Abstract
Medium chain acyl-CoA dehydrogenase deficiency (MCADD) is an autosomal recessive fatty acid β-oxidation defect. The enzyme, medium chain acyl-CoA dehydrogenase is important in the breakdown of medium chain fats into acetyl-CoA to produce ketones. Ketones are used as an alternative energy source when glucose or hepatic glycogen stores become depleted during prolonged fasting. In MCADD during periods of fasting or acute illness, there are insufficient ketones to compensate for the glucose energy deficit, resulting in an hypoketotic hypoglycaemia alongside a build-up of fatty acids. This build-up of fatty acids can be neurotoxic and lead to altered brain function and even unexpected death. Management includes avoiding prolonged periods of starvation, consuming high carbohydrate drinks during periods of illness and in symptomatic patients, reversal of catabolism and sustained anabolism by provision of simple carbohydrates by mouth or intravenously. Coexistence of MCADD and type 1 diabetes (T1D) is rare, there is no causal association though there are some documented cases. A key goal of management in T1D is achievement of good glycaemic control to reduce risk of long-term complications. This can in some cases increase the risk of hypoglycaemia which can be catastrophic in the presence of MCAD
Keywords: paediatrics, endocrinology, diabetes, metabolic disorders
Background
Medium chain acyl-CoA dehydrogenase deficiency (MCADD) is a lifelong, rare metabolic disorder where an enzyme defect restricts the breakdown of medium chain fats into acetyl-CoA to produce ketones as an alternative energy source to glucose. It is present at birth and estimated to affect 1 in every 10 000 babies in the UK.1 It is inherited in an autosomal recessive fashion and is estimated that 1 in every 65 people in the UK could be carriers of the faulty gene and most affected patients have a North-western European ancestry.1 2 MCADD is usually picked up using the new-born blood spot screening test.1 Ordinarily medium chain acyl-CoA dehydrogenase oxidises medium chain fats into short chain fats in the mitochondria to produce acetyl-CoA and eventually ketones. However in MCADD, there is a single missense mutation, an A–G transition at cDNA position 985, which changes a lysine to glutamic acid at residue 329 (K329E), leading to reduced enzyme activity and consequently reduced number of ketones as an energy source.2 This means that during periods of fasting or acute illness, where glucose levels are low, there are insufficient ketones to compensate for the glucose energy deficit, resulting in a hypoketotic hypoglycaemia.2 The substances created when medium chain fats are partially broken down build up in the liver to cause liver dysfunction with hepatomegaly, metabolic acidosis, hyperammonemia and sudden death.2 Therefore, MCADD is a potentially life-threatening condition if not recognised quickly and treated appropriately. Affected patients usually present in the first 3 months to 3 years of life, with episodes of acute illness triggered by prolonged fasting lasting longer than 12–16 hours.2 Signs and symptoms include vomiting and lethargy, which rapidly progress to coma or seizures and cardiorespiratory failure.2 A cornerstone of management of patients with MCADD is the avoidance of long periods of fasting as this can lead to coma and death. As affected individuals get older, their fast tolerance improves. During periods of illness, patients are advised to regularly consume high carbohydrate containing drinks in order to avoid getting into a catabolic state. Type 1 diabetes (T1D) is an autoimmune condition leading to absolute insulin deficiency. Patients require lifelong insulin therapy. The aims of therapy for patients with T1D are to achieve good glycaemic control to reduce risks of long-term chronic complications. However, maintaining a tight glycaemic control in T1D can result in increased risk of hypoglycaemia. The coexistence of MCADD and T1D is rare and there have only been a few case reports.3–6 The need to achieve good glycaemic control with the inherent risk of hypoglycaemia in patients with MCADD creates a management dilemma when both conditions coexist. This is because hypoglycaemic episodes can be catastrophic in the presence of MCADD.7 8
We report the case of an adolescent with both T1D and MCADD and discuss the challenges to management.
Case presentation
We report our experience of managing a 17-year-old male patient with both T1D and MCADD. He was diagnosed with MCADD (homozygous c.985A>G mutation in the ACADM gene) at 16 months of age following an episode of diarrhoea and vomiting. He was managed with a frequent feeding regimen. He was advised to drink a glucose polymer feed (high carbohydrate drink called SOS) every 2–3 hours during periods of illness in order to avoid hypoglycaemia.
He remained well until age 12 when he was diagnosed with T1D after presenting with polyuria, polydipsia and oral thrush. A key challenge was how to avoid catastrophic hypoglycaemic episodes given the two conditions. He was offered sensor augmented insulin pump therapy but declined and opted instead for Dexcom CGM. However, he stopped wearing his CGM devise after 3 months for various reasons; mainly because the device caused localised skin irritation, fell off few times and the notification alarms disrupted his lessons while in school, which he found embarrassing. He did not wish to consider other options as he did not want anything stuck to his body. He was therefore managed with multiple daily injection therapy and regular blood glucose testing.
In order to avoid long periods of starvation and to continue the frequent feeding regimen for his MCADD, he was encouraged to have snacks in between meals. Should he want a snack more than 15 g, we advised him to administer insulin for it. Advice remained to continue to use his emergency regimen SOS 20 (which contained 40 g carbohydrate) during periods of acute illness to avoid developing a catastrophic catabolic state. In addition, he was advised on sick day rules for patients with diabetes which included: frequent monitoring of blood glucose, keeping well hydrated and giving correction doses of insulin to keep blood glucose in target. He managed any hypoglycaemic episodes associated with illness with his emergency SOS regimen, however low blood glucose not associated with illness was managed with 15 g of refined glucose.
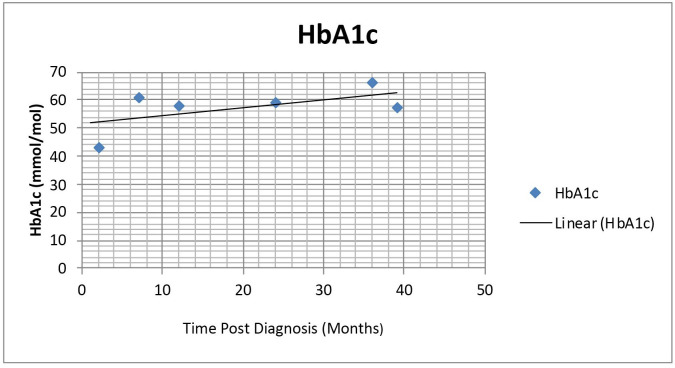
His blood glucose target was initially set at 5–9 mmol/L but this was later reduced to 4–7 mmol/L. His HbA1c varied between 43 mmol/mol (6.1%) and 66 mmol/mol (8.1%) (see figure 1). Although he was unable to consistently maintain the National Institute for Clinical Excellence HbA1c target of 48 mmol/mol (6.5%), he has had no moderate or severe hypoglycaemia.9 His care was shared between the specialist diabetes team and inherited metabolic disease specialists.
Figure 1.
Graph showing patient’s HbA1c in mmol/mol over the first 3 years of managing his type 1 diabetes (T1D) along with his medium chain acyl-CoA dehydrogenase deficiency.
Outcome and follow-up
The patient was successfully followed up every 3–6 months, for 5 years in our paediatric diabetes and endocrine clinic. Over the years, his HbA1c was fairly stable (see figure 1). Occasional slight deterioration of his diabetes was managed with dietician review, education and targeted increase in his insulin regimen. Clinical reviews also reinforced advice on how to manage hypoglycaemia, sick day rules during acute illness, monitoring glucose levels during exercise and safe drinking of alcohol. Given the association of T1D and autoimmune conditions, he had a coeliac screen which was endomysial antibody negative. His annual reviews over the years have been encouraging. His height is currently on the 91st centile and weight on the 75th centile. His blood pressures have always been normal. His feet are in good condition, ankle reflexes are present and vibration sense intact. His injection sites have been satisfactory. He has not had any complications or challenging health issues since treatment. From his initial diagnosis of T1D, he has never had ketoacidosis or significant hypoglycaemia requiring hospital admissions. He is hypoglycaemic aware and able to manage episodes of hypoglycaemia appropriately. He is currently in transition into adult services given his age of 17 years.
Discussion
Our case describes practical aspects of balancing the concurrent risk of hypoglycaemia while trying to achieve good glycaemic control, when T1D and MCADD coexist. The need for frequent snacks would have been easier to manage with an insulin pump therapy but as our patient preferred multiple daily injection regimens, we allowed him 15 g free snacks in between meals. Another challenge was avoiding hypoglycaemic episodes as a complication of insulin therapy. Use of real-time continuous glucose monitoring (CGM) which measures interstitial glucose constantly has been shown to reduce the incidence or severity of hypoglycaemia.10 11 It has alarms which alert the patient when they either have hyperglycaemia or impending hypoglycaemia. An insulin regimen that includes CGM with alert would have been ideal in helping to manage the patient in our case; however as he did not want any devise attached to him, he opted for finger prick testing of blood glucose instead and use of insulin pen injections for corrections. This highlights some of the difficulties with managing adolescents as some become more conscious of body image and do not wish to be different from their peers.12 T1D as a child is managed by adults, mainly by parents. As the child matures, diabetes management task transitions from the parents to the developing teen. Growing up during the teenage years can potentially be an unstable developmental period where the patient may experience changes in lifestyle, education, living situations, shifting relationships with family members and friends. They have to navigate through these transitions while also assuming increasing responsibility for their diabetes care and overall health. Unsuccessful transition of care can lead to deterioration.
Previous studies have shown that even in adolescents that agree to wear the CGM devise, the dropout rates are high. According to a recent prospective study published by Aronson et al, which evaluated the effectiveness of implantable CGM in Canadian adolescents with T1D, 90% of the participants found CGM easy to use and felt confident in the alarm’s ability to warn of blood glucose extremes.12 However, the report also highlighted CGM use was the lowest among adolescents.12 Additionally in the European Prospective Diabetes Follow-up Registry, use among adolescents was significantly lower in comparison to other age groups.12 Adolescents with T1D identified more barriers such as concerns about how the device appeared on their body creating a negative body image. With adolescents, it is important to continue to have open discussions about the benefits of CGM and explore potential barriers in its use.
Acute illness can result in the development of diabetic ketoacidosis (DKA) in patients with T1D if they do not abide by sick day rules. However in the presence of MCADD and T1D, the patient with DKA may not produce ketones, since MCADD blocks the breakdown of medium chain fats into acetyl-CoA to produce ketones. The frequency of DKA in patients with both conditions is unknown, however there are two case reports where patients with both conditions presented with ketonaemia during a diabetic crisis. This is probably because the enzyme deficiency in MCADD was not complete and therefore the fatty acid β-oxidation defect was probably partial, allowing ketones to be produced.2 8 It is therefore very important that patients are educated on the need to test their blood glucose regularly, check for ketonaemia, keep well hydrated and administer extra correction doses of insulin to manage high blood glucose during acute illness while consuming high carbohydrate drinks to avoid developing a catastrophic catabolic state due to the combination of MCADD and T1D. Shared care between the specialist teams including paediatric endocrinologist and specialists in inherited metabolic disease is vital in keeping the patient safe.
Patient’s perspective.
We were initially shocked with the diagnosis of medium chain acyl-CoA dehydrogenase deficiency (MCADD) as he was only 16 months old and had never heard of the condition before. We were not so taken back with the diagnosis of type 1 diabetes (T1D) because the signs were there for a while before the diagnosis. Our main concern with him having both MCADD and T1D was whether he will be able to live a normal life and do the everyday ordinary things his friends do. He has dealt with both conditions amazingly well and we are very pleased with the wonderful care given by all members of the healthcare team.
Learning points.
Coexistence of medium chain acyl-CoA dehydrogenase deficiency and type 1 diabetes (T1D) is rare, and there is no causal association.
A key goal of management in T1D is achievement of good glycaemic control to reduce risk of long-term complications however this can be difficult when balancing the risks of hypoglycaemia in the two conditions.
Managing adolescents can be challenging as they strive for independence.
Shared care between the specialist teams is vital in keeping the patients safe.
Footnotes
Contributors: This case was managed by the second author, JCA, as consultant paediatrician in Diabetes and Endocrinology. The case report was written by DA-M, following discussion with JCA about the key learning points. JCA also worked with DA-M to revise the draft paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.National Health Service (NHS) [online]. Available: https://www.nhs.uk/conditions/mcadd [Accessed 18 August 2020].
- 2.Kliegman R, J SG. Nelson’s textbook of paediatrics. 21st edn. Elsevier, 2019. [Google Scholar]
- 3.Wilson D, Brown A, Gumma AD, et al. Blood glucose control in a pregnant female with type 1 diabetes and medium-chain acyl-CoA dehydrogenase deficiency (MCADD). Endocrine Abstracts 2017;50:EP046. 10.1530/endoabs.50.EP046 [DOI] [Google Scholar]
- 4.Massey H. Puthi V. G170(P) Challenges of managing type1 diabetes mellitus associated with other metabolic disorders. Archives of Disease in Childhood 2017;102:A69. [Google Scholar]
- 5.Hawkins U, Subauste U, Bock H. Not your typical case of DKA: the Intricacies of DKA management in a patient with MCAD deficiency. Available: https://endo.confex.com/endo/2013endo/webprogram/Paper4700.html [Accessed 16thFebruary 2021].
- 6.Moll GW. Diabetes mellitus type 1 in patient with medium-chain acyl-coenzyme A dehydrogenase deficiency. Diabetes 2018;67:2342. 10.2337/db18-2342-PUB [DOI] [Google Scholar]
- 7.Maduemem KE. Medium-Chain acyl-coenzyme A dehydrogenase deficiency (MCADD): a cause of severe hypoglycaemia in an apparently well child. BMJ Case Rep 2016:bcr-2016-217538. 10.1136/bcr-2016-217538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mani S, El-Refee S, MCADD SM. And IDDM-A rare combination. Endocrine Abstracts 2010;24:P36. [Google Scholar]
- 9.National institute for clinical excellence [online].. Available: https://cks.nice.org.uk/topics/diabetes-type-1/management/management-children-young-people [Accessed 18 August 2020].
- 10.Tamborlane WV, Beck RW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–76. [DOI] [PubMed] [Google Scholar]
- 11.Choudhary P, Ramasamy S, Green L, et al. Real-Time continuous glucose monitoring significantly reduces severe hypoglycemia in Hypoglycemia-Unaware patients with type 1 diabetes. Diabetes Care 2013;36:4160–2. 10.2337/dc13-0939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aronson R, Abitbol A, Tweden KS. First assessment of the performance of an implantable continuous glucose monitoring system through 180 days in a primarily adolescent population with type 1 diabetes. Diabetes Obes Metab 2019;21:1689–94. 10.1111/dom.13726 [DOI] [PMC free article] [PubMed] [Google Scholar]