Abstract
Only half of patients with hypertension (HTN) respond to any given antihypertensive medication. Heterogeneity in pathophysiologic pathways underlying HTN is a major contributor. Personalizing antihypertensive therapy could improve blood pressure (BP) reduction. The objective of this study was to assess the effect of pragmatic implementation of a personalized plasma renin activity (PRA)‐based smartphone app on improving BP reduction. Patients with untreated or treated but uncontrolled HTN were recruited. BP and PRA were measured at baseline with final BP measured at 6 months. Patient’s information was entered into the app and treatment recommendations were returned. Clinicians were at liberty to follow or disregard the app’s recommendations. BP levels and percent BP control among patients whose clinicians did and did not follow the app’s recommendations were compared using independent t‐test and Fisher’s exact test, respectively. Twenty‐nine European American patients were included (38% women) with mean age of 52 ± 9 years and median PRA of 1.3 ng/mL/hr (interquartile range 0.5–3.1 ng/mL/hr). Participants whose clinicians followed the app’s recommendations (n = 16, 55%) as compared with those whose clinicians did not (n = 13, 45%), had a greater reduction in 6‐month systolic BP (−15 ± 21 vs. −3 ± 21 mm Hg; adjusted‐P = 0.1) and diastolic BP (−8 ± 8 vs. −1 ± 8 mm Hg; adjusted‐P = 0.04). BP control at 6 months tended to be greater among patients whose clinicians accepted the app’s recommendations vs. those whose clinicians did not (63% vs. 23%, P = 0.06). This pilot study demonstrates that acceptance of the app’s recommendations was associated with a greater BP reduction. Future studies to confirm these pilot findings are warranted.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Only half of the patients with hypertension (HTN) have their blood pressure (BP) controlled. Discordance in the pathophysiologic pathways underlying HTN is likely a contributor to the observed high interindividual variability in BP response to antihypertensive medications.
WHAT QUESTION DID THE STUDY ADDRESS?
☑ Does the plasma renin activity (PRA)‐based smartphone app, which allows for the incorporation of the PRA biomarker into clinical care, affect BP lowering and BP control in patients with untreated or treated but uncontrolled HTN.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ This pilot study demonstrates that there was greater BP reduction and better BP control when the PRA‐based app treatment recommendations were accepted.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL RESEARCH?
☑ Use of this smartphone app and PRA as a biomarker could be used in clinical practice to help clinicians personalize the antihypertensive therapy to improve BP control.
Hypertension (HTN) is one of the most common chronic diseases, affecting ~ 45.6% of the US adults aged 20 years old and over. 1 It is considered a major risk factor for multiple cardiovascular adverse outcomes, including angina, heart failure, stroke, and myocardial infarction, 2 and has been also associated with target organ damage and a high global mortality rate. 2 , 3 Despite the availability of multiple antihypertensive drugs, all of which have blood pressure (BP) lowering efficacy, population‐level BP control is only ~ 50%. 4 , 5 Discordance in the pathophysiologic pathways underlying HTN and pathways targeted by antihypertensive mechanism of action is likely a major contributing factor in the observed interindividual variability in BP response to antihypertensive medications and poor BP control. 6
One of the important physiologic pathways regulating BP is the renin‐angiotensin‐aldosterone system (RAAS). It has been demonstrated that plasma renin activity (PRA)—an indicator of the RAAS activity—is a predictive biomarker of BP response to different antihypertensives. 7 Previous studies have shown that patients with predominantly sodium volume‐mediated HTN (PRA < 0.65 ng/mL/hr) respond better to diuretics and α‐blockers (anti‐volume, or “anti‐V” drugs), whereas those with predominantly renin vasoconstriction‐mediated HTN (PRA ≥ 0.65 ng/mL/hr) respond better to drugs that block the RAAS, such as angiotensin‐converting enzyme (ACE) inhibitors, angiotensin‐receptor blockers (ARBs), and β‐blockers (anti‐renin, or “anti‐R” drugs). 8 , 9 , 10 , 11 , 12 Moreover, it has been suggested that using PRA testing to personalize antihypertensive therapy in treated, but patients with uncontrolled HTN could improve BP control and decrease the number of drugs needed to optimally treat HTN, which would in turn lead to improved adherence, fewer adverse events, and decreased cost associated with treatment. 13
Recently, we demonstrated that a PRA cutoff point of 0.60–0.65 ng/mL/hr is a predictive biomarker of BP response with good sensitivity and specificity in European American (EA) patients with uncomplicated HTN. 14 However, it is important to prospectively assess the value of utilizing PRA as a biomarker in a systematic practical treatment algorithm to improve the precision of antihypertensive therapy in both untreated and treated patients with uncontrolled HTN. Therefore, the objective of this pilot study was to assess the effect of a pragmatic implementation of a personalized antihypertensive treatment smartphone app, used to guide the selection of physiologically optimal HTN treatment based on PRA biomarker and treatment factors, on improving BP reduction and BP control in EA patients with uncomplicated HTN. We provide descriptive results of this pilot pragmatic implementation herein.
METHODS
Study population
The Optimizing Precision of Hypertension Care to Maximize Blood Pressure Control (OPTI‐BP) study was a prospective, multicenter, open‐label, factorial pilot clinical trial (clinicaltrials.gov identifier: NCT02814552). Study participants were recruited from three rural primary care clinics: University of Florida Health Family Medicine‐Crossroads, University of Florida Health Family Medicine‐Old Town, and Tallahassee Memorial Healthcare Physician Partners ‐ Quincy. The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the institutional review boards for all study sites. All participants provided voluntary, written informed consent prior to participation in the study.
Participants aged between 18 and 65 years with untreated HTN or treated but uncontrolled HTN were included. Patients with untreated and treated HTN were included to assess the utility of the PRA biomarker across the spectrum of uncomplicated HTN. Participants were excluded if they had controlled BP, significantly elevated BP, secondary causes of HTN, or intolerance to two or more antihypertensive drug classes. The full list of inclusion and exclusion criteria are presented in Table S1 .
Study procedures
Measurement of BP took place in the clinic and was documented in the electronic health record. Baseline BP was obtained at the first initial visit, or from a previous visit closest to when the baseline PRA measurement was obtained. A blood sample for PRA measurement was collected at baseline and sent to LabCorp for analysis. Upon receipt of the PRA result (usually within 24–48 hours of the visit), the PRA level and current antihypertensive medications (if any) were entered into the PRA smartphone intervention clinical decision support app (Figure 1 ), and treatment recommendations to optimize antihypertensive drug usage were returned via the app to the provider. The treatment recommendations are based on adding and/or subtracting antihypertensive medications (anti‐V or anti‐R) according to PRA level(s) (Table 1 ). 8 , 15 Mechanisms of action of each anti‐V and anti‐R drug class are presented in Table 2 . The app takes into account the type of the current antihypertensive medication(s) (if any) the patient is taking, and Table 1 includes the listing of recommendations based on a patient’s individual clinical scenario. For example, if the patient is taking an ACE inhibitor/ARB and has a PRA < 6.5 ng/mL/hr or is taking a β‐blocker/direct renin inhibitor/central α2‐agonist and has a PRA < 0.65 ng/mL/hr, the app recommends stopping the anti‐R drug and adding an anti‐V drug. In contrast, if a patient is taking an ACE inhibitor/ARB and has a PRA ≥ 6.5 ng/mL/hr or is taking a β‐blocker and has a PRA ≥ 0.65 ng/mL/hr, the app recommends adding a second anti‐R drug. A brief video demonstrating use of the app can be viewed at https://www.laraghmethod.org/pra‐htn‐app/. Data for final BP, determined by the last available BP measurement in the electronic health record obtained within 6 months of study enrollment, was collected. In the case of uncontrolled BP, PRA could be re‐checked as needed during the 6 months of follow‐up, with results entered into the app for refinement of antihypertensive drug therapy as necessary. Clinicians were at liberty to follow or disregard the smartphone app treatment recommendations in accordance with their clinical judgment and preference for antihypertensive medication prescribing based on patient‐specific factors. REDCap was the data collection tool used to collect and store study data. 16
Figure 1.
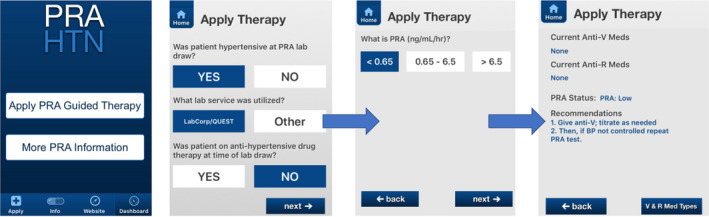
Example screenshots of the personalized PRA‐based smartphone app. Anti‐R, anti‐renin drugs including angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), β‐blockers, direct renin inhibitors and central α2‐agonists; Anti‐V, anti‐volume drugs including diuretics, aldosterone receptor antagonists, calcium channel blockers, vasodilators and α1‐blockers; BP, blood pressure; HTN, hypertension; PRA, plasma renin activity.
Table 1.
PRA‐based app treatment recommendations for untreated and for treated but uncontrolled HTN
| Current drug therapy | PRA, ng/mL/hr | Drug therapy recommendation |
|---|---|---|
| Untreated HTN | ||
| None | < 0.65 | Anti‐V; titrate as needed |
| None | ≥ 0.65 | Anti‐R; titrate as needed |
| Treated, uncontrolled HTN | ||
| ≥ 1 Anti‐V drug; no anti‐R drug | < 0.65 |
Titrate current anti‐V as needed If BP uncontrolled, add another anti‐V; titrate as needed |
| 0.65–6.5 | Add anti‐R; titrate as needed | |
| > 6.5 |
Stop anti‐V if no compelling indication If BP uncontrolled, add anti‐R; titrate as needed |
|
| ≥ 1 Anti‐R drug (ACE inhibitor/ARB), no anti‐V drug | < 6.5 |
Stop anti‐R if no compelling indication If BP uncontrolled, add anti‐V; titrate as needed |
| ≥ 6.5 |
Titrate anti‐R as needed If BP uncontrolled, add another anti‐R; titrate as needed |
|
| ≥ 1 Anti‐R drug (β‐blocker/DRI/central α2‐agonists), no anti‐V drug | < 0.65 |
Stop anti‐R if no compelling indication If BP uncontrolled, add anti‐V; titrate as needed |
| ≥ 0.65 |
Titrate anti‐R as needed If BP uncontrolled, add another anti‐R; titrate as needed |
|
| ≥ 1 Anti‐V drug + ≥ 1 anti‐R drug | < 0.65 |
Titrate current anti‐V as needed If BP uncontrolled, add another anti‐V; titrate as needed |
| ≥ 0.65 |
Stop anti‐V if no compelling indication If BP uncontrolled, titrate current anti‐R as needed If BP uncontrolled, add another anti‐R |
|
ACE, angiotensin converting enzyme; Anti‐R (anti‐renin) drugs including ACE inhibitors, ARBs, β‐blockers, DRIs, and central α2‐agonists; Anti‐V (anti‐volume) drugs including diuretics, aldosterone receptor antagonists, calcium channel blockers, vasodilators, and α1‐blockers; ARB, angiotensin receptor blocker; BP, blood pressure; DRI, direct renin inhibitor; HTN, hypertension; PRA, plasma renin activity.
Table 2.
Mechanisms of action of anti‐V and anti‐R drug classes
| Drug class | Mechanism of action |
|---|---|
| Anti‐V drug classes | |
| Thiazide diuretics | Inhibit the sodium‐chloride transporter in the distal tubule, leading to the inhibition of the reabsorption of about 5% of the filtered sodium. 32 |
| Loop diuretics | Inhibit the sodium‐potassium‐chloride cotransporter in the thick ascending limb of the loop of Henle, leading to the inhibition of the reabsorption of about 25% of the sodium load. 32 |
| Aldosterone receptor antagonists | Block the effect of aldosterone at the distal segment of the distal tubule, leading to the excretion of more sodium and water in the urine. 32 |
| Calcium channel blockers | Inhibit the calcium entry into the cells by binding to the L‐type calcium channels located on the vascular smooth muscles (including those of the preglomerular arterioles) and heart, causing vascular smooth muscle relaxation (vasodilatation) and decreased heart rate. 33 |
| Vasodilators | Relax the smooth muscles in the blood vessels, including those in the renal artery, causing vasodilatation. 34 |
| α1‐Blockers | Block the binding of the norepinephrine to the vascular smooth muscles by blocking the α1‐adrenoreceptors, including those in the renal artery, causing vasodilatation. 35 |
| Anti‐R drug classes | |
| ACE inhibitors | Block the conversion of angiotensin I into angiotensin II by blocking the ACE. 36 |
| ARBs | Block the binding of the angiotensin II to the AT1 receptors on blood vessels and heart. 36 |
| β‐blockers | Block the binding of the norepinephrine and epinephrine to the β‐adrenoreceptors located on the heart and the vascular smooth muscles, including those in kidneys, causing inhibition of the renal renin release. 37 |
| Direct renin inhibitors | Inhibit the binding of renin to angiotensinogen by binding to the active site of renin, causing inhibition of the formation of both angiotensin I and angiotensin II. 36 |
| Central α2‐agonists | Inhibit the renin production via specific renal α2‐adrenoreceptors. 38 |
ACE, angiotensin converting enzyme; Anti‐V (anti‐volume) drug classes increase the renal sodium excretion by one of the mechanisms mentioned in the table. Anti‐R (anti‐renin) drug classes block one of the different sites (mentioned in the table) in the overactive renin angiotensin aldosterone system; ARBs, angiotensin II receptor blockers; AT1, type 1 angiotensin II.
Laboratory analysis
Blood samples for PRA analyses were collected at baseline and as needed during the ensuing 6 months, and sent to LabCorp, a commercial laboratory. To prevent cryoactivation, blood samples were processed at room temperature, then stored frozen. 17 PRA was measured by incubating plasma at physiologic temperature in a buffer that facilitates its enzymatic activity. The PRA results reported are dependent on both renin concentration and the concentration of its substrate in the patient’s plasma. Renin cleaves angiotensinogen to produce a decapeptide, angiotensin‐I. After generation of angiotensin‐I at 37°C in buffering conditions at pH 6, 10% formic acid containing angiotensin‐I internal standard was added. An offline solid phase extraction step was conducted, followed by two wash steps, methanol elution and injection into the liquid chromatography tandem mass spectrometry system, which allows for measurement of the concentration of angiotensin‐I. PRA levels were reported as the amount of angiotensin‐I generated (in ng/mL) per hour. 18
Statistical analyses
Data for continuous variables are presented as means with SDs, except for PRA, which is not normally distributed, thus is presented as median with interquartile range. Data for categorical variables are presented as numbers and percentages. The primary outcome was BP change, defined as the difference between the 6‐month visit and the baseline visit (BP at 6 months subtracted from baseline BP). Data normality for BP change was confirmed using qq plots and Shapiro–Wilk test. Independent t‐test was used to compare the BP change of participants whose clinicians followed the app’s recommendations vs. those whose clinicians did not follow the app’s recommendations. Multivariable linear regression was conducted to estimate the difference in mean BP change comparing participants whose clinicians followed the app’s recommendations and those whose clinicians did not, adjusting for baseline BP. In addition, BP control, defined as systolic BP (SBP) < 140 mmHg and diastolic BP (DBP) < 90 mmHg based on the last available BP was reported and compared using Fisher’s exact test, between participants whose clinicians followed the app’s recommendations and those whose clinicians did not.
RESULTS
Study population
The study population included 29 EA participants, 38% were women. Average age was 52 ± 9 years and the median baseline PRA was 1.3 ng/mL/hr (interquartile range, 0.5–3.1 ng/mL/hr). At baseline, 10% of the participants had untreated HTN, whereas 52%, 21%, and 17% of patients had uncontrolled BP treated with one, two, or three antihypertensive medications, respectively (Table 3 ). The mean ± SD baseline SBP was 153 ± 11 and DBP was 90 ± 7 mmHg (Table 4 ).
Table 3.
Characteristics of the study participants
| Study participants (n = 29) | |
|---|---|
| Age, years | 52 ± 9 |
| Female | 11 (38%) |
| Baseline PRA, ng/mL/hr | 1.3 (0.5–3.1) |
| Number of antihypertensive drug classes at baseline | |
| 1 Anti‐V | 6 (21%) |
| 1 Anti‐R | 9 (31%) |
| 2 Anti‐R | 2 (7%) |
| 1 Anti‐V + 1 anti‐R | 4 (14%) |
| 1 Anti‐V + 2 anti‐R | 4 (14%) |
| 2 Anti‐V + 1 anti‐R | 1 (3%) |
| None | 3 (10%) |
Age is summarized as mean ± SD, PRA as median (interquartile range), and categorical variables as N (percentage).
Anti‐V (anti‐volume) drugs including diuretics, aldosterone receptor antagonists, calcium channel blockers, vasodilators, and α1‐blockers; Anti‐R (anti‐renin) drugs including angiotensin converting enzyme inhibitors, angiotensin receptor blockers, β‐blockers, direct renin inhibitors, and central α2‐agonists; PRA, plasma renin activity.
Table 4.
BP of the study participants
| Study participants (n = 29) | Acceptance of app treatment recommendations (n = 16) | Non‐acceptance of app treatment recommendations (n = 13) | P value* | |
|---|---|---|---|---|
| Baseline SBP, mmHg | 153 ± 11 | 150 ± 12 | 157 ± 9 | 0.1 |
| Baseline DBP, mmHg | 90 ± 7 | 88 ± 7 | 91 ± 6 | 0.2 |
| SBP at 6 months, mmHg | 143 ± 22 | 137 ± 20 | 152 ± 21 | 0.05 |
| DBP at 6 months, mmHg | 85 ± 9 | 81 ± 8 | 89 ± 9 | 0.02 |
| SBP change after 6 months, mmHg | −10 ± 20 | −14 ± 20 | −5 ± 21 | 0.3 |
| DBP change after 6 months, mmHg | −5 ± 9 | −7 ± 9 | −2 ± 9 | 0.1 |
| BP control (SBP < 140 and DBP < 90 mmHg) at 6 months | 13 (45%) | 10 (63%) | 3 (23%) | 0.06 |
All continuous variables are summarized as mean ± SD and compared using independent t‐test. Discrete values are summarized as N (percentage) and compared using Fisher’s exact test.
BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
P values for comparison between participants whose clinicians accepted the app treatment recommendations vs. those whose clinicians did not.
Reduction and control of BP at 6 months according to acceptance of the app’s recommendations
Among the 29 participants included, the app treatment recommendations were accepted for 16 (55%) and were not accepted for 13 (45%). Among those participants whose clinicians accepted the app treatment recommendations, average SBP was lower at 6 months than those whose clinicians did not accept the app’s recommendations (137 ± 20 vs. 152 ± 21 mmHg; P = 0.05). Likewise, DBP was also lower comparing those whose clinicians accepted the app’s recommendations vs. those whose clinicians did not (81 ± 8 vs. 89 ± 9 mmHg; P = 0.02). At 6 months, BP control occurred in 63% 10 , 16 of participants whose clinicians accepted the app treatment recommendations compared with 23% (3 of 13) of participants whose clinicians did not (P = 0.06; Table 4 and Figure 2 ).
Figure 2.
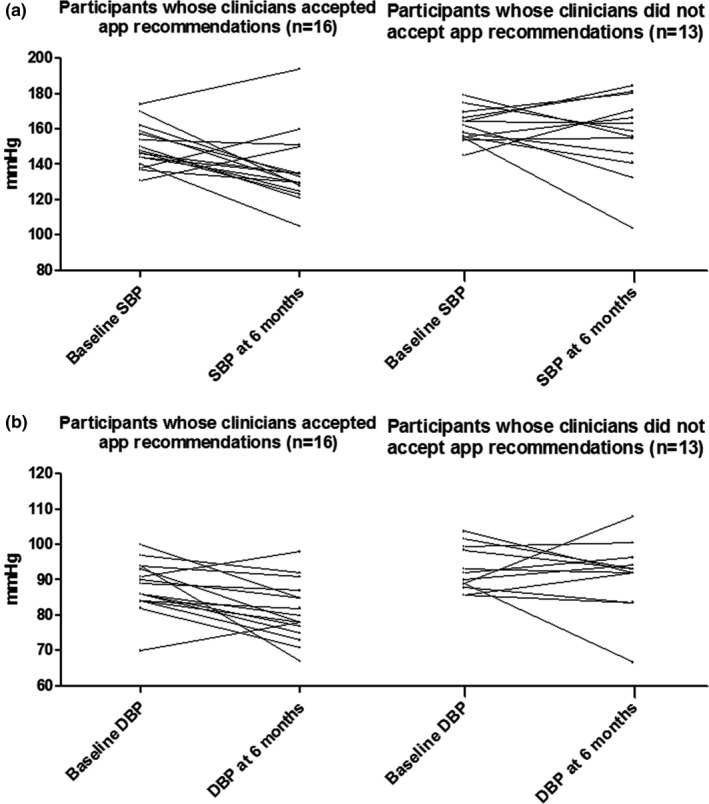
Each line represents a study participant. BP changes of participants whose clinicians accepted the app recommendations and those whose clinicians did not (a) SBP changes of participants whose clinicians accepted the app’s recommendations (n = 16) and those whose clinicians did not accept the app’s recommendations (n = 13). (b) DBP changes of participants whose clinicians accepted the app’s recommendations (n = 16) and those whose clinicians did not accept the app’s recommendations (n = 13). BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Following adjustment for baseline BP, participants whose clinicians accepted the app’s recommendations as compared with those whose clinicians did not, had a greater reduction in 6‐month SBP (−15 ± 21 vs. −3 ± 21 mmHg; adjusted‐P = 0.1) and DBP (−8 ± 8 vs. −1 ± 8 mmHg; adjusted‐P = 0.04; Figure 3 ).
Figure 3.
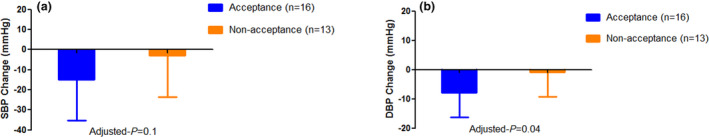
Adjusted BP changes of participants whose clinicians accepted the app recommendations vs those whose clinicians did not (a) Adjusted SBP changes (mean with SD) of participants whose treatment recommendations provided by the app were accepted (n = 16; −15 ± 21 mmHg) vs. those whose treatment recommendations provided by the app were not accepted (n = 13; −3 ± 21 mmHg; adjusted‐P = 0.1). (b) Adjusted DBP changes (mean with SD) of participants whose treatment recommendations provided by the app were accepted (n = 16; −8 ± 8.4 mmHg) vs. those whose treatment recommendations provided by the app were not accepted (n = 13; −1 ± 8 mmHg; adjusted‐P = 0.04). All values were adjusted for baseline SBP/DBP. BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
DISCUSSION
To our knowledge, this is the first study to implement a smartphone app designed to incorporate PRA as a way to personalize antihypertensive medication selection. In this pilot study, we have shown that it is possible to implement use of PRA as a biomarker, along with information on current therapies to further optimize antihypertensive therapy and reduce BP. We believe, if widely applied in clinical setting, this PRA‐based smartphone app could have substantial significant impact on the 116.4 million US adults with HTN through reducing risk of drug adverse events and cardiovascular events. 1
Only half of patients with HTN have controlled BP. 4 , 5 Several studies postulate that restricted access to health care and medication nonadherence are major contributors to poor BP control; yet, there is evidence that even patients who have health insurance and those who do adhere to their antihypertensive medications have uncontrolled BP. 19 In addition to the potential increased cardiovascular risks, uncontrolled BP often leads to the prescription of additional antihypertensive medications, which may increase risk for adverse events and result in additional cost estimated at US $467 million per year among US patients. 20 , 21
Uncontrolled BP likely reflects the high interindividual variability in BP response to various antihypertensive medications, which is mainly due to the heterogeneity in the pathophysiologic pathways underlying HTN. 6 The RAAS pathway plays a significant role in regulating BP. Within this system, renin converts angiotensinogen into angiotensin‐I, which is rapidly converted by the ACE into angiotensin‐II. Angiotensin‐II elevates BP by causing vasoconstriction and by stimulating the aldosterone secretion which leads to sodium retention. 7 RAAS activity is clinically estimated by PRA. 7 Using a personalized approach that is based on PRA—a biomarker that can be aligned with the mechanism of action of many antihypertensive drug classes—could improve BP lowering and control with fewer medications. 8
For a biomarker to be clinically embraced, its utility should be systematically evaluated. 22 Recently, we and others demonstrated that PRA is statistically significantly associated with BP response to multiple antihypertensives and that patients—especially EAs—with higher‐PRA vs. lower‐PRA categories have different BP responses to antihypertensive medications. 9 , 10 , 14 Moreover, we have shown that a PRA cutoff point of 0.60 ng/mL/hr, a cutoff point originally established in a cohort of EA patients decades ago, 23 has a sensitivity of 48.3% and a specificity of 85.1% to predict the BP response to chlorthalidone vs. metoprolol in EA patients with primary uncomplicated HTN. 14
The clinical utility of PRA as a biomarker was evaluated in a prospective study of 73 resistant patients with HTN followed for 1 year. This study showed that PRA‐guided antihypertensive medication selection resulted in significantly fewer antihypertensive medications required to control BP. 24 Additionally, in a randomized controlled clinical trial where 77 patients were treated, but had uncontrolled HTN (mostly white patients) were randomized to either a PRA‐guided treatment approach or to the usual clinical HTN specialists’ care, those treated based on a PRA‐guided approach had a significantly greater reduction in SBP and were treated with fewer anti‐R drugs compared with those treated based on the usual clinical HTN care. 13 In our pilot pragmatic trial, we demonstrated that deployment of a smartphone app that can aid clinicians in interpretation of PRA to optimize BP lowering across the spectrum of patients with uncomplicated HTN is feasible. In addition, we documented that participants whose clinicians accepted the app’s recommendations had on average about 12 mmHg greater 6‐month SBP reduction compared with those whose clinicians did not. This difference is clinically meaningful because a reduction in SBP of just 5 mmHg from baseline has been associated with 27% and 15% declines in risk of coronary heart disease and stroke, respectively. 25 In addition, acceptance of the smartphone app treatment recommendations was associated with a greater BP control.
The antihypertensive medication recommendations triggered by the app were based on adding and/or subtracting anti‐V and anti‐R drugs according to the patient’s PRA. It is possible that PRA is altered based on the prescribed antihypertensive medication. Studies have demonstrated that anti‐V drugs can cause a reactive rise in PRA in response to the reduced sodium content caused by diuresis. 15 Through blocking the formation of angiotensin‐II or its action, ACE inhibitors and ARBs can also increase PRA. 26 Conversely, β‐blockers decrease PRA by directly blocking the release of renin from kidneys. 27 , 28 Although the study protocol allowed clinicians to re‐evaluate PRA during a patient’s HTN treatment course in this pilot study, PRA was not re‐tested.
In addition to reducing BP, improving control, and reducing the number of antihypertensives needed to control BP, PRA has been demonstrated to be cost‐effective. The cost of a PRA test is US $100–150 in the United States and is reimbursed by Medicare and many third‐party insurance providers. 15 , 29 Treating patients based on a PRA‐based treatment strategy has been demonstrated to be associated with a 20% reduction in the total cost of medication per patient. 24 Moreover, using a simulation model, we have previously shown that a PRA‐guided treatment approach increases quality‐adjusted life years and is more cost‐effective than standard treatment care owing to improved BP and a corresponding reduction in complications, such as heart failure, stroke, acute myocardial infarction, and renal disease. 29 Therefore, use of a PRA‐based smartphone app could prevent additional potential costs while improving BP control. Additionally, the PRA‐based smartphone app could be useful in patients with resistant HTN, where the treatment costs are high due to the substantial increase in multiple comorbidities. Use of this app and PRA levels to guide selection of antihypertensive therapy could prevent the need for future procedures like renal denervation, in the high‐risk resistant hypertensive population. 30
Our study adds to the existing literature by further assessing the utility of PRA as a biomarker in clinical practice, using an innovative, systematic, precision‐focused HTN management approach. In our study, our sites were all rural primary care practices where access to specialty care is limited. Implementing this tool in sites like this provides the opportunity for optimized care that might otherwise not be possible. This easy‐to‐use smartphone app can be implemented in any hypertensive population, including those who are treated, untreated, or resistant to antihypertensive therapy, and of any race, to minimize HTN‐related disparities and improve HTN outcomes. However, findings from this pilot study should be considered in light of its limitations. First, our pilot study included a small sample size. Despite the small sample size, when the app’s recommendations were accepted, patients had a statistically significant greater reduction in DBP. Another limitation is the lack of randomization and blinding in this clinical trial. However, this pragmatic pilot trial was conducted to assess the effect of clinical implementation of a personalized PRA‐based smartphone app within the real‐world clinic setting on BP reduction and control. Currently, this app does not function with direct renin but that could be added in a future upgrade to the app software. 31 Additionally, having the smartphone app available to apply the app treatment recommendations when clinical decisions are made is a necessity.
In conclusion, our data suggest that a PRA‐guided smartphone app offering individualized therapy recommendations can be implemented in a real‐world setting, and that HTN management concordant with these recommendations appears to improve BP response. We also believe that findings from this pilot study and others serve as the basis for building a model to personalize the antihypertensive therapy based on demographic, clinical, PRA, and genetic factors rather than implementing the “one size fits all” approach. To assess and confirm the clinical utility of the smartphone app and a PRA‐based treatment strategy, randomized blinded clinical trials with larger sample sizes are needed.
Funding
The OPTI‐BP study was supported in part by the OneFlorida Clinical Data Research Network, funded by the Patient‐Centered Outcomes Research Institute (PCORI) under the grant number CDRN‐1501‐26692, in part by the OneFlorida Cancer Control Alliance, funded by the Florida Department of Health’s James and Esther King Biomedical Research Program #4KB16, and in part by the University of Florida Clinical and Translational Science Institute (UF CTSI), which is supported in part by the NIH National Center for Advancing Translational Sciences under the award number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the PCORI, its Board of Governors or Methodology, the OneFlorida Clinical Research Consortium, the University of Florida’s Clinical and Translational Science Institute, the Florida Department of Health, or the National Institutes of Health.
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
M.M., S.M.S., and R.M.C.‐D. wrote the manuscript. M.M., Y.E.C., Y.G. analyzed the data. E.H., B.R., J.D.L., J.G.H., and R.M.C.‐D. performed the research. R.M.C.‐D. designed the research.
Supporting information
Table S1
Trial Registry Number: NCT02814552
References
- 1. Virani, S.S. et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation 141, e139–e596 (2020). [DOI] [PubMed] [Google Scholar]
- 2. Rapsomaniki, E. et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1·25 million people. Lancet 383, 1899–1911 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO) . World Health Organization Global status report on noncommunicable diseases 2014 (Geneva, Switzerland, World Health Organization, 2015). [Google Scholar]
- 4. Sundström, J. , et al. Effects of blood pressure reduction in mild hypertension: a systematic review and meta‐analysis. Ann. Intern. Med. 162, 184–191 (2015). [DOI] [PubMed] [Google Scholar]
- 5. Clement, D.L. Poor blood pressure control: what can we do? J. Hypertens. 35, 1368–1370 (2017). [DOI] [PubMed] [Google Scholar]
- 6. Materson, B.J. Variability in response to antihypertensive drugs. Am. J. Med. 120 (suppl. 1), S10–S20 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Laragh, J. Laragh's lessons in pathophysiology and clinical pearls for treating hypertension. Am. J. Hypertens. 14, 186–194 (2001). [DOI] [PubMed] [Google Scholar]
- 8. Laragh, J. . Laragh's lessons in pathophysiology and clinical pearls for treating hypertension. Am J Hypertens 14(6 Pt 1), 491–503 (2001). [DOI] [PubMed] [Google Scholar]
- 9. Gharaibeh, K.A. et al. Comparison of blood pressure control rates among recommended drug selection strategies for initial therapy of hypertension. Am. J. Hypertens. 29, 1186–1194 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwartz, G.L. , Bailey, K. , Chapman, A.B. , Boerwinkle, E. & Turner, S.T. The role of plasma renin activity, age, and race in selecting effective initial drug therapy for hypertension. Am. J. Hypertens. 26, 957–964 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Václavík, J. et al. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): a randomized, double‐blind, placebo‐controlled trial. Hypertension 57, 1069–1075 (2011). [DOI] [PubMed] [Google Scholar]
- 12. Canzanello, V.J. et al. Predictors of blood pressure response to the angiotensin receptor blocker candesartan in essential hypertension. Am. J. Hypertens. 21, 61–66 (2008). [DOI] [PubMed] [Google Scholar]
- 13. Egan, B.M. et al. Plasma renin test‐guided drug treatment algorithm for correcting patients with treated but uncontrolled hypertension: a randomized controlled trial. Am. J. Hypertens. 22, 792–801 (2009). [DOI] [PubMed] [Google Scholar]
- 14. Mehanna, M. et al. Plasma renin activity is a predictive biomarker of blood pressure response in European but not in African Americans with uncomplicated hypertension. Am. J. Hypertens. 32, 668–675 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laragh, J.H. & Sealey, J.E. The plasma renin test reveals the contribution of body sodium‐volume content (V) and renin‐angiotensin (R) vasoconstriction to long‐term blood pressure. Am. J. Hypertens. 24, 1164–1180 (2011). [DOI] [PubMed] [Google Scholar]
- 16. Harris, P.A. et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inform. 95, 103208 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sealey, J.E. Plasma renin activity and plasma prorenin assays. Clin. Chem. 37(Pt 2), 1811–1819 (1991). [PubMed] [Google Scholar]
- 18. Van Der Gugten, J.G. & Holmes, D.T. Quantitation of plasma renin activity in plasma using liquid chromatography‐tandem mass spectrometry (LC‐MS/MS). Methods Mol. Biol. 1378, 243–253 (2016). [DOI] [PubMed] [Google Scholar]
- 19. Schroeder, K. , Fahey, T. , Hay, A.D. , Montgomery, A. & Peters, T.J. Relationship between medication adherence and blood pressure in primary care: prospective study. J. Hum. Hypertens. 20, 625–627 (2006). [DOI] [PubMed] [Google Scholar]
- 20. Flack, J.M. et al. Cardiovascular disease costs associated with uncontrolled hypertension. Manag. Care Interface. 15, 28–36 (2002). [PubMed] [Google Scholar]
- 21. Wang, T.J. & Vasan, R.S. Epidemiology of uncontrolled hypertension in the United States. Circulation 112, 1651–1662 (2005). [DOI] [PubMed] [Google Scholar]
- 22. Pletcher, M.J. & Pignone, M. Evaluating the clinical utility of a biomarker: a review of methods for estimating health impact. Circulation 123, 1116–1124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brunner, H.R. et al. Essential hypertension: renin and aldosterone, heart attack and stroke. N. Engl. J. Med. 286, 441–449 (1972). [DOI] [PubMed] [Google Scholar]
- 24. Blumenfeld, J.D. & Laragh, J.H. Renin system analysis: a rational method for the diagnosis and treatment of the individual patient with hypertension. Am. J. Hypertens. 11, 894–896 (1998). [DOI] [PubMed] [Google Scholar]
- 25. Appel, L.J. et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 336, 1117–1124 (1997). [DOI] [PubMed] [Google Scholar]
- 26. Case, D.B. , Wallace, J.M. , Keim, H.J. , Weber, M.A. , Sealey, J.E. & Laragh, J.H. Possible role of renin in hypertension as suggested by renin‐sodium profiling and inhibition of converting enzyme. N. Engl. J. Med. 296, 641–646 (1977). [DOI] [PubMed] [Google Scholar]
- 27. Sim, J.J. et al. Plasma renin activity (PRA) levels and antihypertensive drug use in a large healthcare system. Am. J. Hypertens. 25, 379–388 (2012). [DOI] [PubMed] [Google Scholar]
- 28. Huang, X. et al. Interpreting stimulated plasma renin and aldosterone to select physiologically individualized therapy for resistant hypertension: importance of the class of stimulating drugs. Hypertens. Res. 42, 1971–1978 (2019). [DOI] [PubMed] [Google Scholar]
- 29. Smith, S.M. & Campbell, J.D. Cost‐effectiveness of renin‐guided treatment of hypertension. Am. J. Hypertens. 26, 1303–1310 (2013). [DOI] [PubMed] [Google Scholar]
- 30. Agasthi, P. et al. Renal denervation for resistant hypertension in the contemporary era: a systematic review and meta‐analysis. Sci. Rep. 9, 6200 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lonati, C. , Bassani, N. , Gritti, A. , Biganzoli, E. & Morganti, A. Measurement of plasma renin concentration instead of plasma renin activity decreases the positive aldosterone‐to‐renin ratio tests in treated patients with essential hypertension. J. Hypertens. 32, 627–634 (2014). [DOI] [PubMed] [Google Scholar]
- 32. Roush, G.C. , Kaur, R. & Ernst, M.E. Diuretics: a review and update. J. Cardiovasc. Pharmacol. Ther. 19, 5–13 (2014). [DOI] [PubMed] [Google Scholar]
- 33. Hayashi, K. , Wakino, S. , Sugano, N. , Ozawa, Y. , Homma, K. & Saruta, T. Ca2+ channel subtypes and pharmacology in the kidney. Circ. Res. 100, 342–353 (2007). [DOI] [PubMed] [Google Scholar]
- 34. Loh, Y.C. , Tan, C.S. , Ch'ng, Y.S. , Ahmad, M. , Asmawi, M.Z. & Yam, M.F. Overview of antagonists used for determining the mechanisms of action employed by potential vasodilators with their suggested signaling pathways. Molecules 21, 495 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nerurkar, R.P. & Ved, J.K. Clinical pharmacology of alpha‐1 blockers improving drug‐profile through novel formulations. J. Assoc. Physicians India 62 (suppl.), 9–12 (2014). [PubMed] [Google Scholar]
- 36. Ferrario, C.M. & Mullick, A.E. Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacol. Res. 125(Pt A), 57–71 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holmer, S.R. , Hense, H.W. , Danser, A.H. , Mayer, B. , Riegger, G.A. & Schunkert, H. Beta adrenergic blockers lower renin in patients treated with ACE inhibitors and diuretics. Heart 80, 45–48 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sinclair, M.D. A review of the physiological effects of alpha2‐agonists related to the clinical use of medetomidine in small animal practice. Can. Vet. J. 44, 885–897 (2003). [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


