The severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) enters host cells through the endosomal and nonendosomal pathways. Although the relative contribution of each mechanism could vary depending on host cell types, evidence from studies on related coronaviruses suggest that simultaneous targeting of both pathways could be an efficient therapeutic approach to limit viral infection. This communication highlights the prophylactic potential of combination therapies using TMPRSS2, cathepsin/calpain, and FURIN inhibitors for complete blockade of SARS‐CoV‐2 infection.
Until 2 decades ago, several human coronaviruses were acknowledged, but were given little attention as they only caused mild upper respiratory tract infections with a severity comparable to common cold virus infections. However, the emergence of the severe acute respiratory syndrome (SARS) in China (2002/2003) and Middle East respiratory syndrome (MERS) in Saudi Arabia (2012) changed the overall dynamics of our focus on coronaviruses, both in vaccine development and therapeutics. The current global medical and socioeconomic cost associated with SARS‐CoV‐2 (the newly discovered coronavirus) infection, first reported in the Wuhan region of China in December 2019, is of a magnitude exceeding any other over the last generations. With over 21 million cases reported so far, the ongoing SARS‐CoV‐2 pandemic, referred to as coronavirus disease‐2019 (COVID‐19), has already claimed over 1 million human lives across the globe. Regarding therapeutics for COVID‐19, the 2 main approaches are based on targeting (1) the key viral proteins/enzymes involved in the replication cycle of SARS‐CoV‐2, or (2) the host immune system by enhancing clearance of the virus and/or suppressing the overexaggerated immune reaction leading to multiorgan failure and death. In this paper, the therapeutic potential of targeting the entry mechanisms of the coronavirus is discussed.
Coronaviruses can use two mechanisms of entry into host cells: the endosomal and nonendosomal pathways (Figure 1 ). 1 In view of interrupting the SARS‐CoV‐2 infection cycle by targeting the nonendosomal pathway, the key steps in processing the viral spike (S) proteins, their attachment with host receptors, in particular angiotensin converting enzyme 2 (ACE2), and the viral entry mechanisms have been scrutinized since the outbreak of COVID‐19. Hoffmann et al. highlighted the role of TMPRSS2 priming of the S glycoprotein as part of the nonendosomal SARS‐CoV‐2 cellular entry pathway and the potential application of its inhibitor camostat mesylate as a potential therapeutic agent. 2 Other approaches include using neutralizing antibodies against TMPRSS2. 2 Based on these and other findings, the proteolytic cleavage of inactive S proteins, which is required to generate an active viral ligand suitable for attachment to ACE2, has emerged as a key target of interest. 2
Figure 1.
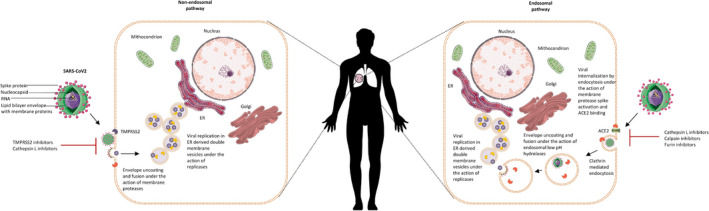
Endosomal and nonendosomal severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) entry into host cells.
The endosomal mechanisms of coronavirus entry into host cells have been well‐established and require the activity of the pH‐dependent cysteine proteases cathepsin B and L. 1 Although further research is required to validate their therapeutic efficacy and overcome certain safety concerns, the claimed benefit of chloroquine and hydroxychloroquine for COVID‐19 is based on targeting the endosomal pathway (pH) of SARS‐CoV‐2 entry. Although these pharmacological mechanisms are proven under in vitro infection models, readers should bear in mind that these antimalarial drugs have multiple mechanisms and their efficacy against COVID‐19 is not yet to be confirmed, as some clinical trials have already been discontinued for various reasons. SARS‐CoV replication was suggested to be significantly higher following entry through the nonendosomal than the endosomal pathway. 3 It is, however, rational to assume that targeting both entry mechanisms simultaneously would offer a superior outcome in effectively disrupting the SARS‐CoV infection cycle. Supporting this notion, combined treatment with camostat (TMPRSS2 inhibitor) and EST (cathepsin L and/or B inhibitor) effectively prevented cell entry and multistep growth of SARS‐CoV in human airway epithelial cells in vitro, which could be attributed to dual inhibition of the endosomal pathway and entry from the cell surface. 4 The combination of camostat and a cathepsin inhibitor ((23,25)‐trans‐epoxysuccinyl‐l‐leucylamindo‐3‐methylbutane ethyl ester) have been studied in relation to MERS‐CoV as well. 5 The results revealed that simultaneous inhibition of TMPRSS2 and cathepsin L completely blocked MERS‐CoV entry into Vero‐TMPRSS2 cells, confirming that, as with for SARS‐CoV, this virus uses both the cell surface and endosomal pathway to infect the host. In addition, camostat was sufficient to block MERS‐CoV entry in human bronchial submucosal gland‐derived Calu‐3 cells, as the combined treatment with the cathepsin inhibitor was no more efficacious than treatment with camostat alone. This latter finding might indicate differential contributions of the cell surface and endosome pathway to viral entry depending on the cell type. These studies further imply that agents inhibiting host serine and/or cysteine proteases, relevant to viral cell entry, could effectively interfere with coronavirus infection. In addition to playing a key role in the endosomal pathway, cathepsin L proteolysis has been shown to contribute to TMPRSS2‐mediated S protein activation and membrane fusion, linking it to cell surface‐mediated SARS‐CoV entry into the host cells. 6 All these promising data based on MERS‐CoV and SARS‐CoV are significant milestones but yet to be translated to SARS‐CoV‐2 therapy through further studies.
Pretreatment of 293/hACE2 cells with E64D (a broad inhibitor for cathepsin B, H, L, and calpain) strongly reduced entry of SARS‐CoV‐2 S pseudovirions (by 92.5%), suggesting the requirement of at least one of the cathepsins or calpain. Further studies using specific inhibitors demonstrated over 76% reduction in cell entry when a cathepsin L (SID‐26681509), but not B inhibitor (CA‐074), was used, implying that particularly cathepsin L is critical for priming the SARS‐CoV‐2 S protein in the lysosome for entry into these cells. 7 Recent study showed simultaneous treatment with camostat mesylate and E64d markedly inhibited SARS‐CoV‐2 entry into Calu‐3 cells, but could not completely abrogate viral entry. 8 The S protein of SARS‐CoV‐2 contains a PRRA motif, which contains four redundant FURIN cleavage sites. 8 Notably, a FURIN‐like cleavage site in the S‐protein of the SARS‐CoV‐2 was identified as unique, as it was lacking in the other SARS‐like coronaviruses. 8 Hoffmann et al. 2 speculated that FURIN may be involved in SARS‐CoV‐2 S protein priming. Luteolin is a well‐known, safe, and potent inhibitor of FURIN with a inhibitor constant value of 58.6 μM, and has been shown to exhibit antiviral activity in mice infected with dengue virus. 9 This small molecule has also potent antiviral effect against wild‐type SARS‐CoV infection in Vero E6 cells (half‐maximal effective concentration: 10.6 µm). 10 Overall, the data discussed in this short communication suggest that simultaneous targeting of endosomal and nonendosomal cellular entry of SARS‐CoV‐2 could represent a highly efficient therapeutic approach to interfere with viral infection. In this context, we feel it would be valuable to test the prophylactic potential of combination therapies of TMPRSS2 inhibitors (such as ambroxol, leupeptin, camostat mesylate, and nafamostat mesylate), Aloxistatin (E64d) (as potent inhibitor of cathepsin L and calpain) in association with FURIN inhibitors (e.g., luteolin) that may lead to complete abrogation of SARS‐CoV‐2 entry into host cells.
Funding
No funding was received for this work.
Conflict of Interest
The authors declared no competing interests for this work.
References
- 1. Zumla, A. , Chan, J.F. , Azhar, E.I. , Hui, D.S. & Yuen, K.Y. Coronaviruses—drug discovery and therapeutic options. Nat. Rev. Drug Discov. 15, 327–347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffmann, M. et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lukassen, S. et al. SARS‐CoV‐2 receptor ACE 2 and TMPRSS 2 are primarily expressed in bronchial transient secretory cells. EMBO J. 39, e105114 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kawase, M. , Shirato, K. , van der Hoek, L. , Taguchi, F. & Matsuyama, S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 86, 6537–6545 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shirato, K. , Kawase, M. & Matsuyama, S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J. Virol. 87, 12552–12561 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simmons, G. , Gosalia, D.N. , Rennekamp, A.J. , Reeves, J.D. , Diamond, S.L. & Bates, P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. 102, 11876–11881 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ou, X. et al. Characterization of spike glycoprotein of SARS‐CoV‐2 on virus entry and its immune cross‐reactivity with SARS‐CoV. Nat. Commun. 11, 1620 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mykytyn, A.Z. et al. The SARS‐CoV‐2 multibasic cleavage site facilitates early serine protease‐mediated entry into organoid‐derived human airway cells. bioRxiv. 10.1101/2020.09.07.286120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peng, M. et al. Luteolin restricts dengue virus replication through inhibition of the proprotein convertase FURIN. Antiviral Res. 143, 176–185 (2017). [DOI] [PubMed] [Google Scholar]
- 10. Yi, L. et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 78, 11334–11339 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]


