024920. [DOI] [PubMed] [Google Scholar]
- 18.Kozawa K, Miura H, Kawamura Y, Tanaka M, Kudo K, Higashimoto Y, et al. Frequency of subclinical herpes zoster in pediatric hematology-oncology patients receiving chemotherapy: A retrospective cohort analysis [published online ahead of print, 2019 Dec 10]. J Med Virol. 2019. doi: 10.1002/jmv.25650. [DOI] [PubMed] [Google Scholar]
- 19.Bonmarin I, Santa-Olalla P, Lévy-Bruhl D. Modelling the impact of vaccination on the epidemiology of varicella zoster virus. Rev Epidemiol Sante Publique. 2008;56(5):323–31. doi: 10.1016/j.respe.2008.07.087. [DOI] [PubMed] [Google Scholar]
- 20.Gao Z, Gidding HF, Wood JG, MacIntyre CR. Modelling the impact of one-dose vs. two-dose vaccination regimens on the epidemiology of varicella zoster virus in Australia. Epidemiol Infect. 2010;138(4):457–68. doi: 10.1017/S0950268809990860. [DOI] [PubMed] [Google Scholar]
- 21.Karhunen M, Leino T, Salo H, Davidkin I, Kilpi T, Auranen K. Modelling the impact of varicella vaccination on varicella and zoster. Epidemiol Infect. 2010;138(4):469–81. doi: 10.1017/S0950268809990768. [DOI] [PubMed] [Google Scholar]
- 22.Siedler A, Arndt U. Impact of the routine varicella vaccination programme on varicella epidemiology in Germany. Euro Surveill. 2010;15:19530. [PubMed] [Google Scholar]
- 23.Quian J, Rüttimann R, Romero C, Dall’Orso P, Cerisola A, Breuer T, Greenberg M, Verstraeten T. Impact of universal varicella vaccination on 1-year-olds in Uruguay: 1997–2005. Arch Dis Child. 2008;93(10):845–50. doi: 10.1136/adc.2007.126243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giammanco G, Ciriminna S, Barberi I, Titone L, Lo Giudice M, Biasio LR. Universal varicella vaccination in the Sicilian paediatric population: rapid uptake of the vaccination programme and morbidity trends over five years. Euro Surveill. 2009;14:19321. [PubMed] [Google Scholar]
- 25.Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med. 2013;159(11):739–45. doi: 10.7326/0003-4819-159-11-201312030-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai K, Yawn BP, Wollan P, Harpaz R. Increasing incidence of herpes zoster over a 60-year period from a population-based study. Clin Infect Dis. 2016;63(2):221–26. doi: 10.1093/cid/ciw296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993–2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52(3):332–40. doi: 10.1093/cid/ciq077. [DOI] [PubMed] [Google Scholar]
- 28.Harpaz R, Leung JW. The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: changing patterns among older adults. Clin Infect Dis. 2019;69(2):341–44. doi: 10.1093/cid/ciy953. [DOI] [PubMed] [Google Scholar]
- 29.Wolfson LJ, Daniels VJ, Altland A, Black W, Huang W, Ou W. The impact of varicella vaccination on the incidence of varicella and Herpes Zoster in the United States: updated evidence from observational databases, 1991–2016. Clin Infect Dis. 2020. March 3;70(6):995–1002. doi: 10.1093/cid/ciz305. [DOI] [PubMed] [Google Scholar]
- 30.MacIntyre R, Stein A, Harrison C, Britt H, Mahimbo A, Cunningham A. Increasing trends of herpes zoster in Australia. PLoS One. 2015;10(4):e0125025. doi: 10.1371/journal.pone.0125025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly HA, Grant KA, Gidding H, Carville KS. Decreased varicella and increased herpes zoster incidence at a sentinel medical deputising service in a setting of increasing varicella vaccine coverage in Victoria, Australia, 1998 to 2012. Euro Surveill. 2014;19(41):20926. doi: 10.2807/1560-7917.ES2014.19.41.20926. [DOI] [PubMed] [Google Scholar]
- 32.Marra F, Chong M, Najafzadeh M. Increasing incidence associated with herpes zoster infection in British Columbia, Canada. BMC Infect Dis. 2016;16(1):589. doi: 10.1186/s12879-016-1898-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell ML, Dover DC, Simmonds KA, Svenson LW. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32(47):6319–24. doi: 10.1016/j.vaccine.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Siedler A, Dettmann M. Hospitalization with varicella and shingles before and after introduction of childhood varicella vaccination in Germany. Hum Vaccin Immunother. 2014;10(12):3594–600. doi: 10.4161/hv.34426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toyama N, Shiraki K. Miyazaki dermatologist society. Universal varicella vaccination increased the incidence of herpes zoster in the child-rearing generation as its short-term effect. J Dermatol Sci. 2018;92(1):89–96. doi: 10.1016/j.jdermsci.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Choi J, Park S, Han K. The trend in the incidence of varicella and herpes zoster in Korea after implementation of universal 1-dose varicella vaccination, 2002–2015. 27th European Congress of Clinical Microbiology and Infectious Diseases; Vienna, Austria; 2017. [Google Scholar]
- 37.Esteban-Vasallo MD, Gil-Prieto R, Dominguez-Berjon MF, Astray-Mojales Y, Gil de Miguel A. Temporal trends in incidence rates of herpes zoster among patients treated in primary care centers in Madrid (Spain), 2005–2012. J Infect. 2014;68(4):378–86. doi: 10.1016/j.jinf.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 38.Wu PY, Wu HD, Chou TC, Sung FC. Varicella vaccination alters the chronological trends of herpes zoster and varicella. PLoS One. 2013;8(10):e77709. doi: 10.1371/journal.pone.0077709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waye A, Jacobs P, Tan B. The impact of the universal infant varicella immunization strategy on Canadian varicella-related hospitalization rates. Vaccine. 2013;31(42):4744–48. doi: 10.1016/j.vaccine.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 41.Gidding HF, Brisson M, Macintyre CR, Burgess MA. Modelling the impact of vaccination on the epidemiology of varicella zoster virus in Australia. Aust N Z J Public Health. 2005;29(6):544–51. doi: 10.1111/j.1467-842X.2005.tb00248.x. [DOI] [PubMed] [Google Scholar]


