Abstract
Introduction
Prehabilitation in colorectal surgery is evolving and may minimise postoperative morbidity and mortality. With many different healthcare professionals contributing to the prehabilitation literature, there is significant variation in reported primary endpoints that restricts comparison. In addition, there has been limited work on patient-related outcome measures suggesting that patients with colorectal cancer needs and issues are being overlooked. The Defining Standards in Colorectal Optimisation Study aims to achieve international consensus from all stakeholders on key standards to provide a framework for reporting future prehabilitation research.
Methods and analysis
A systematic review will identify key standards reported in trials of prehabilitation in colorectal surgery. Standards that are important to patients will be identified by a patient and public involvement (PPI) event. The longlist of standards generated from the systematic review and PPI event will be used to develop a three-round online Delphi process. This will engage all stakeholders (healthcare professionals and patients) both nationally and internationally. The results of the Delphi will be followed by a face-to-face interactive consensus meeting that will define the final standards for prehabilitation for elective colorectal surgery.
Ethics and dissemination
The University of Glasgow College of Medical, Veterinary and Life Sciences Ethics Committee has approved this protocol, which is registered as a study (200190120) with the Core Outcome Measures in Effectiveness Trials Initiative. Publication of the standards developed by all stakeholders will increase the potential for comparative research that advances understanding of the clinical application of prehabilitation.
PROSPERO registration number
CRD42019120381.
Keywords: colorectal surgery, protocols & guidelines, rehabilitation medicine
Strengths and limitations of this study.
There is currently no set of standards for prehabilitation research, limiting evidence synthesis.
This study has international and diverse stakeholders, including patients that have been involved since study inception.
Limitations of online surveys include selection bias.
Introduction
Elective colorectal surgery for benign and malignant conditions is one of the most commonly performed operations in the UK.1 Although mortality is reported as low (3%), postoperative morbidity is common varying from a minor wound infection to a major anastomotic leak that can have significant short-term consequences for the patient. After discharge, a patient’s recovery can be delayed with one in ten requiring emergency readmission within 30 days.2 To improve patients with colorectal cancer outcomes, the development of effective strategies is essential.
Enhanced recovery after surgery (ERAS) programmes seek to optimise perioperative care with the aim of attenuating the stress response to surgery.3 The first ERAS protocol for colorectal surgery was published in 2005 and has since been delivered across the UK, although with variable components, implementation and results.4 5 ERAS protocols focus on intraoperative and postoperative strategies, leaving the preoperative period as a potential opportunity for optimisation. Prehabilitation, the process of physical, nutrition and psychological optimisation prior to surgery, takes advantage of this opportunity and has the potential to successfully augment ERAS. Demonstrated as safe and feasible in predominately patients with colorectal cancer,6 early trial evidence and meta-analyses7 have reported that prehabilitation reduces the number of patients suffering postoperative complications by 51%,8 as well as improving exercise capacity9 and decreasing length of hospital stay.10
Prehabilitation has gained widespread acceptance in recent years with several leading professional bodies showing support: Cancer Research UK; the Clinical Oncology Society of Australia; American College of Sports Medicine (ACSM); International Society of Behavioural Nutrition and Physical Activity (ISBNPA); and Macmillan Cancer Support. Although prehabilitation is being introduced into clinical practice, there are shortcomings in the current evidence base making the next important step the definition of standards for the content, delivery and measurement of outcomes in prehabilitation interventions. One major shortcoming is the lack of research performed in non-cancer populations, including inflammatory bowel disease (IBD), pelvic floor and diverticular disease. The situation is made complex by prehabilitation research spanning different specialty groups: anaesthetics, surgeons, nurse specialists, exercise oncologists/physiologists, nutritionists and psychologists. Currently, this lack of consensus means prehabilitation is varied across the UK and beyond, preventing effective comparison and compilation of results. Development and implementation of standards would encourage homogeneity of data and consequently improve the quality of the evidence base to enhance patients with colorectal cancer care.
To date, there have been limited efforts to involve patients in prehabilitation research. The National Health Service (NHS) advocates for patient-centred care,10 yet often, research is clinician-led and carried out for patients, rather than with them.11 In one of the recent initiatives by the James Lind Alliance with the National Cancer Research Institute (NCRI), over 3500 patients with cancer, their caregivers and healthcare and social care professionals were asked for their key research priorities.12–14 In the final top 10 was ‘what specific lifestyle changes help with recovery from cancer treatment, restore health, and improve quality of life?’. Prehabilitation research clearly addresses this, and the definition of prehabilitation standards will lay important foundations to facilitate research to definitively answer this question.
The Defining Standards in Colorectal Optimisation (DiSCO) Study intends to achieve consensus on key standards for prehabilitation before elective colorectal surgery. DiSCO will involve patients and their caregivers from the start of the process to ensure that results are relevant to service users as well as clinicians. A three-stage study design using multidisciplinary stakeholders will be followed: systematic review and patient and public involvement (PPI) event to develop a standards longlist, standards shortlisting using three rounds of online Delphi and a face-to-face consensus meeting to define the final list of standards for colorectal surgery optimisation.
Aims and objectives
The primary aim of the DiSCO Study is to achieve consensus of prehabilitation standards by all stakeholders that are to be applied in future trials on prehabilitation in elective colorectal surgery.
To achieve this objective, four key questions will be asked:
What are the individual components of prehabilitation?
What type of patient with colorectal cancer should be offered prehabilitation?
Who should deliver prehabilitation?
What outcome measures are important?
Methods and analysis
DiSCO methodology will be guided by the Core Outcome Measures in Effectiveness Trials (COMET) Initiative,15 which provides a handbook instructing the development of core outcome sets. However, the scope of this study is broader than a core outcome set, also seeking to define standards for what prehabilitation should consist of and for whom and how it should be delivered. Accordingly, the COMET recommendations will be modified to facilitate achieving these aims (registered as a study: 200190120).
Patient and public involvement
DiSCO will involve adult patients and their carers/supporters, who have undergone elective resection of a part of their colon or rectum for benign or malignant colorectal conditions. These conditions include, but are not limited to, colorectal cancer, anal cancer, diverticulitis and its complications, IBD and pelvic floor dysfunction. Patients will be invited through social media to allow an international perspective (@DiSCO_study on Twitter and Facebook) and by patient liaison groups of professional bodies and charities, including the Association of Coloproctology of Great Britain and Ireland (ACPGBI) Patient Liaison Group and The Ileostomy and Internal Pouch Support Group.
Stakeholders
From our systematic review and recent work published by Macmillan Cancer Support,16 the study group will identify key stakeholders that have published on prehabilitation. This is likely to include colorectal surgeons, colorectal anaesthetists, colorectal nurse specialists, colorectal oncologist (medical or clinical), exercise oncologists, exercise physiologists, sports scientists, sports medicine specialists, physical exercise/activity specialists, nutritionists/dieticians, psychologists, geriatricians, pharmacists and general practitioners. To ensure inclusivity, specialist associations related to these stakeholders will be approached: ACSM, ISBNPA, Scottish Physical Activity Research Collaboration, Macmillan Cancer Support, Royal College of Anaesthetists, Association of Surgeons of Great Britain and Ireland (ASGBI), ACPGBI, Trainees with an Interest in Perioperative Medicine and ERAS Association.
Members from each stakeholder group will be recruited to form an internationally connected multidisciplinary study team. The three cochief investigators are an expert patient and two consultant colorectal surgeons with research interests in core outcome sets and prehabilitation.
Study design
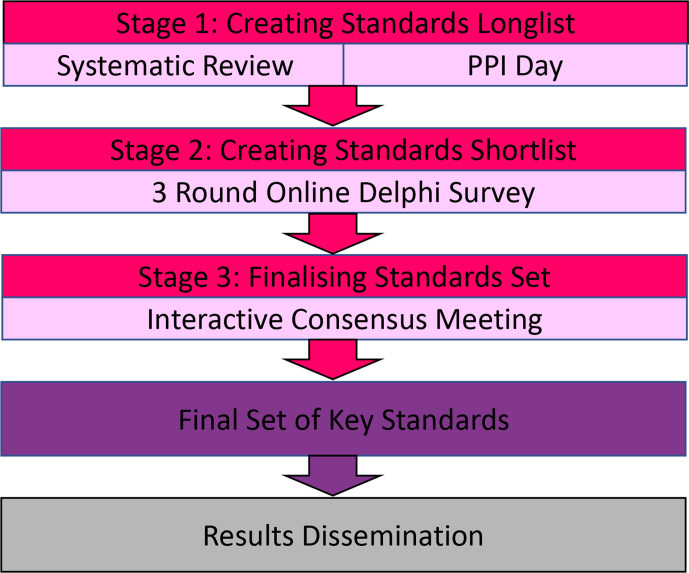
The DiSCO Study will follow a three-stage process (figure 1).
Figure 1.
Diagram of study design.
Stage 1: creating standards longlist
Stage 1 is designed to produce a longlist of standards that will be taken forward into Stage 2. This includes a systemic review and PPI event. At the end of Stage 1, the research team will hold a research meeting to discuss the results and ensure clarity on the longlist of standards that will be used to create the Delphi Survey.
Systematic review
Research aims to determine the range of interventions used in colorectal prehabilitation studies, who delivers the interventions, how patients are selected for the intervention (eligibility criteria) and how/what prehabilitation outcomes are measured.
Method: a systematic review of the current prehabilitation literature in patients undergoing colorectal surgery has been published.17.
PPI event
Research question: what do patients and their family members think is important in a prehabilitation intervention? Specifically: what are the individual components of prehabilitation? What type of patient with colorectal cancer should be offered prehabilitation? Who should deliver prehabilitation? What outcome measures are important, and how should they be measured?
Method
Inclusion criteria:
Adults over 18 years of age.
Patients who have completed or have planned colorectal surgery.
Underlying indication for surgery could be for benign or malignant pathology.
Able to understand, interpret and communicate in English.
Exclusion criteria:
Acute or chronic issues with memory and/or cognition
Sampling
The patient information sheet (PIS) will be sent to colleagues from one hospital department that includes nurses, colorectal nurse specialists (cancer, inflammatory disease, stoma therapists and ERAS specialists), allied health professionals and surgeons. Proposed participants will then be sent the PIS and asked to confirm their attendance by telephone. All patients will be invited to bring family members and/or caregivers to the event. The second strategy to identify patients will be to approach representatives from local community groups that encourage lifestyle change in patients. Appropriate patients will then be discussed with the research team, and the PIS will be sent out accordingly. A target of 20 participants will be sought, with a minimum of 10 of these being patients.
Event location
The PPI event will be held over 4–5 hours at the hospital of one of the surgeons, in a designated quiet and easily accessible room with the capacity to hold 20–30 people comfortably. Participants will be reimbursed for travel expenses. If the event occurs during the COVID-19 pandemic, then the format will be moved to a secure online NHS-approved virtual platform.
Event format
The event will be led by the research team that includes expert patient and colorectal surgeons. The event will be facilitated by colorectal nurse specialist, enhanced recovery nurse specialist, medical students, anaesthetists and surgical/oncological research fellows.
A PowerPoint presentation will be used to give structure to the event. It will include introductions, definitions and explanation of prehabilitation and the aims of the DiSCO Study. Patients will be invited to share their experiences of colorectal surgery and any preparation or prehabilitation they underwent beforehand. To explore the four key aims of the DiSCO Study, the patients will be divided into small groups each facilitated by members of the research team. The whole group will then reconvene for an interactive and patient-led discussion to develop their answers to the four study questions.
Analysis
Comprehensive field notes will be taken by the research team. Thematic analysis will organise the patients’ views into themes that will be incorporated into the longlisting. All patients and family members in attendance will be invited to leave their email address to be contacted for inclusion in Stage 2.
Stage 2: creating standards shortlist
A three-round online modified Delphi process will be performed to develop a shortlist.18 Adhering to recommendations by COMET,15 standards will be split into four domains reflecting the key study questions: content of prehabilitation, recipients of prehabilitation, delivery of prehabilitation and the measurement/assessment of prehabilitation. Participants will be asked to rank the importance of each standard on a validated 9-point Likert scale, which is recommended by the Grading of Recommendations, Assessment, Development and Evaluation working group.19 With this scale, a score of 1–3 signifies that the standard is of little importance, 4–6 some importance and 7–9 critical importance. Round 1 will ask participants to rank every item of the longlist, and differences in rankings between stakeholder groups will be explored. To reduce bias, a predetermined consensus threshold will be used: Standards that are ranked of critical importance (7–9) by >70% or of little importance (1–3) by <15% of each stakeholder group will be deemed to have reached the threshold for consensus for inclusion in the shortlist of key standards. After round 1 of the Delphi, standards reaching the threshold of consensus for inclusion will be directly added to the shortlist and not included in subsequent rounds. All items not reaching this threshold will be taken forward to round 2. The same criteria will be used after round 2 to select items to take forward into round 3. After round 3, any additional items reaching the threshold for consensus for inclusion will be added to the shortlist. Any items that are ranked of critical importance (7–9) by <50% of each stakeholder group or of little importance (1–3) by >50% of each stakeholder group after round 3 will be excluded from the final shortlist. Standards that do not meet the criteria for inclusion or exclusion will be considered borderline. The final shortlist and borderline items will be taken forward for discussion at the final consensus meeting.
The online survey will be powered by COMET DelphiManager software. Representatives from each of the key stakeholder groups will be invited to participate to ensure adequate representation. A target of 100 or more respondents will be sought. Multiple methods will be used for recruitment to maximise the sample size and participant diversity. Study group members who have membership with professional societies will be there for recruitment, including ACPGBI, ACPGBI Patient Liaison Group, ASGBI, ISBNPA and NCRI. Social media networks (Twitter and Facebook) will also be used to advertise the study, hopefully engaging patients with colorectal cancer and members of the public. Patients without access to social media will be targeted through the patient support charities. Participants from the PPI event who left their email addresses will also receive an invitation to partake in the Delphi Study.
Stage 3: Finalising standards set
The shortlist from the Delphi process will be reviewed at a meeting of stakeholder representatives to agree on a final set of standards for publishing, as recommended by the COMET Initiative.15 The meeting is planned to be held face-to-face, but this will depend on COVID-19 restrictions. If a face-to-face meeting is not possible, then it will be held online using videoconferencing software. A random sample of around 50 stakeholders will be invited using contacts from the PPI event and Delphi Survey participants who gave permission to be contacted about the stakeholder event. The shortlist of standards that met the threshold for consensus after each round of the Delphi will be presented and ratified by vote. The borderline standards will be discussed and voted on individually. For each standard, the group will anonymously rank its importance on the same 9-point scale used in the Delphi Study to establish a group baseline. Following this, there will be a group discussion of the standard with arguments for and against its inclusion in the final standards set. A further round of anonymous voting will follow. A result of at least 70% ranking the standard as critically important and fewer than 15% ranking it of little importance will be required for inclusion in the final standards set. There are no universally agreed consensus criteria, and the criteria used here follow published recommendations.20
Ethics and dissemination
This work that includes a wide range of stakeholders, including patients, is performed with robust methodology ensuring that the results accurately reflect the priorities of all stakeholder groups and will be reported using Guidance on Conducting and REporting DElphi Studies.21 Publication in peer-reviewed journals and dissemination through the professional collaborations and associated networks should ensure international adoption of the standards. Such adoption will help to standardise future prehabilitation study design and reporting to optimise the progression of prehabilitation for researchers, clinicians and patients. The University of Glasgow College of Medical, Veterinary and Life Sciences Ethics Committee approved the protocol on 7 July 2020 (200190120).
Supplementary Material
Footnotes
Contributors: SM, SB and RF planned and designed the study. SD, MW, NM, PK, SK and NSF provided advice and guidance. IP drafted the manuscript with all authors reviewing and subsequently approving the final draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer-reviewed.
References
- 1.National Bowel Cancer Audit . Annual report 2019. An audit of the care received by people with bowel cancer in England and Wales v2.0. published online 2020. Available: https://www.nboca.org.uk/content/uploads/2020/01/NBOCA-2019-V2.0.pdf [Accessed 20th November 2020].
- 2.Diers J, Baum P, Matthes H, et al. Mortality and complication management after surgery for colorectal cancer depending on the DKG minimum amounts for hospital volume. Eur J Surg Oncol 2020;20:30796–4. 10.1016/j.ejso.2020.09.024 [DOI] [PubMed] [Google Scholar]
- 3.Fearon KCH, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005;24:466–77. 10.1016/j.clnu.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 4.Paton F, Chambers D, Wilson P, et al. Effectiveness and implementation of enhanced recovery after surgery programmes: a rapid evidence synthesis. BMJ Open 2014;4:e005015. 10.1136/bmjopen-2014-005015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhuang C-L, Ye X-Z, Zhang X-D, et al. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum 2013;56:667–78. 10.1097/DCR.0b013e3182812842 [DOI] [PubMed] [Google Scholar]
- 6.Moug SJ, Mutrie N, Barry SJE, et al. Prehabilitation is feasible in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy and may minimize physical deterioration: results from the Rex trial. Colorectal Dis 2019;21:548–62. 10.1111/codi.14560 [DOI] [PubMed] [Google Scholar]
- 7.Heger P, Probst P, Wiskemann J, et al. A systematic review and meta-analysis of physical exercise prehabilitation in major abdominal surgery (Prospero 2017 CRD42017080366). J Gastrointest Surg 2020;24:1375–85. 10.1007/s11605-019-04287-w [DOI] [PubMed] [Google Scholar]
- 8.Barberan-Garcia A, Ubré M, Roca J, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 2018;267:50–6. 10.1097/SLA.0000000000002293 [DOI] [PubMed] [Google Scholar]
- 9.Minnella EM, Bousquet-Dion G, Awasthi R, et al. Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: a five-year research experience. Acta Oncol 2017;56:295–300. 10.1080/0284186X.2016.1268268 [DOI] [PubMed] [Google Scholar]
- 10.Gillis C, Buhler K, Bresee L, et al. Effects of Nutritional Prehabilitation, With and Without Exercise, on Outcomes of Patients Who Undergo Colorectal Surgery: A Systematic Review and Meta-analysis. Gastroenterology 2018;155:391–410. 10.1053/j.gastro.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 11.Sacristán JA, Aguarón A, Avendaño-Solá C, et al. Patient involvement in clinical research: why, when, and how. Patient Prefer Adherence 2016;10:631–40. 10.2147/PPA.S104259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heneghan C, Goldacre B, Mahtani KR. Why clinical trial outcomes fail to translate into benefits for patients. Trials 2017;18:122. 10.1186/s13063-017-1870-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James Lind Alliance . The James Lind alliance, 2019. Available: http://www.jla.nihr.ac.uk/
- 14.National Cancer Research Institute .  The UK top living with and beyond cancer research priorities. [ONLINE], 2017. Available: https://www.ncri.org.uk/areas-of-interest/living-with-beyond-cancer/ [Accessed 20th November 2020].
- 15.Williamson PR, Altman DG, Bagley H, et al. The comet Handbook: version 1.0. Trials 2017;18:280. 10.1186/s13063-017-1978-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniels SL, Lee MJ, George J, et al. Prehabilitation in elective abdominal cancer surgery in older patients: systematic review and meta-analysis. BJS Open 2020;4:1022–41. 10.1002/bjs5.50347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prehabilitation for people with cancer . Principles and guidance for prehabilitation within the management and support of people with cancer. Macmillan cancer support, 2019. Available: https://be.macmillan.org.uk/be/p-25112-prehabilitation-for-people-with-cancer.aspx
- 18.Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoS Med 2011;8:e1000393. 10.1371/journal.pmed.1000393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GRADE . What is GRADE? [ONLINE], 2019. Available: https://www.gradeworkinggroup.org/ [Accessed 20th November 2020].
- 20.Williamson PR, Altman DG, Blazeby JM, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132. 10.1186/1745-6215-13-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jünger S, Payne SA, Brine J, et al. Guidance on conducting and reporting Delphi studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med 2017;31:684–706. 10.1177/0269216317690685 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.