Abstract
Although pharmacogenetic testing is becoming increasingly common across medical subspecialties, a broad range of utilization and implementation exists across pediatric centers. Large pediatric institutions that routinely use pharmacogenetics in their patient care have published their practices and experiences; however, minimal data exist regarding the full spectrum of pharmacogenetic implementation among children’s hospitals. The primary objective of this nationwide survey was to characterize the availability, concerns, and barriers to pharmacogenetic testing in children’s hospitals in the Children’s Hospital Association. Initial responses identifying a contact person were received from 18 institutions. Of those 18 institutions, 14 responses (11 complete and 3 partial) to a more detailed survey regarding pharmacogenetic practices were received. The majority of respondents were from urban institutions (72%) and held a Doctor of Pharmacy degree (67%). Among all respondents, the three primary barriers to implementing pharmacogenetic testing identified were test reimbursement, test cost, and money. Conversely, the three least concerning barriers were potential for genetic discrimination, sharing results with family members, and availability of tests in certified laboratories. Low‐use sites rated several barriers significantly higher than the high‐use sites, including knowledge of pharmacogenetics (P = 0.03), pharmacogenetic interpretations (P = 0.04), and pharmacogenetic‐based changes to therapy (P = 0.03). In spite of decreasing costs of pharmacogenetic testing, financial barriers are one of the main barriers perceived by pediatric institutions attempting clinical implementation. Low‐use sites may also benefit from education/outreach in order to reduce perceived barriers to implementation.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE OF THE TOPIC?
☑ Implementation of pharmacogenetic testing is well‐described at select institutions; however, it is poorly understood how widespread implementation is, particularly at pediatric hospitals.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ The primary objective of this survey was to characterize pharmacogenetic testing in addition to determining availability, concerns, and barriers to pharmacogenetic testing in pediatric hospitals.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Although this study gives a general sense of pharmacogenetic implementation among pediatric hospitals, it also highlights the considerable differences between sites with limited vs. more extensive implementation, in particular the need for additional education of providers.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The results of this survey provide important information to institutions that have limited implementation of pharmacogenetic testing or for those considering implementation.
The implementation of pharmacogenetic testing has made remarkable progress in recent years, with select institutions introducing preemptive or reactive clinical testing. 1 , 2 Widely available resources, such as PharmVar, 3 the Pharmacogenomics Knowledge Base (PharmGKB), 4 and Clinical Pharmacogenetic Implementation Consortium (CPIC) guidelines, 5 now provide laboratories with consolidated data on pharmacogenetic variants and clinicians with up‐to‐date clinical information in addition to formal dosing recommendations for drug‐gene pairs. The formalization of these resources has undoubtedly contributed to progress in pharmacogenetic implementation. The number of well‐characterized specific drug‐gene interactions (DGIs) is growing exponentially along with our ability to rapidly test for and act on clinically relevant gene variants, allowing for translation of laboratory knowledge into clinical practice. 6
Although not every DGI results in a medication or dosing change, there are institutions where genetic testing for clinically significant variants is part of routine care. CPIC first released guidelines for TPMT and thiopurines in March 2011, with subsequent updates in April 2013 7 and most recently in November 2018, where the guideline was updated to include NUDT15. 8 Within pediatric oncology, patients with acute lymphoblastic leukemia are routinely tested for TPMT and NUDT15 variants prior to initiation of therapy with thiopurines. 9 TPMT is also routinely tested for in pediatric inflammatory bowel disease, which has improved dosing of thiopurines across pediatric centers participating in the ImproveCareNow network. 10 Recently, CPIC has also included pediatric‐specific genotype‐dosing recommendations for voriconazole based on CYP2C19 genotype and atomoxetine based on CYP2D6 genotype. 11 , 12
Implementation requires a collaboration of resources, including laboratories for genotyping, bioinformatics/information technology for interpretation and communication of results, and clinicians to incorporate the results into patient care. Historically, such resources were costly and often not sufficient for incorporation into clinical care, but current and evolving technologies are and will be available to make the process both possible and cost‐efficient. 13 The US Food and Drug Administration (FDA) has also made changes to facilitate the incorporation of pharmacogenetics into clinical practice. The FDA has implemented a voluntary program allowing companies to submit genomics data with new drug applications and has recently released a Table of Pharmacogenomic Associations that includes > 100 medications. The table includes subsections for those associations that they recommend therapeutic changes (dose or medication selection), associations that indicate an impact on safety or response, and associations that have an impact on pharmacokinetics only. Over 200 drug labels have been updated to include pharmacogenetic findings. There are several examples of newly approved targeted therapies with companion diagnostic tests (crizotinib and vemurafenib), drugs with required pharmacogenetic testing (eliglustat, pimozide, and tetrabenazine), and others with potential pharmacogenetic considerations included in the label.
The increase in pharmacogenetic data and progress in implementation are encouraging to physicians and other clinicians eager to provide safer and more effective therapy for their patients through precision medicine. Although discovery and implementation of clinically relevant DGIs require considerable resources and effort, the benefits have the potential to make such expenditures worthwhile, especially as costs decrease with improved technology. Collaborative networks, such as the Pharmacogenomics Research Network (PGRN), 14 the Electronic Medical Records and Genomics (eMERGE) Network, 15 the Implementing Genomics in Practice (IGNITE) 16 and the Canadian Pharmacogenomics Network, 17 allow for distribution of knowledge and effort across multiple institutions. Despite these recent advances in the utility, feasibility, and cost efficacy of pharmacogenetic testing, there are still many institutions that have not implemented routine pharmacogenetic testing into clinical practice. Analysis of the barriers to pharmacogenetic implementation have found that information technology, 18 clinical utility concerns, 19 and training 20 were major barriers to implementation in nonpediatric institutions. However, there has been no analysis of barriers to pharmacogenetic implementation specific to pediatric institutions. In order to assess this, we sought to better understand the landscape of pharmacogenetic testing in pediatric populations, and from there propose solutions to overcome these barriers. Thus, the primary objective of this survey was to characterize pharmacogenetic testing in addition to determining availability, concerns, and barriers to pharmacogenetic testing in pediatric hospitals.
METHODS
This study was reviewed and granted a waiver of Health Insurance Portability and Accountability Act (HIPAA) authorizations by the Institutional Review Board at Nationwide Children’s Hospital. Children’s hospitals were surveyed through REDCap, a secure, encrypted, web‐based application for building surveys and managing survey data, 21 in order to evaluate the availability, concerns, and barriers to pharmacogenetic testing. The survey was developed to assess characteristics about the respondent (training, specialty, and pharmacogenetics background), characteristics of their institution (location, setting, and referral patterns), use of pharmacogenetic testing at the institution (frequency, indications, results reporting, and decision support), and barriers for pharmacogenetic testing. Questions regarding barriers were adapted from a previous publication. 22 Requests were made via the pharmacy director’s listserv of the Children’s Hospital Association (CHA) to identify the appropriate point‐person for each institution regarding current and future pharmacogenetic testing. The survey invitation was distributed to all 248 members of the CHA. Following identification of the appropriate point person for each pediatric institution, a more detailed survey was distributed to identify current practices, future plans, and barriers to future plans at those institutions.
The subsequent detailed survey was distributed through REDCap, with data stored in the secure database ( Supplementary File ). Several questions were designed to characterize the institution, comfort and knowledge level of clinicians, and practices related to pharmacogenetic testing as well as ascertain individual barriers to, concerns of, and availability of pharmacogenetic testing. Participants responded on a numerical scale of 0–100, with 0 defined as not a barrier, 50 as somewhat of a barrier, and 100 as a large barrier.
Statistical analysis was primarily descriptive in nature and compiled in both tabular and narrative form. Sites were categorized as high vs. low pharmacogenetic use sites based upon participant responses regarding experience and use level; respondents who reported that they use pharmacogenetic testing “regularly” were categorized as a high‐use site, whereas those who reported “occasional” use or less were categorized as a low‐use site. Median values for perceived barriers from high‐use and low‐use sites were compared with two‐sample Wilcoxon rank sum tests. P values < 0.05 were considered significant.
RESULTS
Eighteen initial responses identifying a contact person were received from 17 unique institutions ( Figures 1 , 2 ). Of those 17 institutions, complete detailed surveys were submitted by 11 respondents from unique institutions. Two respondents from the same institution submitted complementary partial responses: although institutional demographics and experience of the two respondents were similar, only one respondent supplied data regarding specific DGIs, whereas the other supplied detailed data regarding barriers. For purposes of analysis, the institutional data from these two respondents were condensed into one institutional response, while responses specific to the experience and roles of the individual respondent were kept separate. One additional partially completed survey was obtained, which lacked responses to both barriers and specific DGIs. Responses were received from diverse geographic locations (Figure 2 ). All institutions were noted to be referral centers. Institutional and respondent characteristics, comfort and knowledge level of clinicians, and practices related to pharmacogenetic testing are summarized in Tables 1 , 2 . The majority of respondents were from urban/suburban institutions (92%) and held a Doctor of Pharmacy degree (86%). Half of respondents indicated they used pharmacogenetics regularly, whereas 14% and 36% indicated they either used it occasionally or had considered using it. Eight of the 13 unique institutions (62%) indicated that only general and/or subspecialist pediatricians order pharmacogenetic testing, while 4 institutions (31%) report pharmacists as ordering providers, and 1 institution (8%) also reported nurse practitioners and genetic counselors. Four institutions (31%) offer “in house” testing, and 7 of 13 (54%) sites reported that families had brought pharmacogenetic test results to their institution. One institution reported no pharmacogenetic testing, which is reflected in Table 2 .
Figure 1.
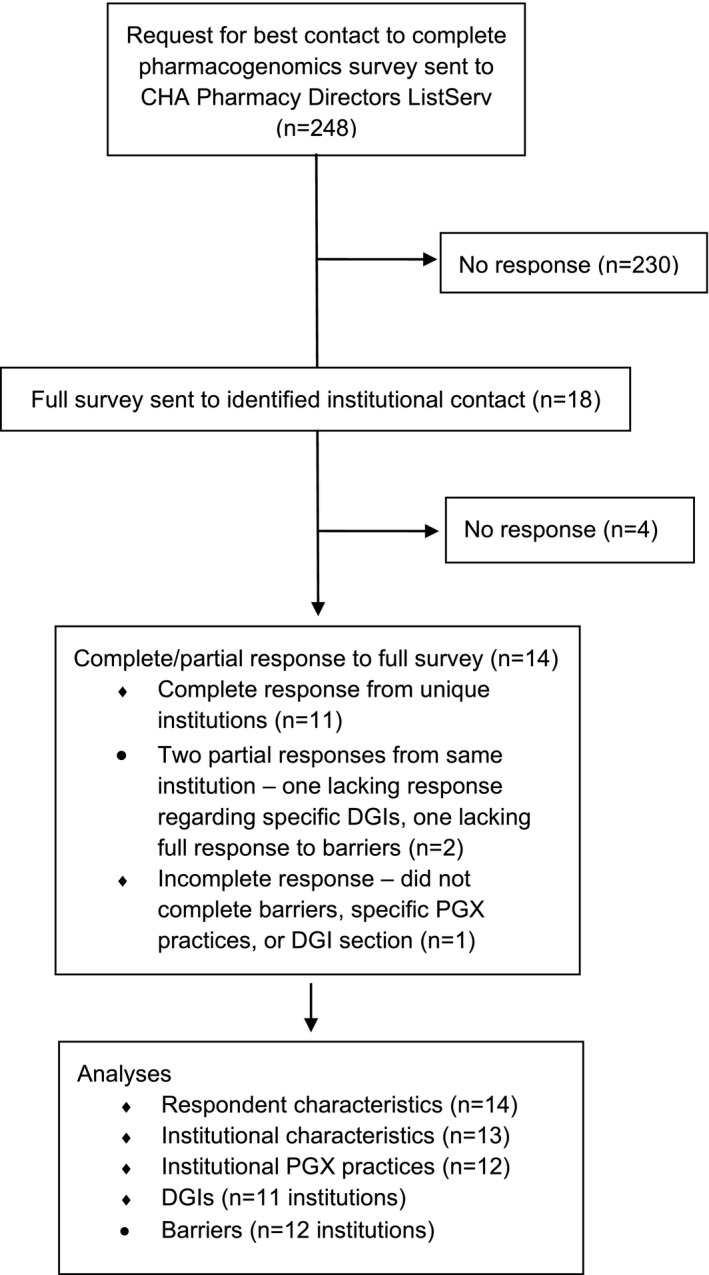
CONSORT flow diagram of survey response. CHA, Children’s Hospital Association; DGI, drug‐gene interaction; PGx, pharmacogenetic.
Figure 2.

Location of sites responding to survey.
Table 1.
Institutional and respondent demographics
| All | High use | Low use | |
|---|---|---|---|
| Setting of institution | (n = 13) | (n = 7) | (n = 6) |
| Urban/suburban | 12 | 7 | 5 |
| Rural | 1 | 0 | 1 |
| Setting of patients/families | (n = 13) | (n = 7) | (n = 6) |
| Urban and suburban | 3 | 1 | 2 |
| Urban, suburban, and rural | 10 | 6 | 4 |
| Institution type | (n = 13) | (n = 7) | (n = 6) |
| Academic | 12 | 6 | 6 |
| Community | 1 | 1 | 0 |
| Respondent type | (n = 14) | (n = 7) | (n = 7) |
| PharmD | 12 | 5 | 7 |
| PhD | 1 | 1 | 0 |
| MD, PhD | 1 | 1 | 0 |
| Years since completion of training | (n = 14) | (n = 7) | (n = 7) |
| 0–5 | 1 | 1 | 0 |
| 6–10 | 2 | 2 | 0 |
| 11–15 | 1 | 0 | 1 |
| ≥ 16 | 10 | 4 | 6 |
| Respondent’s pharmacogenetics education a | (n = 14) | (n = 7) | (n = 7) |
| None | 3 | 1 | 2 |
| Seminar, workshop, CME | 10 | 5 | 5 |
| Online | 3 | 3 | 0 |
| Relevant fellowship or residency | 3 | 3 | 0 |
| Self‐directed education (reading, mentorship) | 2 | 1 | 1 |
| Respondent practice specialties a | (n = 14) | (n = 7) | (n = 7) |
| Administrative | 8 | 1 | 7 |
| Pharmacy | 5 | 2 | 3 |
| Pharmacology | 3 | 3 | 0 |
| Critical care | 1 | 1 | 0 |
| Emergency medicine | 1 | 1 | 0 |
| General pediatrics | 3 | 3 | 0 |
| Genetics | 3 | 3 | 0 |
| Hospital medicine | 1 | 1 | 0 |
| Laboratory | 1 | 1 | 0 |
| Cardiology | 1 | 1 | 0 |
| Hematology/oncology | 2 | 2 | 0 |
| Developmental pediatrics | 1 | 1 | 0 |
| Palliative care | 1 | 1 | 0 |
| Infectious disease | 1 | 0 | 1 |
| Other provider use of pharmacogenetics | (n = 14) | (n = 7) | (n = 7) |
| Yes | 13 | 7 | 6 |
| No | 1 | 0 | 1 |
| Respondent use of pharmacogenetics | (n = 14) | (n = 7) | (n = 7) |
| I have considered using it, but have not yet | 5 | 0 | 5 |
| I use it occasionally | 2 | 0 | 2 |
| I use it regularly | 7 | 7 | 0 |
CME, continuing medical education.
Multiple responses permitted.
Table 2.
Characteristics of pharmacogenetic testing and implementation at each site
| All n (%) | High use | Low use | P value | |
|---|---|---|---|---|
| Pharmacogenetic testing at institution | n = 13 | n = 7 | n = 6 | |
| Yes | 12 (92) | 7 (100) | 5 (83) | |
| No | 1 (8) | 0 | 1 (17) | |
| Ordering provider a | n = 13 | n = 7 | n = 6 | |
| General pediatrician | 6 (46) | 4 (57) | 2 (33) | 0.59 |
| Subspecialty pediatrician | 11 (85) | 7 (100) | 4 (67) | 0.19 |
| Pharmacists | 4 (31) | 4 (57) | 0 | 0.07 |
| Genetic counselor | 1 (8) | 1 (14) | 0 | 1.00 |
| Nurse practitioner | 1 (8) | 1 (14) | 0 | 1.00 |
| N/A (no testing at institution) | 1 (8) | 0 | 1 (17) | |
| Reason for pharmacogenetic testing a | n = 13 | n = 7 | n = 6 | |
| Patient or family request | 5 (38) | 3 (43) | 2 (33) | 1.00 |
| Workup for medication nonresponse | 8 (62) | 5 (71) | 3 (50) | 0.59 |
| Workup for medication side effects | 8 (62) | 5 (71) | 3 (50) | 0.59 |
| Preemptive testing for all patients | 0 | 0 | 0 | |
| Preemptive testing for subset of patients | 7 (54) | 5 (71) | 2 (33) | 0.29 |
| Part of a study protocol | 8 (62) | 6 (86) | 2 (33) | 0.29 |
| N/A | 1 (8) | 0 | 1 (17) | |
| Type of pharmacogenetic testing | n = 13 | n = 7 | n = 6 | |
| Specific gene only | 1 (8) | 1 (14) | 0 | 1.00 |
| Panel‐based only | 2 (15) | 1 (14) | 1 (17) | 1.00 |
| Both specific gene and panel‐based | 6 (46) | 4 (57) | 2 (33) | 0.59 |
| Specific, panel, and targeted Exome | 1 (8) | 1 (14) | 0 | 1.00 |
| No response | 2 (15) | 0 | 2 (33) | |
| N/A | 1 (8) | 0 | 1 (17) | |
| Location of pharmacogenetic testing | n = 13 | n = 7 | n = 6 | |
| In‐house only | 1 (8) | 1 (14) | 0 | 1.00 |
| Send out only | 7 (54) | 4 (57) | 3 (50) | 1.00 |
| Both | 3 (23) | 2 (29) | 1 (17) | 1.00 |
| No response | 1 (8) | 0 | 1 (17) | |
| N/A | 1 (8) | 0 | 1 (17) | |
| Type of in‐house testing a | n = 13 | n = 7 | n = 6 | |
| Genotype | 4 (31) | 3 (43) | 1 (17) | 0.56 |
| Metabolizer status | 4 (31) | 3 (43) | 1 (17) | 0.56 |
| Known DGI | 2 (15) | 2 (29) | 0 | 0.46 |
| No response | 2 (15) | 1 (14) | 1 (17) | |
| N/A | 7 (54) | 4 (57) | 3 (50) | |
| How pharmacogenetic results recorded a | n = 13 | n = 7 | n = 6 | |
| Lab result within EMR | 7 (54) | 5 (71) | 2 (33) | 0.29 |
| Scanned in | 8 (62) | 5 (71) | 3 (50) | 0.59 |
| Discrete result via EMR algorithm | 2 (15) | 2 (29) | 0 | 0.46 |
| No response | 1 (8) | 0 | 1 (17) | |
| N/A | 1 (8) | 0 | 1 (17) | |
| Who interprets pharmacogenetic test results a | n = 13 | n = 7 | n = 6 | |
| Use the report provided | 2 (15) | 2 (29) | 0 | 0.46 |
| Ordering provider | 8 (62) | 4 (57) | 4 (67) | 1.00 |
| Genetic counselor | 1 (8) | 0 | 1 (17) | 0.46 |
| Clinical pharmacist | 6 (46) | 5 (71) | 1 (17) | 0.10 |
| Consultant or pharmacogenetic consult service | 6 (46) | 4 (57) | 2 (33) | 0.59 |
| No response | 1 (8) | 0 | 1 (17) | |
| N/A | 1 (8) | 0 | 1 (17) | |
| Who reports results to patients/families a | n = 13 | n = 7 | n = 6 | |
| Ordering provider | 10 (77) | 6 (86) | 4 (67) | 0.56 |
| Genetic counselor | 1 (8) | 0 | 1 (17) | 0.46 |
| Clinical pharmacist | 5 (38) | 5 (71) | 0 | 0.021* |
| Consultant or pharmacogenetic consult service | 2 (15) | 1 (14) | 1 (17) | 1.00 |
| No response | 1 (8) | 0 | 1 (17) | |
| N/A | 1 (8) | 0 | 1 (17) | |
| Have families brought pharmacogenetic test results to your institution | n = 13 | n = 7 | n = 6 | |
| Yes | 7 (54) | 6 (86) | 1 (17) | 0.07 |
| No | 0 | 0 | 0 | |
| Uncertain | 5 (38) | 1 (14) | 4 (67) | 0.07 |
| No response | 1 (8) | 0 | 1 (17) | |
| Who sent the outside pharmacogenetic testing a | n = 13 | n = 7 | n = 6 | |
| Primary care practitioner | 4 (31) | 4 (57) | 0 | 0.07 |
| Outside subspecialist | 6 (46) | 6 (86) | 0 | 0.005* |
| Patient/family obtained on their own | 3 (23) | 3 (43) | 0 | 0.192 |
| Uncertain | 1 (8) | 0 | 1 (17) | 0.46 |
| No response | 5 (38) | 0 | 5 (83) | |
| What kind of laboratory performed outside pharmacogenetic testing | n = 13 | n = 7 | n = 6 | |
| Commercial/direct‐to‐consumer | 6 (46) | 6 (86) | 0 | 0.005* |
| Academic | 1 (8) | 1 (14) | 0 | 1.00 |
| Uncertain | 1 (8) | 0 | 1 (17) | 0.46 |
| No response | 5 (38) | 0 | 5 (83) | |
| What sources do you consult for interpretation of pharmacogenetic test results a | n = 13 | n = 7 | n = 6 | |
| Literature search | 11 (85) | 7 (100) | 4 (67) | 0.19 |
| CPIC | 9 (69) | 7 (100) | 2 (33) | 0.021* |
| DPWG | 6 (46) | 5 (71) | 1 (17) | 0.10 |
| Google/internet search | 2 (15) | 1 (14) | 1 (17) | 1.00 |
| PharmGKB | 4 (31) | 4 (57) | 0 | 0.07 |
| Other (ClinVar, dbSNP, other variant databases) | 2 (15) | 2 (29) | 0 | 0.46 |
| No response | 1 (8) | 0 | 1 (17) | |
| Respondent need for more pharmacogenetic education | n = 14 | n = 7 | n = 7 | |
| No | 6 (43) | 6 (86) | 0 | 0.005* |
| Yes, a little | 4 (29) | 1 (14) | 3 (43) | 0.56 |
| Yes, a lot | 3 (21) | 0 | 3 (43) | 0.19 |
| No response | 1 (7) | 0 | 1 (14) | |
| Other provider need for more pharmacogenetic education | n = 14 | n = 7 | n = 7 | |
| No | 0 | 0 | 0 | |
| Yes, a little | 2 (14) | 0 | 2 (29) | 0.46 |
| Yes, a lot | 11 (79) | 7 (100) | 4 (57) | 0.19 |
| No response | 1 (7) | 0 | 1 (14) | |
| Institutional plans for pharmacogenetics a | n = 13 | n = 7 | n = 6 | |
| Expand testing | 9 (69) | 6 (86) | 3 (50) | 0.14 |
| Hire a full‐time clinician/pharmacist/other to run a pharmacogenetic program | 3 (23) | 1 (14) | 2 (33) | 0.56 |
| Bring testing “in‐house” | 5 (38) | 3 (43) | 2 (33) | 1.00 |
| Provide increased clinical support via personnel or in EMR | 8 (62) | 6 (86) | 2 (33) | 0.103 |
| Formal education for providers | 2 (15) | 2 (29) | 0 | 0.462 |
| No response | 2 (15) | 0 | 2 (33) |
CPIC, Clinical Pharmacogenetic Implementation Consortium; dbSNP, database‐single‐nucleotide polymorphism; DGI, drug‐gene interaction; DPWG, Dutch Pharmacogenetics Working Group; EMR, electronic medical record; N/A, not applicable; PharmGKB, Pharmacogenomics Knowledge Base.
Multiple responses allowed.
P value < 0.05 when comparing high‐use vs. low‐use institution.
Eleven respondents from 11 unique institutions (4 low‐use and 7 high‐use) supplied data regarding specific DGIs tested at their institution, as well as clinical decision support provided in the electronic prescribing system for these DGIs (Figure 3 ). The most commonly tested DGI among all centers was thiopurine/TPMT, with nine centers reporting use (2 low‐use and 7 high‐use); this was also the only DGI for which a low‐use center reported clinical decision support. The next most frequently tested DGIs were voriconazole/CYP2C19 and codeine/CYP2D6, both of which had seven centers (1 low‐use and 6 high‐use) reporting testing. The most commonly tested DGI among low‐use centers was ivacaftor/CTFR, with two low‐use centers and three high‐use centers reporting routine testing. Aside from the DGIs listed in Figure 3 , two high‐use centers reported additional testing: one center for aripiprazole/CYP2D6, and one center with “too many DGIs to list.”
Figure 3.

Drug‐gene interactions (DGIs) implemented at high‐use and low‐use centers. Black box indicates routine implementation of DGI. “X” indicates that clinical decision support exists for the DGI.
For interpretation of pharmacogenetic results, the majority of respondents cited use of a Literature Search, followed by CPIC, Dutch Pharmacogenetics Working Group (DPWG), PharmGKB, and a Google/Internet search. Notably, none of the low‐use sites indicated they used PharmGKB as a source. Regarding the need for additional pharmacogenetic education for the respondent, nearly half (43%) reported no need for further education (all of whom were from a high‐use site); 21% reported a need for “a lot” of additional education, all of whom were at a low‐use site. However, when asked if other providers at the institution needed more pharmacogenetic education, 79% said yes, a lot, and 7% said yes, a little.
Full results of barriers to implementation are summarized in Figure 4 . Across all sites, the three highest ranked barriers were test reimbursement, test cost, and money. The three lowest ranked barriers were potential for genetic discrimination, sharing results with family members, and availability of tests in certified laboratories. Other items that were considered somewhat of a barrier included electronic health records support, other providers’ experience, knowledge or education, lack of randomized controlled trials documenting superiority of pharmacogenetic‐guided treatment, and genetic exceptionalism for genetic and pharmacogenetic tests.
Figure 4.
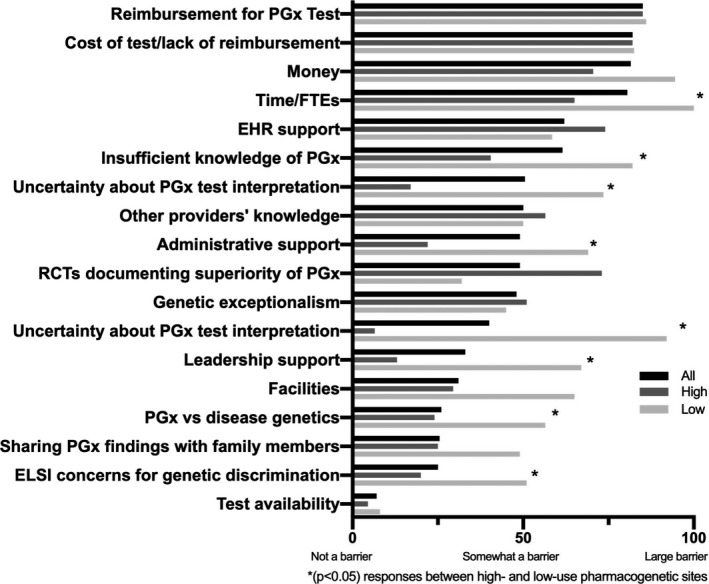
Participant responses to questions regarding availability, knowledge, and barriers to pharmacogenetic testing. *(P < 0.05) Responses between high‐use and low‐use pharmacogenetic sites. EHR, electronic health record; ELSI, ethical, legal, and social implications; FTE, full time equivalent; PGx, pharmacogenetic; RCT, randomized controlled trial.
High‐use and low‐use sites ranked barriers differently. In high‐use sites, test availability, uncertainty about pharmacogenetic test interpretation, and leadership support were the least impactful barriers, whereas reimbursement for pharmacogenetic testing, money, and electronic health record support were seen as the greatest barriers. In low‐use sites, the least impactful barriers were test availability, randomized controlled trials documenting superiority, and genetic exceptionalism, whereas the greatest barriers were time/personnel, money, and uncertainty about pharmacogenetic test interpretation. Low‐use sites rated several barriers significantly higher than the high‐use sites, including support from leadership (P = 0.04), administrative support (P = 0.04), time/personnel (P = 0.01), defining importance of ethical, legal, and social implications (ELSI) in pharmacogenetic vs. disease genetics (P = 0.04), concerns about inclusion of genetic information in the medical record and potential for genetic discrimination (P = 0.02), uncertainty about drug therapy decision based on a pharmacogenetic test (P = 0.03), uncertainty about pharmacogenetic test interpretation (P = 0.04), and insufficient knowledge of pharmacogenetic data (P = 0.03).
DISCUSSION
The results of this survey characterize the state of and elicit the barriers and concerns of pharmacogenetic testing in a sample of pediatric referral centers. Most respondents acknowledged the need for additional pharmacogenetic education, particularly for other providers (not the individual who completed the survey) and all providers (respondent and others) in low‐use sites. The primary concerns identified of survey respondents centered around cost/reimbursement for testing and time/personnel, whereas the availability, uncertainty of interpretation, and concerns about inclusion of genetic information into the medical record were considered lesser barriers. Additionally, low‐use sites viewed leadership/administrative support, time/personnel, ELSI, how to use the information, and insufficient knowledge as significantly greater barriers as compared with high‐use sites. Understanding the issues that are perceived as the greatest barriers will help to inform institutions that treat children seeking to implement pharmacogenetic testing of the perceived challenges that exist specific to the pediatric population.
In terms of the greatest need surrounding pharmacogenetic implementation, additional education of providers is of critical importance. The supplemental pharmacogenetic education acknowledged by respondents at low‐use sites and for other providers is in line with a recent survey of pediatricians surrounding their knowledge and attitudes of pharmacogenetic testing. In this survey of pediatricians in the United States and Japan, few respondents were familiar with pharmacogenetics or CPIC guidelines, whereas most respondents believe pharmacogenetics will improve safety and efficacy and expressed interest in additional pharmacogenetic education. 23 One of the more interesting findings of the survey presented herein were differences noted between high‐use and low‐use sites. Among high‐use sites, uncertainty about pharmacogenetic test interpretation was one of the least impactful barriers, whereas it was one of the largest barriers among low‐use respondents. This uncertainty among low‐use sites highlights the potential utility of widely available resources, such as CPIC guidelines and PharmGKB, to empower and educate clinicians in practical pharmacogenetic use when institutional resources and expertise are not available. In addition to the summary data presented above, review of the survey data by the authors led to incidental observations of discrepancies in the two respondents’ answers from the same institution. Further, some responses were discordant with known practices at individual institutions, also highlighting the need for raised awareness of pharmacogenetics use and resources at the institutional level for many sites. There are many opportunities for education in pharmacogenetics, including several online certificate programs targeted to clinicians, particularly pharmacists. We recommend CPIC’s website and the FDA tables cited above as excellent free resources, as well as PharmGKB’s website and many pathway publications, IGNITE’s website, and the PGRN’s website.
To date, barriers to the clinical implementation of pharmacogenetics have focused more broadly on testing in adults, and are more in line with the low‐use sites surveyed herein, including insufficient knowledge, level of evidence, cost, ELSI barriers, and test‐related barriers. 22 , 24 These barriers are similar to a separate review of pharmacogenetic testing in primarily neuropsychiatric medications, which identified a lack of guidelines, unclear clinical validity, variability in available tests, and cost as additional barriers. 25 Notably, several of the barriers discussed in other publications were listed as either somewhat of a barrier or closer to not a barrier among all the respondents herein (e.g., test availability, ELSI issues, and pharmacogenetics knowledge level). The differences between the results presented herein and prior surveys of clinicians at adult institutions may be partially explained by the fact that the respondents included were deemed the point person for pharmacogenetic testing at their institution, and thus barriers, such as a lack of guidelines, may be less of a concern to them due to a better understanding of what is available. Similar barriers exist globally, as evidenced by a recent literature review, which identified primary barriers to clinical implementation of pharmacogenetics as scientific, educational, ELSI, information technology, and reimbursement. 18 As additional research is completed and further dosing guidelines are updated and developed, it is likely that several of these barriers will become less of a concern over time. Looking more broadly at genetic testing, a survey of physicians from IGNITE sites felt genetic testing is clinically useful, although two‐thirds did not believe they were adequately trained to care for genetically high‐risk patients, 20 emphasizing the need of having adequately trained individuals with the skills necessary to assess these types of patients.
As previously noted, CPIC guidelines have provided clinicians with formal dosing recommendations based on an individual’s genotype for specific drug‐gene(s) pairs. In a prior survey, the Translational Pharmacogenetics Program of the PGRN found that the use of CPIC guidelines allowed for consistent use of pharmacogenetic results across institutions. 26 Although CPIC guidelines do not always contain dosing recommendations specific for children, a recent review highlighted several gene‐drug pairs and dosing relevant to children. 27 In fact, the most commonly reported DGI (thiopurines/TPMT) corresponds to one of the earliest CPIC guidelines, and nearly all reported DGIs herein have published CPIC guidelines.
Among both high‐use and low‐use sites, reimbursement for testing, cost of test/lack of reimbursement, and money were the three largest barriers, while test availability was one of the least concerning barriers. Cost of testing and a lack of reimbursement is consistent with barriers noted elsewhere and emphasizes the need for administrative support and a business plan when considering the introduction of any new form of testing. 18 The minimal concern regarding test availability may be explained in part by the considerable growth of testing companies now offering pharmacogenetic testing to patients. 28 Additionally, the well‐known direct to consumer genetic testing company 23andMe recently announced the addition of pharmacogenetic testing to their panel, making it likely patients and parents/guardians of patients will soon begin arriving to appointments with results in hand.
This survey‐based study has several limitations. Although the initial survey was sent to all 248 CHA members, the initial survey was answered by only 18, and we have complete response data from only 12 pediatric institutions: 11 unique institutional respondents completed the full survey; 2 respondents from the same institution provided complementary partial responses, which were considered a full response for the institution. These 12 centers may not be representative of all children’s hospitals as they are predominantly urban, academic institutions that may vary considerably from those in rural, community settings. Indeed, it is likely that centers where no pharmacogenetic experts were identified are systematically different from respondents. Further, the survey was completed by one individual at each site who may not have an accurate sense of barriers or other data. Insurance coverage decisions for pharmacogenetic testing in psychiatry have changed since the survey was performed, so opinions about financial barriers may change with broader insurance coverage. 29
Currently, few pediatric patients are reaping the benefits of such individually tailored therapy due to a variety of challenges, such as provider knowledge and comfort level, cost, appropriate interpretation of results, and effective communication of these results. 22 Pharmacogenetics has the potential to make a significant impact on pediatric patient care using both the DGIs already identified and those that could be identified in the future. Through pharmacogenetics we have the opportunity to improve drug efficacy and safety, thus improving both morbidity and mortality in this unique patient population. In spite of decreasing costs of pharmacogenetic testing, financial barriers are still perceived as a major barrier by pediatric institutions attempting clinical implementation. Additionally, low‐use sites may also benefit from education/outreach in order to reduce perceived barriers to implementation.
Funding
The authors received no specific funding for this work.
Conflict of Interest
L.B.R. has received research support from BTG, and International Ltd. All other authors declared no competing interests for this work.
Author Contributions
J.T.B., L.B.R., S.L.V.D., I.A., and S.I.C. wrote the manuscript. J.T.B., L.B.R., S.L.V.D., and S.I.C. designed the research. J.T.B., L.B.R., S.L.V.D., and S.I.C. performed the research. J.T.B., L.B.R., S.L.V.D., I.A., and S.I.C. analyzed the data.
Supporting information
Supplementary Material
References
- 1. Dunnenberger, H.M. et al. Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu. Rev. Pharmacol. Toxicol. 55, 89–106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramsey, L.B. et al. Implementation of pharmacogenetics at Cincinnati Children’s Hospital Medical Center: lessons learned over 14 years of personalizing medicine. Clin. Pharmacol. Ther. 105, 49–52 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gaedigk, A. , Whirl‐Carrillo, M. , Pratt, V.M. , Miller, N.A. & Klein, T.E. PharmVar and the landscape of pharmacogenetic resources. Clin. Pharmacol. Ther. 107, 43–46 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whirl‐Carrillo, M. et al. Pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 92, 414–417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Relling, M.V. & Klein, T.E. CPIC: clinical pharmacogenetics implementation consortium of the pharmacogenomics research network. Clin. Pharmacol. Ther. 89, 464–467 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Relling, M.V. & Evans, W.E. Pharmacogenomics in the clinic. Nature 526, 343–350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Relling, M.V. et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin. Pharmacol. Ther. 93, 324–325 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Relling, M.V. et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin. Pharmacol. Ther. 105, 1095–1105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crews, K.R. , Hicks, J.K. , Pui, C.‐H. , Relling, M.V. & Evans, W.E. Pharmacogenomics and individualized medicine: translating science into practice. Clin. Pharmacol. Ther. 92, 467–475 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crandall, W.V. et al. Improved outcomes in a quality improvement collaborative for pediatric inflammatory bowel disease. Pediatrics 129, e1030–e1041 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moriyama, B. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP2C19 and voriconazole therapy. Clin. Pharmacol. Ther. 102, 45–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown, J.T. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 genotype and atomoxetine therapy. Clin. Pharmacol. Ther. 106, 94–102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Altman, R.B. Personal genomic measurements: the opportunity for information integration. Clin. Pharmacol. Ther. 93, 21–23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shuldiner, A.R. et al. The pharmacogenomics research network translational pharmacogenetics program: overcoming challenges of real‐world implementation. Clin. Pharmacol. Ther. 94, 207–210 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wiesner, G.L. et al. Returning results in the genomic era: initial experiences of the eMERGE Network. J. Pers. Med. 10, 30 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weitzel, K.W. et al. The IGNITE network: a model for genomic medicine implementation and research. BMC Med. Genomics 5, 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ross, C.J.D. et al. The Canadian Pharmacogenomics Network for Drug Safety: a model for safety pharmacology. Thyroid 20, 681–687 (2010). [DOI] [PubMed] [Google Scholar]
- 18. Klein, M.E. , Parvez, M.M. & Shin, J.‐G. Clinical implementation of pharmacogenomics for personalized precision medicine: barriers and solutions. J. Pharm. Sci. 106, 2368–2379 (2017). [DOI] [PubMed] [Google Scholar]
- 19. Lee, Y.M. , Manzoor, B.S. , Cavallari, L.H. & Nutescu, E.A. Facilitators and barriers to the adoption of pharmacogenetic testing in an inner‐city population. Pharmacotherapy 38, 205–216 (2018). [DOI] [PubMed] [Google Scholar]
- 20. Owusu Obeng, A. et al. Physician‐reported benefits and barriers to clinical implementation of genomic medicine: a multi‐site IGNITE‐Network Survey. J. Pers. Med. 8, 24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris, P.A. , Taylor, R. , Thielke, R. , Payne, J. , Gonzalez, N. & Conde, J.G. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson, J.A. Pharmacogenetics in clinical practice: how far have we come and where are we going? Pharmacogenomics 14, 835–843 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rahawi, S. et al. Knowledge and attitudes on pharmacogenetics among pediatricians. J. Hum. Genet. 65, 437–444 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Donnell, P.H. et al. The 1200 patients project: creating a new medical model system for clinical implementation of pharmacogenomics. Clin. Pharmacol. Ther. 92, 446–449 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gross, T. & Daniel, J. Overview of pharmacogenomic testing in clinical practice. Ment. Health Clin. 8, 235–241 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luzum, J.A. et al. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: outcomes and metrics of pharmacogenetic implementations across diverse healthcare systems. Clin. Pharmacol. Ther. 102, 502–510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramsey, L.B. , Brown, J.T. , Vear, S.I. , Bishop, J.R. & Van Driest, S.L. Gene‐based dose optimization in children. Annu. Rev. Pharmacol. Toxicol. 60, 311–331 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Filipski, K.K. , Murphy, J.D. & Helzlsouer, K.J. Updating the landscape of direct‐to‐consumer pharmacogenomic testing. Pharmgenomics Pers. Med. 10, 229–232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. United Healthcare Pharmacogenetic Testing . [Internet]. 2020. <https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm‐medical‐drug/pharmacogenetic‐testing.pdf>. Accessed March 27, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


