Abstract
Objectives
The aims of our study were to examine the anticholinergic drug use and to assess the association between anticholinergic burden and cognitive function in the multimorbid elderly patients of the MultiCare cohort.
Setting
MultiCare was conducted as a longitudinal cohort study in primary care, located in eight different study centres in Germany.
Participants
3189 patients (59.3% female).
Primary and secondary outcome measures
Baseline data were used for the following analyses. Drugs were classified according to the well-established anticholinergic drug scale (ADS) and the recently published German anticholinergic burden (German ACB). Cognitive function was measured using a letter digit substitution test (LDST) and a mixed-effect multivariate linear regression was performed to calculate the influence of anticholinergic burden on the cognitive function.
Results
Patients used 1764 anticholinergic drugs according to ADS and 2750 anticholinergics according to the German ACB score (prevalence 38.4% and 53.7%, respectively). The mean ADS score was 0.8 (±1.3), and the mean German ACB score was 1.2 (±1.6) per patient. The most common ADS anticholinergic was furosemide (5.8%) and the most common ACB anticholinergic was metformin (13.7%). The majority of the identified anticholinergics were drugs with low anticholinergic potential: 80.2% (ADS) and 73.4% (ACB), respectively. An increasing ADS and German ACB score was associated with reduced cognitive function according to the LDST (−0.26; p=0.008 and −0.24; p=0.003, respectively).
Conclusion
Multimorbid elderly patients are in a high risk for using anticholinergic drugs according to ADS and German ACB score. We especially need to gain greater awareness for the contribution of drugs with low anticholinergic potential from the cardiovascular system. As anticholinergic drug use is associated with reduced cognitive function in multimorbid elderly patients, the importance of rational prescribing and also deprescribing needs to be further evaluated.
Trial registration number
Keywords: clinical pharmacology, epidemiology, primary care
Strengths and limitations of this study.
The well-selected exclusion criteria create a representative study population for the multimorbid elderly population.
Gaining valid results by the advanced treating of missing values via hot deck imputation and the performance of a multivariate analysis.
As the letter digit substitution test only addresses one aspect of cognition and cognitive impairment is a complex clinical symptom, further analyses are needed.
Introduction
The greater number of people in the population surviving until very late life leads to a challenge to the provision of healthcare, particularly given the proportion of older people that live with multiple comorbidities. These in turn often lead to polypharmacy, which is commonly defined as the coapplication or coprescription of five or more drugs at the same time.1 2 Apart from this, it is also known that multimorbid elderly patients are at a higher risk for taking anticholinergic drugs or drugs that have anticholinergic side effects.3 Besides classic anticholinergic substances—for example, drugs for urinary incontinence, chronic obstructive pulmonary disease or Morbus Parkinson—a lot of drugs lead to anticholinergic adverse drug reactions (ADRs). These ADR and also the intended anticholinergic effects are evoked by the binding of drugs to one of the five muscarinic receptors in autonomous nervous system and especially blocking the parasympathetic nervous system.4 5 Particularly elderly people are more vulnerable towards anticholinergic ADR because of an age-related decreased cholinergic transmission and a poorer metabolism and/or elimination of those substances.6 7 Therefore, there is some evidence that the use of anticholinergic drugs or drugs with anticholinergic activity is associated with a higher risk of falls, hospitalisation and even mortality in elderly patients.4 8–10 Anticholinergic drug use is also associated with cognitive impairments and dementia.11–13 Moreover, the use of anticholinergics leads to less self-dependency and a decrease in functional status.14 Likewise, patients might suffer from typical anticholinergic side effects as mental confusion, tremor, visual disturbances, delirium, dry mouth and urinary retention.15
The anticholinergic burden, the cumulative effect of using multiple drugs with anticholinergic activity simultaneously, can be calculated with the help of different lists.16 In the most common lists, drugs are categorised in none, low, moderate or high anticholinergic activity (zero to three points). The gained scores are summed up, and when the score is greater or equal three points, one should consider to use alternative drugs or a dose reduction. Some lists additionally include the daily dose.17 The number of included drugs varies between the scores and the scoring bases on different methods, for example, with regard to the drug’s potency and efficiency or to its exposure.18 With regard to the association of anticholinergic burdens on the cognitive function in elderly people, conflicting results have been published.19 As the different published tools rate drugs quite differently and on different bases, we decided to use two different tools to evaluate anticholinergic burden of our patient collective.18 The anticholinergic drug scale (ADS) developed by Carnahan et al is validated against serum anticholinergic activity (SAA), and high SAA levels are associated with cognitive impairments.20 21 Furthermore, the ADS score is a well-established tool to identify drugs with anticholinergic activity. Kiesel et al22 developed the German anticholinergic burden score (German ACB) especially for the German drug market in order to improve the routine prescribing in geriatric patients for the German population. To the best of our knowledge, there is no study that compares the ADS score with the German ACB score to investigate the anticholinergic burden of elderly multimorbid patients and pointing out the effect of anticholinergic drug use on the cognitive function. As far as we know, there is still limited data about the influence of anticholinergic drugs on the cognitive function from large European patient cohorts.
The German MultiCare cohort offers ideal conditions, as the study was conducted in order to examine the influence and effects of multimorbidity in multimorbid elderly patients in primary care. Patients and general practitioners (GPs) were interviewed about morbidities, prescribed and over-the-counter (OTC) medication, socioeconomic status, risk factors, health status and functional status, among others.23
The aims of our study were: (1) to identify anticholinergics and drugs with anticholinergic activity with the ADS and the German ACB score (2) and to show the effect of the anticholinergic burden measured with the German ACB score on the cognitive function and compare those findings with the well-established ADS score.
Methods
Study design
The MultiCare study was carried out as a multicentre, observational cohort study in primary care. Baseline data collection started in July 2008, and three follow-ups were performed, and each recruitment wave took 15 months. For our analysis, the baseline assessment of 3189 patients, collected between 21 July 2008 and 6 November 2009, was used. The recruitment took place in eight study centres in Germany (Bonn, Duesseldorf, Frankfurt/Main, Hamburg, Jena, Leipzig, Mannheim and Munich). Multimorbid patients were randomly selected from the GPs’ electronic files in 158 practices. Patients were included if they had at least three diagnosed chronic diseases and were between 65 and 85 years old. Exclusion criteria were: (1) nursing home patients, (2) blindness, (3) deafness, (4) missing capacity to consent, particularly patients with dementia, (5) all patients who had an expected life expectancy of less than 3 months, (6) insufficient ability to read and speak German, (7) patients who participate in other studies and (8) patients poorly known by the physician. A total of 7172 patients out of 50 786 patients from the GPs were contacted for informed consent after screening for inclusion and exclusion criteria. With a total response rate of 46.2%, 3317 patients were included, and after excluding 128 patients because they died before the baseline interview or due to other reasons, 3189 patients remained in the cohort. Standardised interviews and tests, at patients home, were conducted with the remaining 3189 patients to collect data about sex, age, education, income and cognitive skills by using the letter digit substitution test (LDST). Additionally, a brown bag review–capturing all prescription and OTC drugs the patients used on a regular basis or on demand–was performed to collect information about patients’ medication. Information about morbidity was gained with the help of standardised GP interviews. Schäfer et al previously published detailed information on the exact study design23 24 (online supplemental file 1).
bmjopen-2020-044230supp001.pdf (231.9KB, pdf)
Anticholinergic burden classification, descriptive results and subgroup analysis
Prescription and OTC drugs were gathered via brown bag review, and the drugs were classified analogous to the anatomical therapeutic classification (ATC) system.25
We used Excel 2016 (Microsoft Office 2016, Redmond, USA) to rate the anticholinergic drugs according to the German ACB and ADS scores.
The German ACB score was especially developed for the German drug market by Kiesel et al.22 Drugs were classified as drugs with anticholinergic activity with the help of a systematic literature research in PubMed and a subsequently evaluation by experts. The German ACB score comprises 507 substances, whereby 356 drugs have no anticholinergic effect (ACB score=0), 104 drugs are scored as weak (ACB score=1), 18 drugs are scored as moderate (ACB score=2) and 29 drugs are scored as having strong (ACB score=3) anticholinergic effects.
The ADS comprises 413 substances and is based on expert opinions.21 The ADS score categorises drugs into four different levels. Level 0 with no anticholinergic effect (296 substances), 71 level 1 drugs with a weak anticholinergic effect, 12 level 2 drugs with a moderate anticholinergic effect and 34 level 3 drugs with a strong anticholinergic effect.
The anticholinergic burden was calculated by summing up the individual anticholinergic scores of each patient, according to both anticholinergic scores individually.
For gaining the results for the subgroup analysis, a t-test with STATA V.15.1 (StataCorp, College Station, USA) was performed. We defined an alpha-level of 5% (p≤0.05) as statistically significant.
Fit for the Aged (FORTA) classification
For the classification according to FORTA PIM list, drugs were analysed indication based with QlikView 11.20 (QlikTech, Radnor, USA). The FORTA list comprises 296 drugs used in the treatment of 30 diagnoses or indications. FORTA rated drugs indication based as: A (absolute), B (beneficial), C (careful) and D (don’t). Drugs were classified as a potentially inadequate medication (PIM) when they are a FORTA C or D drug.26
FORTA list is used to reveal whether an additional use of an anticholinergic burden classification is necessary or not.
Association of anticholinergic drug use with the cognitive function
We performed a multivariate mixed-effect linear regression to calculate the influence of anticholinergic burden detected by the German ACB and the ADS score on the cognitive skills of the patients. Whereby the LDST, as a speed-depending cognitive task, was used to calculate the cognitive skills of the multimorbid elderly patients.
In LDST, patients have to replace letters by numbers in a specific time to show their ability of processing speed, which is an important cognitive ability and expresses normal cognitive development.27 Sex, age, number of diseases weighted by severity, highest education degree in three groups according to the international CASMIN (comparative analysis of social mobility in industrial nations) classification and household net adjusted disposable income as independent variables into the model were included.28 We adjusted the multilevel linear regression for random effects on the study centre and GP practice in order to minimise the regional effect of prescribing because of the eight different study centres (Bonn, Dusseldorf, Frankfurt/Main, Hamburg, Jena, Leipzig, Mannheim and Munich) and the 158 general practices.
The missing values—in LDST (missing values: 243), number of diseases weighted by severity (152), education standard (3) and the income data sets (258)—were imputed via hot deck imputation. The hot deck imputation has been described elsewhere.24 All analyses were performed with the imputed data sets, and an alpha level of 5% (p≤0.05) was defined as statistically significant. We conducted all statistical test with STATA V.15.1.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Results
Characterisation of the multimorbid elderly patient collective
A total of 3189 patients aged 65–85 years were included in the study. The mean age was 74.4 (±5.2) years and 59.3% of the patients were female. In total, 24 535 drugs including OTC were identified and related to an ATC code. In mean patients used 7.7 (±3.9) drugs (median 7 drugs, range 0–29 drugs). Table 1 summarises the main findings for the ADS and German ACB score. With ADS score, 1764 drugs were identified as anticholinergic for the MultiCare cohort, with a prevalence of anticholinergic drug use of 38.4% (1226). The mean ADS score is 0.8 (±1.3) and 10.5% (334) of all patients had an ADS score of 3 or higher. ForACB, we detected 2750 anticholinergics in total, and the prevalence of anticholinergic drug use is 53.7% (1714). The mean ACB score per patient is 1.2 (±1.6), and 18.1% (567) of all patients had an ACB score of 3 or higher.
Table 1.
Anticholinergic drugs per patient according to anticholinergic drug scale (ADS) and the German anticholinergic burden (German ACB) score and anticholinergic score per patient according to ADS and the German ACB score
| ADS | ACB | |
| Anticholinergic drugs per patient | ||
| Number of anticholinergic drugs | 1764 (7.2%) | 2750 (11.2%) |
| Prevalence of anticholinergic drug use | 38.4% | 53.7% |
| Mean (SD) | 0.6 (±0.9) | 0.9 (±1.0) |
| Median (range) | 0 (0–6) | 1 (0–7) |
| 0 AC per patient | 1963 (61.6%) | 1475 (46.3%) |
| 1 AC per patient | 846 (26.5%) | 1033 (32.4%) |
| 2 AC per patient | 265 (8.3%) | 435 (13.6%) |
| 3 AC per patient | 81 (2.5%) | 172 (5.4%) |
| 4 AC per patient | 26 (0.8%) | 45 (1.4%) |
| 5 AC per patient | 7 (0.2%) | 24 (0.8%) |
| 6 AC per patient | 1 (0.03%) | 4 (0.1%) |
| 7 AC per patient | – | 1 (0.03%) |
| Anticholinergic score per patient | ||
| Mean (SD) | 0.8 (±1.3) | 1.2 (±1.6) |
| Median (range) | 0 (0–11) | 1 (0–11) |
| Score per patient: 0 | 1963 (61.6%) | 1475 (46.3%) |
| Score per patient: 1 | 682 (21.4%) | 802 (25.1) |
| Score per patient: 2 | 210 (6.6%) | 345 (10.8%) |
| Score per patient: 3 | 179 (5.6%) | 272 (8.5%) |
| Score per patient: 4 | 86 (2.7%) | 140 (4.4%) |
| Score per patient: 5 | 36 (1.1%) | 67 (2.1%) |
| Score per patient: 6 | 23 (0.7%) | 45 (1.4%) |
| Score per patient: 7 | 5 (0.2%) | 21 (0.7%) |
| Score per patient: 8 | 2 (0.1%) | 14 (0.4%) |
| Score per patient: 9 | 2 (0.1%) | 5 (0.2%) |
| Score per patient: 10 | – | 1 (0.03%) |
| Score per patient: 11 | 1 (0.03%) | 2 (0.1%) |
AC, anticholinergic.
As the most common ADS drug, we detected furosemide 5.8% (185) as anticholinergic with low potential. Amitriptyline was identified as the most common anticholinergic ADS drug with a high anticholinergic potential, with 2.8% (88). For the ACB score, we identified metformin with 13.7% (436) as an anticholinergic with low potential, as the most reported ACB anticholinergic in the MultiCare cohort. Tramadol with 3.3% (105) and amitriptyline with 2.8% (88) are the most common ACB anticholinergic drugs with a moderate and high anticholinergic potential, respectively. 80.2% of the anticholinergics according to ADS score and 73.4% of the detected anticholinergics according tothe German ACB score are low potential anticholinergic drugs. ADS score most frequently detected drugs from the cardiovascular system (ATC C) with 36.6% (646 drugs, 11 different substances), followed by drugs from the nervous system (ATC N) with 31.9% (563 drugs, 35 different substances). In contrast, drugs from the nervous system make up the largest group of identified ACB anticholinergics, with 50 different substances and 29.5% (812 drugs) in total, followed by the cardiovascular system, with 13 different substances and 25.7% (709 drugs) in total. Considering the distribution of all used drugs within the MultiCare cohort, the proportions of anticholinergic drugs according to ADS and German ACB with regard to drugs from the cardiovascular system are 7.0% and 7.7% and with regard to drugs from the central nervous system are 22.5% and 32.4%, respectively.
In table 2, the top 10 ADS and German ACB anticholinergics and their occurrence in the FORTA PIM list are captured. Two of the top 10 drugs (tiotropium and ipratropium as inhalatives) are not listed in the FORTA list, while only four drugs are classified into the categories C or D.
Table 2.
Top 10 anticholinergics according to anticholinergic drugs scale (ADS) and the German anticholinergic burden (ACB) score and their occurrence in Fit for the Aged (FORTA) list (in brackets: ADS/ACB levels and FORTA categories)
| ADS (level) | ACB (level) | FORTA PIM (categories) | |
| Metformin | – | 436 (1) | 436 (B) |
| Furosemide | 185 (1) | 185 (1) | 185 (B) |
| Tiotropium (inhalative) | – | 125 (1) | – |
| Triamterene | 107 (1) | 107 (1) | 98 (B) |
| Tramadol | 105 (1) | 105 (2) | 105 (C) |
| Theophylline | 104 (1) | 104 (2) | 104 (C) |
| Prednisolone | 103 (1) | – | 103 (B) |
| Digitoxin | 93 (1) | 93 (1) | 32 (C) |
| Amitriptyline | 88 (3) | 88 (3) | 49 (C) und 31 (D) |
| Ipratropium (inhalative) | – | 84 (1) | – |
ADS and German ACB level 1–3 rate drugs in low, middle and high anticholinergic risk. FORTA categories rate drugs as A (absolute), B (beneficial), C (careful), D (don’t), whereby C and D drugs are defined as potentially inappropriate medication.
Subgroup analysis: age, sex and polypharmacy
Tables 3 and 4 summarise the most important results of the subgroup analysis for both scores. Female patients had a significant higher ADS and German ACB score than male patients (ADS: female: 0.82±1.34 male: 0.65±1.15; p<0.001; ACB: female: 1.30±1.73 and male: 1.04±1.42; p<0.001). Patients 80 years old and older had a significant higher ADS score than the patients that are 65 up to 79 years old (p=0.001). In contrast with that, there was no significant effect on the ACB score observed between the two age groups. However, patients using eight drugs or more at the same time had a significant higher ADS and ACB score than patients using less drugs (p<0.001).
Table 3.
The influence of age, sex and the number of taken drugs on the anticholinergic drug use according to anticholinergic drug scale (ADS) score in multimorbid elderly patients (significant p values are marked in bold)
| ADS score | Number of patients | Mean | SD | Range | 95% CI | P value |
| <80 years | 2635 | 0.71 | 1.24 | 0–11 | 0.67 to 0.76 | |
| ≥80 years | 554 | 0.91 | 1.38 | 0–9 | 0.79 to 1.02 | |
| p=0.001 | ||||||
| Male | 1298 | 0.65 | 1.15 | 0–8 | 0.58 to 0.71 | |
| Female | 1891 | 0.82 | 1.34 | 0–11 | 0.76 to 0.86 | |
| p<0.001 | ||||||
| 0–7 drugs | 1688 | 0.36 | 0.82 | 0–9 | 0.33 to 0.4 | |
| 8–29 drugs | 1501 | 1.18 | 1.52 | 0–11 | 1.1 to 1.25 | |
| p<0.001 |
Table 4.
The influence of age, sex and the number of taken drugs on the anticholinergic drug use according to German anticholinergic burden (ACB) score in multimorbid elderly patients (significant p values are marked in bold)
| ACB score | Number of patients | Mean | SD | Range | 95% CI | P value |
| <80 years | 2635 | 1.18 | 1.61 | 0–11 | 1.12 to 1.24 | |
| ≥80 years | 554 | 1.27 | 1.62 | 0–9 | 1.14 to 1.41 | |
| p=0.1992 | ||||||
| male | 1298 | 1.04 | 1.42 | 0–9 | 0.96 to 1.11 | |
| female | 1891 | 1.30 | 1.73 | 0–11 | 1.22 to 1.38 | |
| p<0.001 | ||||||
| 0–7 drugs | 1688 | 0.60 | 1.02 | 0–9 | 0.56 to 0.65 | |
| 8–29 drugs | 1501 | 1.86 | 1.87 | 0–11 | 1.76 to 1.95 | |
| p<0.001 |
Association of anticholinergic drug use with the cognitive function
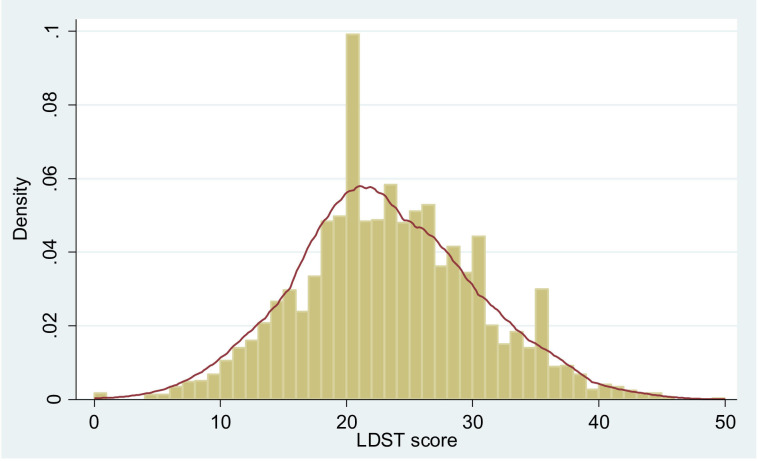
On average, patients achieved a mean LDST score of 23 (±7.1) with a range of 0–50 in the LDST, while 51.9% of the patients gained a score between 20 and 29. Figure 1 shows the kernel density estimator of the baseline results of patients LDST, showing the proportion of patients in each category.
Figure 1.
Kernal density estimator for the baseline assessment of the letter digit substitution test with the purpose of measuring the cognitive function of patients and the number of boxes patients were able to fill out correctly in a defined time. LDST, letter digit substitution test.
We evolved two models to express the influence of anticholinergics on the LDST results. Tables 5 and 6 show the outcomes of the multivariate mixed-effect linear regression for the ADS score and the German ACB score. In the first model, not including FORTA PIM, we detected that with increasing ADS score, the ability to complete the LDST decreases significantly with a regression coefficient of – 0.37 (p≤0.0001). Also, the German ACB score could exhibit the effect of worse LDST results with increasing ACB score with a regression coefficient of – 0.33 (p≤0.0001). According to a sensitivity analysis, we added FORTA PIM as a cofounder to the regression model (ADS score: p=0.257, regression coefficient: −0.04; ACB score: p=0.518; regression coefficient: –0.02). By adding FORTA PIM into the second model, the regression coefficient dropped but was still significant: for ADS score, we measured a regression coefficient of −0.26 (p=0.008) and for the German ACB, a score of −0.24 (p=0.003).
Table 5.
The two linear regression models for the association between cognitive function (LDST) and anticholinergic score according to anticholinergic drugs scale (ADS) score (significant p values are marked in bold)
| LDST | Regression coefficient | P value | 95% CI |
| ADS score per patient | −0.37 | <0.001 | −0.55 to −0.2 |
| Sex | −0.34 | <0.001 | −0.38 to −0.3 |
| Age | 2.57 | <0.001 | 2.11 to 3.04 |
| Casmin3_2 | 2.33 | <0.001 | 1.8 to 2.87 |
| Casmin3_3 | 3.68 | <0.001 | 2.91 to 4.45 |
| Income | 2.45 | <0.001 | 1.92 to 2.98 |
| Number of diseases weighted by severity | −0.13 | <0.001 | −0.18 to −0.09 |
| ADS score per patient | −0.26 | 0.008 | −0.46 to −0.07 |
| Sex | −0.34 | <0.001 | −0.38 to −0.3 |
| Age | 2.58 | <0.001 | 2.12 to 3.04 |
| Casmin3_2 | 2.32 | <0.001 | 1.79 to 2.85 |
| Casmin3_3 | 3.71 | <0.001 | 2.94 to 4.48 |
| Income | 2.44 | <0.001 | 1.91 to 2.97 |
| Number of diseases weighted by severity | −0.12 | <0.001 | −0.16 to −0.07 |
| FORTA PIM | −0.35 | 0.005 | −0.59 to −0.11 |
Dependent variable: results from LDST; independent variable: ADS score; covariables included in the regression model: sex, age, education standard (casmin3_2: comparison between medium and low educational standard; casmin3_3: comparison between high and low educational standard), income, number of diseases weight by severity, used FORTA drugs.
ACB, anticholinergic burden; FORTA, Fit for the Aged; LDST, letter digit substitution test.
Table 6.
The two linear regression models for the association between cognitive function (LDST) and anticholinergic score according to the German anticholinergic burden (ACB) score (significant p values are marked in bold)
| LDST | Regression coefficient | P value | 95% CI |
| ACB score per patient | −0.33 | <0.001 | −0.47 to −0.19 |
| Sex | −0.34 | <0.001 | −0.39 to −0.3 |
| Age | 2.60 | <0.001 | 2.14 to 3.06 |
| Casmin3_2 | 2.33 | <0.001 | 1.8 to 2.86 |
| Casmin3_3 | 3.68 | <0.001 | 2.9 to 4.45 |
| Income | 2.42 | <0.001 | 1.89 to 2.95 |
| Number of diseases weighted by severity | −0.13 | <0.001 | −0,17 to −0,08 |
| ACB score per patient | −0.24 | 0.003 | −0.40 to −0.08 |
| Sex | −0.34 | <0.001 | −0.39 to −0.30 |
| Age | 2.60 | <0.001 | 2.13 to 3.06 |
| Casmin3_2 | 2.32 | <0.001 | 1.79 to 2.85 |
| Casmin3_3 | 3.70 | <0.001 | 2.93 to 4.47 |
| Income | 2.42 | <0.001 | 1.89 to 2.95 |
| Number of diseases weighted by severity | −0.12 | <0.001 | −0.17 to −0.07 |
| FORTA PIM per patient | −0.29 | 0.030 | −0.54 to −0.03 |
Dependent variable: results from LDST; independent variable: ACB score; covariables included in the regression model: sex, age, education standard (casmin3_2: comparison between medium and low educational standard; casmin3_3: comparison between high and low educational standard), income, number of diseases weight by severity, used FORTA drugs.
FORTA, Fit for the Aged; LDST, letter digit substitution test.
Discussion
Statement of principal findings
Our study demonstrates that a huge proportion of multimorbid elderly patients are exposed to anticholinergic drugs or drugs with anticholinergic activity and are consequently affected by the risk of anticholinergic adverse reactions that are associated with decreased cognitive function determined by LDST.
Anticholinergic burden classification and risk factors for anticholinergic drug use
In terms of the ADS score, our findings are in good accordance with the literature.29–31 As there is no publication analysing medication with the German ACB score yet, we compared our findings with the gained results of the ADS score and other well-established anticholinergic scores. The results for the German ACB score (mean anticholinergic burden: 1.2±1.6; prevalence: 53.7%) are comparable with our findings with the ADS score (0.8±1.3; 38.4%) and also other anticholinergic scores described in literature (0.3±0.7 to 1.7±1.5; 17.1%–63.0%).10 29
Even though we determined that drugs from the central nervous system are the most common drugs identified with the German ACB score and the second most common for ADS, our top 10 anticholinergic drugs showed a more diverse spectrum of drugs. Particularly, drugs with low to moderate anticholinergic effects occurred in our top 10 list for both scores. As 80.2% of the anticholinergic drugs according to ADS and 73.4% of the anticholinergic drugs according to German ACB are anticholinergics with a score of 1, it is important to also focus on the drugs with only mild anticholinergic potential during prescribing and reviewing patients’ medications. Furthermore, drugs treating cardiovascular conditions highly contributed to the level 1 anticholinergic drugs in both scores. However, in multimorbid elderly patient, it is common to coprescribe drugs like furosemide, triamterene, digitoxin and captopril to treat multiple conditions.32 Also other studies point out the high prevalence of low potential anticholinergic drug use and especially from the cardiovascular system in elderly patients.32 33 It is stated that especially the cumulative anticholinergic effect contributes to higher anticholinergic scores and even leads to hospitalisation and higher risk for mortality.34–36 A lot of the mentioned and detected level 1 drugs are peripherally acting anticholinergic drugs. However, as the permeability of the blood–brain barrier is increased and at the same time the P-glycoprotein function is decreased with growing age, elderly people are more vulnerable towards anticholinergic ADRs.5
Particularly multimorbid elderly patients are vulnerable for polypharmacy.37 38 So it is not surprising that we identified polypharmacy as risk factor for a high German ACB and ADS score. Besides this, we detected that female patients seem to be more vulnerable towards the exposure with anticholinergic drugs. This gender shift was also observed in several studies, although most studies used different tools to identify the anticholinergic burden.15 30 39 The increased vulnerability of women towards anticholinergic drug exposure might be explainable by the fact that women have a higher health awareness than men.40 In addition, rates of depression are higher in the female population, and we identified drugs from the central nervous system as one of our largest drug groups contributing to the anticholinergic burden.41
Association of anticholinergic drug use with the cognitive function
Multivariate analysis revealed that a higher anticholinergic burden according to ADS and also the German ACB score is associated with a decreased cognitive function according to an increasing poorly performance in the LDST. Interestingly, the newly developed German ACB score showed similar results in our adjusted model in comparison with the already well-established ADS score. A lot of studies prove that a high anticholinergic burden is associated with a decreased cognitive function as well.35 42 However, there are also opposite findings. For example, Kersten et al31 could determine that there was no association between cognitive impairments and anticholinergic drug use according to ADS score. The differences in the outcomes might be explained by several factors. First, it is sometimes difficult to show a homogenous association between anticholinergic drug use and cognitive function, because there is a broad heterogeneity in cholinergic brain reserve in each individuum that leads to differences in the sensitivity to central anticholinergic effects, and second, the used tools for detecting anticholinergic drugs and drugs with anticholinergic activity and measuring the cognitive function of the patients differs between the studies and not always fits the country-specific prescribing habits.43 However, Gray et al44 detected in a prospective cohort study over a time period of 7.3 years that 23% of the patients 65 years old and older develop a dementia and thereof 80% used anticholinergic drugs. As already mentioned, patients were excluded from MultiCare study when they were diagnosed with dementia and/or were living in nursing homes. Even though there was no standardised tool for diagnosing dementia due to the different GPs in the different study centres, we can assume that our patient collective had less cognitive impairments than the collectives in other studies. So it is quite interesting that our patient collective already shows an association between decreased cognitive function based on poorer results in LDST and a high anticholinergic score. That demonstrates the importance of rational prescribing and also deprescribing, even in presumed healthier elderly patients. Drugs with anticholinergic activity are widely prescribed, but we need to evaluate the pros and cons of their usage. On the one hand, alternative treatments are partly not available or appropriate, and on the other hand, there are risk of anticholinergic side effects. That is why we need to weigh the risk between deprescribing and a possible undertreatment of critical conditions. Consequently, we are in need to develop interdisciplinary processes for deprescribing. Ailabouni et al invented a five-step systematic intervention in deprescribing anticholinergic and sedative drugs for a small patient collective. Although they could not report an improvement in cognitive function over a time period of 6 months after deprescribing, they could lower the used medication in mean about 2.1 drugs per patient. They also detected that patients reported significantly less adverse effects, reduced frailty and falls.45 However, for deprescribing, we are in need for validated tools, with regard to anticholinergic drug use and regarding potentially inappropriate medication. In addition, it is interesting to know whether it is necessary to evaluate patients’ medication concerning PIM lists and anticholinergic burden lists. Studies revealed a high proportion of anticholinergics and sedatives within the detected PIM, but there was no analysis with regard to the necessity of using PIM tools and tools to evaluate the anticholinergic burden.46 Although we determined by including FORTA PIM into the regression model a decrease of the regression coefficient for ADS and German ACB score about −0.1, the anticholinergic scores and therefore the use of anticholinergic drugs according to ADS and German ACB score still seemed to have a negative influence on the cognitive function on multimorbid elderly patients. In addition, the FORTA list did not cover all drugs detected with ADS and/or German ACB score. So, we assume that multimorbid elderly patients could benefit from the use of both lists (PIM and anticholinergic burden).
Strength and limitations
Our study has some strengths and limitations. Unfortunately, we could not underline our results by showing an impact of anticholinergic drug use on peripheral anticholinergic ADR (eg, dry mouth, blurred vision, constipation, nausea, urinary retention, impaired sweating or tachycardia) because such parameters were not gained during data collection. However, other studies showed that anticholinergic drug use is associated with significantly increased mouth dryness.31
Most studies had a less healthy patient collective than we had, due to the fact that we excluded patients living in nursing homes. De Vreese et al4 detected that especially patients from nursing homes are at a greater risk of receiving anticholinergic drugs. However, we could demonstrate that even the apparently more healthy elderly patients are in great risk for receiving anticholinergic drugs and thereby suffering from anticholinergic side effects in association with decreased cognitive function. Moreover, we could not evaluate the length of intake of anticholinergic drugs. Further studies, especially longitudinal studies, are necessary to evaluate the decrease in cognitive function over time. As cognitive impairments is a complex clinical symptom and the LDST only addresses one single aspect of cognition, further tests would help to underline our findings. However, a strength of our study is that we performed a multivariate analysis, including among other number of diseases weighted by severity. A sensitivity analysis was performed, revealing that FORTA PIM has to be included as a confounder in the regression model. In contrast to the number of used drugs, which had no significant influence on the results in LDST (ADS score: p=0.257, regression coefficient: −0.04; ACB score: p=0.518; regression coefficient: –0.02). An additional strength is also the advanced treating of missing values via hot deck imputation.
Taken together, anticholinergic drugs and drugs with anticholinergic activity in multimorbid elderly adults appear to be associated with harms that, in certain circumstances, outweigh their potential benefit. We could determine that a high anticholinergic score is associated with a reduced cognitive function, according to increased poorer results in LDST, in multimorbid elderly patients. In addition, we showed that especially drugs with low anticholinergic risk, for example, for treating cardiovascular conditions, contribute to the anticholinergic burden.
Conclusion
Our study demonstrated that it is important to gain greater awareness for the risk of using anticholinergic drugs in multimorbid elderly patients and that there exist tools that are easy to use in medical routine to calculate the anticholinergic burden of this vulnerable patient group. Furthermore, we pointed out that the newly invented German ACB score by Kiesel et al seems to generate comparable results with already validated and established tools. However, it needs to be validated in future in order to gain data about the safe use of this tool.
As shown in our study, it is also important to question lower potential anticholinergic drugs, since cumulative effects of those low potential anticholinergic drugs can lead to high anticholinergic burdens as well.
Further studies are needed, especially showing the effect on patient outcome on deprescribing anticholinergic drugs over a longer time period and longitudinal studies to demonstrate the development of cognitive function under use of anticholinergic drugs over time.
In summary, a high anticholinergic burden and therefore anticholinergic drug use is associated with a decreased cognitive function in multimorbid elderly patients. In order to contribute to an improvement in drug therapy safety, we need to invent strategies for rational prescribing and deprescribing.
Supplementary Material
Acknowledgments
This article is on behalf of the MultiCare Cohort Study Group, which consists of Attila Altiner, Horst Bickel, Wolfgang Blank, Monika Bullinger, Hendrik van den Bussche (principal investigator), Anne Dahlhaus, Lena Ehreke, Michael Freitag, Angela Fuchs, Jochen Gensichen, Ferdinand Gerlach, Heike Hansen, Sven Heinrich, Susanne Höfels, Olaf von dem Knesebeck, Hans-Helmut König, Norbert Krause, Hanna Leicht, Margrit Löbner, Melanie Luppa, Wolfgang Maier, Manfred Mayer, Christine Mellert, Anna Nützel, Thomas Paschke, Juliana Petersen, Jana Prokein, Steffi Riedel-Heller, Heinz-Peter Romberg, Ingmar Schäfer, Martin Scherer (principal investigator), Gerhard Schön, Susanne Steinmann, Sven Schulz, Karl Wegscheider, Klaus Weckbecker, Jochen Werle, Siegfried Weyerer and Birgitt Wiese. We are grateful to the general practitioners in Bonn, Dusseldorf, Frankfurt/Main, Hamburg, Jena, Leipzig, Mannheim and Munich who supplied the clinical information on their patients.
Footnotes
Twitter: @degampraesident
Contributors: All authors provided substantial contributions to study design and implementation. The first draft of the manuscript was written by CK, and all authors commented on previous versions of the manuscript. All authors revised and approved the final manuscript.
Funding: The study was funded by the German Federal Ministry of Education and Research (grant numbers 01ET0725-31 and 01ET1006A-K).
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study is conducted in compliance with the Helsinki Declaration. The study protocol was approved by the Ethics Committee of the Medical Association of Hamburg in February 2008 and amended in November 2008 (Approval-No. 2881).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. The data that support the findings of this study are available from Professor Hendrik van den Bussche, but restrictions apply to the availability of these data, which were used under licence for the current study, and so are not publicly available. Data are however available from the authors on reasonable request and with permission of Professor Hendrik van den Bussche.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.van den Akker M, Vaes B, Goderis G, et al. Trends in multimorbidity and polypharmacy in the Flemish-Belgian population between 2000 and 2015. PLoS One 2019;14:e0212046. 10.1371/journal.pone.0212046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee EA, Brettler JW, Kanter MH. Refining the definition of polypharmacy and its link to disability in older adults: conceptualizing necessary polypharmacy, unnecessary polypharmacy, and polypharmacy of unclear benefit. Perm J 2020;24 10.7812/TPP/18.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villalba-Moreno Angela M, Alfaro-Lara ER, Rodríguez-Pérez A, et al. Association between drug burden index and functional and cognitive function in patients with multimorbidity. Curr Pharm Des 2018;24:3384–91. 10.2174/1381612824666180327154239 [DOI] [PubMed] [Google Scholar]
- 4.De Vreese LP, Mantesso U, De Bastiani E, et al. Anticholinergic burden in adult and elderly people with intellectual disabilities: results from an Italian multicenter cross-sectional study. PLoS One 2018;13:e0205897. 10.1371/journal.pone.0205897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ps N, Ms S, Sn H. Anticholinergics: theoretical and clinical overview [online]. Expert Opin Drug Saf 2016. [DOI] [PubMed] [Google Scholar]
- 6.Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc 2008;56:2203–10. 10.1111/j.1532-5415.2008.02009.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsh TJ, van der Wardt V, Ojo G, et al. Anticholinergic drug burden Tools/Scales and adverse outcomes in different clinical settings: a systematic review of reviews. Drugs Aging 2018;35:523–38. 10.1007/s40266-018-0549-z [DOI] [PubMed] [Google Scholar]
- 8.Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr 2015;15:31. 10.1186/s12877-015-0029-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrade C. Anticholinergic drug exposure and the risk of dementia: there is modest evidence for an association but not for causality. J Clin Psychiatry 2019;80. [DOI] [PubMed] [Google Scholar]
- 10.Green AR, Reifler LM, Bayliss EA, et al. Drugs contributing to anticholinergic burden and risk of fall or fall-related injury among older adults with mild cognitive impairment, dementia and multiple chronic conditions: a retrospective cohort study. Drugs Aging 2019;36:289–97. 10.1007/s40266-018-00630-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell NL, Perkins AJ, Bradt P, et al. Association of anticholinergic burden with cognitive impairment and health care utilization among a diverse ambulatory older adult population. Pharmacotherapy 2016;36:1123–31. 10.1002/phar.1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu M, Hughes T. Anticholinergic drug usage and cognitive impairment: findings from three large European cohort studies. J Neurol 2019;266:1816–8. 10.1007/s00415-019-09415-9 [DOI] [PubMed] [Google Scholar]
- 13.Bishara D, Harwood D, Sauer J, et al. Anticholinergic effect on cognition (AEC) of drugs commonly used in older people. Int J Geriatr Psychiatry 2017;32:650–6. 10.1002/gps.4507 [DOI] [PubMed] [Google Scholar]
- 14.Boccardi V, Baroni M, Paolacci L, et al. Anticholinergic burden and functional status in older people with cognitive impairment: results from the ReGAl project. J Nutr Health Aging 2017;21:389–96. 10.1007/s12603-016-0787-x [DOI] [PubMed] [Google Scholar]
- 15.Byrne CJ, Walsh C, Cahir C, et al. Anticholinergic and sedative drug burden in community-dwelling older people: a national database study. BMJ Open 2018;8:e022500. 10.1136/bmjopen-2018-022500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali S, Peterson GM, Bereznicki LR, et al. Association between anticholinergic drug burden and mortality in older people: a systematic review. Eur J Clin Pharmacol 2020;76:319–35. 10.1007/s00228-019-02795-x [DOI] [PubMed] [Google Scholar]
- 17.Hilmer SN, Mager DE, Simonsick EM, et al. Drug burden index score and functional decline in older people. Am J Med 2009;122:1142–9. 10.1016/j.amjmed.2009.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer T, Haefeli WE, Seidling HM. Different methods, different results—how do available methods link a patient's anticholinergic load with adverse outcomes? Eur J Clin Pharmacol 2015;71:1299–314. 10.1007/s00228-015-1932-x [DOI] [PubMed] [Google Scholar]
- 19.Salahudeen MS, Chyou T-Y, Nishtala PS. Serum anticholinergic activity and cognitive and functional adverse outcomes in older people: a systematic review and meta-analysis of the literature. PLoS One 2016;11:e0151084. 10.1371/journal.pone.0151084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ms S, Sn H, Ps N. Comparison of anticholinergic risk scales and associations with adverse health outcomes in older people [Internet]. J Am Geriatr Soc 2015. [DOI] [PubMed] [Google Scholar]
- 21.Carnahan RM, Lund BC, Perry PJ, et al. The anticholinergic drug scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol 2006;46:1481–6. 10.1177/0091270006292126 [DOI] [PubMed] [Google Scholar]
- 22.Kiesel EK, Hopf YM, Drey M. An anticholinergic burden score for German prescribers: score development. BMC Geriatr 2018;18:239. 10.1186/s12877-018-0929-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schäfer I, Hansen H, Schön G, et al. The German multicare-study: patterns of multimorbidity in primary health care – protocol of a prospective cohort study. BMC Health Serv Res 2009;9:145. 10.1186/1472-6963-9-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schäfer I, Hansen H, Schön G, et al. The influence of age, gender and socio-economic status on multimorbidity patterns in primary care. first results from the multicare cohort study. BMC Health Serv Res 2012;12:89. 10.1186/1472-6963-12-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DIMDI - ATC/DDD anatomisch-therapeutisch-chemische Klassifikation mit definierten Tagesdosen [online]. Available: http://www.dimdi.de/static/de/klassi/atcddd/index.htm [Accessed 20 Jul 2017].
- 26.Pazan F, Weiss C, Wehling M, et al. The EURO-FORTA (fit fOR the aged) list: international consensus validation of a clinical tool fOR improved drug treatment in older people. Drugs Aging 2018;35:61–71. 10.1007/s40266-017-0514-2 [DOI] [PubMed] [Google Scholar]
- 27.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev 1996;103:403–28. 10.1037/0033-295X.103.3.403 [DOI] [PubMed] [Google Scholar]
- 28.Educational reform in France, West-Germany and the United Kingdom: updating the CASMIN educational classification [online]. Available: https://www.ssoar.info/ssoar/handle/document/20816 [Accessed 4 Apr 2020].
- 29.Dauphinot V, Mouchoux C, Veillard S, et al. Anticholinergic drugs and functional, cognitive impairment and behavioral disturbances in patients from a memory clinic with subjective cognitive decline or neurocognitive disorders. Alzheimers Res Ther 2017;9:58. 10.1186/s13195-017-0284-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swami S, Cohen RA, Kairalla JA, et al. Anticholinergic drug use and risk to cognitive performance in older adults with questionable cognitive impairment: a cross-sectional analysis. Drugs Aging 2016;33:809–18. 10.1007/s40266-016-0400-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kersten H, Molden E, Willumsen T, et al. Higher anticholinergic drug scale (ADS) scores are associated with peripheral but not cognitive markers of cholinergic blockade. Cross sectional data from 21 Norwegian nursing homes. Br J Clin Pharmacol 2013;75:842–9. 10.1111/j.1365-2125.2012.04411.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green AR, Reifler LM, Boyd CM, et al. Medication profiles of patients with cognitive impairment and high anticholinergic burden. Drugs Aging 2018;35:223–32. 10.1007/s40266-018-0522-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson K, Fox C, Maidment I. Anticholinergic drugs and risk of dementia: case-control study. BMJ 2018;29:k1315. 10.1136/bmj.k1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mate KE, Kerr KP, Pond D, et al. Impact of multiple low-level anticholinergic medications on anticholinergic load of community-dwelling elderly with and without dementia. Drugs Aging 2015;32:159–67. 10.1007/s40266-014-0230-0 [DOI] [PubMed] [Google Scholar]
- 35.Pfistermeister B, Tümena T, Gaßmann K-G, et al. Anticholinergic burden and cognitive function in a large German cohort of hospitalized geriatric patients. PLoS One 2017;12:e0171353. 10.1371/journal.pone.0171353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gnjidic D, Hilmer SN, Hartikainen S, et al. Impact of high risk drug use on hospitalization and mortality in older people with and without Alzheimer's disease: a national population cohort study. PLoS One 2014;9:e83224. 10.1371/journal.pone.0083224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy C, Clyne B, Corrigan D, et al. Supporting prescribing in older people with multimorbidity and significant polypharmacy in primary care (SPPiRE): a cluster randomised controlled trial protocol and pilot. Implement Sci 2017;12:99. 10.1186/s13012-017-0629-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Buedingen F, Hammer MS, Meid AD, et al. Changes in prescribed medicines in older patients with multimorbidity and polypharmacy in general practice. BMC Fam Pract 2018;19:131. 10.1186/s12875-018-0825-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suehs BT, Caplan EO, Hayden J, et al. The relationship between anticholinergic exposure and falls, fractures, and mortality in patients with overactive bladder. Drugs Aging 2019;36:957–67. 10.1007/s40266-019-00694-5 [DOI] [PubMed] [Google Scholar]
- 40.Barrenberg E, Garbe E. Use of over-the-counter (OTC) drugs and perceptions of OTC drug safety among German adults. Eur J Clin Pharmacol 2015;71:1389–96. 10.1007/s00228-015-1929-5 [DOI] [PubMed] [Google Scholar]
- 41.Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol 2009;5:363–89. 10.1146/annurev.clinpsy.032408.153621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ancelin ML, Artero S, Portet F, et al. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ 2006;332:455–9. 10.1136/bmj.38740.439664.DE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collamati A, Martone AM, Poscia A, et al. Anticholinergic drugs and negative outcomes in the older population: from biological plausibility to clinical evidence. Aging Clin Exp Res 2016;28:25–35. 10.1007/s40520-015-0359-7 [DOI] [PubMed] [Google Scholar]
- 44.Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia. JAMA Intern Med 2015;175:401–7. 10.1001/jamainternmed.2014.7663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ailabouni N, Mangin D, Nishtala PS. DEFEAT-polypharmacy: deprescribing anticholinergic and sedative medicines feasibility trial in residential aged care facilities. Int J Clin Pharm 2019;41:167–78. 10.1007/s11096-019-00784-9 [DOI] [PubMed] [Google Scholar]
- 46.Harrison SL, Kouladjian O’Donnell L, Bradley CE, et al. Associations between the drug burden index, potentially inappropriate medications and quality of life in residential aged care. Drugs Aging 2018;35:83–91. 10.1007/s40266-017-0513-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-044230supp001.pdf (231.9KB, pdf)