Abstract
Obesity is a prevalent childhood condition and the degree of adiposity appears likely to be an important covariate in the pharmacokinetics (PKs) of many drugs. We undertook these studies to facilitate the evaluation and, where appropriate, quantification of the covariate effect of body fat percentage (BF%) on PK parameters in children. We examined two large databases to determine the values and variabilities of BF% in children with healthy body weights and in those with obesity, comparing the accuracy and precision of BF% estimation by both clinical methods and demographically derived techniques. Additionally, we conducted simulation studies to evaluate the utility of the several methods for application in clinical trials. BF% was correlated with body mass index (BMI), but was highly variable among both children with healthy body weights and those with obesity. Bio‐impedance and several demographically derived techniques produced mean estimates of BF% that differed from dual x‐ray absorptiometry by < 1% (accuracy) and a SD of 5% or less (precision). Simulation studies confirmed that when the differences in precision among the several methods were small compared with unexplained between‐subject variability of a PK parameter, the techniques were of similar value in assessing the contribution of BF%, if any, as a covariate for that PK parameter. The combination of sex and obesity stage explained 68% of the variance of BF% with BMI. The estimation of BF% from sex and obesity stage can routinely be applied to PK clinical trials to evaluate the contribution of BF% as a potential covariate.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ The presence and degree of obesity is an important factor in the pharmacokinetics (PKs) of some drugs. Estimates of body fat percentage (BF%) or other quantitative assessments of the degree of obesity have rarely been included in PK studies in children.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ When considering BF% as a potential covariate in clinical PK studies in children, how do fat mass estimation methods compare in accuracy, precision, and clinical practicality?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Methods of BF% estimation in children vary in accuracy and precision, but are insignificant compared with typical between‐subject variability of PK parameters observed in clinical studies. For drugs in which BF% is an important determinant of alterations in drug disposition in children with obesity, the estimation of BF% from routine clinical demographics provides necessary accuracy and precision.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ To quantify the effect of adiposity on drug PK in children with obesity, BF% can be estimated from one of several anthropometric equations, or even more easily from the estimates of BF% based on obesity stage.
Approximately one‐third of children in the United States are overweight or obese, and this proportion has continued to increase over the last decade. 1 , 2 Fat and nonfat tissues have many distinct physical, chemical, and metabolic characteristics, so differences in drug disposition can occur relative to a child’s obesity status. Nevertheless, differences in drug pharmacokinetics (PKs) between children with and without obesity have been described for only a few drugs. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 For most drugs, the effects of obesity on PKs in children are poorly characterized, 11 , 12 , 13 , 14 leaving clinicians with inadequate information on how to design appropriate dosage regimens for this population. Because the fraction of body mass represented by adipose tissue may vary considerably among children with obesity, it is likely that the degree of adiposity might be an important covariate in understanding changes in drug PKs in children with obesity.
Body fat percentage (BF%) can be measured clinically by a variety of methods, such as bio‐impedance and dual energy x‐ray absorptiometry (DEXA), 15 , 16 , 17 or it can be estimated from subject demographics and anthropomorphic data. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 These methods differ in accuracy and precision, and their comparative suitability for studying the effect of BF% on drug disposition has not been explored.
To evaluate the different approaches for estimating BF% in clinical PK trials in children, we characterized the variability of body fat in children with healthy body weights and children with obesity. We then compared the accuracy and precision of commonly used clinical means of fat mass estimation, including the development of a new anthropometric equation, and assessed the utility and desirable precision of fat percentage measurement methodologies for clinical PK studies in children.
METHODS
BF% and its variability in children and adolescents
BF% and its variability during childhood and adolescence was quantified in two data sets: (i) a cross‐sectional survey of American children (the National Health and Nutrition Examination Survey (NHANES) 2003–2004) 27 ; and (ii) data from Chicago‐area youth being seen for weight management services and enrolled in the Pediatric Obesity Weight Evaluation Registry (POWER), which is a multi‐institutional, prospective effort established in 2013 to improve diagnosis and treatment of children with obesity. 28
The NHANES study included children and adolescents aged 8–17 years and collected anthropometric data, such as age, weight, height, sex, history of current or past pregnancy, and fat mass measured by both bio‐impedance and DEXA. NHANES 2003–2004 is the most recent NHANES survey that utilized both DEXA and bio‐impedance for body composition determination. Bio‐impedance was measured using the Hydra ECF/ICF Spectrum Analyzer (Model 4200) without standardization for time of day or time from activities of daily life. 27 , 28 Demographic data, including self‐identified race and menarche status, were also recorded.
The Chicago POWER data were collected at the Wellness and Weight Management Program at the Ann & Robert H. Lurie Children’s Hospital of Chicago in 2014–2017 and de‐identified for use in this study. Data collected on children aged 8–17 years included age, sex, weight, height, self‐identified race, pubertal stage by clinical examination, and fat mass by bio‐impedance. When different aspects of the clinical pubertal examination were discordant in an individual, the breast examination in girls and the genital examination in boys was used to designate the subject’s pubertal stage. Bio‐impedance was measured using the Tanita SC‐331S Body Composition Analyzer without standardization for time of day or time from activities of daily life. No girls were pregnant at the time of evaluations; past pregnancy history was not available. Body mass index (BMI) percentiles were calculated from the 2000 Centers for Disease Control and Prevention growth references, 29 , 30 smoothed with lambda‐mu‐sigma (LMS) methodology. 31 , 32 , 33 Correspondingly, obesity stage was assigned using the conventional definitions: underweight, BMI less than fifth percentile; healthy weight, 5–< 85th percentile; overweight, 85–< 95th percentile; obesity stage 1, 100–< 120% of the age‐specific and sex‐specific 95th percentile BMI (BMI95); obesity stage 2, 120–< 140% BMI95; obesity stage 3, ≥ 140% BMI95. 1 , 34 , 35
The datasets were examined separately and as a single, combined dataset. Most fields of the two databases could be combined directly (age, weight, etc.). Because pubertal stage was not recorded in the NHANES dataset, analysis of fat mass by puberty stage could only be assessed from the Chicago POWER dataset. Nonetheless, NHANES recorded post‐menarche status in girls, so a menarche variable was created for the POWER dataset girls and recorded as post‐menarche if Tanner stage was 4 or higher.
Methodologies for estimating BF%
Clinical methods
As described above, DEXA and bio‐impedance were the direct clinical estimations of BF% that were performed using the NHANES dataset (both DEXA and bio‐impedance) and POWER (bio‐impedance only) dataset.
Demographically derived estimates
We calculated BF% from anthropometric variables using four of the most commonly cited equations in the literature used to estimate fat mass or lean body mass. 18 , 20 , 23 , 25 In addition, in an attempt to maximize the accuracy of a predictive equation approach, we used the combined NHANES 2003–2004 and POWER datasets to generate a new equation for BF%. The methodology for developing this equation and its final form are detailed in the Supplementary Material s. Finally, we also estimated BF% using the individual’s sex, obesity status (present: BMI = or > 95‰, or absent: BMI < 95‰) and the combination of sex and obesity stage, as described above. With these techniques, an estimated BF% for each subject was assigned to be the corresponding mean BF% determined by DEXA for that sex, obesity status, or obesity stage from the NHANES 2003–2005 survey.
Accuracy and precision of body fat percentage estimation
An additional dataset, the NHANES 2005–2006 study, was used as a validation dataset to assess the accuracy and precision of each demographic‐derived methodology for predicting body fat percentage. The NHANES study, conducted in 2005–2006, 40 included identical variables as the 2003–2004 study, except that bio‐impedance was not measured. Each of the above‐described methods was used to predict body fat percentage for each subject in this dataset, and the paired comparisons of predicted and DEXA‐measured body fat percentages were generated, summarized, and tested statistically by t‐tests.
The accuracy of each approach was deemed to be the mean of paired differences and the precision was taken as the corresponding SD. Bio‐impedance was not measured in the NHANES 2005–2006 survey, so accuracy and precision for this technique were examined by comparing DEXA and bio‐impedance in each subject using the NHANES 2003–2004 survey. For each estimation technique and for each categorical covariate relating to BF% (sex, obesity status, and obesity stage), the percentage of the variance (η2) in BF% that was explained was calculated by the decrease in variance (SD2) of the BF% estimation for the database population from before to after the estimation technique or covariate was added to the equation.
Drug PK simulations and precision of BF% estimation methodology
The suitability of each BF% estimation technique for application to clinical PK studies was then assessed in simulation studies. We postulated a group of hypothetical drugs for which the between‐subject variability (BSV) of a PK parameter varied within a range typically found in pediatric clinical PK studies (15–80%). 41 , 42 , 43 Of course, for many drugs, BF% would not be an important covariate regardless of how accurately it was measured, yet limiting ourselves to situations in which BF% was an important covariate, one would expect to account for some measurable fraction of the BSV in the PK analysis. Correspondingly, we were interested in studying the situation in which the BSV (due to BF%) was significant and varied this contribution to be in the range of 10–60%. Our interest was then focused on whether the difference in precision in the measurement of BF% between the several techniques (with corresponding differing SDs between measured and estimated BF%) would identify one or more techniques as superior for identifying this covariance. To accomplish this, we calculated the SD of an observed variance of BSV, due to fat mass as the square root of the sum of the variance (SD2) of actual BSV due to fat mass and the variance of the measurement of BF%. Then, for every hypothetical combination of overall BSV, BSV due to observed BF%, and variance of the BF% methodology, we calculated the error in apparent BF% proportion of overall BSV and plotted it as a function of observed BSV. We expected that a difference of 10% or less between observed and actual BSV attributed to BF% or less in the common variance would identify a BF% measurement approach of suitable precision for application to clinical trials.
Data and statistical analyses
Data processing, graphic visualization, and statistical analyses, including t‐tests, analysis of variance, and F‐tests for comparison of variances, were performed using R (version 3.3.1; R Foundation for Statistical Computing) and RStudio (version 0.99.489). The specific techniques for each analysis are identified along with their results.
RESULTS
Fat mass distribution and variability in children
The demographic and anthropometric summary of the childhood databases is provided in Table 1 . There were a total of 2,260 children (1,763 in the NHANES 2003–2004 survey and 497 in the Chicago POWER study) between the ages of 8 and 17 years. There were 2,014 children in the NHANES 2005–2006 dataset used for the subsequent validation analysis.
Table 1.
Demographics of the subjects in the datasets
| NHANES 2003–2004 | POWER (Chicago) | NHANES 2005–2006 | |
|---|---|---|---|
| Unique subjects, n | 1,763 | 497 | 2014 |
| Observations, n | 1,763 | 1,002 a | 2014 |
| Male, % | 50.9 | 53.0 | 50.1 |
| Age, months | 159 (132–180) | 143 (123–168) | 157 (130–182) |
| Weight, kg | 53.4 (42.2–65.1) | 77.1 (58.0–95.9) | 53.0 (41.4–65.5) |
| BMI, kg/m2 | 21.1 (18.2–24.6) | 31.1 (27.0–37.1) | 20.9 (18.1–24.9) |
| Body fat, % | |||
| Bio‐impedance | 30.2 (23.1–36.6) | 41.4 (35.4–47.5) | Not performed |
| DEXA | 29.4 (23.2–35.6) | Not performed | 29.9 (23.4–36.7) |
| Obesity stage, no. boys/no. girls) | |||
| 5th–< 85th percentile BMI | 543/535 | 3/0 | 619/563 |
| 85th–< 95th percentile BMI | 166/159 | 25/35 | 162/165 |
| 1.0–< 1.2 × 95th percentile BMI | 121/106 | 149/144 | 135/173 |
| 1.2–< 1.4 × 95th percentile BMI | 48/44 | 177/165 | 69/76 |
| ≥ 1.4 × 95th percentile BMI | 19/22 | 177/127 | 24/27 |
| Race, % | |||
| Black | 36.2 | 9.3 | 32.2 |
| Hispanic | 33.5 | 64.6 | 36.6 |
| Other | 4.3 | 5.8 | 5.2 |
| White | 26.0 | 20.4 | 25.9 |
| Pubertal stage 1 (% boys/% girls) b | Not performed | 44.7/16.3 | Not performed |
| Pubertal stage 2 (% boys/% girls) b | Not performed | 26.4/22.7 | Not performed |
| Pubertal stage 3 (% boys/% girls) b | Not performed | 11.5/20.2 | Not performed |
| Pubertal stage 4 (% boys/% girls) b | Not performed | 10.6/16.3 | Not performed |
| Pubertal stage 5 (% boys/% girls) b | Not performed | 6.7/24.6 | Not performed |
| Post‐menarche b (% of girls) | 54.6 | 40.9 | 52.9 |
BMI, body mass index; DEXA, dual energy x‐ray absorptiometry; NHANES, National Health and Nutrition Examination Survey; POWER, Pediatric Obesity Weight Evaluation Registry.
Data in each dataset limited to children between 96 and 204 months of age with body fat determined by bioimpedance and exclude underweight (BMI < 5th percentile) children and girls who were known to have been or were pregnant. Distribution data presented as median (interquartile range).
Median of two observations per subject (interquartile range: 1–3); summary data based on status at time of observations.
Pubertal stage determined in 208 boys and puberty stage/post‐menarche status in 203 girls in Chicago POWER study.
BF% among all children as determined by DEXA (NHANES 2003–2004) varied widely (coefficient of variation (CV) = 21% among girls, 32% among boys). Even among children with healthy body weights (BMI 5–< 85th percentile for age and sex), BF% varied considerably (CV = 16% for girls and CV = 22% for boys). In this healthy weight subset, BF% was only weakly correlated with BMI normalized for BMI95 (BMI/BMI95; r 2 = 0.048) and was even more poorly correlated with weight (r 2 = 0.0149) and BMI (r 2 = 0.037). By contrast, the correlation between BF% and BMI95 was stronger (relative to peers with healthy weight) among children who were overweight or had obesity (r 2 = 0.343, P < 0.0001). The mean BF% in boys by obesity stage were as follows: healthy weight, 21.4%; overweight, 29.9%; obesity stage 1, 35.6%; obesity stage 2, 39.2%; and obesity stage 3, 44.2%. For girls, the mean BF% were: healthy weight, 29.4%; overweight, 36.3%; obesity stage 1, 40.6%; obesity stage 2, 44.6%; and obesity stage 3, 47.5%. The distribution and variability of BF% by obesity stage is depicted in Figure 1 . Table S1 provides the values of BF%s and variabilities for both NHANES 2003–2004 and NHANES plus POWER for the entire population, subdivided by sex, obesity status, and obesity stage.
Figure 1.
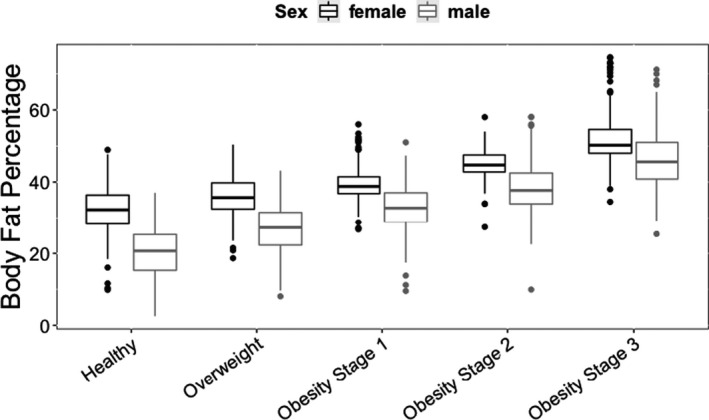
BF% measured by bio‐impedance as a function of obesity stage, separated by sex. Datasets are NHANES 2003–2004 plus POWER, filtered to eliminate underweight (BMI < 5th percentile) and previously or currently pregnant subjects. Obesity stages: healthy (5th–< 85th percentile BMI), overweight (85th–< 95th percentile BMI), obesity stage 1 (BMI = 1.0–<1.2 × 95th percentile BMI), obesity stage 2 (BMI = 1.2–< 1.4 × 95th percentile BMI), and obesity stage 3 (BMI = or >1.4 × 95th percentile BMI). Each box and central line represents median and IQR. Whiskers are the highest and lowest values up to 1.5 × IQR, with outliers depicted beyond. Girls had a higher fat percentage in all BMI groups. Variability of fat percentage was similar across BMI groups and in both sexes. BF%, body fat percentage; BMI, body mass index; IQR, interquartile range; NHANES, National Health and Nutrition Examination Survey; POWER, Chicago Pediatric Obesity Weight Evaluation Registry.
There was similar variability in BF% among children in each obesity stage, including those with healthy body weight. The mean fat percentage as a function of obesity stage was highly significant by analysis of variance. For both sexes, the difference in BF% between each obesity stage and its adjacent categories was significant (P < 0.001).
Methodologies for estimating BF%: DEXA vs. bio‐impedance
Comparison of fat percentage measurements by DEXA with estimation by bio‐impedance in the NHANES 2003–2004 survey demonstrated that bio‐impedance provided an accurate and precise estimate of BF% in this population. The median difference between the techniques was 0.12% (interquartile range (IQR): −3.4 to 3.5%, SD of paired comparisons: 0.052), similar to previous comparisons of the two techniques. 15 , 17 , 44 The concordance between BF% determined by bio‐impedance and DEXA is illustrated in Figure S1 a, and the difference in the measurements as a function of DEXA‐determined BF% is provided in Figure S1 b.
Methodologies for estimating BF%: DEXA vs. demographic estimation methods
The accuracy and precision of the several demographic methods of estimating BF% were evaluated using a dataset not used to generate the predictive equations (NHANES 2005–2006 survey, Table 1 ) by comparing the predicted BF% with that value measured by DEXA. The distributions of the observed and predicted value differences are provided for each method in Figure 2 (new demographic equation, equations of Al‐Sallami, and obesity stage) and in Figure S4 (additional anthropometric equations from the literature). The precisions of estimation of BF% (SD of paired comparison with BF% by DEXA) for obesity stage, bio‐impedance, and the new demographic equation are provided in Table 2 . The new equations were similar in precision, superior in accuracy to the equations of Al‐Sallami, and superior in both accuracy and precision to the remaining anthropometric equations that were studied. The obesity stage approach provided similar accuracy and precision to the new equations. The accuracy and variability of the new equations using the NHANES 2005–2006 dataset (median, −0.00230; IQR, −0.034 to 0.029) appeared similar to the concordance of observed and predicted values in the developmental dataset, NHANES 2003–2004, as cited above (median, −0.000095; IQR, −0.034 to 0.034). For comparison with other methods, the comparison of BF% measured by DEXA and estimated by bio‐impedance in the NHANES 2003–2004 dataset is provided in Figure 2d . Validation of bio‐impedance using the NHANES 2005–2006 dataset was not possible because bio‐impedance was not measured in this survey.
Figure 2.
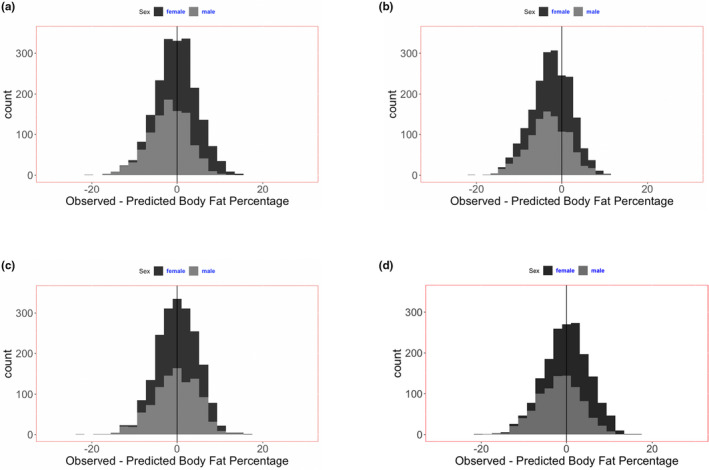
Accuracy and precision of several methods of estimating body fat mass percentage in children. (a–c)The subject population was the NHANES survey of 2005–2006, in which fat percentage was measured by DEXA. (d) The subject population was the NHANES survey of 2003–2004, in which fat percentage was measured by both DEXA and bio‐impedance. Additional analyses of other anthropometric equations previously reported in the literature are provided in FigureS4. (a) New anthropometric equation to estimate body fat mass percentage: mean difference (SD) of DEXA observed and equation predicted values: −0.000300 (0.0487). (b) Anthropometric equations for estimation of BF% reported by Al‐Sallami (2015): mean difference (SD) of DEXA observed and equation predicted values: −0.0249 (0.0467). Less accurate (P < 0.001), but not different in precision (P > 0.05) than new predictive equations. (c) BF% estimation from the mean population values by obesity stage: mean difference (SD) of DEXA observed and mean value of the respective obesity stage: −0.00033 (0.0481). Not different in accuracy or precision (P > 0.05) than new predictive equations. (d) BF% measured by bio‐impedance: mean difference (SD) of DEXA observed and bio‐impedance predicted values: −.0016 (0.0524). Statistical comparison with new predictive equations not performed because these equations were generated using these data in the NHANES 2003–2004 survey. BF%, body fat percentage; DEXA, dual energy x‐ray absorptiometry; NHANES, National Health and Nutrition Examination Survey.
Table 2.
Percentage of variance in BF% estimation explained by several prediction methods
| BF% Prediction | NHANES (n = 1,763) a | NHANES plus POWER (n = 2,765) b | ||||
|---|---|---|---|---|---|---|
| SD c | Variance | Variance explained (η 2) | SD | Variance | Variance explained (η 2) | |
| All subjects, no prediction method | 0.085 | 0.00722 | 0 | 0.011 | 0.0121 | 0 |
| By sex | 0.077 | 0.00593 | 17.9% d | 0.103 | 0.0106 | 12.3% d |
| By obesity status, present or absent and sex | 0.057 | 0.00325 | 55.0% e | 0.076 | 0.00578 | 52.3% e |
| By obesity stage and sex | 0.048 | 0.00230 | 68.1% f | 0.064 | 0.00410 | 66.1% f |
| By bio‐impedance | 0.052 | 0.00270 | 62.6% f | NA | – | – |
| By demographic equation | 0.049 | 0.00240 | 66.8% f | 0.058 | 0.00336 | 72.2% f |
BF%, body fat percentage; NA, not applicable; NHANES, National Health and Nutrition Examination Survey; POWER, Pediatric Obesity Weight Evaluation Registry.
The expanded summary data of body fat mass estimation by sex, obesity status, and obesity stage are provided in Table S1 in the Supplementary Materials.
Predicted BF% compared with clinically measured dual x‐ray absorptiometry values in NHANES 2003–2004 survey.
Predicted BF% compared with clinically measured values bio‐impedance values in combined NHANES 2003–2004 survey, plus POWER survey.
SD of paired dual x‐ray absorptiometry measured values minus predicted values: mean values of all comparisons for all methods < 0.003.
Improvement in variance of BF% estimation using sex group mean compared with using population mean alone (P < 0.001 by analysis of variance).
Improvement in variance of BF% estimation by obesity status (present or absent) plus sex group mean compared using sex mean alone (P < 0.001 by analysis of variance).
Improvement in variance of BF% estimation by these techniques compared using obesity status plus sex mean (P < 0.001 by analysis of variance).
Methodologies for estimating BF%: Explanation of variance
Because considerable variability (variance = SD2) in BF% measured by DEXA was found among subjects in the databases in this study, we calculated the proportion of that variance (η 2) that could be identified by methods of estimating BF% and by several categorical covariates that appeared to explain some differences in BF% (sex, obese status, and obesity stage) and summarized the results in Table 2 . When compared with DEXA in the NHANES 2003–2004 database, the new equations and bio‐impedance appeared similarly powerful for the estimation of BF% (η 2 = 62.6% and 66.8%, respectively). The combination of sex and obesity stage had similar strength (η 2 = 68.1%) to both bio‐impedance and the new equations. On the other hand, sex alone explained only 17.9% of the variance, whereas sex together with obesity status (presence or absence of obesity) explained 55.0% of the variance. Similar results were found when the same estimation methods other than bio‐impedance were compared with DEXA using the NHANES 2003–2004 plus POWER dataset (Table 2 ).
Drug PK simulations and precision of BF% estimation methodology
Figure 3 illustrates the impact of the precision differences among the techniques on fat mass estimation on the assessment of fat mass contribution to BSV. Using our initial criteria that sought to identify fat percentage contribution to BSV within 10%, all fat mass measurement techniques with a SD of ~ 0.05 (i.e., 2 SD = 10%) or less yielded accurate and nearly identical assessments of fat mass contribution to BSV, regardless of the underlying contribution of body fat percentage to BSV. Therefore, this analysis identifies DEXA, bio‐impedance, the new demographic equations, or obesity categories as being of similar value in quantifying the covariance of BF% on a PK parameter, provided the overall BSV is 20% or more.
Figure 3.
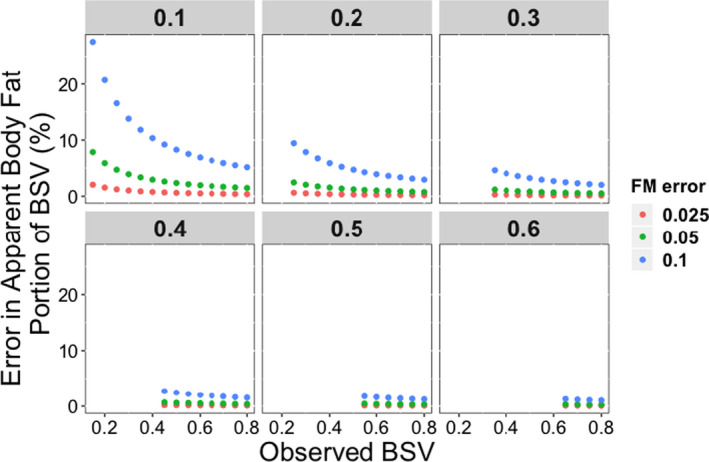
The impact of error in BF% estimation methodology on the attribution of observed PK parameter BSV to subject fat percentage. Each panel represents a hypothetical value of PK parameter BSV and each color of dots represent typical values of error (CV%) in fat percentage estimation of the several methodologies in this report. The only circumstances where error is > 10% is if observed BSV is very small (< 0.2) and the actual portion of BSV attributable to subject fat mass is less than the error in fat percentage estimation methodology. No such error is seen if fat percentage estimation error is ≤ 5%. BF%, body fat percentage; BSV, between‐subject variability; CV, coefficient of variation; PK, pharmacokinetic.
DISCUSSION
In order to improve PK studies of drugs in children with and without obesity, we evaluated several clinically applicable methods of estimating BF%. We found that BF% varied substantially among children with healthy body weights, as well as in children with obesity. Among those with healthy body weights, BF% was only weakly correlated with weight, BMI, or BMI/BMI95 (r 2 < 0.05 for all comparisons). Among children with obesity, the correlation between BF% was stronger with BMI/BMI95 (r 2 = 0.343) than with weight or BMI.
In the NHANES 2003–2004 dataset, bio‐impedance was found to be accurate and precise (SD = 5.2%) in estimating BF% compared with DEXA. Furthermore, equations based on a child’s age, sex, and BMI95 were found to be similarly precise (SD = 4.9%). The strong correlation between BF% and BMI/BMI95 in children with obesity supported the observation that the combination of a subject’s sex and obesity stage yielded similar precision (SD = 4.8%) in estimating BF% in the combined NHANES and POWER datasets.
The precision of the several methods for measuring or estimating BF% was small compared with the usual values of BSV and the residual error of PK parameter values found in clinical studies (typically 25–40% or higher for BSV and 10% or higher for residual error). 41 , 42 , 43 Critically important, however, was that the differences in precision among bio‐impedance, new BF% equations, or the combination of sex and obesity stage (0.3%) are trivial compared with the much larger variabilities (BSV and residual variability) for PK parameters in clinical studies. Furthermore, the difference in precision between these methods and DEXA (≤ 5.2%), which is the standard for measuring BF% in children, were not large enough to influence estimation of the contribution of BF% for any PK study unless the sum of parameter variabilities was unusually small (< 10%). As illustrated in Figure 3 , if total BSV and residual error is < 20%, then the precision of the fat mass determination method becomes somewhat more important, although identifying sources of BSV for drugs with such low variability is likely to have minimal clinical significance.
The findings from our study should facilitate the consideration of BF% as a covariate in clinical trials in children. The new anthropometric equations are straightforward to apply, but the application of an estimate from a subject’s obesity stage is even simpler. PK analyses can utilize the mean BF% found for each combination of sex and obesity stage or, alternatively, sex and obesity stage can be tested concurrently as categorical covariates.
Our study has several important limitations. First, we limited our studies to children 8–17 years of age, because the databases that were available for the generation of equations for prediction of fat mass were limited to this age group. Fat mass is highly variable in children of all ages, and extending these studies to other ages will yield valuable information. Second, neither the NHANES nor POWER surveys were designed to be true cross‐sectional representations of the child and adolescent populations of the United States. NHANES intentionally oversamples racial and ethnic minorities and the POWER dataset was largely limited to children with obesity; however, together, the datasets included broad representation of American children and there was no statistical evidence of differences in our analyses between children of different ethnicities. Third, the use of BMI95 as a normalizing measure in the description of fat mass has been adopted for children with obesity, but has not been previously used in children with healthy body weights. We chose BMI/BMI95 as the independent variable in our modeling of BF% because of its strong correlation with fat mass in children with obesity. 1 , 16 , 34 , 35 , 36 , 37 , 38 Additionally, we found that fat mass varied widely in children of healthy body weights and the application of BMI/BMI95 performed as well, if not better, than weight or BMI. 37 Finally, the utility of the BF% in clinical PK trials requires experimental verification. Obesity will not be associated with altered PK for every drug, yet studies done primarily in adults have identified some physical drug properties, as well as mechanisms of metabolism and elimination, that are more likely associated with altered PK in children and adolescents with obesity. 4 , 7 , 8 , 13
Understanding the impact of obesity on the disposition of drugs in children is an urgent priority, due to the increased prevalence of obesity, as well as the paucity of clinical drug trials that include obese children. Obesity is associated with changes in body composition and organ system function changes that have been shown to alter the PK of some drugs. 4 , 5 , 7 , 13 BF% has seldom been measured in PK trials in children. Furthermore, the relationships between BF% and drug PK values have not been experimentally explored. Identifying the sources of variability in drug clearance and distribution volumes is important to improve the safety and efficacy of drug therapy in all children, so an accurate estimation of the degree of adiposity will provide a potentially valuable covariate in clinical PK studies. In order to improve drug therapy for all children, clinical PK studies should include adequate representation of children with obesity and include estimates of the degree of adiposity in these analyses.
Funding
This work was funded under National Institute of Child Health and Human Development (NICHD) contract HHSN275201000003I for the Pediatric Trials Network (PI Danny Benjamin). H.W. receives salary support for research from the National Institutes of Health (NIH) Clinical and Translational Science Award (5UL1TR001117‐05). C.P.H. receives salary support for research from the NICHD (K23HD090239) and the US government for his work in pediatric and neonatal clinical pharmacology (contract HHSN267200700051C, PI D. Benjamin under the Best Pharmaceuticals for Children Act). M.C.‐W. receives support for research from the NIH (5R01‐HD076676 and HHSN275201000003I), National Institute of Allergy and Infectious Diseases (HHSN272201500006I), US Food and Drug Administration (1U18‐FD006298), and Biomedical Advanced Research and Development Authority (HHSO100201300009C). The design and conduct of the study, the interpretation of data, the preparation, review, and decision to submit the manuscript were all solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Conflict of Interest
C.P.H. and M.C.‐W. receive support from industry for drug development in children and adults. All other authors declared no competing interests for this work.
Author Contributions
T.P.G., H.J.B., H.W., A.J.A., E.M.P., M.Q., C.P.H., and M.C.‐W. wrote the manuscript. T.P.G., H.J.B., H.W., A.J.A., E.M.P., M.Q., C.P.H., and M.C.‐W. designed the research. T.P.G., H.J.B., H.W., C.P.H., and M.C.‐W. performed the research. T.P.G., H.W., and C.P.H. analyzed the data.
Supporting information
Figure S1‐S4‐Table S1
References
- 1. Kelly, A.S. et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 128, 1689–1712 (2013). [DOI] [PubMed] [Google Scholar]
- 2. Skinner, A.C. , Ravanbakht, S.N. , Skelton, J.A. , Perrin, E.M. & Armstrong, S.C. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics 141, e20173459 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson, B.J. & Holford, N.H. Getting the dose right for obese children. Arch. Dis. Child. 102, 54–55 (2017). [DOI] [PubMed] [Google Scholar]
- 4. Brill, M.J.E. , Diepstraten, J. , van Rongen, A. , van Kralingen, S. , van den Anker, J.N. & Knibbe, C.A.J. Impact of obesity on drug metabolism and elimination in adults and children. Clin. Pharmacokinet. 51, 277–304 (2012). [DOI] [PubMed] [Google Scholar]
- 5. Cheymol, G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin. Pharmacokinet. 39, 215–231 (2000). [DOI] [PubMed] [Google Scholar]
- 6. Duffull, S.B. , Dooley, M.J. , Green, B. , Poole, S.G. & Kirkpatrick, C.M. A standard weight descriptor for dose adjustment in the obese patient. Clin. Pharmacokinet. 43, 1167–1178 (2004). [DOI] [PubMed] [Google Scholar]
- 7. Hanley, M.J. , Abernethy, D.R. & Greenblatt, D.J. Effect of obesity on the pharmacokinetics of drugs in humans. Clin. Pharmacokinet. 49, 71–87 (2010). [DOI] [PubMed] [Google Scholar]
- 8. Harskamp‐van Ginkel, M.W. , Hill, K.D. , Becker, K.C. et al. Drug dosing and pharmacokinetics in children with obesity: a systematic review. JAMA Pediatr. 169, 678–685 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shakhnovich, V. et al. Obese children require lower doses of pantoprazole than nonobese peers to achieve equal systemic drug exposures. J. Pediatr. 193, 102–108.e1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith, M.J. , Gonzalez, D. , Goldman, J.L. et al. Pharmacokinetics of clindamycin in obese and nonobese children. Antimicrob. Agents Chemother. 61, e02014–e02016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rowe, S. , Siegel, D. & Benjamin, D.K. Jr Best Pharmaceuticals for Children Act – Pediatric Trials Network Administrative Core Committee. Gaps in drug dosing for obese children: a systematic review of commonly prescribed emergency care medications. Clin. Ther. 37, 1924–1932 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sampson, M.R. , Cohen‐Wolkowiez, M. , Benjamin, D.K. Jr , Capparelli, E.V. & Watt, K.M. Pharmacokinetics of antimicrobials in obese children. GaBI J. 2, 76–81 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaughns, J.D. et al. Obesity and pediatric drug development. J. Clin. Pharmacol. 58, 650–661 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiong, Y. , Fukuda, T. , Knibbe, C.A.J. & Vinks, A.A. Drug dosing in obese children: challenges and evidence‐based strategies. Pediatr. Clin. North Am. 64, 1417–1438 (2017). [DOI] [PubMed] [Google Scholar]
- 15. Duren, D.L. et al. Body composition methods: comparisons and interpretation. J. Diabetes Sci. Technol. 2, 1139–1146 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Freedman, D.S. , Wang, J. , Maynard, L.M. et al. Relation of BMI to fat and fat‐free mass among children and adolescents. Int. J. Obes. (Lond). 29, 1–8 (2005). [DOI] [PubMed] [Google Scholar]
- 17. Sinha, J. , Duffull, S.B. & Al‐Sallami, H.S. A review of the methods and associated mathematical models used in the measurement of fat‐free mass. Clin. Pharmacokinet. 57, 781–795 (2018). [DOI] [PubMed] [Google Scholar]
- 18. Al‐Sallami, H.S. , Goulding, A. , Grant, A. , Taylor, R. , Holford, N. & Duffull, S.B. Prediction of fat‐free mass in children. Clin. Pharmacokinet. 54, 1169–1178 (2015). [DOI] [PubMed] [Google Scholar]
- 19. Cortés‐Castell, E. , Juste, M. , Palazón‐Bru, A. , Monge, L. , Sánchez‐Ferrer, F. & Rizo‐Baeza, M.M. A simple equation to estimate body fat percentage in children with overweightness or obesity: a retrospective study. PeerJ 5, e3238 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Forbes, G.B. Relation of lean body mass to height in children and adolescents. Pediatr. Res. 6, 32–37 (1972). [DOI] [PubMed] [Google Scholar]
- 21. Foster, B.J. , Platt, R.W. & Zemel, B.S. Development and validation of a predictive equation for lean body mass in children and adolescents. Ann. Hum. Biol. 39, 171–182 (2012). [DOI] [PubMed] [Google Scholar]
- 22. Holford, N.H.G. & Anderson, B.J. Allometric size: the scientific theory and extension to normal fat mass. Eur. J. Pharm. Sci. 109S, S59–S64 (2017). [DOI] [PubMed] [Google Scholar]
- 23. Janmahasatian, S. , Duffull, S.B. , Ash, S. , Ward, L.C. , Byrne, N.M. & Green, B. Quantification of lean bodyweight. Clin. Pharmacokinet. 44, 1051–1065 (2005). [DOI] [PubMed] [Google Scholar]
- 24. McCarthy, H.D. , Cole, T.J. , Fry, T. , Jebb, S.A. & Prentice, A.M. Body fat reference curves for children. Int. J. Obes. (Lond). 30, 598–602 (2006). [DOI] [PubMed] [Google Scholar]
- 25. Peters, A.M. , Snelling, H.L. , Glass, D.M. & Bird, N.J. Estimation of lean body mass in children. Br. J. Anaesth. 106, 719–723 (2011). [DOI] [PubMed] [Google Scholar]
- 26. Peterson, C.M. et al. Tri‐ponderal mass index vs body mass index in estimating body fat during adolescence. JAMA Pediatr. 171, 629 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Centers for Disease Control and Prevention National Center for Health Statistics . National Health and Nutrition Examination Survey, NHANES 2003–2004. <https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2003> (2006). Accessed April 21, 2020.
- 28. Kirk, S. , Armstrong, S. , King, E. et al. Establishment of the Pediatric Obesity Weight Evaluation Registry: a national research collaborative for identifying the optimal assessment and treatment of pediatric obesity. Child Obes. 13, 9–17 (2017). [DOI] [PubMed] [Google Scholar]
- 29. Kuczmarski, R.J. et al. CDC growth charts for the United States: methods and development. Vital Health Stat. 11, 1–190 (2000). [PubMed] [Google Scholar]
- 30. Ogden, C.L. , Kuczmarski, R.J. , Flegal, K.M. et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 109, 45–60 (2002). [DOI] [PubMed] [Google Scholar]
- 31. Flegal, K.M. , Cole, T.J. Construction of LMS parameters for the Centers for Disease Control and Prevention growth charts. Natl. Health Stat. Report 2013, 1–3 (2000). [PubMed] [Google Scholar]
- 32. Flegal, K.M. , Wei, R. , Ogden, C.L. , Freedman, D.S. , Johnson, C.L. & Curtin, L.R. Characterizing extreme values of body mass index‐for‐age by using the 2000 Centers for Disease Control and Prevention growth charts. Am. J. Clin. Nutr. 90, 1314–1320 (2009). [DOI] [PubMed] [Google Scholar]
- 33. Ogden, C.L. , Li, Y. , Freedman, D.S. , Borrud, L.G. & Flegal, K.M. Smoothed percentage body fat percentiles for U.S. children and adolescents, 1999–2004. Natl. Health Stat. Rep. 43, 1–7 (2011). [PubMed] [Google Scholar]
- 34. Freedman, D.S. et al. BMI z‐Scores are a poor indicator of adiposity among 2‐ to 19‐year‐olds with very high BMIs, NHANES 1999–2000 to 2013–2014. Obesity (Silver Spring) 25, 739–746 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gulati, A.K. , Kaplan, D.W. & Daniels, S.R. Clinical tracking of severely obese children: a new growth chart. Pediatrics 130, 1136–1140 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Freedman, D.S. & Berenson, G.S. Tracking of BMI z scores for severe obesity. Pediatrics 140, e20171072 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Freedman, D.S. , Butte, N.F. , Taveras, E.M. , Goodman, A.B. & Blanck, H.M. Longitudinal changes in BMI z‐scores among 45 414 2–4‐year olds with severe obesity. Ann. Hum. Biol. 44, 687–692 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Freedman, D.S. , Woo, J.G. , Ogden, C.L. , Xu, J.H. & Cole, T.J. Distance and percentage distance from median BMI as alternatives to BMI z score. Br. J. Nutr. 124, 493–500 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Logistic Equation . WolframMathWorld web site <http://mathworld.wolfram.com/LogisticEquation.html>. Accessed April 21, 2020.
- 40. Centers for Disease Control and Prevention National Center for Health Statistics . National Health and Nutrition Examination Survey, NHANES 2005–2006. <https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2005> (2006). Accessed April 21, 2020.
- 41. Al‐Sallami, H.S. et al. Between‐subject variability: should high be the new normal? Eur. J. Clin. Pharmacol. 70, 1403–1404 (2014). [DOI] [PubMed] [Google Scholar]
- 42. Wright, D.F.B. , Hasegawa, C. & Al‐Sallami, H.S. Population pharmacokinetics and pharmacokinetic‐pharmacodynamics in clinical pharmacology. In: Drug Discovery and Evaluation: Methods in Clinical Pharmacology ( Hock, F.J. and Gralinski, M.R. , eds.), pp. 1–26. (Springer International Publishing, Berlin, Germany, 2018). [Google Scholar]
- 43. Holford, N.H. & Buclin, T. Safe and effective variability‐a criterion for dose individualization. Ther. Drug Monit. 34, 565–568 (2012). [DOI] [PubMed] [Google Scholar]
- 44. Weber, D.R. , Leonard, M.B. & Zemel, B.S. Body composition analysis in the pediatric population. Pediatr. Endocrinol. Rev. 10, 130–139 (2012). [PMC free article] [PubMed] [Google Scholar]
- 45. Loomba‐Albrecht, L.A. & Styne, D.M. Effect of puberty on body composition. Curr. Opin. Endocrinol. Diabetes Obes. 16, 10–15 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1‐S4‐Table S1


