ABSTRACT
Outbreaks of infectious diseases cause great fear and a desire to avoid infection. One of the most effective outbreak containment methods is vaccination. However, in order for this strategy to be effective, a majority of the susceptible population should be vaccinated in a short time. This may require changing the practice of immunization execution and changing attitudes toward vaccination. In the survey on the attitudes of Polish parents and guardians toward vaccinations, we asked about the acceptance of vaccination in places other than health-care facilities in both non-epidemic and epidemic conditions. The study was conducted using an anonymous questionnaire in two Warsaw hospitals between August 2018 and February 2019 and was addressed to parents and legal guardians of children. At the time of the survey, “epidemic” was a hypothetical term. Two hundred fifty respondents participated in the study. The pharmacy was the most accepted non-healthcare facility vaccination location, both normally and during an outbreak, with 54.4% (123/226) and 75.2% (170/226) of respondents finding pharmacies an acceptable location, respectively. A gas station had the lowest acceptance: 5.8% (13/226) and 28.8% (65/226), respectively. The only statistically significant demographic factors affecting acceptance of each vaccination location were male sex (p = .001) and higher education level (p = .001). Of those surveyed, 58.5% (131/224) would approve of vaccination in front of a hospital or outpatient clinic during an outbreak; 70.5% (43/61) of men versus 54.0% (88/163) of women, p = .026. In conclusion, during an outbreak, people would be more likely to accept vaccination at locations other than a health-care facility.
KEYWORDS: Acceptance, epidemic, immunization, vaccination execution
Introduction
Infectious diseases are a permanent and inseparable companion of mankind. The most effective method of preventing many of them is vaccination. Thanks to worldwide vaccination, smallpox has been eradicated, poliomyelitis is nearly eliminated, and the incidence of many other infectious diseases has been significantly reduced. The World Health Organization (WHO) estimates that vaccines prevent 3 million deaths and many millions of cases of disease annually, including diseases with long-term health sequelae.1 Currently, the incidence of many infectious diseases is low, especially in developed countries, where national immunization programs have existed for many years and children are routinely vaccinated; thus, there is little fear of these diseases. However, the number of parents who refuse to vaccinate their children has recently increased. In Poland, there were approximately 2500 cases of vaccination refusal in 2009, and almost 40,000 in 2018.2 There are many reasons for vaccine refusal, including psychological reasons (i.e., fear), philosophical beliefs (i.e., veganism), or religious background.3,4 The range of parents’ concerns about vaccinations – as research shows – is very large. Parents are afraid of their children receiving too many shots during one visit, 5 as well as fever after vaccination5 and other side effects that they have heard about from other people or from the media.6,7 There are parents who believe that the obligation to vaccinate violates their civil rights, as evidenced by the clear decline in vaccinations in Italy, France, and Australia, where the governments have in recent years decided to require vaccination of children who are to attend public education facilities.8 Parents declare that their fear of vaccination is caused by the lack of information on vaccine components9 and about vaccines in general.10 Moreover, people who declare that they have too little (not enough) information about vaccines tend to present a negative attitude toward vaccination.11 Some people who are generally not opposed to vaccination may not be sufficiently motivated to set a date for and attend a vaccination visit.12 Paradoxically, many parents refuse to vaccinate their children because they want to protect them against diseases, damage, and malformations; nonetheless, they base their knowledge on pseudoscientific reports by homeopaths and naturopaths, among others, on half-truths taken from unverified sources on the Internet, and on so-called anecdotal evidence, or overheard stories of the harmfulness of vaccines.13 Vaccine hesitancy is an increasing and global problem, as reported by the WHO12 and the European Centre for Disease Prevention and Control (ECDC).14
Occasionally, for reasons that are not fully understood, the number of people suffering from a specific infectious disease increases rapidly and an epidemic occurs.15 In the past, outbreaks of cholera and bubonic plague regularly decimated the population of Europe. Several epidemics also occurred in the twenty-first century: severe acute respiratory syndrome (SARS) in 2003, avian influenza in 2003–2006, influenza A/H1N1pdm in 2009–2010, Ebola virus disease in 2013–2016, and, currently, a novel coronavirus disease (COVID-19). Epidemics invariably cause great fear and a desire to avoid infection. If a vaccine is available, universal vaccination is the most effective way to contain an outbreak. Examples are the control of invasive meningococcal disease caused by serogroup C in Great Britain in the late 1990s by introducing universal vaccination of infants and catching-up vaccination of other age groups16 or reducing the incidence of Ebola fever by ring vaccination in some African countries a few years ago.17
The threat of disease during an outbreak may change attitudes toward vaccination. People who have not been vaccinated previously (for various reasons) may decide to get vaccines. In an epidemic especially, the effectiveness of vaccination depends on its coverage. This means that large numbers of people must be vaccinated in a short time. High coverage of polio vaccination in Africa was achieved in large part because vaccination teams reached individual villages and children were vaccinated in open areas, such as the village’s main square.
As part of the survey on attitudes of Polish parents toward vaccination, we asked whether the respondents would accept vaccinations in locations other than health-care facilities both in normal conditions and in case of an outbreak. The study was conducted in the years 2018 and 2019; thus, at the time of the survey, “outbreak” was a hypothetical term.
Methods
The study was conducted using an anonymous questionnaire in two Warsaw hospitals (Regional Hospital for Infectious Diseases and Pediatric Teaching Clinical Hospital) between August 2018 and February 2019. The survey, in the form of a questionnaire consisting of closed questions, was addressed to parents and legal guardians of children. It was collected directly by students or medical staff during inpatient stays or outpatient visits. Only one parent of a patient (the mother or the father) submitted the survey.
Questionnaire
In addition to demographic data, such as sex and gender, number of children, and education level, the questionnaire contained 21 closed questions concerning attitudes toward both vaccination in general and vaccination of the respondents’ children. The survey included two questions about acceptance of vaccination executed in a non-healthcare facility (nHCF; at a pharmacy, gas station, or shopping mall and in front of a hospital, outpatient clinic, or town hall) in normal conditions and in an epidemic. The possible answers ranged from 1 (“definitely not”) to 6 (“definitely yes”). This report presents an analysis of the answers to those two questions, together with demographic data and answers to a question regarding a self-assessment of knowledge about vaccination. For this question, the possible answers ranged from 1 (“definitely poor”) to 6 (“definitely high”). Analysis of the rest of the survey will be the subject of another report.
Statistical analysis
For the purposes of correlation analysis, answers >3/6 points were defined as positive. To estimate the influence of different variables on the attitude toward vaccination logistic regression was used. The statistical significance threshold was set at p < .05.
Results
In total, 250 respondents participated in the study, and 9.6% (24/250) of questionnaires were rejected due to the lack of answers to at least two questions. There was a statistically significant positive correlation between the number of children and the age of the respondents (p < .001). The detailed characteristics of the respondents are presented in Table 1.
Table 1.
Characteristics of the study group
| Age (y) (%, n) | <36 |
≥36 |
|---|---|---|
| 34.1 (77) | 65.9 (149) | |
| Sex (%, n) | Male | Female |
| 27.0 (61) | 73.0 (165) | |
| Education level (%, n) | Primary or secondary | Higher |
| 27.4 (62) | 72.6 (164) | |
| Number of children in family | 1 | >1 |
| 31.9% (72) | 68.1% (154) |
The pharmacy achieved the greatest acceptance from the respondents as a nHCF vaccination site, both normally and during the epidemic, with acceptance rates of 54.4% (123/226) and 75.2% (170/226), respectively. In contrast, the gas station had the lowest rate of acceptance: 5.8% (13/226) and 28.8% (65/226), respectively. In all cases, there was a clear trend to increase the acceptance of nHCF locations for vaccination during an outbreak (Figure 1). The majority of respondents (52.4%, 54 of the 103 who had expressed a negative attitude toward the pharmacy) changed their attitude toward the pharmacy from negative to positive, and only 25.4% (54/213) changed their attitude toward the gas station (Figure 2).
Figure 1.
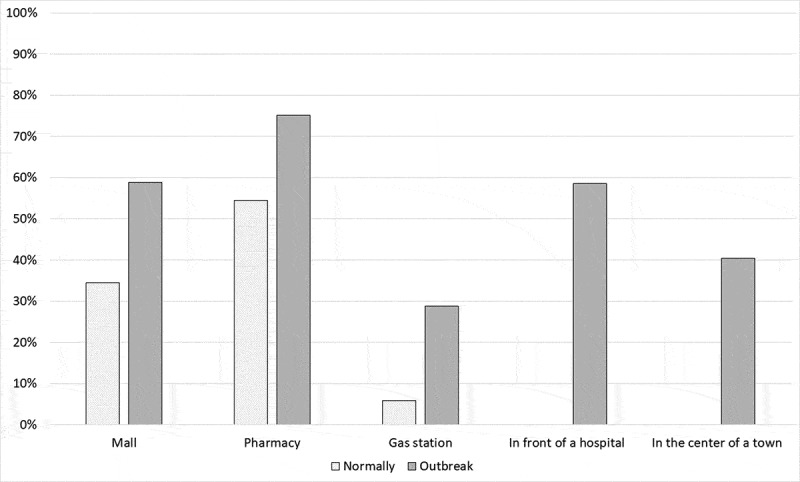
Acceptance of vaccination at an nHCF location normally and during an outbreak
Figure 2.
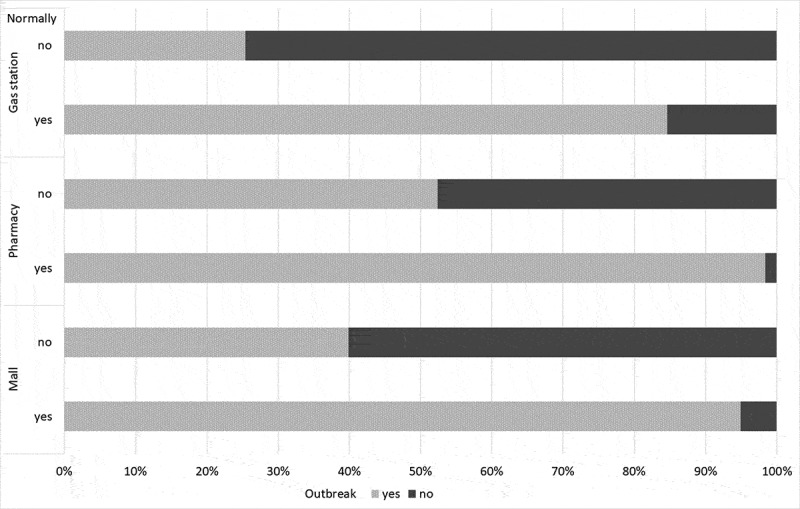
Change of acceptance of vaccination at nHCF locations depending on the epidemiological situation. The colors on the horizontal bars reflect the change in acceptance of vaccination at nHCF locations during the epidemic, depending on the initial acceptance or non-acceptance of vaccination
The only statistically significant factors affecting acceptance of nHCF were male sex and – in cases of an epidemic – having attended post-secondary education (higher education level). In addition, acceptance was more common among respondents <36 y old and among those with only one child (Table 2); however, the differences were not statistically significant.
Table 2.
Acceptance of vaccination at nHCF places according to demographic characteristics
| Sex | Male (n = 61) | Female (n = 165) | β | OR [CI 95%] | p | |
|---|---|---|---|---|---|---|
| Shopping mall | Normally | 52.5% (32) | 27.9% (46) | 1.052 | 2.864 [1.542–5.317] | 0.001 |
| Outbreak | 77.1% (47) | 52.1% (86) | 1.124 | 3.076 [1.540–6.141] | 0.001 | |
| Pharmacy | Normally | 67.2% (41) | 49.7% (82) | 0.717 | 2.049 [1.099–3.818] | 0.024 |
| Outbreak | 88.5% (54) | 70.3% (116) | 1.216 | 3.375 [1.419–8.025] | 0.006 | |
| Gas station | Normally | 8.2% (5) | 4.8% (8) | 0.621 | 1.861 [0.567–6.108] | 0.306 |
| |
Outbreak |
41.0% (25) |
24.2% (40) |
0.780 |
2.182 [1.140–4.174] |
0.018 |
| Education level |
Primary and secondary (n = 62) |
Higher (n = 164) |
β |
OR [CI 95%] |
p |
|
| Shopping mall | Normally | 27.4% (17) | 37.2% (61) | 0.344 | 1.410 [0.726–2.738] | 0.310 |
| Outbreak | 40.3% (25) | 65.9% (108) | 0.984 | 2.674 [1.440–4.966] | 0.001 | |
| Pharmacy | Normally | 46.8% (29) | 57.3% (94) | 0.354 | 1.425 [0.784–2.590] | 0.246 |
| Outbreak | 67.7% (42) | 78.0% (128) | 0.425 | 1.529 [0.786–2.975] | 0.211 | |
| Gas station | Normally | 6.5% (4) | 5.5% (9) | −0.311 | 0.733 [0.210–2.553] | 0.625 |
| |
Outbreak |
12.9% (8) |
34.8% (57) |
1.188 |
3.280 [1.445–7.442] |
0.004 |
| Age (y) |
<36 (n = 77) |
≥36 (n = 149) |
β |
OR [CI 95%] |
p |
|
| Shopping mall | Normally | 40.3% (31) | 31.5% (47) | −0.332 | 0.718 [0.382–1.349] | 0.303 |
| Outbreak | 65.0% (50) | 55.7% (83) | −0.381 | 0.683 [0.362–1.290] | 0.240 | |
| Pharmacy | Normally | 58.4% (45) | 52.3% (78) | −0.220 | 0.802 [0.440–1.464] | 0.473 |
| Outbreak | 80.5% (62) | 72.5% (108) | −0.622 | 0.537 [0.258–1.117] | 0.096 | |
| Gas station | Narmally | 9.1% (7) | 4.0% (6) | −0.687 | 0.503 [0.148–1.713] | 0.272 |
| |
Outbreak |
36.4% (28) |
24.8% (37) |
−0.612 |
0.542 [0.280–1.049] |
0.069 |
| Number of children in family |
1 (n = 72) |
>1 (n = 154) |
β |
OR [CI 95%] |
p |
|
| Shopping mall | Normally | 41.7% (30) | 31.2% (48) | −0.333 | 0.717 [0.380–1.351] | 0.304 |
| Outbreak | 62.5% (45) | 57.1% (88) | −0.071 | 0.932 [0.491–1.769] | 0.829 | |
| Pharmacy | Normally | 58.3% (42) | 52.6% (81) | −0.138 | 0.871 [0.474–1.600] | 0.656 |
| Outbreak | 73.6% (53) | 76.0% (117) | 0.378 | 1.460 [0.721–2.958] | 0.294 | |
| Gas station | Normally | 9.7% (7) | 3.9% (6) | −0.725 | 0.484 [0.144–1.634] | 0.243 |
| Outbreak | 29.2% (21) | 28.6% (44) | 0.217 | 1.242 [0.627–2.461] | 0.535 | |
A majority of respondents 58.5% (131/224) would agree to vaccination in front of hospital or outpatient clinic during an epidemic; 70.5% (43/61) of men versus 54.0% (88/163) of women, p = .026.
Discussion
The results of our questionnaire study indicate that during an epidemic, people would be much more likely to agree to be vaccinated at a public place that is not a health-care facility. There are no data concerning this or similar issues in the literature; therefore, we can only discuss our results.
Both in normal conditions and during an outbreak, the pharmacy was the most accepted location among respondents (54.4% and 75.2%, respectively) and the least accepted was a gas station (55.8% and 28.8%, respectively). This is justified because there is a correlation between trust in health care (including pharmacies) and the positive impact of this system on people’s behavior and attitudes, including those related to vaccinations.18,19 This may be a result of the centuries-old, well-established belief that in the pharmacy, one can not only buy and order medicines but also receive advice on health and disease management. In many countries all over the world, pharmacists are becoming advisors on vaccines and even co-create vaccination calendars and organize vaccine-related information campaigns.20 In addition, the design of the pharmacy itself (white color; neatness; lack of decorations; often, the presence of a blood pressure meter; leaflets on healthy lifestyle or medicines; etc.) usually suggests a close relationship with medicine. Trust in health care may also explain our finding that during an epidemic, respondents would significantly more often choose vaccination on the street in front of a medical facility (hospital or outpatient clinic) than in front of, for example, a town hall or a church.
Interestingly, men more often declared acceptance of nHCF. This applied to all three locations (pharmacy, gas station, shopping mall). This is likely due to the fact that men tend to be more task-oriented and make decisions faster, so they are ready to accept unusual ways to solve the problem.
Respondents with a higher education level were more likely to accept vaccination at a nHCF. This was true in both non-epidemic and epidemic conditions. The probable explanation is that highly educated people, who had defined their knowledge about vaccination as great, are generally more convinced of the benefits of vaccination and, therefore, more willingly accept the facilitation of vaccination, which may be achieved, for example, via vaccination ‘on the go’ at the pharmacy or shopping center nearby, as this does not require an appointment or other procedures associated with a routine visit in a health-care facility. Moreover, the speed of processing new information, including in the context of vaccination, health threats resulting from non-vaccination, and threats connected with an epidemic, is strongly correlated with the level of education; in this sense, people with a higher education level may make decisions about vaccination faster.21 Furthermore, those with a higher education level may also know that very little is needed to properly carry out vaccination. (Apart from a well-trained person, only a small amount of equipment is required.) Our results are consistent with the results of many studies that have assessed the execution of vaccination, both in developing22 and in developed countries such as Spain or the USA.23,24 In those studies, higher education was associated with better implementation of the vaccination program. However, some studies do not confirm this relationship.23,25 It is very difficult to directly compare all of these studies since they concern different regions of the world and various vaccines.
It is also worth noting that the tendency to accept nHCF as a place for vaccination was slightly more common among younger respondents and those with only one child, even taking into account the statistically significant correlation between those groups. On the basis of evolutionary principles, this can be explained by the tendency to bring the only child to the foreground of life, even facing the potential risk of vaccination in nonstandard places.
Our study was conducted when the risk of any outbreak in Europe, including Poland, seemed very low. However, its results illustrate the readiness of people to change their mind and accept solutions, that they did not previously approve, during unusual circumstances. Almost 60% of the respondents declared that in an epidemic, they would agree to vaccination ‘out in the open’ (i.e., in front of the hospital or outpatient clinic). This indicates that people who want protection against infection are willing to tolerate various inconveniences. This is important for the management of outbreaks, as mass vaccination is extremely effective. This was shown, for example, by the polio vaccination campaign in Africa, where vaccinators went from house to house vaccinating children.
Limitations of this study include the small number of respondents and the risk of sample bias, as those who participated in our study did not necessarily represent the view of the general population. The survey was collected among parents and legal guardians of children; the attitude of persons not having children can be different. Persons with higher educational level were overrepresented among the parents participating in the study.
Due to the current situation – the pandemic caused by the novel coronavirus SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) – our survey takes on new meaning. Polish experiences with vaccination carried out in epidemic conditions come from the twentieth century. In the 1950s, the national vaccination against poliomyelitis virus limited the outbreak of acute infantile paralysis within a few years.26 In 1963, compulsory mass vaccination against smallpox controlled the outbreak in Wroclaw.27 These vaccinations were carried out in completely different sociopolitical conditions. During the pandemic of influenza in the 2009/2010 season, a vaccine against A/H1N1pdm was not available in Poland, so we do not have any data on the acceptance of mass vaccinations carried out in case of an outbreak. There is currently no SARS-CoV-2 vaccine, but many research teams are working intensively to create one. It is very likely that immunization will be the main weapon in the fight against this pandemic, and its effectiveness will depend on vaccination coverage. Execution of mass vaccination might require to use other locations in addition to health-care facilities if their capacity would be limited. We hope that our results would help to plan vaccination execution taking into considerations that some public places as vaccination location are more acceptable than other.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.World Health Organization . Immunization highlights. 2010. [accessed 2020 April25]. http://www.who.int/immunization/newsroom/highlights
- 2.Czarkowski MP, Kondej B, Staszewska-Jakubik E, Cielebąk E. Szczepienia ochronne w Polsce w 2018 roku. Narowy Instytut Zdrowia Publicznego – Państwowy Zakład Higieny, Warszawa 2019, 85. [accessed 2020 April25] http://wwwold.pzh.gov.pl/oldpage/epimeld/2018/Sz_2018.pdf
- 3.McKee C, Bohannon K.. Exploring the reasons behind parental refusal of vaccines. J Pediatr Pharmacol Ther. 2016;21(2):104–09. doi: 10.5863/1551-6776-21.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson HJ, Clarke RM, Jarrett C, Eckersberger E, Levine Z, Schulz WS, Paterson P. Measuring trust in vaccination: a systematic review. Hum Vaccin Immunother. 2018;14(7):1599–609. doi: 10.1080/21645515.2018.1459252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy A, Lavail K, Nowak G, Basket M, Landry S. Confidence about vaccines in the United States: understanding parents’ perception. Health Aff (Milwood). 2011;30(6):1151–59. doi: 10.1377/hlthaff.2011.0396. [DOI] [PubMed] [Google Scholar]
- 6.Fredrickson DD, Davis TC, Arnould CL, Kennen EM, Hurniston SG, Cross JT, Bocchini JA. Childhood immunization refusal: provider and parents perceptions. Fam Med. 2004;36:431–39. [PubMed] [Google Scholar]
- 7.Gualano MR, Olivero E, Voglino G, Corezzi M, Rossello P, Vicentini C, Bert F, Siliquini R. Knowledge, attitudes and beliefs towards compulsory vaccination: a systematic review. Hum Vaccin Immunother. 2019;15(4):918–31. doi: 10.1080/21645515.2018.1564437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Ambrogi M. Why do parents not want to vaccinate their children? Lancet. 2018;18(8):844. doi: 10.1016/S1473-3099(18)30442-0. [DOI] [Google Scholar]
- 9.Saada A, Lieu TA, Morain SR, Zikmund-Fisher BJ, Wittenberg E. Parents’ choices and rationales for alternative vaccination schedules: a qualitative study. Clin Pediatr (Phila). 2015;54(3):236–43. doi: 10.1177/0009922814548838. [DOI] [PubMed] [Google Scholar]
- 10.Harmsen IA, Mollema L, Ruiter RA, Paulussen TG, de Melker HE, Kok G. Why parents refuse childhood vaccination: a qualitative study using online focus groups. BMC Pub Health. 2013;13:1183. doi: 10.1186/1471-2458-13-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gust DA, Kennedy A, Shiu I, Smith PJ, Nowak G, Pickering LK. Parent attitudes toward immunizations and healthcare providers the role of information. Am J Prev Med. 2005;29(2):105–12. doi: 10.1016/j.amepre.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Lane S, MacDonald NE, Marti M, Dumolard L. Vaccine hesitancy around the globe: analysis of three years of WHO/UNICEF Join reporting Form data 2015-2017. Vaccine. 2018;36(26):3861–67. doi: 10.1016/j.vaccine.2018.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . Six common misconceptions about immunization. https://www.who.int/vaccine_safety/initiative/detection/immunization_misconceptions/en/index6.html [accessed 2020 April25].
- 14.European Centre for Disease Prevention and Control . Vaccine hesitancy among healthcare workers and their patients in Europe – a qualitative study. Stockholm, Sweden: ECDC; 2015. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/vaccine-hesitancy-among-healthcare-workers.pdf accessed 17June 2020 [Google Scholar]
- 15.Porta M. A Dictionary of epidemiology. Oxford, The United Kingdom: Oxford University Press; 2008. p. 79. [Google Scholar]
- 16.Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunization campaign against meningococcal serogroup C diseases in the UK: a success story. Vaccine. 2001;20(suppl.10):558–67. doi: 10.1016/S0264-410X(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 17.Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, Carroll MW, Dean NE, Diatta I, Doumbia M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet. 2017;389(100068):505–18. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mora T, Trapero-Bertran M. The influence of education on the access to childhood immunization: the case of Spain. BMC Pub Health. 2018;18(1):893. doi: 10.1186/s12889-018-5810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozawa S, Stack ML. Public trust and vaccine acceptance – international perspectives. Hum Vaccin Immunother. 2013;9(8):1774–78. doi: 10.4161/hv.24961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosado H, Bates I. An overview of current impact on immunization. Global report, International Pharmaceutical Federation, 2016
- 21.Schober T, Effect of measles outbreak on vaccination uptake, 2018, Working Paper, 1818 [DOI] [PubMed]
- 22.Acharya K, Paudel YR, Dharel D. The trend of full vaccination coverage in infants and inequalities by wealth quintile and maternal education: analysis from four recent demographic and health surveys in Nepal. BMC Pub Health. 2019;19(1):1673. doi: 10.1186/s12889-019-7995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rencken CA, Dunsiger S, Gjelsvik A, Amanullah S. Higher education associated with better national tetanus vaccination coverage: A population-based assessment. Prev Med. 2020;17:106063. doi: 10.1016/j.ypmed.2020.106063. [DOI] [PubMed] [Google Scholar]
- 24.Lu PJ, Srivastav A, Santibanez TA, Christopher Stringer M, Bostwick M, Dever JA, Stanley Kurtz M. Williams WW Knowledge of influenza vaccination recommendation and early vaccination uptake during the 2015-16 season among adults aged ≥18years – United States. Vaccine. 2017;35(34):4346–54. doi: 10.1016/j.vaccine.2017.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faisal-Cury A, Levy RB, Tourinho MF, Grangeiro A, Eluf-Neto J. Vaccination coverage rates and predictors of HPV vaccination among eligible and non-eligible female adolescents at the Brazilian HPV vaccination public program. BMC Pub Health. 2020;20(1):458. doi: 10.1186/s12889-020-08561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Przesmycki F, Dobrowolska H, Mirski B, Wior H, Stanczyk R, Zaleska H. Vaccination against poliomyelitis in Poland with types 1 and 3 attenuated viruses of Koprowski. Bull World Health Org. 1962;26:733–43. [PMC free article] [PubMed] [Google Scholar]
- 27.Arendzikowski B, Kocielska W, Przestalska H. Smallpox epidemic in Wroclaw in 1963. Przegl Epidemiol. 1964;18:153–63. [PubMed] [Google Scholar]


