ABSTRACT
In 2015, the world witnessed the resurgence and global spread of Zika virus (ZIKV). This arbovirus infection is associated with Guillain-Barré syndrome in adults and with devastating congenital malformations during pregnancy. Despite scientific efforts, the development of a vaccine capable of inducing long-term protection has been challenging. Without a safe and efficacious licensed vaccine, control of virus transmission is based on vector control, but this strategy has been shown to be inefficient. An effective and protective vaccine relies on several requirements, which include: (i) induction of specific immune response against immunodominant antigens; (ii) selection of adjuvant-antigen formulation; and (iii) assessment of safety, effectiveness, and long-term protection. In this commentary, we provide a brief overview about the current efforts for the development of an efficacious ZIKV vaccine, covering the most important preclinical trials up to the formulations that are now being evaluated in clinical trials.
KEYWORDS: Zika virus, vaccine, clinical trials
Zika virus (ZIKV) is a mosquito-borne flavivirus first isolated from a rhesus macaque in 1947.1 Before 2015, ZIKV infection was reported in a few countries and associated mostly with a mild disease. Thenceforth, ZIKV outbreaks spread across more than 80 countries, and the disease has been associated with severe complications.2 Although ZIKV shares many structural features with other flavivirus such as dengue virus (DENV), its ability to cause congenital malformations during pregnancy3, and rare neurological disorders in adults, such as Guillain-Barré syndrome,4 makes this virus uniquely dreadful. Given the effectiveness of some vaccines against flaviviruses like yellow fever virus (YFV) and Japanese encephalitis virus (JEV), the pursuit of an effective vaccine candidate against ZIKV is attainable and has been the subject of intensive research. Currently, there are several ongoing clinical trials (Phases I and II) to develop a vaccine to prevent ZIKV infection (Table 1) using different strategies (Figure 1).
Table 1.
ZIKV-vaccine candidates in clinical trial
| Vaccine strategy | Candidate name | Antigen | Sponsor | Status | Phase I | Phase II | References |
|---|---|---|---|---|---|---|---|
| GLS-5700 | GeneOne Life Science, Inc./ Inovio Pharmaceuticals vio | Completed | NCT02809443 | 13 | |||
| DNA | VRC5283 | prM-E | NIAID/VRC | Completed | NCT02996461 | NCT03110770 | 14,15 |
| VRC5288 | Completed | NCT02840487 | 14 | ||||
| mRNA | mRNA-1325 | prM-E | Moderna Therapeutics | Completed | NCT03014089 | 21 | |
| mRNA-1893 | Active, not recruiting | NCT04064905 | 23 | ||||
| Completed or | NCT02963909 | ||||||
| ZPIV | NIAID/WRAIR/BIDMC | Active, not recruiting* |
NCT02952833 NCT02937233 NCT03008122 |
6,8,24 | |||
| Whole inactivated | PIZV (TAK-426) | Virus | Takeda Pharmaceuticals | Active, not recruiting |
NCT03343626 | 26 | |
| BBV121 | Bharat Biotech International | Completed | CTRI/2017/05/008539 | 27 | |||
| VLA1601 | Valneva Austria GmbH/Emergent Biosolutions | Completed | NCT03425149 | - | |||
| Live attenuated | rZIKV/D4130-713 | Virus | NIAID | Completed | NCT03611946 | 30,31 | |
| MV-ZIKA | Themis Bioscience GmbH | Completed | NCT02996890 | 38 | |||
| Viral vectored | MV-ZIKA RSP | Recruiting | NCT04033068 | 38 | |||
| ChAdOx1 Zika | prM-E | University of Oxford | Recruiting | NCT04015648 | 34 |
*Only NCT03008122 is active, not recruiting.
Figure 1.
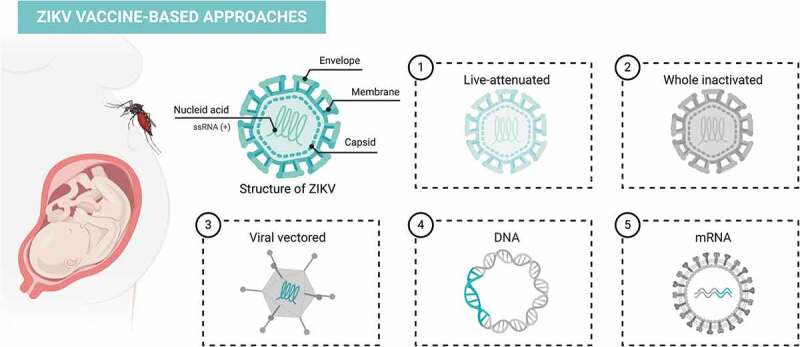
ZIKV vaccine-based approaches
(1) Live-attenuated vaccine: in this approach, the virus is attenuated by different methods and loses its effectivity to replicate and promote disease. The live-attenuated virus induces a potent immune response, but despite of being active the virus in the formulation is not able to induce sickness in an immunocompetent individual. The attenuated vaccine usually guarantees long-term protection and only requires a single dose, for example, in the widely described YF vaccine. (2) Whole inactivated: the virus becomes noninfectious using chemical agents such as formalin, β-propiolactone or heat; therefore, the virus is unable to cause disease nor to infect the cells or replicate. (3) Viral vector: viral vectors are genetically engineered viruses without pathogenicity, that retains their capacity to infect host cells but not causing any disease. Through genetic engineering techniques, it is possible to add antigens of interest into the genetic material of the virus. There are many viral vectors like adenovirus and vaccinia virus. (4) DNA vaccine: this vaccine is based on delivery of genes encoding a specific antigen that is subsequently transcribed and translated in proteins by host cells. Furthermore, DNA vaccines are able to induce cellular and humoral immune responses safely, with low cost and are easily manufactured. (5) mRNA: as DNA vaccines, the strategy of vaccination with mRNA can induce a potent cellular and humoral immune responses. Figure created with BioRender.com.
DNA-based vaccine formulations are one of the most promising candidates tested in humans due to their ability to induce humoral and cellular immune responses, low cost, high stability, and safety profile,5 without infection or replication capacity. Since ZIKV outbreaks, researchers have evaluated the immunogenicity and protection profiles of different DNA-based vaccines encoding E and prM-E proteins.6 In preclinical studies, constructs encoding the full-length prM-E sequence showed to be the most promising candidates to induce neutralizing antibodies, T-cell immunity, and protection in mice6,7 and nonhuman primates.8 Passive transfer of antibodies induced by DNA vaccines provided sterile protection in a lethal challenge model.9 Recently, we showed that a recombinant protein and a plasmid DNA based on the ZIKV E protein induced a robust humoral and polyfunctional CD4+ T cell response.10 In order to increase the immunogenicity of DNA vaccines, several strategies have been described,11 such as the use of in vivo electroporation, combination with adjuvants, and heterologous prime-boost immunization.12 Inovio Pharmaceuticals developed the first ZIKV DNA vaccine candidate (GLS-5700) tested in clinical trials (NCT02809443 and NCT02887482). GLS-5700 was administered via intradermal injection followed by electroporation, and 62% of the volunteers developed neutralizing antibodies against ZIKV after receiving three doses of the vaccine candidate.13
Other two DNA vaccines are being tested in humans: VRC5283 and VRC5288, developed by the Vaccine Research Center14 of the National Institute of Allergy and Infectious Diseases (NIAID). Unlike GLS-5700, modifications have been made to improve protein-expression and subviral particle release from transduced cells. To create the VRC5283 vaccine, the ZIKV prM signal sequence was replaced with the analogous region of JEV. In VRC5288, besides the modification in the signal sequence, the carboxyterminal stem-anchor region of ZIKV protein E was also exchanged to the equivalent JEV sequence.15 Both vaccine-formulations elicited high titers of neutralizing antibodies that protected mice and nonhuman primates after challenge.15 For this reason, both DNA vaccines were selected for immunogenicity and safety evaluation in humans (NCT02840487 and NCT02996461). Recent functional analysis revealed that despite the capacity to induce neutralizing antibodies, the ability to bind to the mature virion better predicts vaccine-induced protection and should be considered to assay new candidates.16 VRC5283 was shown to be safe, well-tolerated and induced T-cell immune response and neutralizing antibodies,14 moving forward to a Phase II clinical trial (NCT03110770).
Another promising, low-cost, and safe vaccine approach is based on non-replicating mRNA. The main advantage is that the mRNA vaccine can be directly translated in the cytoplasm upon cell transfection, contrary to a DNA vaccine which needs to enter the nucleus to start transcription.17 In recent years, lipid-encapsulated or naked forms of sequence-optimized mRNA candidates elicit potent immunity against several pathogens and cancer.18–21 A single dose of lipid-nanoparticle-encapsulated mRNA encoding prM- E-induced potent neutralizing antibodies and protected mice and nonhuman primates from viremia.21 Similarly, other encapsulated mRNA vaccine-conferred neutralizing antibodies and consequently sterilizing immunity in mice. This engineered vaccine encodes mutations into the conserved fusion-loop epitope in the E sequence that reduces the production of antibodies enhancing DENV infection.22 Two mRNA vaccine candidates for ZIKV developed by Moderna Therapeutics are being tested in Phase I clinical trials, named mRNA-1325 (NCT03014089) and mRNA-1893 (NCT04064905). In preclinical trials, mRNA-1893 protected against ZIKV transmission during pregnancy in mice.23
Efforts to develop a whole inactivated virus vaccine against the ZIKV vaccine began immediately after the 2015 outbreak. This platform has been successfully developed against other flaviviruses such as Tick-borne encephalitis virus (TBEV) and JEV. The first preclinical studies using a purified inactivated ZIKV vaccine (named as ZPIV) were described by Larocca et al.6 A single dose of formalin-inactivated ZIKV vaccine, adjuvanted with aluminum hydroxide, protected mice from different ZIKV challenge strains (Brazil and Puerto Rico ZIKV isolates).6 In addition, an extra dose of the ZPIV vaccine was also effective in rhesus macaques,8 and afforded robust protection even after 1 year of vaccination.24 The safety and immunogenicity evaluation of this vaccine candidate conducted by NIAID/WRAIR/BIDMC was confirmed in three clinical trials (NCT02963909, NCT02952833 and NCT02937233). Fourth trial in an endemic area is still ongoing (NCT03008122).
In a collaboration between WRAIR and Sanofi Pasteur, the vaccine was optimized using Pasteur’s experience in flavivirus vaccine development. A modified and optimized ZIKV-vaccine (ZPIV-SP) showed improved immunogenicity compared with the first-generation vaccine in mice,25 supporting advancement of the ZPIV-SP candidate toward clinical development. Other formalin-inactivated ZIKV candidates were developed by Takeda Pharmaceuticals, Valneva Austria GmbH/Emergent BioSolutions and Bharat Biotech, Hyderabad (NCT03343626, NCT03425149, and CTRI/2017/05/008539, respectively). In preclinical trials, TAK-426 (alum-adjuvanted PIZV) by Takeda Pharmaceuticals induced high levels of neutralizing antibodies that were able to confer passive protection to naive mice against lethal challenge.26 Similarly, an alum-adjuvanted inactivated-vaccine (BBV121. Bharat Biotech) conferred protection against Asian and African ZIKV strains in immunodeficient mice.27
First-generation live-attenuated vaccines (LAV) against other flavivirus diseases, like YFV and JEV, have also been evaluated as potential ZIKV-vaccine candidates. There are few ways to reduce the virulence of the pathogen for vaccine production – differently from that used for the 17D YF vaccine, genetic manipulation of the viral genome has been used for ZIKV attenuation. Strategies are based on the removal of specific carbohydrate addition sites, site-directed deletions on 3′-UTR region or production of chimeric-attenuated flaviviruses that encode the ZIKV prM and E sequences.28 A ZIKV- 3′UTR-LAV candidate induced protective immunity in mice and rhesus macaques, also preventing pregnancy transmission and testis damage in mice.29 Similarly, a single-dose of plasmid-launched live-attenuated ZIKV vaccine-induced seroconversion, T-cell immune response, and sterile immunity in mice.30
Furthermore, a chimeric-attenuated vaccine swapping the prM-E sequence between DENV-2 and ZIKV into DENV-2 backbone or into ZIKV backbone was highly immunogenic and prevented viral infection by DENV-2 or ZIKV after challenge, respectively.31 Another chimeric-attenuated candidate using ZIKV prM-E in a DENV-4 backbone has been developed by NIAID, and recently completed a Phase 1 trial (NCT03611946). Different viral vectors that express ZIKV genes have been tested as a delivery platform in pursuit to develop an effective ZIKV-vaccine. Adenovirus-based vaccine vectors have been tested in preclinical settings and demonstrated high immunogenic potential.32–36 A single-shot of a rhesus adenovirus serotype 52 vector vaccine candidate expressing the ZIKV prM-E elicited neutralizing antibodies and long-term protection against viral challenge in rhesus monkeys.8,24 A replication-deficient chimpanzee adenoviral (ChAdOx1) ZIKV-vaccine candidate also provided protection and long-lasting anti-envelope immunity in mice, and will be next evaluated in a clinical trial (NCT04015648).34 Other strategies using a vaccinia-based construct against both ZIKV and Chikungunya virus (CHIKV) induced neutralizing antibodies in mice and protected against viremia and arthritis or fetal/placental infection and testis damage after CHIKV or ZIKV challenges, respectively.35 Furthermore, a vesicular stomatitis virus (VSV) vector expressing ZIKV prM-E induced strong cellular and humoral immune responses that protected mice from lethal challenge.37 Preclinical evaluation with a measles virus-based vaccine candidate expressing the ZIKV prM-E reduced plasma viremia and ZIKV load in distinct organs, preventing fetal infection during pregnancy.38 Now, two measles-based ZIKV-vaccine candidates developed by Themis Bioscience have been tested in Phase I clinical trial (NCT02996890 and NCT04033068).
Until now, substantial breakthroughs have been achieved toward the development of vaccine platforms to prevent ZIKV infection and effectively limit congenital syndrome. Without an effective-licensed ZIKV-vaccine, we are still susceptible to another epidemic equal or even worse than the 2015 outbreak, reminding that we are still dealing with the consequences of children born with neurological problems from the previous outbreak.
For this reason, the pursuit of a safe, effective, and long-term immunogenic vaccine against ZIKV continues.
Acknowledgments
The authors want to thank Prof. Silvia Beatriz Boscardin for the critical reading of the manuscript. JSA received fellowship from CAPES; VASL received fellowship from FAPESP (grant number 2018/05320-7); ERF received fellowship from AFIP and DSR received fellowship from CNPq.
Funding Statement
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico; Fundação de Amparo à Pesquisa do Estado de São Paulo [2017/17471-7].
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
References
- 1.Dick SFK GWA, Haddow AJ.. 1952. Zika virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg. 46:509–20. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 2.Organization WH . 2017. Zika virus, microcephaly, Guillain-Barré syndrome.
- 3.de Araujo TVB, Ximenes RAA, Miranda-Filho DB, Souza WV, Montarroyos UR, de Melo APL, Valongueiro S, de Albuquerque MFPM, Braga C, Filho SPB, et al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case-control study. Lancet Infect Dis 2018;18:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Styczynski AR, Malta J, Krow-Lucal ER, Percio J, Nobrega ME, Vargas A, Lanzieri TM, Leite PL, Staples JE, Fischer MX, et al. 2017. Increased rates of Guillain-Barre syndrome associated with Zika virus outbreak in the Salvador metropolitan area, Brazil. PLoS Negl Trop Dis. 11:e0005869. doi: 10.1371/journal.pntd.0005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorritsma SHT, Gowans EJ, Grubor-Bauk B, Wijesundara DK. 2016. Delivery methods to increase cellular uptake and immunogenicity of DNA vaccines. Vaccine. 34:5488–94. doi: 10.1016/j.vaccine.2016.09.062. [DOI] [PubMed] [Google Scholar]
- 6.Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, Boyd M, Ng’ang’a D, Kirilova M, Nityanandam R, et al. 2016. Vaccine protection against Zika virus from Brazil. Nature. 536:474–78. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin BD, Muthumani K, Warner BM, Majer A, Hagan M, Audet J, Stein DR, Ranadheera C, Racine T, De La Vega M-A, et al. 2017. DNA vaccination protects mice against Zika virus-induced damage to the testes. Nat Commun. 8:15743. doi: 10.1038/ncomms15743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Nganga D, Nanayakkara O, et al. 2016. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 353:1129–32. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muthumani K, Griffin BD, Agarwal S, Kudchodkar SB, Reuschel EL, Choi H, Kraynyak KA, Duperret EK, Keaton AA, Chung C, et al. 2016. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMEnv DNA vaccine. NPJ Vaccines. 1:16021. doi: 10.1038/npjvaccines.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amaral MP, Apostolico JS, Tomita N, Coirada FC, Lunardelli VAS, Fernandes ER, Souza HFS, Astray RM, Boscardin SB, Rosa DS, et al. 2020. Homologous prime-boost with Zika virus envelope protein and poly (I:C) induces robust specific humoral and cellular immune responses. Vaccine. 38:3653–64. doi: 10.1016/j.vaccine.2020.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Kutzler MA, Weiner DB. 2008. DNA vaccines: ready for prime time? Nat Rev Genet. 9:776–88. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kardani K, Bolhassani A, Shahbazi S. 2016. Prime-boost vaccine strategy against viral infections: mechanisms and benefits. Vaccine. 34:413–23. doi: 10.1016/j.vaccine.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 13.Tebas P, Roberts CC, Muthumani K, Reuschel EL, Kudchodkar SB, Zaidi FI, White S, Khan AS, Racine T, Choi H, et al. 2017. Safety and Immunogenicity of an Anti-Zika virus DNA vaccine - Preliminary report. N Engl J Med. doi: 10.1056/NEJMoa1708120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudinski MR, Houser KV, Morabito KM, Hu Z, Yamshchikov G, Rothwell RS, Berkowitz N, Mendoza F, Saunders JG, Novik L, et al. 2018. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet. 391:552–62. doi: 10.1016/S0140-6736(17)33105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, et al. 2016. Rapid development of a DNA vaccine for Zika virus. Science. 354:237–40. doi: 10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.:Maciejewski S, Ruckwardt TJ, Morabito KM, Foreman BM, Burgomaster KE, Gordon DN, Pelc RS, DeMaso, CR, Ko SY, Fisher BE, et al. Distinct neutralizing antibody correlates of protection among related Zika virus vaccines identify a role for antibody quality. Sci Transl Med. 2020;12.. doi: 10.1126/scitranslmed.aaw9066. [DOI] [PubMed] [Google Scholar]
- 17.Pardi N, Hogan MJ, Porter FW, Weissman D. 2018. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 17:261–79. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollard C, Rejman J, De Haes W, Verrier B, Van Gulck E, Naessens T, De Smedt S, Bogaert P, Grooten J, Vanham G, et al. 2013. Type I IFN counteracts the induction of antigen-specific immune responses by lipid- based delivery of mRNA vaccines. Mol Ther. 21:251–59. doi: 10.1038/mt.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinon F, Krishnan S, Lenzen G, Magne R, Gomard E, Guillet JG, Lévy J-P, Meulien P. 1993. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur J Immunol. 23:1719–22. doi: 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- 20.Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H. 2016. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 21.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, et al. 2017. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 543:248–51. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, et al. 2017. Modified mRNA vaccines protect against Zika virus infection. Cell. 168:1114–25 e10. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagger BW, Dowd KA, Chen RE, Desai P, Foreman B, Burgomaster KE, Himansu S, Kong W-P, Graham BS, Pierson TC, et al. 2019. Protective efficacy of nucleic acid vaccines against transmission of Zika virus during pregnancy in mice. J Infect Dis. 220:1577–88. doi: 10.1093/infdis/jiz338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbink P, Larocca RA, Visitsunthorn K, Boyd M, De La Barrera RA, Gromowski GD, Kirilova M, Peterson R, Li Z, Nanayakkara O, et al. 2017. Durability and correlates of vaccine protection against Zika virus in rhesus monkeys. Sci Transl Med. 9:eaao4163. doi: 10.1126/scitranslmed.aao4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecouturier V, Bernard MC, Berry C, Carayol S, Richier E, Boudet F, Heinrichs J. 2019. Immunogenicity and protection conferred by an optimized purified inactivated Zika vaccine in mice. Vaccine. 37:2679–86. doi: 10.1016/j.vaccine.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Baldwin WR, Livengood JA, Giebler HA, Stovall JL, Boroughs KL, Sonnberg S, Bohning KJ, Dietrich EA, Ong YT, Danh HK. 2018. Purified inactivated Zika vaccine candidates afford protection against lethal challenge in mice. Sci Rep. 8:16509. doi: 10.1038/s41598-018-34735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sumathy K, Kulkarni B, Gondu RK, Ponnuru SK, Bonguram N, Eligeti R, Gadiyaram S, Praturi U, Chougule B, Karunakaran L, et al. 2017. Protective efficacy of Zika vaccine in AG129 mouse model. Sci Rep. 7:46375. doi: 10.1038/srep46375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng WC, Soto-Acosta R, Bradrick SS, Garcia-Blanco MA, Ooi EE. 2017. The 5ʹ and 3ʹ untranslated regions of the flaviviral genome. Viruses. 9:137. doi: 10.3390/v9060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shan C, Muruato AE, Jagger BW, Richner J, Nunes BTD, Medeiros DBA, Xie X, Nunes JGC, Morabito KM, Kong W-P, et al. 2017. A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage. Nat Commun. 8:676. doi: 10.1038/s41467-017-00737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou J, Xie X, Luo H, Shan C, Muruato AE, Weaver SC, Wang T, Shi P-Y. 2018. A single-dose plasmid-launched live-attenuated Zika vaccine induces protective immunity. EBioMedicine. 36:92–102. doi: 10.1016/j.ebiom.2018.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie X, Yang Y, Muruato AE, Zou J, Shan C, Nunes BT, Medeiros DBA, Vasconcelos PFC, Weaver SC, Rossi SL. 2017. Understanding Zika virus stability and developing a chimeric vaccine through functional analysis. mBio. 8(1). doi: 10.1128/mBio.02134-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox F, van der Fits L, Abbink P, Larocca RA, van Huizen E, Saeland E, Verhagen J, Peterson R, Tolboom J, Kaufmann B, et al. 2018. Adenoviral vector type 26 encoding Zika virus (ZIKV) M-Env antigen induces humoral and cellular immune responses and protects mice and nonhuman primates against ZIKV challenge. PLoS One. 13(8):e0202820. doi: 10.1371/journal.pone.0202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Q, Chan JF, Poon VK, Wu S, Chan CC, Hou L, Yip CCY, Ren C, Cai J-P, Zhao M, et al. 2018. Immunization with a novel human type 5 adenovirus-vectored vaccine expressing the premembrane and envelope proteins of Zika virus provides consistent and sterilizing protection in multiple immunocompetent and immunocompromised animal models. J Infect Dis. 218(3):365–77. doi: 10.1093/infdis/jiy187. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Camacho C, Abbink P, Larocca RA, Dejnirattisai W, Boyd M, Badamchi-Zadeh A, Wallace ZR, Doig J, Velazquez RS, Neto RDL, et al. Rational Zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nat Commun. 2018;9:2441. doi: 10.1038/s41467-018-04859-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prow NA, Liu L, Nakayama E, Cooper TH, Yan K, Eldi P, Hazlewood JE, Tang B, Le TT, Setoh YX, et al. 2018. A vaccinia- based single vector construct multi-pathogen vaccine protects against both Zika and chikungunya viruses. Nat Commun. 9:1230. doi: 10.1038/s41467-018-03662-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu K, Song Y, Dai L, Zhang Y, Lu X, Xie Y, Zhang H, Cheng T, Wang Q, Huang Q, et al. Recombinant Chimpanzee Adenovirus Vaccine AdC7-M/E Protects against Zika Virus Infection and Testis Damage. J Virol. 2018;92.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emanuel J, Callison J, Dowd KA, Pierson TC, Feldmann H, Marzi A. 2018. A VSV- based Zika virus vaccine protects mice from lethal challenge. Sci Rep. 8:11043. doi: 10.1038/s41598-018-29401-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nurnberger C, Bodmer BS, Fiedler AH, Gabriel G, Muhlebach MD. A measles virus-based vaccine candidate mediates protection against Zika virus in an allogeneic mouse pregnancy model. J Virol. 2019;93. [DOI] [PMC free article] [PubMed] [Google Scholar]


