Abstract
Non‐alcoholic fatty liver disease (NAFLD) is an increasing problem in pediatrics with limited treatment options. We prospectively assessed outcomes in patients managed in a hepatology clinic (HC) alone vs. those managed in combination with a multidisciplinary weight management program (MWMP). We describe each group’s readiness to change at the time of NAFLD diagnosis. Patients diagnosed with NAFLD were given a modified Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) at enrollment (T1) to assess readiness to change. They were then followed at 3–9 months (T2) and at 10–15 months (T3). Linear mixed models were used to evaluate changes in body mass index (BMI), BMI z‐score, and transaminases over time and between the two groups. There were no significant treatment group main effects or treatment × time interactions for our primary end points for HC alone (n = 75) or with MWMP (n = 18). There was a significant main effect for time for BMI z‐score, with BMI z‐scores declining on average by 0.0568 (P = 0.004) from visit to visit. Low SOCRATES subscales scores in HC alone (n = 33) or with MWMP (n = 4) suggested a patient population with low recognition of disease and likelihood of taking steps for change. Patients with obesity and NAFLD had low scores on all three SOCRATES subscales. Despite this, both groups had improvement in BMI z‐score without significant difference between the two treatment groups in other primary end points. Further study is needed to identify the most effective patient selection and treatment strategies for pediatric patients with NAFLD, including pharmacotherapy and surgery.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Multidisciplinary clinics are often used in the treatment of obesity. Parental readiness to change is described in the literature.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ What are the baseline characteristics, readiness to change, and prospective outcomes for patients seen in a hepatology clinic (HC) alone to those seen in combination with multidisciplinary weight management program (MWMP)? Were there baseline characteristics that accounted for differences in clinical outcomes?
WHAT DOES THE STUDY ADD TO OUR KNOWLEDGE?
☑ Patients seen in an HC at a tertiary care clinic alone or with a MWMP are both able to achieve a reduction in body mass index z‐score without significant differences between groups. Readiness to change scores are low in a pediatric population newly diagnosed with nonalcoholic fatty liver disease.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Our study highlights an area where further development of pharmacotherapy is needed to improve modest outcomes.
Despite ongoing efforts to improve the public awareness and care of children in the United States, rates of obesity are increasing with 18.5% of US children and adolescents ages 2–19 years classified as obese in 2015–2016. 1 , 2 Decades of study have demonstrated interactions among genetics, nutrition, physical activity, culture, and public health policy in addition to emerging theories of infectobesity, the microbiome, circadian rhythm, and other potential contributors. 3 , 4 As a result, children are at increasing risk of developing metabolic syndrome, nonalcoholic fatty liver disease (NAFLD), and premature mortality, and complication rates increase as they become adolescents. 5 Treatment options vary, but a staged approach is recommended for children and adolescents with obesity. This begins with counseling in the primary care setting for stage 1 and progresses to multidisciplinary services, medications, and bariatric surgery for those in stage 4 treatment. 6 Multidisciplinary weight management programs (MWMPs) have demonstrated success in weight reduction across a range of clinical settings and are frequently used to combat obesity at the patient level. 7 , 8 , 9 Assessment of readiness to change behaviors contributing to obesity is recommended at initiation of MWMP and is often informally performed as part of an overall motivational interviewing approach by the clinician during office visits. 10
With the increasing rates of obesity have come increasing rates of NAFLD, which is widely expected to become the most common liver disease of childhood. 11 NAFLD is a spectrum of disease that ranges from simple steatosis to nonalcoholic steatohepatitis and advanced fibrosis. The etiology of NAFLD is similar to obesity; however, not all patients with obesity develop NAFLD and there are additional intrinsic and extrinsic factors involved. 12 It is currently the second leading cause of liver transplantation in adults and is expected to eventually become the most common. 13 The mainstay of treatment is weight management prior to the onset of advanced fibrosis. Medications such as vitamin E and metformin have been trialed, but results have been inconclusive and newer therapies are still under investigation. 14
Patients with obesity and NAFLD are often followed by a gastroenterologist or hepatologist to monitor complications and provide treatment. Depending on patient interest and program availability, patients may be seen in a pediatric hepatology clinic (HC) alone or concurrently with an MWMP. Although the success of MWMP in weight management has been demonstrated, outcomes have not been prospectively compared with those resulting from care received in an HC alone. In this study, our aims were to describe and compare baseline characteristics, readiness to change, and prospective outcomes for patients seen in an HC alone to those seen in combination with MWMP. In addition, we sought to determine whether baseline characteristics accounted for differences in clinical outcomes.
METHODS
This was an observational prospective study of pediatric patients with NAFLD. All research activities were approved by our institutional internal review board and proceeded in accordance with the Helsinki Declaration of 1975. Subjects were identified for study eligibility at their diagnostic visit for NAFLD in the HC (T1). Subsequently, subjects were grouped into an HC alone condition or HC with MWMP condition. Patients were considered part of the HC with MWMP condition if they were also seen in our center’s MWMP within 6 months of their initial HC visit. Given the naturalistic design of this study, patients were not randomized into either treatment arm; instead, referral to the MWMP was based on provider discretion and patient interest. Age of enrollment could be 2 to 17 years. Those aged 13–17 years were given an adapted paper‐and‐pencil version of the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) questionnaire (SOCRATES version 8A) 15 , 16 to fill out after their initial clinic visit to assess for readiness to change at diagnosis. Originally developed in the setting of alcohol use, items were adapted by the authors of this study to reflect patient attitudes toward their obesity and potential health consequences (see Supplementary Material). This adapted SOCRATES questionnaire was used because no other age appropriate questionnaire measuring readiness to change was available at the time of study development (Figure S1 ). (Note: There is now an obesity‐specific and validated SOCRATES‐OO for adults, but the current study began before its development 17 .) Subjects were then followed for 2 follow‐up visits in the HC at 3–9 months (T2) and 10–15 months (T3). Patients who attended all appointments as prescribed were given a US $25 dollar gift card for study completion. Enrollment stopped when the wait list for the MWMP at our center grew to > 6 months. Patients could no longer qualify for the HC with MWMP condition as they could not be seen within 6 months of their diagnosis of NAFLD. Primary end points included body mass index (BMI), BMI z‐score, alanine aminotransferase (ALT), and aspartate aminotransferase (AST).
All patients referred to an HC with elevated transaminases and obesity completed an evaluation according to current recommendations. 18 This includes screening laboratory work and abdominal imaging to demonstrate steatosis. Screening tests obtained per clinic protocol included antinuclear antibody, total IgG, antismooth muscle antibody, antiliver kidney microsomal antibody, perinuclear antineutrophil cytoplasmic antibodies, serology for hepatitis B and C, ceruloplasmin, ferritin, total IgA, anti‐tissue transglutaminase antibody IgA level, and alpha‐1‐antitrypsin phenotype with other tests ordered as needed per patient history, family history, and clinical findings. Liver biopsy is typically reserved for cases where the laboratory work is concerning for alternative diagnosis or for persistently elevated transaminases (> 3× upper limit of normal over 6 months) to confirm diagnosis and stage fibrosis, similar to EPSGHAN guidelines. 18 Results from noninvasive techniques, such as FibroScan to measure steatosis and fibrosis) were introduced to our clinic after the start of the study and therefore were unable to be included in the dataset. After the initial visit, patients were scheduled for follow‐up every 3–6 months in the HC to set weight management goals, monitor changes, and assess the progression of liver disease.
The MWMP at our center is titled Promoting Health in Teens and Kids (PHIT Kids). The multidisciplinary team includes physicians, nurse practitioners, psychologists, dietitians, and social workers. Patients are seen for monthly clinic visits for treatment and monitoring of progress. Beyond comorbidity assessment, family education, and motivational interviewing, medications, and bariatric surgery are treatment options for qualifying patients, although surgery was not available at our institution at the start of the study. Patients can also attend a 12‐session weekly group meeting in the evening for more intensive dietary, behavioral, and physical activity interventions.
Statistical analysis
Participant demographic information was summarized using descriptive statistics, and mean SOCRATES subscale scores were compared between groups using independent‐samples t‐tests. Preliminary analyses were conducted to compare patients who attended routine monthly visits in the MWMP to those who also attended the weekly evening group meetings. We determined that patients who attended routine monthly visits in the MWNP were not significantly different than those who also attended weekly group meetings; as such, these two groups were considered as one (i.e., MWMP) for all analyses. Linear mixed effects models 19 , 20 were used to evaluate main effects for treatment group (HC alone and HC with MWMP) and time (T0, T1, and T2), as well as group differences in changes because the baseline visit (group × time interaction effect), for each of the primary outcome variables (BMI, BMI z‐score, and ALT and AST laboratory values). Models were specified to control for baseline differences between participants in the outcome variables when evaluating changes over time. Linear mixed effects modeling was selected for analyses due to its capacity to validly model change in outcome variables when participants vary on the number and spacing of completed measurement occasions. 21 The linear mixed model equations were initially set up as follows: , where the outcome variable for timepoint (t) and individual (i) is modeled as a function of the rate of change in the outcome variable for the HC alone reference group () plus any difference in rate of change for those in the HC + MWMP group (), while controlling for baseline values and group differences in those values (, ) and allowing for unaccounted for within‐subject and between‐subject error (). Covariates of age at diagnosis, sex, race (coded as white vs. all others), and insurance status (public vs. private or self‐pay) also were explored in models and removed if nonsignificant.
RESULTS
Sample characteristics
The mean age of initial HC visit was 12.6 (n = 75, SD = 2.9, range 5–17) and 11.0 (n = 18, SD = 3.3, range 5–17) for the HC alone and HC with MWMP treatment groups, respectively. This difference was statistically significant (t[91] = 2.049, P = 0.043). Over half of the entire sample (54.8%) self‐identified as white, and over three‐fourths of patients (76.3%) were men; this was roughly similar in each of the two treatment groups. Seventy‐two percent of all patients had public insurance.
Of the 93 total patients, 75 were seen in an HC alone and 18 were seen in both the HC and MWMP. The mean number of visits attended was 2.09 (n = 75, SD = 0.841) and 2.56 (n = 18, SD = 0.616) for the HC alone and HC with MWMP treatment groups, respectively. This difference was statistically significant (t[91] = −2.191, P = 0.031). Only 30 (40.0%) of the HC alone group and 11 (61.1%) of the HC with MWMP attended clinical visits at all 3 timepoints. Additionally, 13 of 75 (17.3%) HC patients were referred to the MWMP but did not attend their MWMP visit. Of the 18 who were seen in both the HC and MWMP, 5 (27.8%) subjects only attended 1 visit in the MWMP. For a more detailed summary of these results, see Table 1 . No patients received bariatric surgery (not available at the start of the study) and only one patient in the MWMP condition received pharmacotherapy (topiramate) at a single timepoint.
Table 1.
Sample characteristics
| Total sample | HC | MWMP | Significance, P value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | n | % | M | SD | n | % | M | SD | n | % | |||
| Age, years at NAFLD Diagnosis | 12.25 | 3.06 | 93 | ‐ | 12.56 | 2.93 | 75 | ‐ | 10.94 | 3.32 | 18 | ‐ | 0.043* | |
| Number of visits attended | 2.18 | 0.82 | 93 | ‐ | 2.09 | 0.84 | 75 | ‐ | 2.56 | 0.62 | 18 | ‐ | 0.031* | |
| Attended 1 visit | ‐ | ‐ | 24 | 25.8 | ‐ | ‐ | 23 | 30.7 | ‐ | ‐ | 1 | 5.6 | ‐ | |
| Attended 2 visits | ‐ | ‐ | 28 | 30.1 | ‐ | ‐ | 22 | 29.3 | ‐ | ‐ | 6 | 33.3 | ‐ | |
| Attended 3 visits | ‐ | ‐ | 41 | 44.1 | ‐ | ‐ | 30 | 40 | ‐ | ‐ | 11 | 61.1 | ‐ | |
| Referral patterns | Referred to MWMP, but did not attend | ‐ | ‐ | 13 | 14 | ‐ | ‐ | 13 | 17.3 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Attended only 1st MWMP visit | 5 | 5.4 | ‐ | ‐ | 5 | 27.8 | ||||||||
| Attended weekly MWMP intervention | 6 | 6.5 | ‐ | ‐ | 6 | 33.3 | ||||||||
| Sex | Male | ‐ | ‐ | 71 | 76.3 | ‐ | ‐ | 56 | 74.7 | ‐ | ‐ | 15 | 83.3 | ‐ |
| Female | 22 | 23.7 | 19 | 25.3 | 3 | 16.7 | ||||||||
| Race | White | ‐ | ‐ | 51 | 54.8 | ‐ | ‐ | 44 | 58.7 | ‐ | ‐ | 7 | 38.9 | ‐ |
| Asian | 1 | 1.1 | 1 | 1.3 | 0 | 0 | ||||||||
| Black | 4 | 4.3 | 4 | 5.3 | 0 | 0 | ||||||||
| Hispanic/Latino | 31 | 33.3 | 25 | 33.3 | 6 | 33.3 | ||||||||
| More than one race reported | 5 | 53.8 | 0 | 0 | 5 | 27.8 | ||||||||
| Unknown | 1 | 1.1 | 1 | 1.3 | 0 | 0 | ||||||||
| Insurance status | Private | ‐ | ‐ | 25 | 26.9 | ‐ | ‐ | 17 | 22.7 | ‐ | ‐ | 8 | 44.4 | ‐ |
| Public | 67 | 72 | 57 | 76 | 10 | 55.6 | ||||||||
| Self‐pay | 1 | 1.1 | 1 | 1.3 | 0 | 0 | ||||||||
HC, hepatology clinic; MWMP, multidisciplinary weight management program; NAFLD, nonalcoholic fatty liver disease.
*P < 0.05.
SOCRATES
There were 37 SOCRATES questionnaires filled out in the HC group and 4 filled out in the MWMP group. There were no significant differences between the two treatment groups on any of the three SOCRATES subscales (i.e., recognition, ambivalence, or taking steps). Participants reported very low recognition, indicating very low recognition of their obesity as a problem and low desire to change. Participants also, on average, scored low on ambivalence, suggesting that they were not ambivalent about making changes that would result in weight loss. In other words, because the subjects did not recognize a problem or need for change, they were relatively certain about maintaining their current lifestyle behaviors and equally certain about not engaging in weight loss behaviors. Finally, participants scored low on taking steps, reflecting a relative lack of making current changes in order to decrease their weight. For a summary of these results, see Table 2 .
Table 2.
SOCRATES subscale scores
| Total sample (n = 37) |
HC (n = 33) |
MWMP (n = 4) |
Significance, P‐value | |||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |||
| Subscale | Recognition | 24.32 | 20.21 | 24.55 | 20.01 | 22.50 | 25.00 | 0.852 |
| Ambivalence | 39.73 | 24.21 | 40.91 | 25.05 | 30.00 | 14.14 | 0.402 | |
| Taking Steps | 37.57 | 25.76 | 38.18 | 26.63 | 32.50 | 18.93 | 0.683 | |
HC, hepatology clinic; MWMP, multidisciplinary weight management program; SOCRATES, Stages of Change Readiness and Treatment Eagerness Scale.
Primary outcome variables – main effects and interaction effects
There were no significant main effects for treatment group for any of the four primary outcomes. Likewise, there were no significant group × time interaction effects. There was, however, a significant main effect of time for BMI z‐score, but not for AST, ALT, or raw BMI. On average, BMI z‐score decreased at a rate of −0.0625 from visit to visit for those in the HC alone group and by −0.0408 for those in the HC with MWMP group. Because the main effect for treatment group and the group × time interaction term were nonsignificant in this model, the treatment group variable was removed from a final model of BMI z‐score. In this model, the average rate of linear decrease in BMI z‐score across all participants, regardless of treatment group, was −0.0568 (P = 0.004) per visit. For a summary of these results, see Table 3 , Figure 1 , and Figure S2 . Covariates (age, sex, race, and insurance status) had no significant effect in any of the models.
Table 3.
Results of linear mixed effects model for primary outcome variables
| Outcome |
Initial status [95% CI] |
Group effect [95% CI] |
Time effect [95% CI] |
Group × time interaction [95% CI] |
|---|---|---|---|---|
| BMI | 34.74* [32.51, 36.96] | −3.80 [−8.84, 1.24] | −0.64 [−1.77, 0.50] | 1.73 [−4.8, 3.94] |
| BMI z‐score | 2.38* [2.30, 2.46] | 0.11 [−0.08, 0.29] | −0.06* [−0.11, −0.02] | 0.02 [−0.07, 0.11] |
| ALT | 107.37* [92.53, 122.20] | −2.44 [−36.60, 31.72] | −2.33 [−20.43, 15.78] | −8.11 [−43.67, 27.45] |
| AST | 57.62* [49.75, 65.48] | 11.59 [−11.66, 34.84] | 3.20 [−8.56, 14.96] | −12.10 [−35.82, 11.63] |
*Statistically significantly different from 0, P < 0.05. Initial status is the estimated average baseline value of the outcome variable for the HC group, and the group effect represents the estimated difference in this average baseline value associated with the MWMP group. The time main effect is the estimated average change in the outcome variable from visit to visit for the HC group, and the group × time interaction effect is the estimated difference in this average rate of change associated with the MWMP group.
ALT, alanine aminotransferase; ALT, aspartate aminotransferase; BMI, body mass index; CI, confidence interval.
Figure 1.
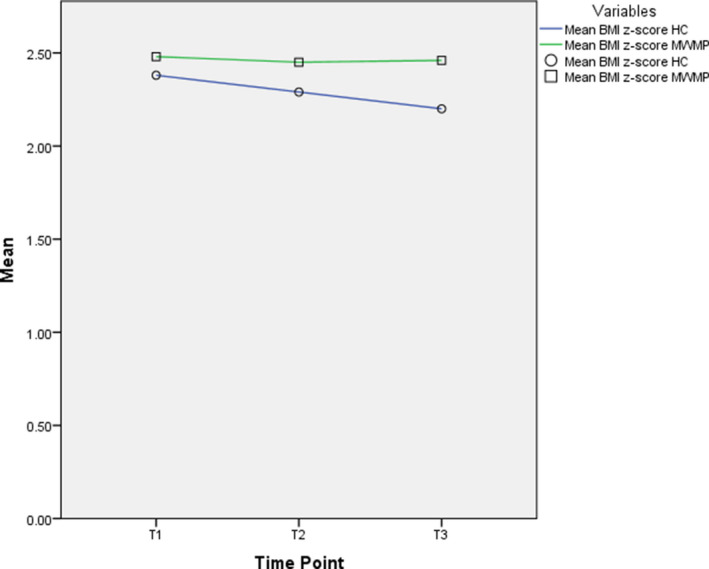
Comparison of mean body mass index (BMI)z‐score changes over time for the hepatology clinic alone (HC, ○) and those seen with a multidisciplinary weight management program (MWMP, □).
DISCUSSION
Our study found small but statistically significant improvement in BMI z‐score over time for patients with NAFLD seen either in a pediatric HC alone or concurrently with an MWMP. Both conditions offered regular medical follow‐up visits to provide education, monitor adherence, and reassess response to therapy. There were no significant differences found among these treatment conditions. This may indicate that some patients do not receive a benefit from the additional resources of our MWMP if already seen in the HC or that improved patient selection is needed to determine who is best served by the MWMP. However, this study does not address access to bariatric surgery (available after the study began) or pharmacotherapy (only a single timepoint in the MWMP condition), which would be important for future research.
A reduction in BMI z‐score is an important treatment goal in both obesity and NAFLD. This may be a better reflection of our groups’ outcomes than raw BMI alone, as healthy BMI ranges change with age, and suggests that both conditions achieve an important treatment outcome. Raw BMI and transaminases were found to not decrease significantly regardless of group or to vary in amount of change between the two conditions. Several reasons for this may exist. The BMI z‐score may better reflect patients’ weight outcomes than BMI alone. In addition, it has also been demonstrated that transaminase elevations themselves do not accurately predict fibrosis in pediatric NAFLD. 22 With a reduction in BMI z‐score, our patients may have a reduction in steatosis or slowing of fibrosis not seen with noninvasive testing. Further improvements in steatosis may eventually lead to a clinically significant normalization of transaminases. It is important to note that the change in BMI z‐score was small, but our results reflect a linear reduction in BMI z‐score per unit of time. Based on our model, BMI z‐score was predicted to continue to improve with each visit and cumulative effects may be more clinically significant. Given the difficulty achieving successful weight reduction with nonsurgical treatment, we felt that this result was still clinically significant despite the small change per visit over time.
In our study, the amount of readiness to change could not be distinguished between the two groups. However, it remains an important characteristic in this population. Readiness to change was found in Brazilian child and adolescent patients to be associated with increased improvement in anthropometrics, hemodynamics, and cardiorespiratory fitness. 23 National organization recommendations 10 and literature reviews 24 , 25 support assessment of this construct as well. Our results may reflect a patient population less prepared to actively change their lifestyle behaviors than clinicians perceive, and more realistic behavioral goals may need to be collaboratively set with the family.
The current study possesses several strengths worthy of mention. It is the first study to prospectively compare treatment outcomes of pediatric NAFLD with HC alone or in combination with an MWMP over an extended period. Other published studies have followed children with obesity and NAFLD over similar time frames, but have not compared hepatology follow‐up alone and MWMP treatments. 26 Additionally, we formally evaluated patient readiness to change in our sample, an important construct in the obesity treatment literature. 25 We found that BMI z‐score improved in our population with regular follow‐up despite having lower readiness to change, reinforcing that regular treatment sessions are associated with improved weight outcomes, perhaps due to monitoring alone 27 or regression to the mean. 28 As providers establish their clinic structure, our study reveals the possibility that BMI z‐score can improve either in HC alone or in combination with an MWMP. These results can impact program infrastructure as providers assess their available local resources to expand either their HC or MWMP. The naturalistic nature of our study design also offered us the ability to describe our patient’s real‐time appointment adherence patterns. Attrition played a significant role in both groups, and—along with assessment of readiness for change—needs to be a target of future studies. 25 Increasing patient retention is an important target for future therapies.
These strengths notwithstanding, our study has limitations that warrant discussion. Treatment groups were based on provider or patient preference without randomization or controlling for therapies. This naturalistic design may lead to unknown confounders normally addressed through randomization. There may be inherent characteristics in those who choose or do not choose to attend an MWMP that could affect results. Effects of this can be seen in the total number of SOCRATES questionnaires filled out in the HC alone and MWMP groups. Parental readiness to change was not assessed in our study, although it may influence our results. The role of caregivers in the pediatric obesity population is increasingly garnering clinical and research interest. Parents have been found to be more ready for dietary changes in children but less so for physical activity changes in older children. 29 Parental perception of their own weight affects parental readiness to change both positively and negatively. 29 , 30 Interestingly, parental readiness to change, readiness for treatment, and ratings of importance have not been found to predict success in the treatment of obesity. 31 , 32 Given that caregivers frequently control the access to food and physical activities, future studies should be undertaken to identify the most effective parental factors and interventions for success.
Attrition was significant in our study, with many patients lost to follow‐up in the HC or to their MWMP appointments despite enrollment into our study (Table 1 ). This attrition furthered the divide in sample sizes between the two groups as well as SOCRATES questionnaire completion and may introduce bias. Attrition is a known feature of this population. 33 Previous studies have shown that as many as 58.8% of patients do not attend their initial referral to an MWMP. 34 After evaluation, 47% of patients do not return for a second MWMP visit. 35 Our center uses a combination of electronic, physical mail, and telephone reminders in attempts to decrease attrition. However, further study is needed to identify effective strategies to improve retention in this population, which can improve access to care.
BMI and BMI z‐score are frequent indicators of outcome in MWMP, but it is important to consider that these measures are not the sole target. A recent study by Luca et al. of the SickKids Team Obesity Management Program found that BMI did not decrease significantly compared with a control adolescent group with obesity not seen in an MWMP. 36 However, the group seen in the MWMP did have improvements in cardiometabolic, psychological, and health behavior outcomes. 36 Although our study did not find significant differences in transaminases, findings such as Luca et al.’s highlight the need for comprehensive and multidisciplinary evaluation of clinical outcomes in this population. Additionally, our study was limited by lack of access to noninvasive techniques to monitor steatosis and fibrosis, such as FibroScan, which will be important in future studies, as serial liver biopsy is difficult to clinically justify in this patient population.
As obesity rates in the United States continue to worsen, further study is required to address the optimal treatment strategies in this patient group. The US Preventive Services Task Force reported that moderate to high intensity (> 26 hours) comprehensive behavioral interventions are associated with durable improvements in weight status. To aid in weight management, there is emerging interest in the development of pharmacotherapy to treat pediatric obesity. 37 Although adult pharmacotherapy is already in use, pediatric medications are increasingly utilized for weight management. Indeed, our results may be part of a call for the further development of pharmacologic intervention given the modest improvement in outcomes of both HC and MWMP. Bariatric surgery is becoming more widespread in the United States to treat pediatric obesity for those who need further treatment. 35 It has been found to be safe and effective and is most often offered to pediatric patients in the setting of a tertiary care MWMP. These treatment options may be among the most important factors in determining a referral to an MWMP. Last, patient level therapies may be ineffective to manage this crisis across the nation. The surge in obesity reflects a complex interplay among patient level characteristics, culture, and public policy that influence patient behaviors, such as agricultural subsidies. 38 It may be more cost effective to promote public health policies to demonstrate cost savings and prevention of cases of childhood obesity. 39
Our results suggest that, despite low readiness to change, our pediatric patients with NAFLD demonstrated statistically significant improvement in BMI z‐score over time without collateral changes in BMI, AST, and ALT. This argues that future study is needed to identify the most effective means of engaging and maintaining adolescents with obesity and NAFLD in treatment, including refinement of HC, MWMP, pharmacotherapy, and bariatric surgery.
Funding
No funding was received for this work.
Conflicts of Interest
All authors declared no competing interests for this work.
Author Contributions
All authors wrote the manuscript. V.S., R.T.F., A.D., and S.H. designed the research. V.S. and H.W. performed the research. V.S., A.D., and M.C. analyzed the data.
Supporting information
Fig S1
Fig S2
References
- 1. Skinner, A.C. , Ravanbakht, S.N. , Skelton, J.A. , Perrin, E.M. , Armstrong, S.C. Prevalence of obesity and severe obesity in US children, 1999‐2016 [published correction appears in Pediatrics. 2018 Sep;142(3)]. Pediatrics 141, e20173459 (2018).29483202 [Google Scholar]
- 2. Hales, C.M. , Carroll, M.D. , Fryar, C.D. & Ogden, C.L. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief 288, 1–8 (2017). [PubMed] [Google Scholar]
- 3. Baranowski, T. , Motil, K.J. & Moreno, J.P. Multi‐etiological perspective on child obesity prevention. Curr. Nutr. Rep. https://doi.org/ 10.1007/s13668-019-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reilly, J.J. Evidence‐based obesity prevention in childhood and adolescence: critique of recent etiological studies, preventive interventions, and policies. Adv. Nutr. 3, 636S–641S (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reilly, J.J. & Kelly, J. Long‐term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int. J. Obes. (Lond). 35, 891–898 (2011). [DOI] [PubMed] [Google Scholar]
- 6. Spear, B.A. et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics 120 (suppl. 4), S254–S288 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Pona, A.A. , Dreyer Gillette, M.L. , Odar Stough, C. , Gerling, J.K. & Sweeney, B.R. Long‐term outcomes of a multidisciplinary weight management intervention for youth with disabilities. Child Obes. 13, 455–461 (2017). [DOI] [PubMed] [Google Scholar]
- 8. Hampl, S.E. et al. Patient attendance and outcomes in a structured weight management program. J. Pediatr. 176, 30–35 (2016). [DOI] [PubMed] [Google Scholar]
- 9. Hampl, S. , Odar Stough, C. , Poppert Cordts, K. , Best, C. , Blackburn, K. & Dreyer Gillette, M.L. Effectiveness of a hospital‐based multidisciplinary pediatric weight management program: two‐year outcomes of PHIT kids. Child Obes. 12, 20–25 (2016). [DOI] [PubMed] [Google Scholar]
- 10. Krebs, N.F. , Himes, J.H. , Jacobson, D. , Nicklas, T.A. & Guilday, P. Assessment of child and adolescent overweight and obesity. Pediatrics 120, S193–S228 (2007). [DOI] [PubMed] [Google Scholar]
- 11. Patton, H.M. , Sirlin, C. , Behling, C. , Middleton, M. , Schwimmer, J.B. & Lavine, J.E. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J. Pediatr. Gastroenterol. Nutr. 43, 413–427 (2006). [DOI] [PubMed] [Google Scholar]
- 12. Nobili, V. et al. Nonalcoholic fatty liver disease: a challenge for pediatricians. JAMA Pediatr. 169, 170–176 (2015). [DOI] [PubMed] [Google Scholar]
- 13. Wong, R.J. et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 148, 547–555 (2015). [DOI] [PubMed] [Google Scholar]
- 14. Alkhouri, N. & Feldstein, A.E. The TONIC trial: a step forward in treating pediatric nonalcoholic fatty liver disease. Hepatology 55, 1292–1295 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maisto, S.A. , Conigliaro, J. , McNeil, M. , Kraemer, K. , O'Connor, M. & Kelley, M.E. Factor structure of the SOCRATES in a sample of primary care patients. Addict. Behav. 24, 879–892 (1999). [DOI] [PubMed] [Google Scholar]
- 16. Miller, W.R. & Tonigan, J.S. Assessing drinkers’ motivation for change: The Stages of Change Readiness and Treatment Eagerness Scales (SOCRATES). Psychol. Addict. Behav. 10, 81–89 (1996). [Google Scholar]
- 17. Vieira da Silva, R. , de Oliveira, I.R. & Lopes Velasquez, M. Stages of change readiness and treatment eagerness scale in overweight and obesity's psychometric properties (SOCRATES‐OO). J. Clin. Psychol. Med. Settings. https://doi.org/ 10.1007/s10880-019-09672-w. [DOI] [PubMed] [Google Scholar]
- 18. Vajro, P. et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J. Pediatr. Gastroenterol. Nutr. 54, 700–713 (2012). [DOI] [PubMed] [Google Scholar]
- 19. Krueger, C. & Tian, L. A comparison of the general linear mixed model and repeated measures ANOVA using a dataset with multiple missing data points. Biol. Res. Nurs. 6, 151–157 (2004). [DOI] [PubMed] [Google Scholar]
- 20. Singer, J.D. & Willett, J.B. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence (Oxford University Press, New York, 2003). [Google Scholar]
- 21. Gibbons, R.D. , Hedeker, D. & DuToit, S. Advances in analysis of longitudinal data. Annu. Rev. Clin. Psychol. 6, 79–107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jackson, J.A. et al. Performance of fibrosis prediction scores in paediatric non‐alcoholic fatty liver disease. J. Paediatr. Child Health 54, 172–176 (2018). [DOI] [PubMed] [Google Scholar]
- 23. da Silva, D.F. et al. Impact of readiness to change behavior on the effects of a multidisciplinary intervention in obese Brazilian children and adolescents. Appetite 87, 229–235 (2015). [DOI] [PubMed] [Google Scholar]
- 24. Brown, C.L. & Perrin, E.M. Obesity prevention and treatment in primary care. Acad. Ped. 18, 736–745 (2018). [DOI] [PubMed] [Google Scholar]
- 25. McMaster, C.M. , Gow, M.L. , Neal, R. , Alexander, S. , Baur, L.A. & Cohen, J. Acceptability of hospital‐based pediatric weight management services among patients and families: a narrative synthesis. Child Obes. 16, 129–140 (2020). [DOI] [PubMed] [Google Scholar]
- 26. Reinehr, T. , Schmidt, C. , Toschke, A.M. & Andler, W. Lifestyle intervention in obese children with non‐alcoholic fatty liver disease: 2‐year follow‐up study. Arch. Dis. Child. 94, 437–442 (2009). [DOI] [PubMed] [Google Scholar]
- 27. O'Connor, E.A. , Evans, C.V. , Burda, B.U. , Walsh, E.S. , Eder, M. & Lozano, P. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA 317, 2427–2444 (2017). [DOI] [PubMed] [Google Scholar]
- 28. Cockrell Skinner, A. , Goldsby, T.U. & Allison, D.B. Regression to the mean: a commonly overlooked and misunderstood factor leading to unjustified conclusions in pediatric obesity research. Child Obes. 12, 155–158 (2016). [DOI] [PubMed] [Google Scholar]
- 29. Rhee, K.E. , McEachern, R. & Jelalian, E. Parent readiness to change differs for overweight child dietary and physical activity behaviors. J. Acad. Nutr. Diet 114, 1601–1610 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rhee, K.E. , De Lago, C.W. , Arscott‐Mills, T. , Mehta, S.D. & Davis, R.K. Factors associated with parental readiness to make changes for overweight children. Pediatrics 116, e94–e101 (2005). [DOI] [PubMed] [Google Scholar]
- 31. Gunnarsdottir, T. , Njardvik, U. , Olafsdottir, A.S. , Craighead, L.W. & Bjarnason, R. The role of parental motivation in family‐based treatment for childhood obesity. Obesity (Silver Spring) 19, 1654–1662 (2011). [DOI] [PubMed] [Google Scholar]
- 32. Anderson, Y.C. et al. Caregiver's readiness for change as a predictor of outcome and attendance in an intervention programme for children and adolescents with obesity: a secondary data analysis. BMJ Open 9, e023195 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skelton, J.A. & Beech, B.M. Attrition in paediatric weight management: a review of the literature and new directions. Obes. Rev. 12, e273–e281 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shaffer, L.A. , Brothers, K.B. , Burkhead, T.A. , Yeager, R. , Myers, J.A. & Sweeney, B. Factors associated with attendance after referral to a pediatric weight management program. J. Pediatr. 172, 35–39 (2016). [DOI] [PubMed] [Google Scholar]
- 35. Kyler, K.E. , Bettenhausen, J.L. , Hall, M. , Fraser, J.D. & Sweeney, B. Trends in volume and utilization outcomes in adolescent metabolic and bariatric surgery at children's hospitals. J. Adolesc. Health 65, 331–336 (2019). [DOI] [PubMed] [Google Scholar]
- 36. Luca, P. et al. Adolescents with severe obesity: outcomes of participation in an intensive obesity management programme. Pediatr. Obes. 10, 275–282 (2015). [DOI] [PubMed] [Google Scholar]
- 37. Kelly, A.S. , Fox, C.K. , Rudser, K.D. , Gross, A.C. & Ryder, J.R. Pediatric obesity pharmacotherapy: current state of the field, review of the literature and clinical trial considerations. Int. J. Obes. (Lond). 40, 1043–1050 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siegel, K.R. et al. Association of higher consumption of foods derived from subsidized commodities with adverse cardiometabolic risk among US adults. JAMA Intern. Med. 176, 1124–1132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gortmaker, S.L. et al. Three interventions that reduce childhood obesity are projected to save more than they cost to implement. Health Aff. 34, 1932–1939 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2


