ABSTRACT
Immunoscore can accurately predict the prognosis of patients with stage I–III colorectal cancer. However, whether it can be used to predict the prognosis of colorectal cancer peritoneal metastases (CRCPM) remains to be validated. We analyzed peritoneal and ovarian metastases in 68 patients with CRCPM. The immunoscore (IS) was based on the infiltration level of CD3+ and CD8+ T cells, whereas the TBM score was derived from the infiltration level of CD3+, CD8+, CD20+ and CD163+ cells to tumor microenvironment (TME). The predictive value of IS and TBM scores for relapse-free survival (RFS) and overall survival (OS) of patients with CRCPM was analyzed using Kaplan Meier curve and Cox multivariate models. Significant difference in the infiltration levels of different immune cell subtypes in primary lesions, peritoneal metastasis and ovarian metastasis were compared using t-test.CRCPM patients with high IS (>1), high TBM1 score (≥2) or high TBM2 score (≥2) had a significantly longer OS (IS: median OS, not reached vs 23 months, p = .0078; TBM1: not reached vs 21.5 months, p = .013; TBM2: 39.3 months vs 15.2 months, p = .001). On the other hand, patients with high IS had a trend of improved RFS (13.4 months vs 11.0 months, p = .067). However, TBM1 and TBM2 score has no predictive utility for RFS. Multivariate analysis revealed that IS, TBM1 and TBM2 can accurately predict OS, but not RFS. Finally, the infiltration level of CD3+ T cells, CD8+ T cells, CD20+ B cells, and CD68+ macrophage was significantly higher in peritoneal metastatic tissue and ovarian metastatic tissue, relative to primary tumor tissues.The IS and TBM score of peritoneal metastases could effectively predict OS of patients with CRCPM. Peritoneal metastasis of colorectal cancer decreased the infiltration level of T and B cells.
KEYWORDS: Colorectal cancer, peritoneal metastases, immunoscore, tumor infiltrating immune cell, prognosis
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors, and the third leading cause of cancer-related morbidity and mortality worldwide.1 Besides, peritoneal metastasis (PM) exacerbates the CRC-related deaths.2 Previously, CRCPM was thought to be a terminal and an incurable cancer stage, requiring only palliative treatment.3 Due to its unique biological properties, PM is associated with very poor prognosis, relative to other organ metastases, such as liver or lung metastasis.4 However, recent advances in surgical technology and other treatment interventions have improved the overall survival (OS) of patients with M1c CRC.5,6 The advent of monoclonal antibody therapies (Programmed Cell Death 1, PD-1; Programmed Cell Death 1 Ligand 1, PDL-1; Cytotoxic T Lymphocyte Antigen 4, CTLA-4) that kill cancer cells by activating the adaptive immune system,7 have revolutionized cancer treatment. However, this treatment module is only effective in few patients with high mutation burden, characterized by under-expression of DNA mismatch repair proteins (dMMR) and microsatellite instability-high (MSI-high).8 On the other hand, tumors with low mutation burden or lower infiltration of immune cells usually exhibit immune resistance.9,10
Tumor microenvironment (TME) comprises of malignant and nonmalignant cells (immune cells, tumor blood vessels, lymphatic vessels, fibroblasts, adipocytes, vascular endothelial cells, etc.). The immune cells perform different function at various stages of tumor development and progression, resulting into a dynamic immune system.11 Infiltration of CD8+ T lymphocytes to TME can directly mount an immune response against the tumor. Other critical anti-tumor cells include tumor associated macrophages (TAMs) and B lymphocytes.11,12 Presence of tumor infiltrating lymphocytes (TILs) in TME influence the prognosis of head, neck, lung, kidney, ovarian, breast, colorectal and pancreatic cancer.13–19 Infiltration pattern of TILs, particularly CD8+ and CD3+ T cells both in early and advanced stage CRC strongly and independently predicts the prognosis of CRC.18,20 In addition, high-density B lymphocyte infiltration also indicates improved prognosis of CRC.21 Given their plasticity, of TAMs are polarized into different subtypes.22 Research shows that pan-TAMs are associated with a poor prognosis of most solid tumors.23 However, the prognostic value of TAMs in CRC remains controversial.23,24
The immunoscore based on the infiltration density of CD3+ and CD8+ TILs is used independently in predicting the prognosis of patients with stage II and III colon cancer.18 Based on this traditional immune scoring system, the prognostic value of TB scoring system (CD8+ T cells/CD20+ B cells) has also been validated for advanced CRC (liver metastasis, lung metastasis).21 However, very few studies have explored the immune cell infiltration profile of CRCPM. Here, we derived an accurate immune scoring system for predicting the prognosis of CRCPM. Thus we provide a theoretical basis for screening patients with CRCPM who are more suitable for immunotherapy. We analyzed the subtypes and infiltrating density of the immune cells in TME of primary CRC and PM. We also analyzed the immunological changes in TME following metastasis.
Methods and materials
Research participants
This study involved patients with CRCPM, who underwent abdominal metastasis resection at the Second Affiliated Hospital, Zhejiang University School of Medicine from January 2011 to December 2018. We included patients with pathologically diagnosed with CRCPM, fit for tumor cytoreductive surgery as a treatment option as well as those with sufficient pathological data and specimens of PM for analysis. Patients who underwent preoperative radiotherapy for abdominal tumors and those with autoimmune diseases were excluded from the study. The protocol for this study was approved by Institutional Review Board (IRB). All study participants consented to this research in writing.
Data collection
Patients were followed up to October 2019. The survival as well as recurrence status of patients were obtained using an outpatient system, inpatient records or telephone follow-up. Clinical and pathological data of those patients were collected and recorded. The data included pathological and histological type, T stage of the primary tumor, number of lymph node metastases, neurovascular invasion, among others. Clinical information, such as the anatomical site of the primary tumor, whether of the patients received hyperthermic intraperitoneal chemotherapy (HIPEC), presence of ascites, peritoneal cancer index (PCI) and completeness of cytoreduction (CC) were also recorded.
Immunohistochemistry
Paraffin-embedded slides of peritoneal metastatic lesion specimens were stained and labeled using anti CD3+ T cells (BOSTER, No. PB0112), CD8+ T cells (BOSTER, No. PB0235) and CD20+ B cells (BOSTER, No. PB0028) and CD68+ (BOSTER, No.M00602) as well as CD163+ macrophages (BOSTER, No.BA13856) using specific antibodies. The slides were stained using hematoxylin and eosin (HE). The CD3+, CD8+, CD20+, CD68+ and CD163+ cells were identified by a qualified pathologist. Two pathologists blinded to patient’s clinical information divided the immunohistochemical section into invasive margin (IM) or central area of tumor (CT). To confirm the location of the CT/IM, the CT area was defined to be at least a distance of 20x fields from the border of normal mucosa. The IM area was defined as a 20x field within the most distal tumor cells.25,26 The hot spots with positive staining in the two regions were obtained. Computer-assisted calculations of density of positively stained immune cells in both the CT and the IM of the primary focus of CRC were performed using Image J software (National Institute of Health, Bethesda, MD, USA). The same method was used in counting the positively stained immunocytes in the CT area of the PM and ovarian metastasis.
Image acquisition and quantification
The tissue sites with the highest density of tumor infiltrating immune cells in the CT and IM images were captured under high-power magnification (20×) using a Leica dm6000 B molecular microscope. A high-density image of positive cell was captured in the CT area and IM of primary focus, while for noninvasive margin staining sections, only the CT image of high-density infiltrating positive cells was taken. Computer assisted image analysis was performed using ImageJ software V1.48. Five built-in functions of ImageJ were used to calculate the number of positively stained cells per high power field of view (HP). The cutoff value of the infiltrating density for each tumor-associated immune cell subtype was calculated using the X-tile software V. 3.6.1. For relapse-free survival (RFS), the best cutoff values for high infiltration of CD3+, CD8+, CD20+, CD68+ and CD163+ immune cells were 110, 117, 143, 268 and 99 cells/HP, respectively. On the other hand, for OS, the best cutoff points for high infiltration of CD3+, CD8+, CD20+, CD68+ and CD163+ immune cells were 117.5, 88, 92, 158 and 40 cells/HP, respectively. Each immune cell subtype was assigned a dichotomous score (high infiltration density of CD3+, CD8+, CD20+ cells were scored 1, low density was scored 0; low infiltration density of CD163+ was scored 1, high infiltration density was scored 0) based on the set cutoff point. The traditional Immunoscore was derived using the infiltration density of CD3+ and CD8 + T cells. Patients with an Immunoscore = 2 were classified in to high-score group (IS-high), whereas those with an Immunoscore <2 were classified into a low-score group (IS-low). We also established a TBM scoring system 1 (TBM1), based on the infiltration density of CD8+, CD20+ and CD163+ immune cells. Patients with TBM1 ≥ 2 were classified in to high-score group (TBM1-high), TBM1 < 2 was defined as low-score group (TBM1-low). TBM scoring system 2 (TBM2) was established according to infiltration density of CD3+, CD8+, CD20+ and CD163+ immune cells. Patients with TBM2 ≥ 2 were classified into the high-score group (TBM2-high), whereas those with TBM2 < 2 were classified into the low-score group (TBM2-low).
Statistical analysis
The example of computer-assisted analysis of the densities of the positively stained cells is provided in Figure 1a to 1d (a→b→c→d). This method has been used in previous studies.20,27
Figure 1.
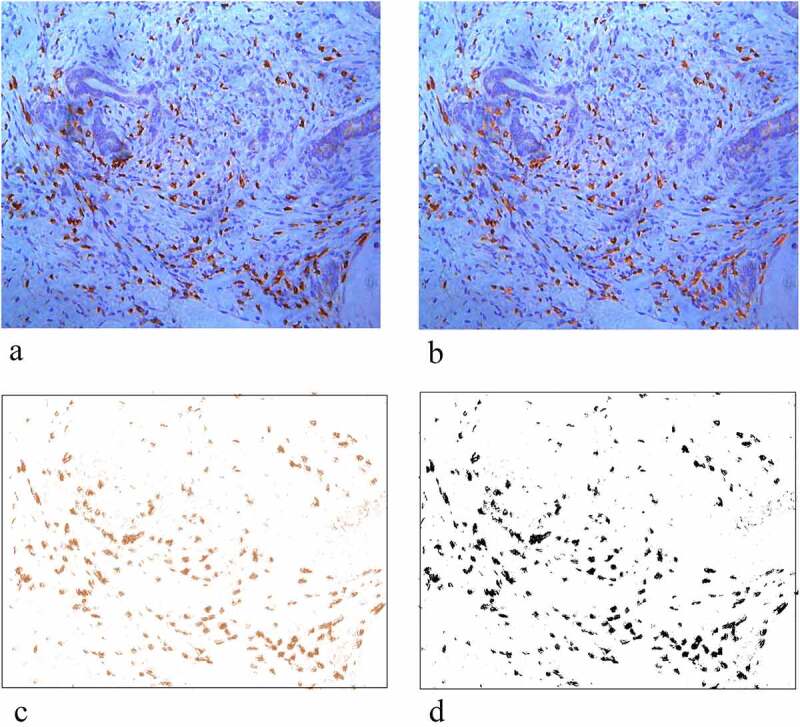
Example of computer-assisted analysis of the densities of the positively stained cells. (a) Image of a peritoneal metastatic tumor center with CD3+ staining. (b) Image after conducting rolling-ball method for background subtraction. (c) Image after performing color deconvolution for separating DAB (brown) and hematoxylin. (d) Image after thresholding to obtain a binary image
RFS was defined as the period from the date of peritoneal metastasectomy to the date of first relapse at any site, or death due to any cause instead of relapse. OS was calculated from the peritoneal metastasectomy to death due to any cause. The prognostic utility of the Immuno-score, TBM immune score and various clinicopathological factors was analyzed using Kaplan Meier curve and log rank test. Cox multivariate models were used for multivariate analysis to determine independent prognostic factors affecting the survival of patients with CRCPM. T-test was used to analyze the difference in infiltration density of the tumor-associated immune cells in primary tissue, peritoneal metastatic tissue and ovarian metastatic tissue of CRC. All statistical examinations were conducted using GraphPad Prism (version 8.0.1) and IBM SPSS software (version 22). P < .05 was considered statistically significant.
Results
Basic characteristics of patients with CRCPM
Overall, we recruited 68 patients with a mean age of 55 years. Of these, 38 (55.88%) were female whereas 30 (44.12%) were male. All the patients underwent cytoreductive surgery, in which 51.5% of the patients presented with primary lesions of CRC. There were 36 cases (52.9%) of right colon cancer and 22 cases (32.4%) of left colon cancer, 10 (14.7%) cases of rectal cancer. Thirteen patients (19.1%) had ovarian metastasis. Moreover, 50% of the CRCPM patients presented with histologically confirmed for mucinous adenocarcinoma or signet ring cell carcinoma, whereas the rest was presented with non-mucinous adenocarcinoma/signet ring cell carcinoma. The G1 histological grade had 4 cases (5.9%), G2 had 23 cases (33.8%), while G3 had 41 cases (60.3%). Intraoperative ascites was identified in 41 patients (60.3%). However, metastatic lesions for 11 patients were not quantitatively analyzed for having less tumor cells or poor staining. The median infiltrating density of CD3+ cells in CT was 205/HP (Interquartile Range, IQR, 152.0–295.8/HP), whereas that of CD8+ T cells was 58.5/HP (IQR, 29.0–140.5/HP). The median infiltrating density of CD20+ B cells was 55/HP (IQR, 33–130.3/HP), that of CD68+ macrophages was 148/HP (IQR, 72.8–215.0/HP), while that of CD163+ macrophages was 45/HP (IQR, 25.8–88.5/HP). These clinical characteristics are summarized in Table 1.
Table 1.
Clinical characteristics of patients with colorectal cancer peritoneal metastasis
| Variables | N (%) |
|---|---|
| Age (mean) | 55 (25–87) |
| Sex | |
| Female | 38 (55.9) |
| Male | 30 (44.1) |
| PCI | |
| PCI<10 | 42 (61.8) |
| PCI≥10 | 26 (38.2) |
| Primary cancer | |
| Right colon cancer | 36 (52.9) |
| Left colon cancer | 22 (32.4) |
| Rectal cancer | 10 (14.7) |
| Pathological type | |
| Non-Adenocarcinoma/Signet-ring cell cancer | 34 (50.0) |
| Adenocarcinoma/Signet-ring cell cancer | 34 (50.0) |
| Grade | |
| G1-2 | 27 (39.7) |
| G3 | 41 (60.3) |
| T stage | |
| T1-3 | 48 (70.6) |
| T4 | 20 (29.4) |
| N stage | |
| unknown | 15 (22.1) |
| N0 | 17 (25.0) |
| N1-2 | 36 (52.9) |
| Ovarian metastasis | |
| yes | 13 (19.1) |
| no | 55 (80.9) |
| CC | |
| CCR0-1 | 55 (80.9) |
| CCR2-3 | 13 (19.1) |
| HIPEC | |
| Yes | 61 (89.7) |
| No | 7 (11.5) |
| Ascites | |
| Yes | 41 (60.3) |
| No | 27 (39.7) |
| Recurrence | |
| Yes | 56 (82.4) |
| No | 12 (17.6) |
| Survival status | |
| Yes | 39 (57.4) |
| No | 29 (42.6) |
Comparison of immune status of primary and metastatic focus in patients with CRCPM
The comparative infiltration densities of the immune cells in four patients with primary lesions, PM and ovarian metastasis are shown in Figure 2a–2e. The ovarian metastasis showed an immune desert state, extremely deficient in each subtype immune cells. Compared with the primary lesions, the infiltration of CD3+ T cells, CD8+ T cells and CD20+ B cells, associated with better prognosis, were substantially lower both in peritoneal and ovarian metastases. In addition, the infiltration of immune cells in ovarian metastases were substantially low, relative to peritoneal metastases.
Figure 2.
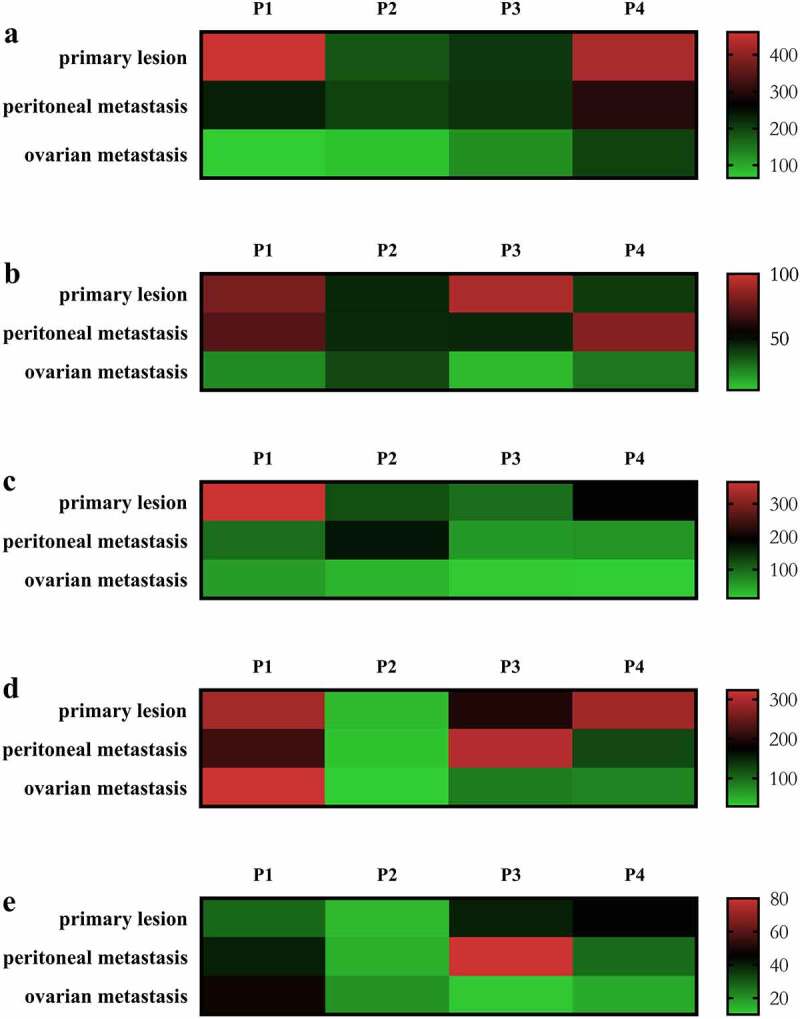
Infiltrating densities (cells/HP) of CD3 (a), CD8 (b), CD20 (c), CD68 (d) and CD163 (e) were quantified using hotspot in the center of tumor. immune densities were scaled from low (green) to high (red) in four patients with primary tumor, peritoneal metastasis and ovarian metastasis
The infiltration of CD3+, CD8+, CD20+, CD68+ and CD163+ immune cells in the IM area was substantially higher than that of CT area in 34 CRCPM patients (CD3: p = .002, CD8: p = .007, CD20: p = .005, CD68: p = .01, CD163: p = .008) (Figure 3a–3e). On the other hand, the infiltrating density of CD8 + T cells and CD20 + B cells in 27 patients with CRCPM was significantly lower than in the corresponding primary lesions (CD8: p = .0061; CD20: p = .0036) (Figure 4a–4e).
Figure 3.
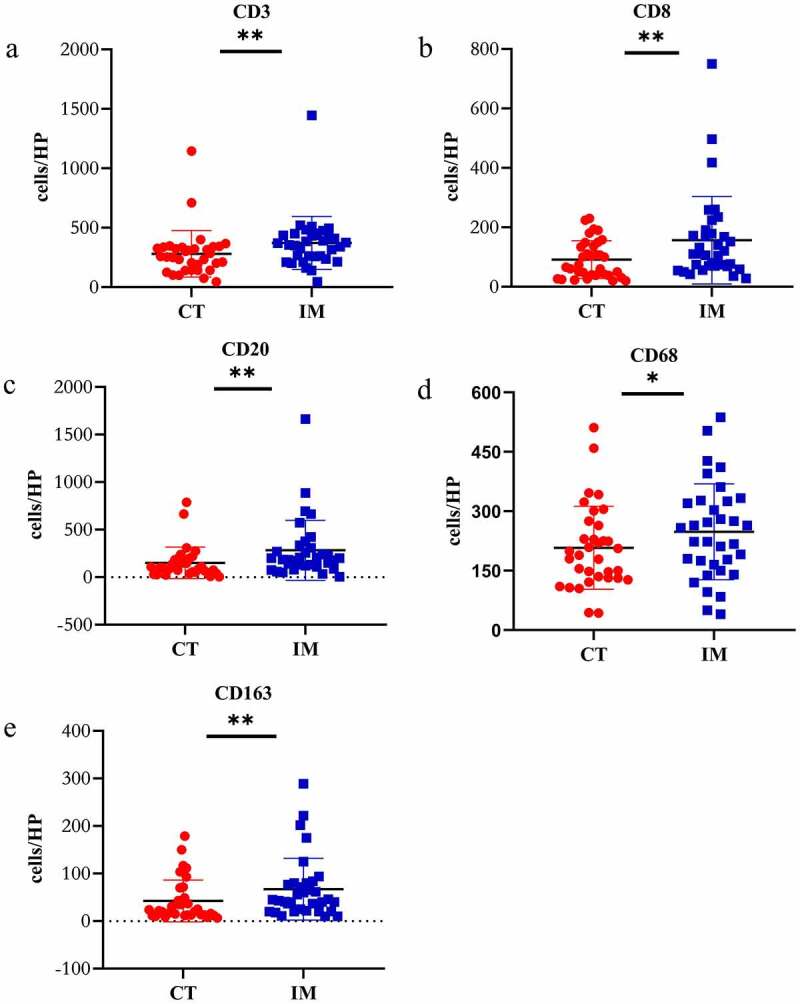
The density was calculated as the number of positive cells/HP (Y-axis), X-axis represents different infiltrating sites of primary lesions. A two-sided paired t test was applied to compare CT with IM. a: CD3 + T cell, b: CD8 + T cell, c: CD20 + B cell, d: CD68+ macrophage, e: CD163+ macrophage. CT: central area of tumor, IM: invasive margin, HP: high power field of view. (*represents P < .05, ** represents P < .005, ns: no statistical difference)
Figure 4.
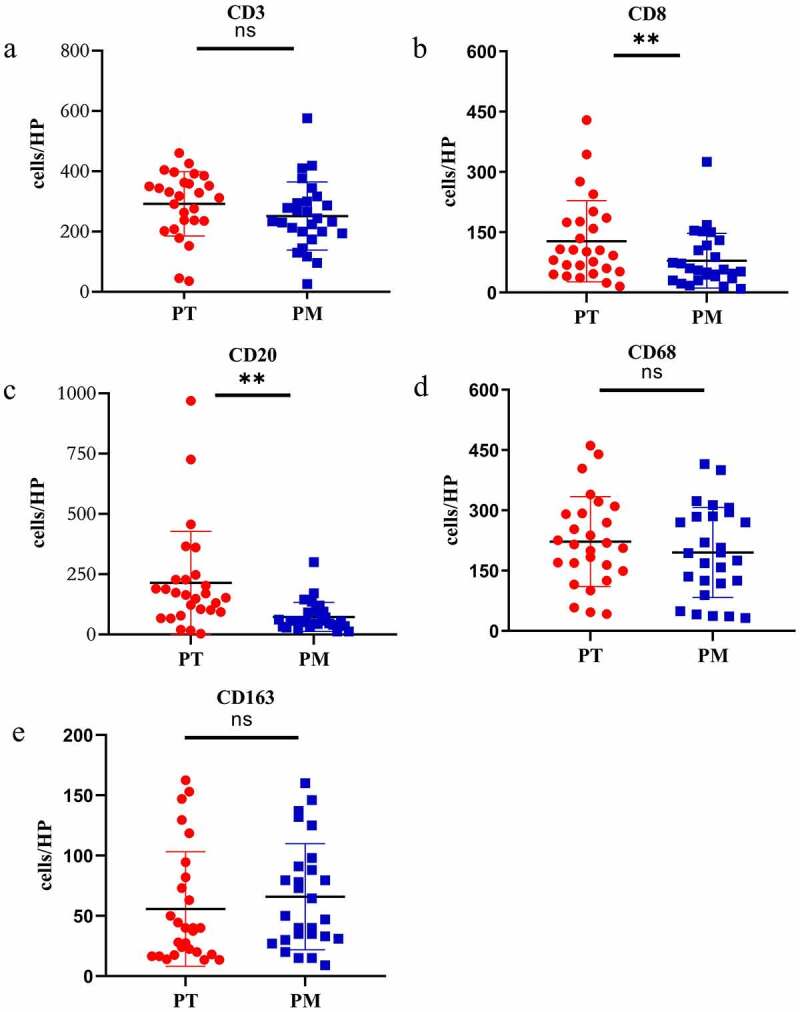
The density was calculated as the number of positive cells/HP (Y-axis), X-axis represents the lesions in different parts. A two-sided paired t test was applied to compare the infiltrating density of each subtype immunocyte in PM with the corresponding primary lesions. a: CD3 + T cell, b: CD8 + T cell, c: CD20 + B cell, d: CD68+ macrophage, e: CD163+ macrophage. PT: primary tumor (red), PM: peritoneal metastasis (blue), HP: high power field of view, (*represents P < .05, ** represents P < .005, ns: no statistical difference)
The infiltrating density of CD3+ T cells, CD8+ T cells, CD20+ B cells and CD68+ macrophages in PM or ovarian metastasis was significantly lower compared to the primary lesions (peritoneal metastasis vs primary lesion: CD3, p = .0004; CD8, p = .01; CD20, p < .0001; CD68, p = .0015; ovarian metastasis vs primary lesion: CD3, p = .0001; CD8, p = .0117; CD20, p = .011; CD68, p = .0245). However, there was no difference in the expression level of CD163+ macrophages in PM or ovarian metastases and primary focus (peritoneal metastasis vs primary lesion: CD163, p = .65; ovarian metastasis vs primary lesion: CD163, p = .86). The immune status of the lesions with multiple PMs was similar, and infiltrating density of the immune cells in each subtype showed no statistical difference (second metastasis vs first metastasis: CD3, p = .69; CD8, p = .69; CD20, p = .16; CD68, p = .86; CD163, p = .67). Our data showed that the density of CD3+ T cells and CD20+ B cells in PM was significantly different from that of ovarian metastasis, but the infiltrating density of other subtype immune cells was similar (ovarian metastasis vs peritoneal metastasis: CD3, p = .0003; CD8, p = .21; CD20, p = .002; CD68, p = .76; CD163, p = .60) (Figure 5a–5e).
Figure 5.
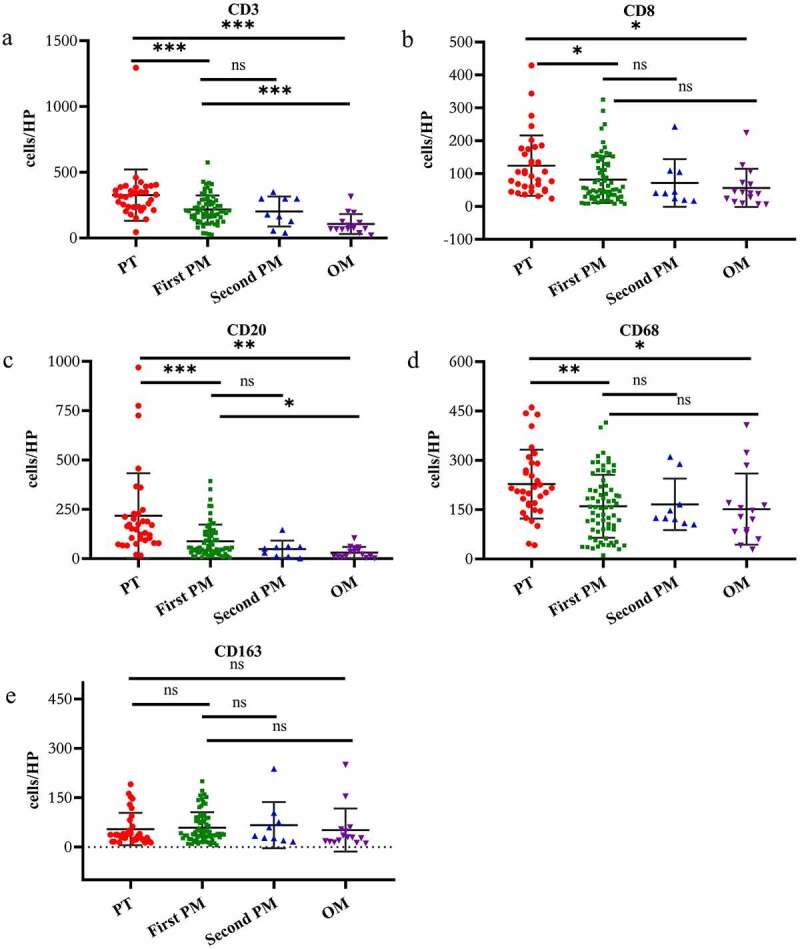
The density was calculated as the number of positive cells/HP (Y-axis), X-axis represents the lesions in different parts. A two-sided paired t test was applied to compare PT with first PM, PT with OM, first PM with second PM, first PM with OM, respectively. a: CD3 + T cell, b: CD8 + T cell, c: CD20 + B cell, d: CD68+ macrophage, e: CD163+ macrophage. PT: primary tumor (red), first PM: first peritoneal metastasis (green), second PM: second peritoneal metastasis (blue), OM: ovarian metastasis (purple), HP: high power field of view, (*represents P < .05, ** represents P < .005, ns: no statistical difference)
The infiltrating level of immune cell of different histological types was shown in Figure S1. We only observed a differential infiltration of T cells (CD3+ T cell, CD8+ T cell) between mucinous adenocarcinoma/signet ring cell carcinoma and non-mucinous adenocarcinoma/signet ring cell carcinoma.
Prognostic value of the different immune scoring systems in PM
Out of the 57 CRCPM patients who were followed up to October 2019, 84.2% had tumor recurrence while 56.1% patients died. The median follow-up time was 24 months (IQR, 13.2–36.9 months). All three immune scoring systems predicted 3-year OS (66% IS-high vs 27% IS-low, p = .0078; 62% TBM1-high vs 32% TBM1-low, p = .013; 55% TBM2-high vs 12% TBM2-low, p = .001). However, the immune scoring system did not demonstrate good predictive values for 3-year RFS (33% IS-high vs 7% IS-low, p = .067; 28% TBM1-high vs 12% TBM1-low, p = .30; 16% TBM2-high vs 0% TBM2-low, p = .75).
Univariate and multivariate analysis of prognostic factors
Univariate analysis of the various clinicopathological factors and subsets of tumor-infiltrating immune cells revealed that ascites (non-existent vs existent, HR, 0.35, 95% CI (0.16–0.75), p = .0017), PCI (<10 vs > 10, HR, 0.43, 95% CI (0.20–0.94), P = .033), CD3+ T cell infiltrating density (high vs low, HR, 0.44, 95% CI (0.15–1.34), p = .05), CD8+ T cell infiltrating density (high vs low, HR, 0.27, 95% CI (0.14–0.55), p = .0009), IS (IS-high vs IS-low, HR, 0.36, 95% CI (0.18–0.72), p = .008), TBM1 score (TBM1-high vs TBM1-low, HR, 0.36, 95% CI (0.18–0.73) and TBM2 score (TBM2-high vs TBM2-low, HR, 0.33, 95% CI (0.14–0.81), p = .001) had prognostic value. There was no difference in the prediction of OS using the other univariate factors, such as age, gender, location of primary tumor, histological grade, pathological type, T stage of primary tumor, infiltrating density of CD20+ B cells, infiltrating density of CD68+ macrophages, and infiltrating density of CD163+ macrophages. Furthermore, data from the univariate survival analysis indicated that only the T stage of primary tumor (T2-3 vs T4, HR, 0.48, 95% CI (0.27–0.87) p = .03), ascites (non-existent vs existent, HR,0.44, 95% CI (0.33–0.84), p = .002), PCI (<10 vs ≥10, HR, 0.57, 95% CI (0.32–1.04), p = .04) and infiltration density of CD8+ T cell (high vs low, HR, 0.52, 95% CI (0.29–0.94), p = .04) had predictive value in RFS. The prognostic value of other factors was unsatisfactory (Table 2, OS: Figure 6a–6c; RFS: Figure 6d–6e).
Table 2.
Univariate analysis of the prognosis of patients with colorectal cancer peritoneal metastasis
| Variables |
OS |
|
RFS |
|
|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||
| HR (95% CI) | P Value | HR (95% CI) | P Values | |
| Age (year) | ||||
| >65 vs ≤65 | 0.64 (0.28–1.46) | P = .23 | 1.02 (0.54–1.93) | P = .95 |
| Sex | ||||
| Female vs male | 1.22 (0.61–2.46) | P = .58 | 0.94 (0.54–1.66) | P = .83 |
| Primary site | ||||
| Right colon vs left colon | 0.85 (0.42–1.72) | P = .65 | 1.41 (0.80–2.48) | P = .24 |
| Grade | ||||
| G1-2 vs G3 | 0.98 (0.47–2.08) | P = .97 | 0.72 (0.40–1.28) | P = .28 |
| Pathological type | ||||
| Non-mucinous vs mucinous/signet-ring cancer | 0.68 (0.34–1.36) | P = .28 | 0.87 (0.50–1.54) | P = .63 |
| T stage | ||||
| T2-3 vs T4 | 0.59 (0.28–1.24) | P = .2 | 0.48 (0.27–0.87) | P = .03 |
| Ascites | ||||
| no vs yes | 0.35 (0.16–0.75) | P = .0017 | 0.44 (0.33–0.84) | P = .002 |
| PCI | ||||
| <10 vs ≥10 | 0.43 (0.20–0.94) | P = .033 | 0.57 (0.32–1.04) | P = .04 |
| CD3+ cell(/HP) | ||||
| High infiltration vs low infiltration | 0.44(0.15–1.34) | P = .05 | 0.52 (0.18–1.46) | P = .09 |
| CD8+ cell(/HP) | ||||
| High infiltration vs low infiltration | 0.27(0.14–0.55) | P = .0009 | 0.52 (0.29–0.94) | P = .04 |
| CD20+ cell(/HP) | ||||
| High infiltration vs low infiltration | 0.68 (0.33–1.40) | P = .31 | 0.59 (0.33–1.05) | P = .09 |
| CD68+ cell(/HP) | ||||
| High infiltration vs low infiltration | 1.61 (0.8–3.23) | P = .18 | 0.68 (0.34–1.33) | P = .31 |
| CD163+ cell(/HP) | ||||
| High infiltration vs low infiltration | 1.59 (0.79–3.19) | P = .21 | 0.47 (0.24–0.91) | P = .07 |
| IS | ||||
| High vs low | 0.36 (0.18–0.72) | P = .008 | 0.56 (0.31–0.99) | P = .067 |
| TBM1 | ||||
| High vs low | 0.36 (0.18–0.73) | P = .01 | 0.71 (0.39–1.31) | P = .3 |
| TBM2 | ||||
| High vs low | 0.33 (0.14–0.81) | P = .001 | 0.87 (0.35–2.15) | P = .75 |
Figure 6.
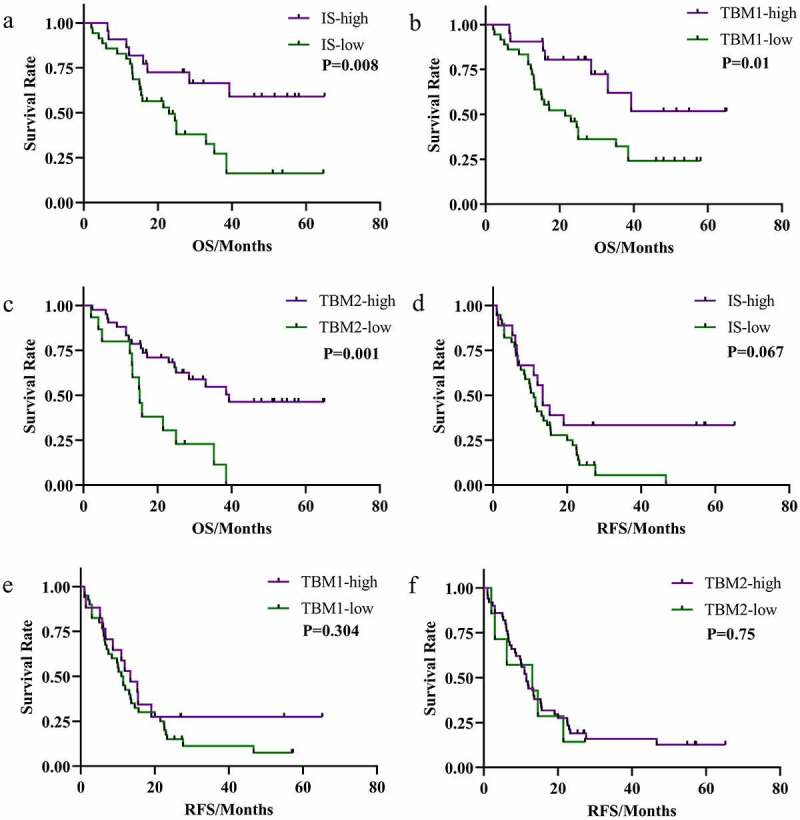
Kaplan-Meier estimates and log-rank test OS according to the IS (a), TBM1 score (b) and TBM2 score (c); Kaplan-Meier estimates and log-rank test RFS according to the IS (d), TBM1 score (e) and TBM2 score (f). The number of patients at risk for each group is shown. (a, d) patients with IS = 2 (IS-high, purple) are grouped. Similarly, patients with IS 0 and IS 1 are grouped (IS-low, green). (b, e) patients with TBM1 ≥ 2 and TBM1 < 2 are shown in purple and green, respectively. (c, f) patients with TBM1 ≥ 2 and TBM1 < 2 are shown in blue and black, respectively. IS = immunoscore
As such only T stage of the primary tumor, presence of ascites and PCI were included in the multivariable analysis. We combined IS and TBM1 score or TBM2 score to establish three risk proportion models. Immunoscore (IS-high vs IS-low, HR, 0.35, 95% CI (0.15–0.80), p = .013), TBM1 score (TBM1-high vs TBM1-low, HR, 0.307, 95% CI (0.14–0.67), p = .003) and TBM2 score (TBM2-high vs TBM2-low, HR, 0.29, 95% CI (0.12–0.69), p = .005) had independent predictive value for OS. In addition, existence of ascites was an independent risk factor in the first and third multivariate risk proportion models. However, none of the three immune scoring systems indicated independent predictive value for RFS. In the three risk proportion models, only T stage of primary tumor and presence of ascites could independently predict RFS (Table 3).
Table 3.
Multivariate analyses of prognostic factors of patients with CRCPM. Bold value indicates P < .05, HR hazard ratio, CI confidence interval, T stage of primary tumor, ascites, PCI score and Immunoscore were included in the Multivariate analysis 1; T stage of primary tumor, ascites, PCI score and TBM1 were included in the Multivariate analysis 2; T stage of primary tumor, ascites, PCI score and TBM2 were included in the Multivariate analysis 3
| OS | Multivariate analysis 1 | Multivariate analysis 2 | Multivariate analysis 3 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| T Stage | ||||||
| T4 vs T2-3 | 1.72 (0.72–4.10) | P = .219 | 2.09 0.86–5.07) | P = .103 | 2.07 (0.86–4.98) | P = .106 |
| Ascites | ||||||
| Yes vs no | 2.63 (1.28–5.40) | P = .009 | 1.96 (0.91–4.19) | P = .085 | 2.52 (1.21–5.25) | P = .014 |
| PCI | ||||||
| ≥10 vs <10 | 1.694 (0.81–3.56) | P = .163 | 1.91 (0.89–4.13) | P = .099 | 1.97 (0.92–4.22) | P = .079 |
| IS | ||||||
| High vs low | 0.35 (0.15–0.80) | P = .013 | ||||
| TBM1 | ||||||
| High vs low | 0.307 (0.14–0.67) | P = .003 | ||||
| TBM2 | ||||||
| High vs low | 0.29 (0.12–0.69) | P = .005 | ||||
| RFS | Multivariate analysis 1 | Multivariate analysis 2 | Multivariate analysis 3 | |||
| T Stage | ||||||
| T4 vs T2-3 | 2.13 (1.03–4.41) | P = .041 | 2.20 (1.06–4.55) | P = .034 | 2.30 (1.12–4.70) | P = .023 |
| Ascites | ||||||
| Yes vs no | 2.17 (1.17–4.01) | P = .014 | 2.19 (1.18–4.06) | P = .013 | 2.20 (1.18–4.08) | P = .013 |
| PCI | ||||||
| ≥10 vs <10 | 1.68 (0.91–3.08) | P = .095 | 1.78 (0.96–3.30) | P = .068 | 1.72 (0.93–3.19) | P = .085 |
| IS | ||||||
| High vs low | 0.69 (0.35–1.35) | P = .28 | ||||
| TBM1 | ||||||
| High vs low | 0.80 (0.40–1.58) | P = .521 | ||||
| TBM2 | ||||||
| High vs low | 0.93 (0.39–2.23) | P = .87 | ||||
Discussion
In this study, immunoscore and TBM score were used to evaluate peritoneal metastasis in patients with CRC. Our data demonstrated that TBM scoring system could accurately predict the prognosis of patients with CRCPM after cytoreductive surgery. We successfully established a traditional IS scoring model based on the infiltration density of CD3+ T cells and CD8+ T cells in PM, TBM1 scoring system based on the infiltration density of CD8+ T cells, CD20+ B cells and CD163+ macrophages, as well as TBM2 scoring system based on infiltration density of CD3+ T Cells, CD8+ T cells, CD20+ B cells and CD163+ macrophages. The three scoring systems were associated with good prognostic value for OS. Although the three scoring systems did not display statistical significance in predicting RFS, it was observed that the IS-high or TBM-high group had improved RFS.
Compared with other related studies, we used infiltration of multiple immune cell subtypes in TME to predict the prognosis of CRCPM. Comparatively, the predictive value of TBM score was superior to that of clinical factors-based model. In this study, Image J was used to quantify the status of immune cell infiltration in tumor tissue. Compared with other methods, this software had simple operation steps and strong practicability. However, the methodology and calculations were not fully automated, which consumed a lot of time and was easy to mix in subjective factors.
Many studies have reported that the density of T-cell subtypes and the ratio of each subtype in the primary lesions strongly predict the OS and recurrence of CRC.9,28–33 Besides, the influence of T cell infiltration density or ratio of each subtype on prognosis has also been reported in many CRC studies with liver metastasis.34–36 Recent studies have quantitatively analyzed immune status of primary lesions or liver metastasis to predict prognosis of early CRC and liver metastasis. The results showed that high immune scores are effective index to predict neoplastic outcome.18,20,21,37 For instance, Mlecnik et al. reported that traditional high immunoscore can predict disease-free survival and OS of CRC with liver and lung metastases, and the established TB scoring system in this study can improve DFS and OS (TB3-4 vs TB0–2; 5-year OS, 63.7% vs 21.4%, p < .001; 5-year DFS, 25.7% vs 5.0%, p < .001) (I3–4). The TB scoring system combines both the infiltrating densities of B cells and CD8 + T cells in the liver and lung metastases.21 Kwak et al. used the M2 phenotype tumor associated macrophages to conduct an immunoquantitative analysis of advanced CRC, using only CD163+ macrophages in the primary lesions. The results showed that high-density infiltration of CD163+ macrophages was associated with poor prognosis. Besides, they observed that IS-ma scoring system based on the infiltration density of CD3+ T cells and CD8+ T cells in metastases, and the infiltration density of CD163+ macrophages in the primary lesions could also predict prognosis (OS: high IS-ma vs low IS-ma, p = .005).26 However, only few studies have explored the value of immune cell infiltrating status in predicting prognosis of peritoneal metastases in patients with CRCPM. Here, we evaluated the tumor infiltrating T lymphocytes (CD3+ T cells, CD8+ T cells), tumor infiltrating B lymphocytes (CD20+ B cells) and TAMs (CD163+ macrophages) in peritoneal metastases of CRC. We then established a novel immune scoring system – TBM scoring system, and proved its prognostic value in patients with CRCPM. It was equivalent to the prognostic value of a single immune marker (especially CD20, CD68, CD163), and the immune score composed of multiple immune indexes had more independent prognostic value. For patients with CRC who could not obtain or resect the primary tumor, the analysis of the immune status of CRCPM could help to predict the prognosis of these patients. However, one of the shortcomings of this study was that the short follow-up period dilutes the credibility of this study.
CD3+ T cells and CD8+ T cells are important immune components in TME of CRC and determine the anti-tumor immune response.18,20,38 Higher infiltration of CD8+ T cells in the epithelium and stroma of tumor tissue is indicative of an effective immune response and indicates improved survival.39 Previous meta-analysis indicated that high-density infiltrating CD3+ T cells and CD8+ T cells in the CT of CRC do not project improved OS and DFS. However, in the IM, the high-density infiltration of CD8+ T cells was associated with improved OS. In addition, high-density CD3+ T cells predicted the prolongation of OS and DFS in the IM .40 Contrarily, a recent meta-analysis showed that high-density infiltration of CD8+ T cells in CT depicts improved OS.41 Our study also demonstrated that the infiltration density of T and B lymphocytes as well as TAMs was higher in IM CT in primary CRC.
In previous studies, CD68 and CD163 were found to be independent prognostic markers in patients with solid tumors. These markers hold great potential to be therapeutic targets for the treatment of solid tumors.42–44For intestinal tumors, infiltration level of CD163+ macrophages is associated with perineural invasion, MSI and TIL densities. Stromal CD68+ and CD163+ macrophage infiltration levels are significantly related to the infiltration level of CD3+ and CD8+ T cells.43 During tumorigenesis and development, the polarization of TAM subtypes affects disease prognosis. Previous studies have confirmed that the TME of most solid tumors is acidified, a condition detected by macrophage G Protein-Coupled Receptor (GPCR), resulting in the expression of macrophage transcription factor-ICE (inducible cyclic adenosine monophosphate (cAMP) early repressor). Acidified TME triggers polarization of macrophages to non-inflammatory subtypes, leading to immune evasion. Unlike melanoma and lung cancer with strong acidic TME, the microenvironment of colon cancer is less acidic.45 This might explain why the polarization of the pan-macrophages to non-inflammatory macrophages in the less acidic intestinal tumors is weaker than in other solid tumors. Thus, high infiltrating pan-macrophages predict good OS in CRC,23,46 and high-density TAMs infiltration correlate with high density CD8+ T cell infiltration, lack of distant and lymph node metastasis, high microsatellite instability, and non-mucinous adenocarcinoma.46 Although CD68+ macrophages could not predict the OS of CRCPM (p = .18), the low-density CD68+ macrophages infiltration group showed an improved OS. This contradicted with the results from a recent meta-analysis which showed that the high-density infiltration of pan-macrophages in primary lesions of CRC was associated with prolonged OS. This may be due to the fact that CRCPM does not exhibit the same low acid TME as the primary tumor. Although low-density infiltration CD163+ macrophage group did not significantly influence the prognosis, it tended to prolong the OS (p = .21). This matched with a previous observation that overexpression of CD163+ macrophages in tumor tissues is associated with poor prognosis.47,48
TIL subpopulations in human solid tumors contain different proportions of infiltrating B cells. B cells are considered to be the main effector cells of humoral immunity. They secrete immunoglobulins, promote T cell responses or directly kill tumor cells.49 Tumor infiltrating B lymphocytes (TIBS) exist in many solid tumors, but the prognostic value of TIBS in malignant tumors is controversial. High-density TIBS/tumor infiltrating plasma cells in TME of some malignant solid tumors, such as CRC or lung cancer has been associated with good prognosis.50,51 On the contrary, high-density TIBS is associated with high recurrence risk or poor prognosis in prostate and ovarian cancer.52 Existing evidence shows that TIBs control tumor progression by secreting immunoglobulins, promoting T cell responses, or directly killing tumor cells.53 In addition, TIBS act as antigen presenting cells and promotes immune response of T cells in TME. For example, in non-small cell lung cancer and metastatic CRC, T cells with high infiltration density were located in the high-density B cell infiltration area, and the high-immunoscore based on infiltrating density of TILs and B cells predicted good prognosis.14,21 Another subtype called regulatory B cell (Bregs) in the TME of solid tumors enhances tumor activity through immunosuppressive factors such as IL-10, TGF-β, and inhibits CTL response by inducing polarization of TAMs and differentiation of regulatory T cells (Tregs).54–57 Our findings demonstrate that the infiltrating level of B cells in peritoneal metastases does not affect prognosis of tumors. The impact of different subtypes of B cells on the prognosis should be further investigated.
In addition, our results showed that, compared to primary tumors, the tumor infiltrating T or B cells are downregulated in peritoneal or ovarian metastasis. This state of immune cell desertification may explain why patients with CRCPM are prone to relapse and metastasis, thus poor disease prognosis. Among the clinical factors, PCI and ascites are independent prognostic factors. Similar to previous studies, we demonstrate that high-PCI is associated with poor prognosis in advanced cancer patients.6,58–60 Therefore, quantification of tumor burden coupled with the quantification of immune effector and suppressor cells in TME may be more accurate in predicting the long-term prognosis of cancer patients.
Conclusion
The microenvironment of peritoneal and ovarian metastases usually exhibits low expression and desertification of immune cells compared to the primary lesions. Here, we demonstrate that the IS scoring system constructed by combining the infiltration density of CD3+ and CD8+ T cells in CRCPM, and TBM scoring system constructed by combining CD3+ T cells, CD8+ T cells, CD20+ B cells and CD163+ macrophages, could successfully predict the OS of patients with CRCPM. In addition, clinical factors such as high PCI and ascites are independent risk factors.
Supplementary Material
Acknowledgments
Our special acknowledgments to Mr. Xiang Zhou for helping us with editing and to BOSTER for providing antibodies for immunohistochemistry.
Funding Statement
The study was funded by National Natural Science Foundation of China [81472819,81672342]; Zhejiang Provincial Key R&D Program of China [2019C03018]; Zhejiang Provincial Natural Science Foundation of China [LY20H160038].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethics approval and consent to participate
This study was approved by the local institutional ethical committee of the Second Affiliated Hospital, Zhejiang University School of Medicine
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Siegel RL, Kd M, Jemal A.. Cancer statistics. CA Cancer J Clin. 2019 2019;69(1):7–13. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Van Gestel YR, De Hingh IH, Van Herk-sukel MP, Van Erning FN, Beerepoot LV, Wijsman JH, Slooter GD, Rutten HJ, Creemers GJ, Lemmens VE. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol. 2014;38(4):448–454. doi: 10.1016/j.canep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Lambert LA. Looking up: recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J Clin. 2015;65(4):284–298. doi: 10.3322/caac.21277. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Cervantes A, Adam R,Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. [DOI] [PubMed] [Google Scholar]
- 5.Shida D. ASO author reflections: long-term outcomes after r0 resection of colorectal peritoneal metastasis. Ann Surg Oncol. 2018;25(S3):832–833. doi: 10.1245/s10434-018-6782-1. [DOI] [PubMed] [Google Scholar]
- 6.Verwaal VJ, Van Ruth S, De Bree E, van Sloothen G, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2003;21(20):3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 7.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganesh K, Stadler ZK, Cercek A, Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16(6):361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages, C, Tosolini, M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 10.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. New York, NY. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125(23):5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee A, Chabria Y, Kanna NRR, Gopi J, Rowlo P, Sun XF, Pathak S. Role of tumor specific niche in colon cancer progression and emerging therapies by targeting tumor microenvironment. Advances in experimental medicine and biology 2019. [DOI] [PubMed]
- 13.Spector ME, Bellile E, Amlani L, Zarins K, Smith J, Brenner JC, Rozek L, Nguyen A, Thomas D, McHugh JB, et al. Prognostic value of tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2019;145(11):1012. doi: 10.1001/jamaoto.2019.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinoshita T, Muramatsu R, Fujita T, Nagumo H, Sakurai T, Noji S, Takahata E, Yaguchi T, Tsukamoto N, Kudo-Saito C, et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann Oncol. 2016;27(11):2117–2123. doi: 10.1093/annonc/mdw319. [DOI] [PubMed] [Google Scholar]
- 15.Hotta K, Sho M, Fujimoto K, Shimada K, Yamato I, Anai S, Konishi N, Hirao Y, Nonomura K, Nakajima Y. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer. 2011;105(8):1191–1196. doi: 10.1038/bjc.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Wang J, Chen R, Bai Y and Lu X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget. 2017;8(9):15621–15631. doi: 10.18632/oncotarget.14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadvand S, Faghih Z, Montazer M, Safaei A, Mokhtari M, Jafari P, Talei AR, Tahmasebi S Ghaderi A. Importance of CD45RO+ tumor-infiltrating lymphocytes in post-operative survival of breast cancer patients. Cell Oncol (Dordr). 2019;42(3):343–356. doi: 10.1007/s13402-019-00430-6. [DOI] [PubMed] [Google Scholar]
- 18.Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I. Rau Tilman T, Berger Martin D, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. The Lancet. 2018;391(10135):2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 19.Nejati R, Goldstein JB, Halperin DM, Wang H, Hejazi N, Rashid A, Katz MH, Lee JE, Fleming JB, Rodriguez-Canales J, et al. Prognostic significance of tumor-infiltrating lymphocytes in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemotherapy. Pancreas. 2017;46(9):1180–1187. doi: 10.1097/MPA.0000000000000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Lin HC, Huang MY, Shao Q, Wang ZQ, Wang FH, Yuan YF, Li BK, Wang DS, Ding PR, et al. The Immunoscore system predicts prognosis after liver metastasectomy in colorectal cancer liver metastases. Cancer Immunol Immunother. 2018;67(3):435–444. doi: 10.1007/s00262-017-2094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mlecnik B, Van Den Eynde M, Bindea G, Church SE, Vasaturo A, Fredriksen T, Lafontaine L, Haicheur N, Marliot F, Debetancourt D, et al. Comprehensive intrametastatic immune quantification and major impact of immunoscore on survival. J Natl Cancer Inst. 2018;110(1):110. doi: 10.1093/jnci/djx123. [DOI] [PubMed] [Google Scholar]
- 22.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 23.Zhang QW, Liu L, Gong CY, Zhang QW, Liu L, Gong CY, Shi HS., Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7(12):e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, Oldenborg PA, Palmqvist R. The distribution of macrophages with a m1 or m2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PloS One. 2012;7(10):12. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232(2):199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwak Y, Koh J, Kim DW, Kang SB, Kim WH, Lee HS. Immunoscore encompassing CD3+ and CD8+ T cell densities in distant metastasis is a robust prognostic marker for advanced colorectal cancer. Oncotarget. 2016;7(49):81778–81790. doi: 10.18632/oncotarget.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabrielson A, Wu Y, Wang H, Jiang J, Kallakury B, Gatalica Z, Reddy S, Kleiner D, Fishbein T, Johnson L, et al. Intratumoral CD3 and CD8 T-cell densities associated with relapse-free survival in HCC. Cancer Immunol Res. 2016;4(5):419–430. doi: 10.1158/2326-6066.CIR-15-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuwahara T, Hazama S, Suzuki N, Yoshida S, Tomochika S, Nakagami Y, Matsui H, Shindo Y, Kanekiyo S, Tokumitsu Y, et al. Intratumoural-infiltrating CD4 + and FOXP3 + T cells as strong positive predictive markers for the prognosis of resectable colorectal cancer. Br J Cancer. 2019;121(8):659–665. doi: 10.1038/s41416-019-0559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang CY, Chiang SF, Ke TW, Chen TW, You YS, Chen WT, Chao KSC. Clinical significance of programmed death 1 ligand-1 (CD274/PD-L1) and intra-tumoral CD8+ T-cell infiltration in stage II-III colorectal cancer. Sci Rep. 2018;8(1):15658. doi: 10.1038/s41598-018-33927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svensson MC, Leandersson K, Nodin B, Micke P, Larsson AH, Eberhard J, Jirström K. The clinical impact of tumour-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: a cohort study. editor^, editors”. Int J Cancer. 2017;141(8):1654–1666. City. doi: 10.1002/ijc.30869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu P, Fan W, Zhang Z, Wang J, Wang P, Li Y, Yu M. The clinicopathological and prognostic implications of foxP3(+) regulatory T cells in patients with colorectal cancer: a meta-analysis. Front Physiol. 2017;8:950. doi: 10.3389/fphys.2017.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eriksen AC, Sorensen FB, Lindebjerg J,, Hager H, dePont Christensen R, Kjaer-Frifeldt S, Hansen TF. The prognostic value of tumor-infiltrating lymphocytes in stage ii colon cancer. A Nationwide Population-Based Study Transl Oncol. 2018;11:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato, E, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22(6):679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 34.Katz SC, Bamboat ZM, Maker AV, Shia J, Pillarisetty VG, Yopp AC, Hedvat CV, Gonen M, Jarnagin WR, Fong, Y, et al. Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann Surg Oncol. 2013;20(3):946–955. doi: 10.1245/s10434-012-2668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sideras K, Galjart B, Vasaturo A, Pedroza-Gonzalez A, Biermann K, Mancham S, Nigg AL, Hansen BE, Stoop HA, Zhou G, et al. Prognostic value of intra-tumoral CD8(+) /FoxP3(+) lymphocyte ratio in patients with resected colorectal cancer liver metastasis. J Surg Oncol. 2018;118(1):68–76. doi: 10.1002/jso.25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa K, Tanaka K, Homma Y, Nojiri K, Kumamoto T, Takeda K, Endo, I. Low infiltration of peritumoral regulatory T cells predicts worse outcome following resection of colorectal liver metastases. Ann Surg Oncol. 2015;22(1):180–186. doi: 10.1245/s10434-014-3974-1. [DOI] [PubMed] [Google Scholar]
- 37.Sun G, Dong X, Tang X, Qu H, Zhang H, Zhao E. The prognostic value of immunoscore in patients with colorectal cancer: a systematic review and meta-analysis. Cancer Med. 2019;8(1):182–189. doi: 10.1002/cam4.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berntsson J, Svensson MC, Leandersson K, Nodin B, Micke P, Larsson AH, Eberhard J, Jirstrom K. The clinical impact of tumour-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: a cohort study. Int J Cancer. 2017;141(8):1654–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nazemalhosseini-Mojarad E, Mohammadpour S, Torshizi Esafahani A, Gharib E, Larki P, Moradi A, Amin Porhoseingholi M, Asadzade Aghdaei H, Kuppen PJK, Zali MR. Intratumoral infiltrating lymphocytes correlate with improved survival in colorectal cancer patients: independent of oncogenetic features. J Cell Physiol. 2019;234(4):4768–4777. doi: 10.1002/jcp.27273. [DOI] [PubMed] [Google Scholar]
- 40.Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, Peng H, Cui L, Li C. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110(6):1595–1605. doi: 10.1038/bjc.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Ge X, He J, Cheng Y, Wang Z, Wang J, Sun L. The prognostic value of tumor-infiltrating lymphocytes in colorectal cancer differs by anatomical subsite: a systematic review and meta-analysis. World J Surg Oncol. 2019;17(1):85. doi: 10.1186/s12957-019-1621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang M, Li Z, Ren M, Li S, Zhang L, Zhang X, Liu F. Stromal infiltration of tumor-associated macrophages conferring poor prognosis of patients with basal-like breast carcinoma. J Cancer. 2018;9(13):2308–2316. doi: 10.7150/jca.25155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim Y, Wen X, Bae JM, Kim JH, Cho NY, Kang GH. The distribution of intratumoral macrophages correlates with molecular phenotypes and impacts prognosis in colorectal carcinoma. Histopathology. 2018;73(4):663–671. doi: 10.1111/his.13674. [DOI] [PubMed] [Google Scholar]
- 44.Oike N, Kawashima H, Ogose A, Hotta T, Hatano H, Ariizumi T, Sasaki T, Yamagishi T, Umezu H, Endo N. Prognostic impact of the tumor immune microenvironment in synovial sarcoma. Cancer Sci. 2018;109(10):3043–3054. doi: 10.1111/cas.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bohn T, Rapp S, Luther N, Klein M, Bruehl TJ, Kojima N, Aranda Lopez P, Hahlbrock J, Muth S, Endo S, et al. Tumor immunoevasion via acidosis-dependent induction of regulatory tumor-associated macrophages. Nat Immunol. 2018;19(12):1319–1329. doi: 10.1038/s41590-018-0226-8. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Ge X, Xu X, Yu S, Wang J, Sun L. Prognostic value and clinicopathological roles of phenotypes of tumour-associated macrophages in colorectal cancer. J Cancer Res Clin Oncol. 2019;145(12):3005–3019. doi: 10.1007/s00432-019-03041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, Lei XF, Beppu T, Baba H, Takeya M. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101(8):1913–1919. doi: 10.1111/j.1349-7006.2010.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shabo I, Olsson H, Elkarim R, Sun XF, Svanvik J. Macrophage infiltration in tumor stroma is related to tumor cell expression of CD163 in colorectal cancer. Cancer Microenvironment: Official Journal of the International Cancer Microenvironment Society. 2014;7(1–2):61–69. doi: 10.1007/s12307-014-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tokunaga R, Naseem M, Lo JH, Battaglin F, Soni S, Puccini A, Berger MD, Zhang W, Baba H, Lenz HJ. B cell and B cell-related pathways for novel cancer treatments. Cancer Treat Rev. 2019;73:10–19. doi: 10.1016/j.ctrv.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189(7):832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 52.Woo JR, Liss MA, Muldong MT, Palazzi K, Strasner A, Ammirante M, Varki N, Shabaik A, Howell S, Kane CJ, et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J Transl Med. 2014;12(1):30. doi: 10.1186/1479-5876-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang SS, Liu W, Ly D, Xu H, Qu L, Zhang L. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol. 2019;16(1):6–18. doi: 10.1038/s41423-018-0027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J Clin Invest. 2011;121(11):4268–4280. doi: 10.1172/JCI59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011;71(10):3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25(6):809–821. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, et al. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 2016;6(3):270–285. doi: 10.1158/2159-8290.CD-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Giorgio A, De Iaco P, De Simone M, Garofalo A, Scambia G, Pinna AD, Verdecchia GM, Ansaloni L, Macri A, Cappellini P, et al. Cytoreduction (Peritonectomy Procedures) combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in advanced ovarian cancer: retrospective Italian multicenter observational study of 511 cases. Ann Surg Oncol. 2017;24(4):914–922. doi: 10.1245/s10434-016-5686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park EJ, Baik SH, Hur H, Min BS, Kang J, Han YD, Cho MS, Lee KY, Kim NK. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for appendiceal and colorectal cancer with peritoneal carcinomatosis: clinical outcomes at 2 tertiary referral centers in Korea. Medicine. 2017;96(21):e6632. doi: 10.1097/MD.0000000000006632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alzahrani N, Ferguson JS, Valle SJ, Liauw W, Chua T, Morris DL. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: long-term results at St George hospital, Australia. ANZ J Surg. 2016;86(11):937–941. doi: 10.1111/ans.13152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


